Abstract
This paper evaluates the relative contribution of vibration and noise from railway on physiological sleep outcomes. Vibration from railway freight often accompanies airborne noise, yet is almost totally absent in the existing literature. In an experimental investigation, 23 participants, each sleeping for six nights in the laboratory, were exposed to 36 simulated railway freight pass-bys per night with vibration alone (aWd,max = 0.0204 ms−2), noise alone (LAF,max = 49.8 dB), or both vibration and noise simultaneously. A fourth exposure night involved 52 pass-bys with concurrent vibration and noise. Sleep was measured with polysomnography. Cardiac activity was measured with electrocardiography. The probability of cortical arousals or awakenings was greater following all exposures, including vibration alone, than spontaneous reaction probability (p < 0.05). The effects of vibration exposure and noise exposure on changes of sleep stage and arousals were directly additive. Vibration and noise exposure both induced heart rate acceleration above spontaneously expected fluctuations at baseline. The results indicate that vibration and noise are processed in the brain separately yet in parallel, with both contributing towards the likelihood of sleep disruption. The findings show that vibration is of importance when considering the impact of railway freight on sleep.
I. INTRODUCTION
Sleep is a vital biological event that is required by all animals.1 Sleep can be disrupted by environmental noise, and nighttime noise exposure from traffic can contribute to impaired endothelial function and adrenaline release,2 increased awakenings and arousals,3 increased wakefulness and reduced REM sleep,4 disrupted sleep architecture,5 changes in cardiovascular activity and blood pressure,6 increased morning tiredness,7 and decreased self-reported sleep quality.8 Such noise-induced sleep disturbance accounts for the annual loss of almost 1 × 106 healthy life years in Western Europe alone.9,10 Railway noise in particular has been suggested to cause more deleterious effects than other traffic sources,11 with freight trains having a greater impact on sleep than other train types.12
With respect to exposure from railways, and railway lines carrying freight in particular, noise is often accompanied by vibration. While much previous research has investigated both the psychological and physiological effects of railway noise on sleep,3,4,11–15 the impact of vibration is less well understood. Previous work, both with laboratory and cross-sectional study designs, has reported adverse effects of nocturnal vibration, including both self-reported sleep disturbances and objectively measured sleep disruption.16–20 Nocturnal railway freight vibration has been shown to be particularly problematic, with increasing vibration levels resulting in a greater likelihood of cortical arousals, awakenings and sleep stage changes,20 stronger cardiac activations,21 and negative effects on self-reported sleep parameters.19 However, studies on railway vibration typically include concurrent nocturnal noise exposure, and as such they are limited in the conclusions regarding vibration per se. To the author's knowledge, only one previous small study has examined the effect of unaccompanied traffic vibration on sleep, in which four study subjects were exposed to simulated heavy road traffic, with exposures comprised of vibration only, noise only, and vibration and noise simultaneously.22 Arousals were found to occur more frequently when the noise was accompanied with vibration than when the noise was presented alone. Interestingly, vibration-only was found to elicit more sleep stage changes than when accompanied by noise, with the authors suggesting that vibration alone may be perceived as more threatening without the noise providing a source identification cue. This study is, however, limited by its small sample size and furthermore does not address the particular case of railway vibration. Other experimental studies investigating vibration and noise on sleep have generally not focused on whole-body vibration, nor specifically on traffic-induced vibration exposure, but have, nevertheless, reported that vibration and noise exposure during sleep induce more arousals and awakenings than noise alone, and that responsiveness increases with stimulus intensity.23
Noise is received via the auditory system, and vibration is received via the somatosensory system. Although the body uses these disparate systems, as well as the separate channels involved with other sensory inputs (e.g., visual, olfactory, and gustatory), at higher brain levels the inputs interact and are merged together to create a coherent perception of the external environment.24 For instance, it has been shown that neural response to visual input is modulated by auditory stimuli as early as 46 ms after stimulus onset.25 Such cross-modal interactions also exist between audition and mechanoreception and at optimal sound levels, the presence of auditory noise enhances the perceptual sensitivity to vibratory stimuli.26 Previous investigations in monkeys have found that neuronal oscillations in response to low intensity combined auditory and somatosensory stimulation are super-additive, whereby the response amplitude was greater than the sum of the responses to each individual stimulus. At sound intensities higher than 50 dB, however, the effect was directly additive.24 Processing of environmental cues continues during sleep,27 and in studies involving concurrent environmental exposures it is, therefore, problematic to identify the contribution that each separate exposure makes towards effects on sleep. Knowledge of such mechanisms, hence, requires measuring sleep when the sleeping body is reacting to noise alone, vibration alone, and noise and vibration together.
In summary, there is evidence that environmental vibration contributes to sleep disturbance, but the mechanisms involved are unclear. Residents living in close proximity to freight railway lines are frequently subjected to both the associated vibration and noise, yet previous work into resulting physiological outcomes has almost exclusively focused on the noise component of the exposure. Railway vibration, therefore, represents a significantly neglected yet potentially deleterious exposure in the existing literature. Furthermore, the effect of increasing the number of nocturnal events relative to previous work20 will be examined, since the frequency of trains has been shown to influence physiological reaction probability.3 The aim of the present laboratory study was, therefore, to investigate the effect of separate and combined vibration and noise exposure on sleep.
II. METHODS AND MATERIALS
A. Experimental procedure
1. Study subjects and setting
Twenty-four young healthy adults were initially recruited via advertisement posters in public areas of university campuses throughout Gothenburg. One volunteer dropped out due to illness before the start of the study. Of the remaining 23 participants (age range 19–30, mean 23.7 SD ± 3.1 yr), 13 were female and 10 were male. All applicants were screened prior to acceptance on the study, with self-reported information collected on: age; sex; tobacco use; caffeine consumption; noise sensitivity and vibration tolerance, both measured on a five-point semantic scale (Not at all sensitive/tolerant–Extremely sensitive/tolerant); habitual sleep and wake times during normal and free (e.g., weekends, holidays) periods; hearing acuity; sleep quality; snoring; restless legs; breathing; allergies; medicine use; and body mass index (BMI). Participants reporting noise sensitivity as 3–5 on the response scale (i.e., Quite, Very, or Extremely sensitive) were classed as being sensitive to noise. The exclusion criteria were self-reported sleep problems, medication that might affect sleep, tobacco use or caffeine dependence and a BMI <18.5 or >25. If an applicant was suitable, their hearing was tested with pure tone audiometry to a level of ≤20 dB hearing level (HL). During the trial, they were prohibited from caffeine after 15:00 and alcohol at all times. All volunteers provided informed written consent and were financially compensated for participation.
One female participant left the study after three nights for personal reasons. Data are available for this person during the control and V36 nights. Due to a technical fault, data are not available for one female participant during V36.
Our sound environment laboratory was furnished to resemble a residential apartment.28 The communal space included a private entrance, kitchenette, living area, and washing facilities. Three private noise and vibration isolated rooms (background LAEq ≤ 13 dB) were equipped as bedrooms with a bed, small chest of drawers, desk, and chairs. Each bedroom had eighty-eight 10 in. loudspeakers concealed within the ceiling to reproduce low frequencies, and two loudspeaker cabinets in the upper corners of the room for higher frequencies (crossover frequency 250 Hz). Band-pass filtered pink noise (LAEq = 25 dB) with a spectrum resembling ventilation sound was introduced throughout the study periods. Electrodynamic shakers (Quake Q10B) driven by 1000 W power amplifiers (BKA1000–4 A) were mounted directly to the underside of the bed frames. Enclosures for the vibration transducers were designed and installed to ensure minimal airborne operational noise, and the headboards of the beds were removed to prevent re-radiation of structurally transmitted noise.
2. Vibration and noise exposure
Individuals slept between 23:00 and 07:00 for six consecutive nights. The first night allowed for adaptation to the environment and sleep measurement apparatus. The second night served as a quiet control night, allowing a baseline measurement of normal sleep in the absence of noise or vibration. Night time noise and/or vibration were introduced during nights 3–6, which were arranged in a Latin square design to minimize ordering effects. Exposure nights could involve either 36 trains with corresponding noise and vibration (NV36), 36 trains with noise exposure only (N36), 36 trains with vibration exposure only (V36), or 52 trains with both noise and vibration (NV52). Audio recordings of freight train pass-bys filtered to represent a closed window (ISO 717–1:1997)29 were used as noise exposure (Table I).19 Train vibration was synthesized using an amplitude modulated 10 Hz sinusoid horizontally along the length of the bed with maximum amplitude 0.0204 ms−2 (Wd weighted).30 Vibration began when the noise level of the train reached 35 dBA, corresponding to a delay tvib start of between 2.9 and 7.8 s from the noise onset (Table I). The vibration rise time did not vary between the different trains, with the first peak occurring 5.6 s after vibration onset.
TABLE I.
Acoustic and temporal characteristics of the individual trains. Measured at the pillow.
| Train | LAEq,pb (dB) | LAFmax,pb (dB) | t > 35 dB (s) | t10%-90% (s) | tvib start (s) |
|---|---|---|---|---|---|
| 1 | 44.0 | 48.4 | 11.5 | 8.9 | 7.7 |
| 2 | 42.7 | 47.2 | 46.2 | 9.8 | 7.3 |
| 3 | 44.5 | 49.8 | 23.7 | 8.4 | 7.8 |
| 4 | 45.6 | 49.8 | 29.2 | 7.9 | 2.9 |
| 5 | 42.4 | 47.2 | 56.9 | 9.2 | 6.3 |
LAEq,pb Equivalent A-weighted noise level for the train pass-by. LAFmax,pb Maximum A-weighted noise level for the train pass-by. t > 35 dB Time that the noise level exceeded 35 dB, here used as the train duration. t10%−90% Time for the noise level to progress from 10% of the maximum to 90% of the maximum, here used as the noise rise time. tvib start Time between noise onset and vibration onset.
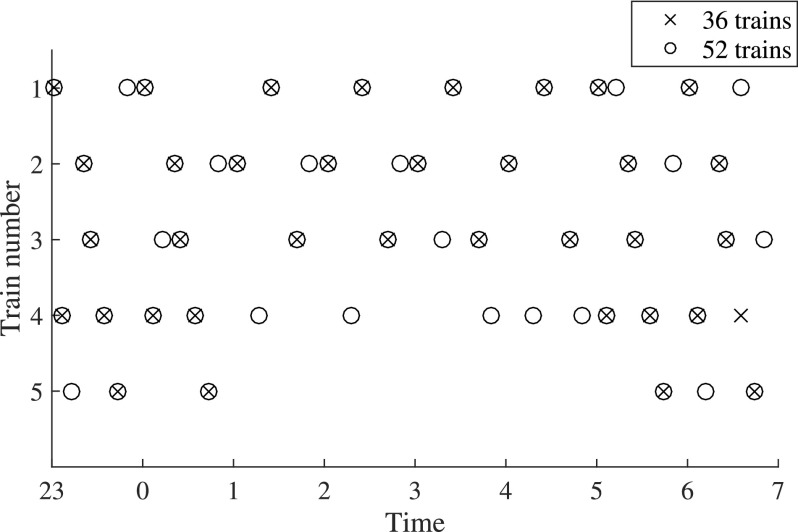
Trains were arranged to minimize differences in noise levels between different hours while maintaining distribution patterns typical similar to those in the field (Fig. 1). The lowest level was between 02 and 03 (LAEq,1h = 29.7 dB) and the highest was between 00 and 01 (LAEq,1h = 32.8 dB). These values correspond to −1.8 to +1.3 dB relative to the 8-h noise level. The maximum noise level was the same across all hours, LAFmax = 49.8 dB.
FIG. 1.
Distribution of different events throughout 36 and 52 train nights.
3. Sleep measurement
Sleep was measured using ambulatory polysomnography (PSG) devices (SOMNOscreen plus, SOMNOmedics GmbH, Germany). Electroencephalogram (EEG) surface electrodes were positioned according to the international 10–20 system at CZ, F3, F4, C3, C4, O1, O2, M1, and M2. Electrooculogram (EOG) and submental electromyogram (EMG) electrodes were positioned as per the American Academy of Sleep Medicine specifications.31 All electrode impedances were ≤5 kΩ following attachment. Data were downloaded offline each morning after sleep cessation. EEG, EOG, and EMG signals were used to determine sleep stage throughout each measurement period in 30 s epochs, manually scored by a trained sleep technologist according to pertinent criteria.31 The technologist was blind to the study design, and blind to the experimental intervention status (control or exposure) of any of the sleep recordings. Arousals were identified using appropriate guidance.32 Arousals of >15 s duration were classified as awakenings.33 Cardiac activity was recorded using torso electrode placement electrocardiogram (ECG) lead II. Respiratory movements were recorded with thorax and abdomen effort belts, and pulse, plethysmogram and peripheral oxygen saturation were recorded with a finger pulse oximeter, but these data are not reported here.
B. Analysis
Sleep macrostructure was determined from the scored PSG data. Time in “light” sleep (stage N1), “intermediate” sleep (stage N2), “deep” sleep (stage N3), and rapid eye movement (REM) sleep were determined from the respective sum of the 30 s epochs scored for each stage. A sleep stage change (SSC) was defined as transferring to a “lighter” sleep stage, excluding wake. REM sleep was defined as occupying the sleep depth position between Wake and N1.6 Sleep onset latency (SOL) is the time taken to enter the first non-wake sleep stage from lights out at 23:00. N3 latency and REM latency are the first occurrence of a N3 and REM epoch, respectively, following sleep onset. Wakefulness after sleep onset (WASO) is the total time spent in wake stages following sleep onset. Sleep period time (SPT) is the time between sleep onset and the final awakening, which at the latest corresponds to the 0700 alarm call. Sleep efficiency (SE) is the percentage of time asleep relative to the time in bed. Macrostructure effects were statistically analyzed using a linear mixed model with study night (five levels) as the explanatory variable and individuals included as a random effect, accounting for repeated measurements on the same participants. Each macrostructure outcome was in a preliminary step analyzed using logarithmic, square root and untransformed data. The transformation that gave the best compliance with the model assumptions of normality was used for further analysis.34
PSG data were analyzed to determine the probabilities of event-related physiological outcomes of SSCs, EEG arousals (3–15 s), EEG awakenings (>15 s) and combined EEG reactions (arousals and awakenings together) in a manner described previously20 using a time window following noise onset, or the point where noise would have started had it accompanied vibration in the night with vibration only. The time window length was 60 s in duration, as used previously in analysis of event-related analysis of nocturnal railway vibration and noise.3,20 Furthermore, in line with previous recommendations,35 empirical analysis revealed that a 60 s window length maximized the reaction probability.34 The probability of EEG reactions and SSCs occurring spontaneously was obtained by analyzing the quiet control nights at times corresponding to trains in the exposure nights. The spontaneous probabilities were found to be independent of the frequency of these “phantom trains” by comparing 52 phantom trains with 36 phantom trains (paired sample t-test, combined EEG p = 0.660; SSC: p = 0.901).
Analysis of these phantom trains thus yields the probabilities of reactions occurring spontaneously, independently of the presence of stimulus (Pspontaneous). The probabilities obtained from analysis of events in the exposure nights could conceivably include such spontaneous reactions, and are, therefore, only the probability of observing a reaction in the time window (Pobserved). Hence, the additional reaction probability Padditional attributable to the event is given by Eq. (1)35
| (1) |
Differences in probabilities between study nights were statistically analyzed within the general linear mixed model (GLMM) framework with participants included as a random effect and assuming a binomial distribution. If main effects were observed, the following post hoc comparisons were tested: Control:N36, V36, NV36, and NV52; N36:V36 and NV36; V36:N36 and NV36; NV36:NV52, N36, and V36. Tukey corrections were used for sleep macro- and micro-structure objective data to account for multiple hypothesis testing.
Event-related heart rate amplitude (HRA) analysis was performed by determining the difference between maximum heart rate acceleration and deceleration in the 60 s time window starting at noise onset or the point where noise would have started had it accompanied vibration in the night with vibration only. Spontaneous HRA variation was obtained by analyzing phantom trains in the control night. HRA data were positively skewed and, therefore, log-transformed before analysis.34 The results were analyzed in a mixed model with a random intercept with experiment night as the predictor variable, a random effect variable accounting for repeated measurements on the same individuals, and a repeated effect variable accounting for multiple measurements within each night. A number of different models including sex, noise sensitivity and with variations of covariance structure were built, and subsequently compared using the Akaike's information criterion36 to determine the model best describing the data.34 Post hoc comparisons between different nights were performed with Bonferroni corrections applied.
III. RESULTS
A. Polysomnogram data
1. Macrostructure
No statistically significant effects were observed for SOL, N3 latency, number of SSCs, arousals or awakenings, SPT, WASO, sleep efficiency, first awakening time, REM or N3 continuity, or total time spent in N1, N2, N3, or REM. Sleep macrostructure results are presented in Supplemental Table S2.34
2. Event related data
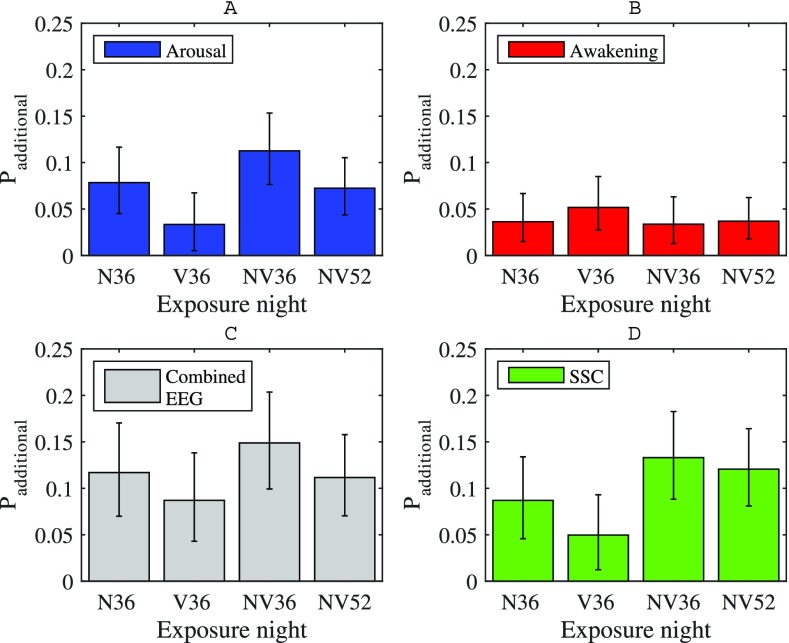
The total number of event-related reactions across all participants in each night is presented in Table II, and the additional probabilities of EEG reactions or sleep stage change during an individual event relative to the spontaneous reaction probability are presented in Fig. 2.34 EEG reaction probability was significantly higher in all exposure nights than the spontaneous probability determined from the control night to at least the p < 0.05 level (GLMM). Across all nights with 36 trains, the highest number of event-related awakenings occurred in the vibration-only condition, corresponding to an increased awakening probability of around 0.05 compared to the spontaneous awakening frequency. The additional awakening probability in V36 was not, however, significantly different from other exposure nights.
TABLE II.
Total number of event-related shifts to lighter sleep stage, arousals, and awakenings across all participants each study night.
| Response | “Phantom” trains (52) | Event-related reactions | |||
|---|---|---|---|---|---|
| Control | N36 | V36 | NV36 | NV52 | |
| Total arousals (n) | 124 | 132 | 101 | 159 | 190 |
| Total awakenings (n) | 36 | 48 | 59 | 47 | 72 |
| Total EEG reactions (n) | 160 | 180 | 160 | 206 | 262 |
| Total SSCs (n) | 129 | 127 | 109 | 155 | 207 |
SSC: Sleep stage changes. V36: Vibration only, 36 trains. N36: Noise only, 36 trains. NV36: Noise and vibration, 36 trains. NV52: Noise and vibration, 52 trains.
FIG. 2.
(Color online) Event-related EEG reaction probabilities for each study night. (A) Arousals. (B) Awakenings. (C) Combined EEG reactions. (D) Sleep stage changes (SSC). Probabilities were calculated with the pooled data from all participants using all events within the full study night. V36: Vibration only, 36 trains. N36: Noise only, 36 trains. NV36: Noise and vibration, 36 trains. NV52: Noise and vibration, 52 trains. Error bars indicate 95% confidence intervals.
The pattern of increased awakenings in V36 was not mirrored by an increase in arousals. Here, the combined conditions induced more arousals than with vibration alone. This was especially true in the NV36 night, where the arousal probability was around 0.08 higher than in V36 (p = 0.003). Arousal probabilities did not significantly differ between NV36 and NV52, where trains occurred more frequently.
Since event-related arousals, with the exception of V36, occurred more than twice as frequently as awakenings, the combined EEG reaction additional probability followed a similar pattern to arousals, in that the combined vibration and noise exposure had a higher probability than vibration alone. However, none of the observed differences in combined EEG reaction probability between exposure nights were statistically significant.
For sleep stage changes, again the combined vibration and noise stimulation showed the greatest effect, and SSC probabilities were higher in N36, NV36, and NV52 nights relative to the spontaneous probability to at least the p < 0.01 level. Although SSC probability was higher in both nights with noise and vibration than vibration-only (p < 0.05), as seen in Fig. 3 the vibration component of the exposure nevertheless contributed to an increased likelihood of sleep stage changes. Furthermore, changes to a wake stage were not included in the calculation of SSCs, so it is unsurprising that nights with the highest awakening probability, in this case the vibration-only condition, partially contributes to a slightly lower probability of sleep stage change.
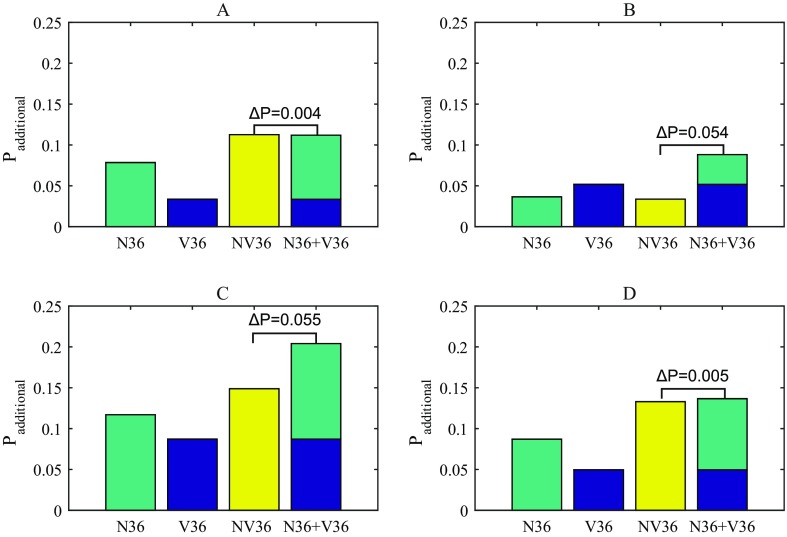
FIG. 3.
(Color online) Contributions of noise and vibration exposure to different physiological reaction probabilities. (A) Arousals. (B) Awakenings. (C) Combined EEG reactions. (D) Sleep stage changes (SSC). N36: Noise only, 36 trains. V36: Vibration only, 36 trains. NV36: Noise and vibration, 36 trains.
The additional probabilities are presented in Fig. 3 along with the sum of the noise-only and vibration-only nights. The difference between this sum and the probabilities from the dual stimuli night (NV36) is calculated according to Eq. (2). For sleep stage change and arousal probabilities, the sum of Padditional from the noise-only and vibration-only nights is close to the probability from the combined exposure night, agreeing to within 0.4 percentage points. This close agreement is not observed for awakenings or consequently combined EEG reactions, which have differences of ΔP = 0.054 and 0.055, respectively:
| (2) |
B. Heart rate data
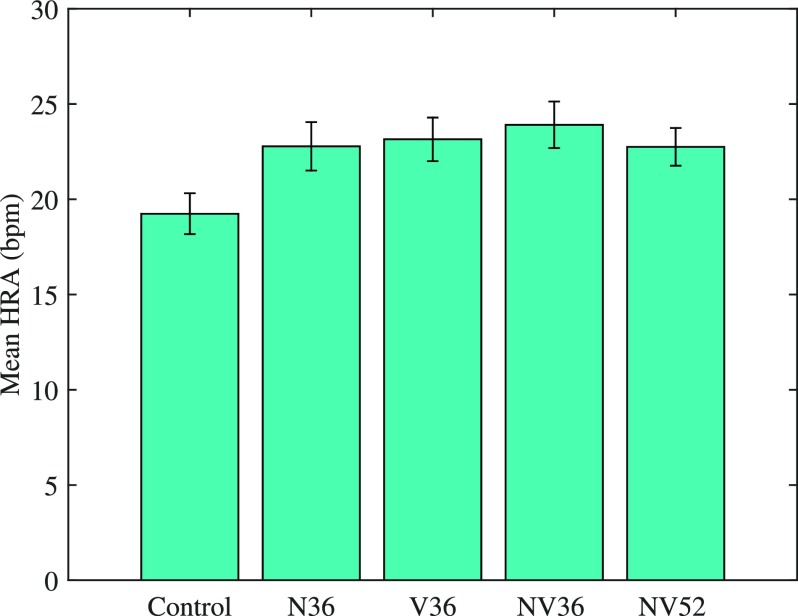
HRA was greater when the participants were awake before the train onset, or awoke during the train pass-by itself, than when they continued to sleep [F(1,4096) = 368, p < 0.001]. Data where participants were awake were subsequently excluded from the analysis. The mixed model with random intercept that best described the data used a first order autoregressive covariance matrix structure, which accounts for the time-varying nature of the data.34 A first model included experiment night, sex and sensitivity. Sex (p = 0.159) and sensitivity (p = 0.153) were found to be non-significant and were thus excluded from analysis. The final model, therefore, included experimental night as the predictor variable and the intercept and the repeated effect terms (participant and event number within night), and employed a first order autoregressive covariance structure. A significant main effect of night was found [p < 0.001, F(4,852) = 5.13, see Fig. 4]. Post hoc tests revealed that HRA was higher in all exposure nights (N36, V36, NV36, NV52) than in the control night (p < 0.05). HRA did not differ significantly between the exposure nights.
FIG. 4.
(Color online) Mean HRA in all study nights. Error bars indicated 95% confidence intervals.
IV. DISCUSSION
For the first time, this study has investigated how noise and vibration from railway traffic interact regarding their effects on human sleep. The controlled laboratory design allowed for nights with simultaneous noise and vibration, and nights with only noise or vibration. The comparisons of physiological reactions between nights, therefore, enables determination of the degree of response attributable to each separate exposure stimulus, and the interactions thereof. In summary, both vibration and noise contributed to event-related EEG reactions, sleep stage changes and increases in cardiac activity. The effects of vibration and noise were directly additive for EEG arousals and SSCs. Heart rate accelerated following vibration, noise, and combined exposure, but the degree of acceleration did not differ between these different exposure scenarios. No effects on overall sleep architecture were observed between 36-event nights with noise only, vibration only, or combined noise and vibration. Increasing nocturnal noise and vibration exposure from 36 trains to 52 trains had no significant effect on sleep macrostructure or event-related cortical or autonomic response.
A. Effects on sleep physiology
The effects of noise and vibration were directly additive for event-related EEG arousals and SSCs. This has previously only been observed in monkeys for sound intensities above 50 dB.24 In contrast, a very small study had previously reported that changes of sleep depth were more frequent following exposure to heavy road traffic vibration-only than when noise was presented alongside.22 The probability for awakenings, which are the strongest form of activation during sleep,37 was slightly higher during vibration-only than noise-only or noise and vibration together in the present study, although the difference was not statistically significant. Nevertheless, taken together with the fact that the effect on awakenings within V36 differed from the effect on arousals and SSCs, we assume that the absence of acoustic information hinders classification of the specific nature of the exposure source. Auditory information continues to be processed during sleep,27 and could enable identification of the exposure as railway vibration. An unidentified exposure, hence, could indicate a potential threat, with the sleeping brain preparing the body to react by waking up. The sleeping brain's spontaneous rhythms preclude its ability to maintain stable sleep in the presence of noise.38 The brain may, however, be less resilient to vibration than noise, and, hence, may more readily awaken from sleep following vibration, as seen here, although the effect was modest. In the opposite direction to awakenings, arousal probability was lower during vibration-only trains than noise-only or combined vibration and noise. These EEG arousals are a mechanism by which the body maintains vigilance towards the external environment even while maintaining a sleeping state,39 so the lower arousal probability implies that the brain is less able to evaluate the vibration and maintain sleep, being more likely instead to lead to an awakening. However, if vibration does not lead to an awakening, it still contributes towards the overall impact of exposure on sleep fragmentation, reflected by the additive effect on arousals and SSCs.
The only exposure night where SSC probability was not significantly higher than the baseline was the vibration-only condition. However, in this V36 night, awakening probability was greatest, and such awakenings were not included in SSC derivation. Therefore, in the exposure nights involving noise, the exposure had a significant probability to lead to a reduction of sleep depth, while maintaining a sleeping state. In the vibration-only night, however, rather than sleep purely becoming more shallow, the body was more likely to fully awaken.
Increasing the number of events with both vibration and noise from 36 to 52 resulted in a decrease of around 0.04 in additional arousal probability, and subsequently combined EEG reaction probability, although this decrease was not statistically significant. Within-night habituation to railway noise, whereby reaction probability decreased with an increasing number of nocturnal events, has been reported previously,3 but this habituation was not observed when investigating 20 and 36 nocturnal vibration and noise exposures.20 It is, therefore, possible that when traffic frequency exceeds a certain threshold, a slight habituation to the repeated exposure manifests. However, compared to noise exposure, any such habituation effects in the present work are rather small when vibration and noise are presented simultaneously, supporting the notions that vibration may be more indicative of threat than identifiable noise, that sleep stability is less effectively maintained following vibration than noise, or indeed a combination of the two.
Whereas, noise and vibration were found to lead to event-related EEG reactions, overall sleep architecture was found to be generally unaffected during the full night. These biological responses are, therefore, occurring at the expense of spontaneous changes in sleep microstructure, as has been observed previously for noise3 and combined noise and vibration.20 It is, however, unclear whether these disruptions of the natural rhythm of sleep are relevant for negative outcomes associated with interference of vital processes including memory consolidation,40 daytime sleepiness,41 hormone regulation42 and clearance of toxins that accumulate during wakefulness.43
B. Effects on cardiac activity
Heart rate amplitude was higher during trains in all exposure nights relative to changes occurring spontaneously, indicating that vibration, noise, or a combination thereof are capable of inducing autonomic activations. Increased heart rate has previously been shown to occur following exposure to air, road and railway noise,3,44 and simultaneous railway noise and vibration,21 but to our knowledge this is the first time such a response has been shown for vibration alone. Scenarios where vibration, but not noise, occur are uncommon, but homes in the vicinity of railway tunnels represent such a situation if the ground properties are such that ground-borne noise is not generated simultaneously. Although not statistically significant, HRA was somewhat higher in the NV36 night than either of the N36 and V36 nights, suggesting that the degree of autonomic response is related to multiple salient sensory inputs, rather than to the brains reaction to a single preponderant stimulus.
The current study found that increases of HRA were lower during sleep than when an individual awoke or was awake prior to stimulus onset, as expected. Some previous work has reported that HRA in response to traffic noise was lower during sleep than wakefulness,45 but such studies do not account for the large heart rate elevations that accompany awakenings.46 Furthermore, the HRA did not differ between nights with 36 and 52 trains. If autonomic habituation following repeated exposure were to occur within the night, as has been reported previously for railway noise,3 then it would be expected that HRA would be lower in the 52-train night. The data in the present study, therefore, do not suggest that there is a habituation to vibration exposure. The relevance of such cardiac activations in the long term, either with or without awakenings, is not known, but has been suggested to be a risk factor for the development of cardiovascular disease, including arterial hypertension.13
C. Relevance for situation in the field
In areas with strong vibration, self-reported annoyance to railway noise was higher than in areas with no or low vibration levels,47 with the authors proposing that vibration may modulate the psychological response to noise, rather than evoking a response itself. In the present work we were intrigued by the possibility of effects on sleep following similar cross-modal mechanisms. Stochastic resonance is the phenomenon whereby the addition of random signal noise, in this instance not necessarily auditory noise, to a signal causes it to move above threshold, evoking a response.48 For instance, low level auditory noise increases vibration perception.26 We found instead that rather than the introduction of a second modality increasing the response to a first, vibration and auditory noise were, at least in terms of arousal and sleep stage changes, directly additive. Annoyance, such as that reported to be modulated by exposure to vibration,47 is by definition a psychological phenomenon, and physiological reactions to noise during sleep occur independently of annoyance.49 The additive effects of vibration and noise do not, therefore, necessarily disagree with the cross-modal field findings.
Since both environmental noise and vibration contribute separately to physiological response, reducing one single exposure, typically noise, may not be sufficient to provide adequate protection for exposed populations. The vibration amplitudes used in the current work correspond to the exposure of around 7300 individuals in Sweden alone.50 Sleep disturbance following nighttime environmental noise can be highly variable between individuals,51 and care should, therefore, be taken when considering the implications of the present work in the context of response in the field.
V. CONCLUDING REMARKS
Vibration and noise from railway freight, both differentially and coincidentally, contributed towards sleep disturbance, reflected by cardiac activations, EEG arousals and awakenings, and changes of sleep depth. The effects of noise and vibration were directly additive for EEG arousals and sleep stage changes, indicating that both inputs are processed in the brain separately yet in parallel, with both contributing towards the likelihood of arousal. There was no indication that either cardiac or autonomic habituation occurred within nights. However, the rather homogeneous age distribution in the present work limits the conclusions that can be drawn regarding older or younger populations. With increasing age, there is a monotonic increase in arousal frequency and a decrease in the proportion of deep sleep.52,53 Arousal from sleep following environmental noise intrusion is more likely during shallower sleep stages,37 so it follows that an older population may be more reactive to noise and vibration than is demonstrated in the present study. Further work could, therefore, seek to examine response to vibration in noise among not only older but also younger populations, with children requiring more sleep and as such representing a potentially vulnerable group.
ACKNOWLEDGMENTS
The work was supported by grant 266248 from the Seventh Framework Programme (FP7). We gratefully thank Agneta Agge, Sofie Fredriksson, and Josefine Erlingsdotter Larsson for their invaluable assistance in performing the experimental study.
References
- 1. Cirelli C. and Tononi G., “ Is sleep essential?,” PLoS Biol. 6(8), e216 (2008). 10.1371/journal.pbio.0060216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schmidt F. P., Basner M., Kroger G., Weck S., Schnorbus B., Muttray A., Sariyar M., Binder H., Gori T., Warnholtz A., and Munzel T., “ Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults,” Eur. Heart J. 34(45), 3508–3514 (2013). 10.1093/eurheartj/eht269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Basner M., Muller U., and Elmenhorst E. M., “ Single and combined effects of air, road, and rail traffic noise on sleep and recuperation,” Sleep 34(1), 11–23 (2011). 10.1093/sleep/34.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aasvang G. M., Overland B., Ursin R., and Moum T., “ A field study of effects of road traffic and railway noise on polysomnographic sleep parameters,” J. Acoust. Soc. Am. 129(6), 3716–3726 (2011). 10.1121/1.3583547 [DOI] [PubMed] [Google Scholar]
- 5. Basner M., Glatz C., Griefahn B., Penzel T., and Samel A., “ Aircraft noise: Effects on macro- and microstructure of sleep,” Sleep Med. 9(4), 382–387 (2008). 10.1016/j.sleep.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 6. Carter N., Henderson R., Lal S., Hart M., Booth S., and Hunyor S., “ Cardiovascular and autonomic response to environmental noise during sleep in night shift workers,” Sleep 25(4), 444–451 (2002). 10.1093/sleep/25.4.444 [DOI] [PubMed] [Google Scholar]
- 7. de Kluizenaar Y., Janssen S. A., van Lenthe F. J., Miedema H. M., and Mackenbach J. P., “ Long-term road traffic noise exposure is associated with an increase in morning tiredness,” J. Acoust. Soc. Am. 126(2), 626–633 (2009). 10.1121/1.3158834 [DOI] [PubMed] [Google Scholar]
- 8. Griefahn B., Marks A., and Robens S., “ Noise emitted from road, rail and air traffic and their effects on sleep,” J. Sound Vib. 295(1–2), 129–140 (2006). 10.1016/j.jsv.2005.12.052 [DOI] [Google Scholar]
- 9. Basner M., Babisch W., Davis A., Brink M., Clark C., Janssen S., and Stansfeld S., “ Auditory and non-auditory effects of noise on health,” Lancet 383(9925), 1325–1332 (2013). 10.1016/S0140-6736(13)61613-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization, “Burden of disease from environmental noise. Quantification of healthy life years lost in Europe,” WHO Regional Office for Europe, Copenhagen, Denmark, 2011, p. 101. [Google Scholar]
- 11. Elmenhorst E. M., Pennig S., Rolny V., Quehl J., Mueller U., Maass H., and Basner M., “ Examining nocturnal railway noise and aircraft noise in the field: Sleep, psychomotor performance, and annoyance,” Sci. Total Environ. 424, 48–56 (2012). 10.1016/j.scitotenv.2012.02.024 [DOI] [PubMed] [Google Scholar]
- 12. Saremi M., Greneche J., Bonnefond A., Rohmer O., Eschenlauer A., and Tassi P., “ Effects of nocturnal railway noise on sleep fragmentation in young and middle-aged subjects as a function of type of train and sound level,” Int. J. Psychophysiol. 70(3), 184–191 (2008). 10.1016/j.ijpsycho.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 13. Münzel T., Gori T., Babisch W., and Basner M., “ Cardiovascular effects of environmental noise exposure,” Eur. Heart J. 35(13), 829–836 (2014). 10.1093/eurheartj/ehu030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aasvang G. M., Moum T., and Engdahl B., “ Self-reported sleep disturbances due to railway noise: Exposure-response relationships for nighttime equivalent and maximum noise levels,” J. Acoust. Soc. Am. 124(1), 257–268 (2008). 10.1121/1.2932074 [DOI] [PubMed] [Google Scholar]
- 15. Miedema H. M. and Vos H., “ Associations between self-reported sleep disturbance and environmental noise based on reanalyses of pooled data from 24 studies,” Behav. Sleep Med. 5(1), 1–20 (2007). 10.1207/s15402010bsm0501_1 [DOI] [PubMed] [Google Scholar]
- 16. Peris E., Woodcock J., Sica G., Moorhouse A. T., and Waddington D. C., “ Annoyance due to railway vibration at different times of the day,” J. Acoust. Soc. Am. 131(2), EL191–EL196 (2012). 10.1121/1.3679390 [DOI] [PubMed] [Google Scholar]
- 17. Klaeboe R., Thurunen-Rise H., Harvik L., and Madshus C., “ Vibration in dwellings from road and rail traffic—Part II: Exposure-effect relationships based on ordinal logit and logistic regression models,” Appl. Acoust. 64(1), 89–109 (2002). 10.1016/S0003-682X(02)00053-1 [DOI] [Google Scholar]
- 18. Öhrström E., Gidlöf-Gunnarsson A., Ögren M., and Jerson T., “ Effects of railway noise and vibration in combination: Field and laboratory studies,” Euronoise 2009, Edinburgh (2009), Paper 270. [Google Scholar]
- 19. Smith M. G., Croy I., Ogren M., and Persson Waye K., “ On the influence of freight trains on humans: A laboratory investigation of the impact of nocturnal low frequency vibration and noise on sleep and heart rate,” PLoS One 8(2), e55829 (2013). 10.1371/journal.pone.0055829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith M. G., Croy I., Hammar O., and Persson Waye K., “ Vibration from freight trains fragments sleep: A polysomnographic study,” Sci. Rep. 6, 24717 (2016). 10.1038/srep24717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Croy I., Smith M. G., and Persson Waye K., “ Effects of train noise and vibration on human heart rate during sleep: An experimental study,” BMJ Open 3(5), e002655 (2013). 10.1136/bmjopen-2013-002655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arnberg P. W., Bennerhult O., and Eberhardt J. L., “ Sleep disturbances caused by vibrations from heavy road traffic,” J. Acoust. Soc. Am. 88(3), 1486–1493 (1990). 10.1121/1.400305 [DOI] [PubMed] [Google Scholar]
- 23. Kato T., Montplaisir J. Y., and Lavigne G. J., “ Experimentally induced arousals during sleep: A cross-modality matching paradigm,” J. Sleep Res. 13(3), 229–238 (2004). 10.1111/j.1365-2869.2004.00409.x [DOI] [PubMed] [Google Scholar]
- 24. Lakatos P., Chen C. M., O'Connell M. N., Mills A., and Schroeder C. E., “ Neuronal oscillations and multisensory interaction in primary auditory cortex,” Neuron 53(2), 279–292 (2007). 10.1016/j.neuron.2006.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Molholm S., Ritter W., Murray M. M., Javitt D. C., Schroeder C. E., and Foxe J. J., “ Multisensory auditory-visual interactions during early sensory processing in humans: A high-density electrical mapping study,” Brain Res. Cogn. Brain Res. 14(1), 115–128 (2002). 10.1016/S0926-6410(02)00066-6 [DOI] [PubMed] [Google Scholar]
- 26. Lugo E., Doti R., and Faubert J., “ Ubiquitous crossmodal Stochastic Resonance in humans: Auditory noise facilitates tactile, visual and proprioceptive sensations,” PLoS One 3(8), e2860 (2008). 10.1371/journal.pone.0002860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Portas C. M., Krakow K., Allen P., Josephs O., Armony J. L., and Frith C. D., “ Auditory processing across the sleep-wake cycle: Simultaneous EEG and fMRI monitoring in humans,” Neuron 28(3), 991–999 (2000). 10.1016/S0896-6273(00)00169-0 [DOI] [PubMed] [Google Scholar]
- 28.Sound Environment and Health research group homepage www.amm.se/soundenvironment (Last viewed March 2017).
- 29.ISO 717-1:1997 “ Acoustics—Rating of sound insulation in buildings and of building elements—Part 1: Airborne sound insulation” (International Organization for Standardization, Geneva, Switzerland, 1997).
- 30.ISO 2631-1 “ Mechanical vibration and shock—Evaluation of human exposure to whole-body vibration Part 1: General Requirements” (International Organization for Standardization, Geneva, Switzerland, 1997).
- 31. Iber C., Ancoli-Israel S., Chesson A., and Quan S. F., The AASM Manual for the Scoring of Sleep and Associated Events; Rules, Terminology and Technical Specifications, 1st edition ( American Academy of Sleep Medicine, Westchester, IL, 2007), pp. 1–59. [Google Scholar]
- 32. Bonnet M., Carley D., Carskadon M., Easton P., Guilleminault C., Harper R., Hayes B., Hirshkowitz M., Ktonas P., Keenan S., Pressman M., Roehrs T., Smith J., Walsh J., Weber S., and Westbrook P., “ EEG arousals: Scoring rules and examples: A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association,” Sleep 15(2), 173–184 (1992). 10.1093/sleep/15.2.173 [DOI] [PubMed] [Google Scholar]
- 33. Ekstedt M., Akerstedt T., and Soderstrom M., “ Microarousals during sleep are associated with increased levels of lipids, cortisol, and blood pressure,” Psychosom. Med. 66(6), 925–931 (2004). 10.1097/01.psy.0000145821.25453.f7 [DOI] [PubMed] [Google Scholar]
- 34.See supplementary material at http://dx.doi.org/10.1121/1.4983302 E-JASMAN-141-037705 for additional methods and results.
- 35. Brink M., Basner M., Schierz C., Spreng M., Scheuch K., Bauer G., and Stahel W. A., “ Determining physiological reaction probabilities to noise events during sleep,” Somnologie 13(4), 236–243 (2009). 10.1007/s11818-009-0437-1 [DOI] [Google Scholar]
- 36. Akaike H., “ Information theory and an extension of the maximum likelihood principle,” in Selected Papers of Hirotugu Akaike, edited by Parzen E., Tanabe K., and Kitagawa G. ( Springer; New York, 1998), pp. 199–213. [Google Scholar]
- 37. Basner M., Samel A., and Isermann U., “ Aircraft noise effects on sleep: Application of the results of a large polysomnographic field study,” J. Acoust. Soc. Am. 119(5 Pt 1), 2772–2784 (2006). 10.1121/1.2184247 [DOI] [PubMed] [Google Scholar]
- 38. Dang-Vu T. T., McKinney S. M., Buxton O. M., Solet J. M., and Ellenbogen J. M., “ Spontaneous brain rhythms predict sleep stability in the face of noise,” Curr. Biol. 20(15), R626–R627 (2010). 10.1016/j.cub.2010.06.032 [DOI] [PubMed] [Google Scholar]
- 39. Halasz P., Terzano M., Parrino L., and Bodizs R., “ The nature of arousal in sleep,” J. Sleep Res. 13(1), 1–23 (2004). 10.1111/j.1365-2869.2004.00388.x [DOI] [PubMed] [Google Scholar]
- 40. Rasch B. and Born J., “ About sleep's role in memory,” Physiol. Rev. 93(2), 681–766 (2013). 10.1152/physrev.00032.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pepin J.-L., Launois S. H., Tamsier R., and Levy P., “ Sleepiness due to sleep-related breathing disorders” in Sleepiness: Causes, Consequences and Treatment, edited by Thorpy M. J. and Billiard M. ( Cambridge University Press, Cambridge, UK: ), pp. 154–167 (2011). [Google Scholar]
- 42. Spiegel K., Tasali E., Leproult R., and Van Cauter E., “ Effects of poor and short sleep on glucose metabolism and obesity risk,” Nat. Rev. Endocrinol. 5(5), 253–261 (2009). 10.1038/nrendo.2009.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xie L., Kang H., Xu Q., Chen M. J., Liao Y., Thiyagarajan M., O'Donnell J., Christensen D. J., Nicholson C., Iliff J. J., Takano T., Deane R., and Nedergaard M., “ Sleep drives metabolite clearance from the adult brain,” Science 342(6156), 373–377 (2013). 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tassi P., Rohmer O., Schimchowitsch S., Eschenlauer A., Bonnefond A., Margiocchi F., Poisson F., and Muzet A., “ Living alongside railway tracks: Long-term effects of nocturnal noise on sleep and cardiovascular reactivity as a function of age,” Environ. Int. 36(7), 683–689 (2010). 10.1016/j.envint.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 45. Di Nisi J., Muzet A., Ehrhart J., and Libert J. P., “ Comparison of cardiovascular responses to noise during waking and sleeping in humans,” Sleep 13(2), 108–120 (1990). 10.1093/sleep/13.2.108 [DOI] [PubMed] [Google Scholar]
- 46. Griefahn B., Brode P., Marks A., and Basner M., “ Autonomic arousals related to traffic noise during sleep,” Sleep 31(4), 569–577 (2008). 10.1093/sleep/31.4.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gidlöf-Gunnarsson A., Ögren M., Jerson T., and Öhrstrom E., “ Railway noise annoyance and the importance of number of trains, ground vibration, and building situational factors,” Noise Health 14(59), 190–201 (2012). 10.4103/1463-1741.99895 [DOI] [PubMed] [Google Scholar]
- 48. Faisal A. A., Selen L. P. J., and Wolpert D. M., “ Noise in the nervous system,” Nat. Rev. Neurosci. 9(4), 292–303 (2008). 10.1038/nrn2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Frei P., Mohler E., and Roosli M., “ Effect of nocturnal road traffic noise exposure and annoyance on objective and subjective sleep quality,” Int. J. Hyg. Environ. Health 217(2–3), 188–195 (2014). 10.1016/j.ijheh.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 50. Arnesson M., Ekblad A., and Ögren M., “ Residential vibration exposure from railway traffic in Sweden,” Internoise 2016, Hamburg, Germany (2016), pp. 2131–2137. [Google Scholar]
- 51. McGuire S., Muller U., Elmenhorst E. M., and Basner M., “ Inter-individual differences in the effects of aircraft noise on sleep fragmentation,” Sleep 39(5), 1107–1110 (2016). 10.5665/sleep.5764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Boselli M., Parrino L., Smerieri A., and Terzano M. G., “ Effect of age on EEG arousals in normal sleep,” Sleep 21(4), 351–357 (1998). 10.1093/sleep/21.4.361 [DOI] [PubMed] [Google Scholar]
- 53. Bonnet M. H. and Arand D. L., “ EEG arousal norms by age,” J. Clin. Sleep Med. 3(3), 271–274 (2007). [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1121/1.4983302 E-JASMAN-141-037705 for additional methods and results.