Abstract
It has been proposed that protein supplementation during resistance exercise training enhances muscle hypertrophy. The degree of hypertrophy during training is controlled in part through activation of satellite cells and myonuclear accretion.
Purpose
To determine the efficacy of protein supplementation (and the type of protein) during traditional resistance training on myofiber cross-sectional-area, satellite cell content and myonuclear addition.
Methods
Healthy young men participated in supervised whole body progressive resistance training 3d/wk for 12 weeks. Participants were randomized to one of three groups ingesting a daily 22g macronutrient dose of soy-dairy protein blend (PB, N=22), whey protein isolate (WP, N=15) or an isocaloric maltodextrin placebo (MDP, N=17). Lean mass, vastus lateralis myofiber-type-specific cross-sectional-area, satellite cell content and myonuclear addition were assessed pre and post resistance training.
Results
PB and the pooled protein treatments (PB+WP=PRO) exhibited a greater whole body lean mass %change compared to MDP (p=0.057 for PB) and (P=0.050 for PRO), respectively. All treatments demonstrated similar leg muscle hypertrophy and vastus lateralis myofiber-type-specific cross-sectional-area (P<0.05). Increases in myosin heavy chain I and II myofiber satellite cell content and myonuclei content were also detected following exercise training (P<0.05).
Conclusion
Protein supplementation during resistance training has a modest effect on whole body lean mass as compared to exercise training without protein supplementation and there was no effect on any outcome between protein supplement types (Blend vs Whey). However, protein supplementation did not enhance resistance exercise-induced increases in myofiber hypertrophy, satellite cell content or myonuclear addition in young healthy men. We propose that as long as protein intake is adequate during muscle overload the adaptations in muscle growth and function will not be influenced by protein supplementation.
Keywords: Muscle, hypertrophy, myofiber, protein type, whey, soy, resistance, exercise, training
Introduction
It has been reported that the amount of protein in the diet does not influence muscle hypertrophy following resistance exercise training (45). Despite this information, the notion that protein supplements can enhance muscle growth at rest or during exercise training is very popular (39). One reason for this popularity is that several acute studies have shown protein or amino acids ingestion following a bout of resistance exercise (RE; see Document, Supplemental Digital Content 1, List of abbreviations) can enhance muscle protein synthesis (39). On the other hand, there are a limited number of longitudinal studies that have examined whether protein supplementation during resistance exercise training (RET) enhances muscle growth and strength as compared to RET without protein supplementation (8, 31, 35, 41).
The prevailing theory for contraction-induced myofiber growth posits that acute and periodic increases in protein synthesis lead to the accumulation of protein and expansion of myofiber volume. Expansion of the myofiber strains the myonuclear domain, the volume of a myofiber maintained by one myonucleus to regulate essential cell function. The addition of myonuclei to a growing myofiber occurs by inducing satellite cell proliferation and migration to target myofibers. These proliferating satellite cells may then undergo terminal differentiation and fuse to current myofibers as myonuclei, (i.e. myonuclear addition) to facilitate additional hypertrophy (16). Additional functions of satellite cells include the maintenance of the myofiber environment and the repair/remodeling of myofibers. Since muscle hypertrophy includes the addition of new proteins and myofiber expansion the idea of myonuclear addition during exercise training to support muscle growth seems logical, however the exact role and necessity for satellite cells to support hypertrophy through myonuclear addition is controversial (14).
Olsen et al. first demonstrated that RET with protein supplementation may provide enhancement of the satellite cell pool compared to RET alone (32). Recently, Farup and colleagues have expanded upon these findings by demonstrating that this effect is muscle fiber-type-specific, as reported in results from both acute (11) and chronic studies (12). These findings in human skeletal muscle have been supported by basic and pre-clinical approaches demonstrating that nutrient provision (in particular the leucine metabolite HMB) enhances myogenic proliferation via mTORC1 signaling (27, 40). Farup et al. also conducted studies to assess the separate effects of contraction mode (concentric vs. eccentric) and protein supplementation on myofiber growth and expansion of the satellite cell and the myonuclear pools (12). However, no study has determined and/or reported the effect of protein supplementation during traditional RET, with concurrent concentric and eccentric muscle action, on expansion of the satellite cell (SC) pool and myonuclear addition at the fiber-type-specific level. Using a large cohort of young men, our goal was to determine the role of protein supplementation (and the type of protein used as supplementation) during RET on fiber-type-specific myofiber growth, satellite cell content, and myonuclear addition. We hypothesized that protein supplementation (independent of the type protein used) would enhance myofiber growth and satellite cell and myonuclear content during RET.
Methods
Participants
We recruited healthy male participants for this double-blind, randomized clinical trial. Participant were of similar age (PB: 24.1±0.6; WP: 24.6±1.0; MDP 25.2±1.1), height (PB: 178.6.1±1.5; WP: 180.0.6±2.1; MDP 176.0±1.6) weight (PB: 77.51±2.3; WP: 83.5±3.4; MDP 76.3±1.3), BMI (PB: 24.3±0.6; WP: 25.7±0.9; MDP 24.5±0.8), whole body lean mass (PB: 56.2±1.3; WP: 58.9±2.3; MDP 55.4±1.7), and leg lean mass (PB: 19.0±0.4; WP: 20.7±1.0; MDP 18.7±0.8) at pre. The subjects included in this study were a subset of a larger clinical trial (38), where screening and enrollment details can be found alongside information concerning treatment compliance, dietary intake and strength testing. There were no differences between treatments at pre for any of the descriptive characteristics (p>0.10). As our previous report indicated (38), the habitual protein intake for participants was ∼1.3 g/kg/d and the participants increased protein intake in both protein supplement groups above this level.
The participants were healthy and recreationally active, but were not engaged in any regular exercise-training program (<2 sessions of high-intensity aerobic or resistance exercise/week) at the time of enrollment. All participants gave written informed consent before enrollment in the study. The study was approved by the UTMB Institutional Review Board, and is in compliance with the Declaration of Helsinki as revised in 1983. Of the 70 participants who underwent pre testing, 2 withdrew before undergoing exercise training (WP: n = 1, PB: n = 1), 4 withdrew during the first 6 weeks (MDP: n = 3, WP: n = 1), and 6 withdrew during the last 6 weeks (MDP: n = 4, WP: n = 2). Of the 58 study completers, snap frozen muscle samples necessary for immunohistochemical analysis could not be obtained for 4 participants. Thus all the data provided herein are from the 54 completers for whom we have data on the primary immunohistochemical outcomes (PB: n = 22, WP: n = 15, MDP: n = 17).
Study Design
The study design can be found in more detail elsewhere (38), briefly, following enrollment, completion of a run-in period consisted of the pre-training study day at UTMB and then 3 non-consecutive days of exercise familiarization and pre 1-repetition max (1-RM) strength testing at the University of Texas Medical Branch Alumni Fieldhouse. The pre-training study day included assessment of body composition, a muscle biopsy and a isometric and isokinetic strength test of the thigh as we have previously described in the initial clinical trial (38). Soon after, the participants reported to the UTMB Alumni Fieldhouse for familiarization/testing before beginning 12 weeks of training. After 12 weeks of training, participants were re-tested exactly 3 days following the final exercise session of the training program. For the post-testing, participants reported to the ITS-CRC at the same time in the morning as for the pre-training study day to repeat the same laboratory tests and sample collection.
Pre and Post-testing Study Days
Participants reported to the ITS-CRC at UTMB in the morning following an overnight fast. They were instructed to refrain from any medication that affects muscle metabolism, and also caffeine, supplements, and alcohol for several days before testing. After arrival on the unit, anthropometric tests and a DXA scan (dual-energy X-ray absorptiometry) were completed (Hologic ADR 4500W, Bedford, MA).
All the pre-testing exercise familiarization and 1-RM testing was conducted after the muscle biopsy. A percutaneous biopsy sample of the VL muscle was performed using a 5 mm Bergström biopsy needle with suction, under sterile procedure and local anesthesia (1% lidocaine). The sample was aliquoted and snap-frozen in liquid nitrogen and stored at −80°C for future analysis. Suitable muscle samples where orientation was apparent were carefully laid on Tissue Tek Optimal Cutting Temperature (OCT; Thermo Fisher Scientific, Rockford, IL) affixed to cork, submerged in liquid nitrogen-cooled isopentane, and then placed on dry ice until they could be stored at −80°C until subsequent immunohistochemical analysis. Following isometric and isokinetic knee extension and flexion strength testing on a dynamometer, participants were fed a meal before leaving the unit. All testing was repeated on the post-testing day in the same order.
Resistance Exercise Training
Following familiarization and 1-repetition maximum (1-RM) strength testing, participants began a 12-week whole-body progressive resistance exercise-training (RET) program as we have previously described in the initial clinical trial (38). All exercise-training sessions were performed at the UTMB Alumni Fieldhouse. Exercise sessions were performed on non-consecutive days, 3 times weekly, with 4 rest days per week, under supervision of qualified personal trainers. Participants were allowed to maintain their recreational physical activity, but instructed not to do any other strength training outside of the study. To allow for unforeseen life events, participants were given 13 weeks following the familiarization period to complete 36 exercise sessions. This allowed for 100% exercise compliance.
Supplementation
Participants were randomized (20 per group) to the Placebo (MDP), Whey (WP) or Blend (PB) isoclaoric treatments as previously described (38). The PB and WP treatments were isonitrogenous and pooled to reflect protein supplementation (PRO) overall. Immediately following each workout, under direct observation of the study personnel, the participants ingested either the placebo beverage or one of the protein supplements to which they were assigned. On the four resting (non-exercise) days each week, the participants ingested the placebo or supplement one time between meals. Participants were instructed to refrain from any other food or macronutrient-containing beverage for 2 hours before or after exercise or supplementation.
Whey and protein blend samples were provided by DuPont Nutrition & Health (St. Louis, MO) and were independently tested for amino acid profile. The soy-dairy blend (PB) was composed of 25% soy protein isolate, 25% whey protein isolate, and 50% sodium caseinate. The whey (WP) treatment consisted of 100% whey protein isolate, and carbohydrate placebo (MDP) was an isocaloric maltodextrin mixture. The dose for the two protein nutritional supplements was ∼22 g protein/day, thus the leucine content was 2.00 g for the PB and 2.31 g for the WP. Supplements were separated into individual ready-made packets for daily consumption, and participants were given a 2-week supply. The personal trainer collected the empty supplement packets from each subject every 2 weeks. Supplements and placebo were given in powder form and dissolved in 300 ml water to ensure a rapid and predictable absorption.
RNA Concentration
Total RNA was isolated by homogenizing 10–20 mg of tissue with a hand-held homogenizing dispenser (T10 Basic Ultra Turrax, IKA, Wilmington, NC) in 1 ml of Tri reagent. The RNA was separated into an aqueous phase using 0.2 ml of chloroform and subsequently precipitated from ∼475µl of aqueous phase using 0.5 ml of isopropanol. Total RNA was quantified by measuring the total volume of the aqueous phase. RNA was washed twice with 1 ml of 75% ethanol, air-dried, and suspended in a known amount of nuclease-free water. RNA concentration was determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE).
Immunohistochemistry
Immunohistochemical techniques were conducted as previously described (15). Samples were removed from the cork at −25°C in a ThermoFisher Cryostat (Fisher Scientific HM 525X) where they were cut in 7 µm cross-sections. Pre and post samples for the same subject were placed on the same slides Fisherbrand Superfrost®/Plus microscope slides (Fisher Scientific, USA). Two slides were generated per subject, one for analysis of myofiber myosin heavy chain (MHC) typing and cross-sectional area (CSA) and the other for fiber-type-specific satellite cell and myonuclear content. Following cutting, a hydrophobic marker (Vector, H-4000, Burlingame, CA) separated the sections, which were dried at room temperature (RT) and then stored at −20°C until analysis.
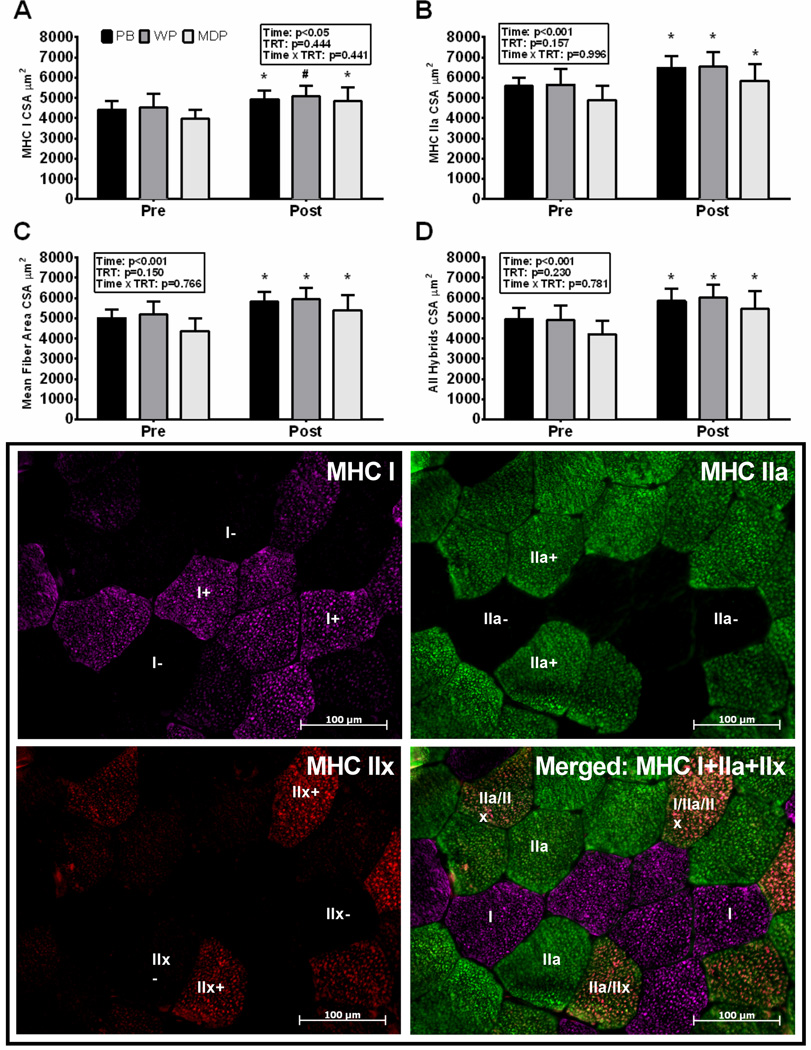
Myofiber MHC type and CSA was determined as following. Sections were rehydrated in phosphate buffered saline (PBS) for 2 × 5 minutes at RT. Slides were incubated for at least 1 h at RT and then overnight at 4°C with primary antibodies, mouse anti-myosin heavy chain (MHC) type I (BA.D5 IgG2b, 1:50, Developmental Studies Hybridoma Bank, Iowa City, IA) in a 1:1 ratio of supernatant with mouse anti-MHC IIa (SC.71 IgG1, Developmental Studies Hybridoma Bank) and mouse anti-MHC IIx (6H1 IgM, Developmental Studies Hybridoma Bank). Slides were rinsed 3 times for 5 min each with PBS followed by 1 hour incubation with secondary antibodies diluted in PBS, Alexa Fluor 488 conjugated goat anti-mouse IgG1 (for MHC IIa: 1:500, #A21121, Invitrogen, Carlsbad, CA), Alexa Fluor 647 conjugated goat anti-mouse IgG2b (for MHC I: 1:250, #A21242, Invitrogen) and Alexa Fluor 594 goat anti-mouse IgM (for MHC IIx: 1:250, #A21044, Invitrogen) at RT in the dark. Slides were rinsed 3 × 5 minutes each with PBS, before and after a 5 minute post-fix in methanol. Slides were mounted with fluorescent mounting media (Vector, H-4000) and dried before imaging. Staining procedures resulted in MHC IIa staining green, MHC I staining purple, and MHC type IIx staining red (Figure 1). Images for fiber typing were captured at 100x magnification using a fluorescence microscope (Axio Imager.M1m, Carl Zeiss, Toronto, Ontario, Canada) and AxioCam MRm camera (Carl Zeiss). Image processing and analysis was done using AxioVision 4.8.2 software. For each image, the number of muscle fibers for pure MHC type I, IIa, IIx and hybrid type I, I/IIa, I/IIx, IIa/IIx and I/IIa/IIx fibers were counted, and cross sectional areas (CSA) for MHC type I, IIa, IIa/IIx and I/IIa/IIx fibers were measured. Fibers with frequencies less than 1–2% (pure IIx and hybrid I/IIa and I/IIx) were removed from further analysis due to insufficient data for analysis. Hybrid denotes all hybrid groups combined. T2 represents all MHC II (IIa+IIa/IIx) isoforms pooled together. Approximately 250 muscle fibers were analyzed for fiber type distribution and 200 for CSA in each sample.
Figure 1.
Fiber-type specific and mean (MFA) vastus lateralis cross-sectional area by treatment. Protein blend (PB) or whey protein (WP) or maltodextrin placebo (MDP) and representative immunohistochemical image for identification of fiber typing and cross-sectional area quantification in vastus lateralis. MHC I stained purple (top left), MHC IIa stained green (top right), and MHC type IIx stained red (bottom right) and merged image (bottom left).Data are mean ± SEM, n=15 (WP), 22 (PB) & 17 (MDP). Units are µm2. Significant change * (p<0.05).
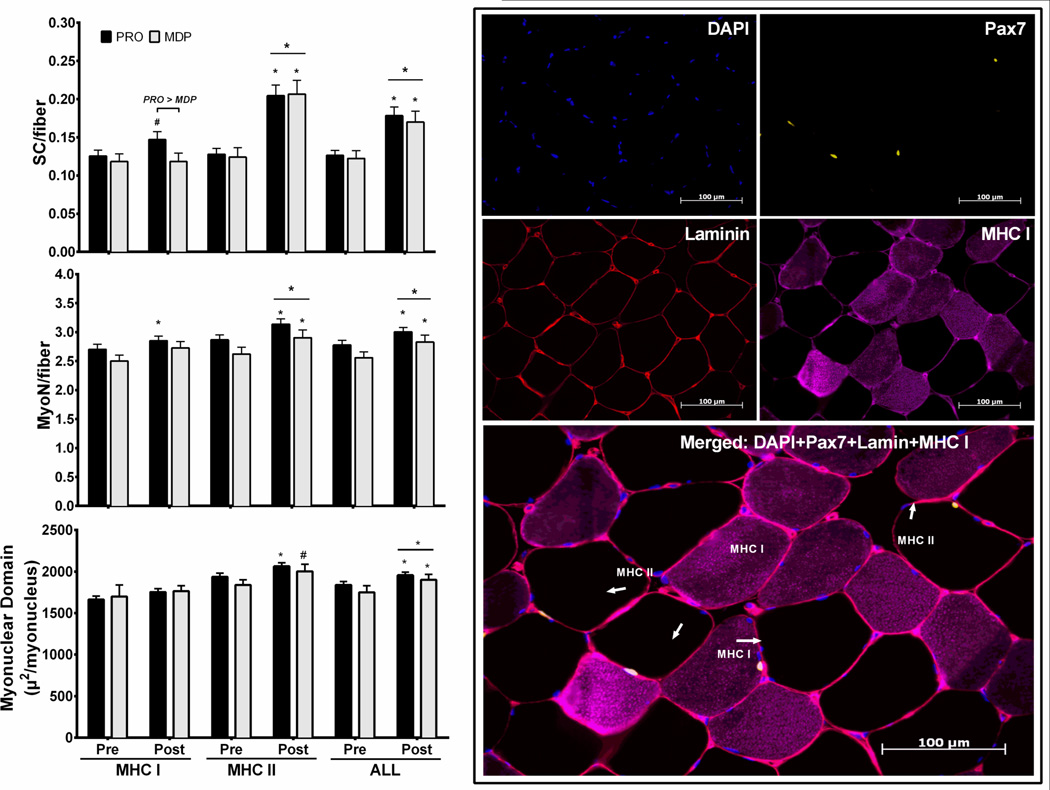
Fiber-type-specific satellite cells and myonuclei were determined as follows. Sections were fixed in ice cold acetone for 3 minutes followed by three 3-minute rinses in PBS. Sections were incubated for at least an hour at RT and then overnight at 4°C with primary antibodies against MHC I (BA.D5 IgG2b, 1:50, Developmental Studies Hybridoma Bank) and Laminin (L9393, 1:200, Sigma-Aldrich, St. Louis, MO). On day 2, three 5-minute washes in PBS preceded a 7 min HO treatment (3% in PBS) to block endogenous peroxidases. After three 3- minute rinses in PBS, sections were incubated for 1 hour with secondary antibodies: Alexa Fluor 647 conjugated goat anti-mouse IgG2b (for MHC I: 1:250, #A21242, Invitrogen) and Alexa Fluor 594 goat anti-rat IgG1 (for laminin: 1:500, #A11034, Invitrogen) diluted in PBS at RT in the dark. After three 3-minute rinses in PBS, sections were blocked for 1 hour in 2.5% normal horse serum (NHS) (Vector, S-2012) at RT. Sections were incubated for at least an hour at RT and then overnight at 4°C with a primary antibody against mouse anti-Pax7 (1:100, Developmental Studies Hybridoma Bank). On day 3 of staining, sections were rinsed 4 × 5 min with PBS before and after 1 hour incubation with goat anti-mouse IgG biotin –SP-conjugated (1:1000) (Jackson Immuno Research, Cat #115-065-205) in 2.5% NHS (for Pax7) at RT. Sections were exposed to a 1 hour incubation of Streptavidin-horseradish peroxidase conjugate (1:100) in PBS, washed, 3 × 5min in PBS, and incubated for 20 min in Alexa Fluor 488 (1:200, Tyramide signal amplification kit, #T20932, Invitrogen) in amplification diluents. Following three 5-min washes in PBS, sections were mounted in 4’,6-diamidino-2-phenylindole (DAPI) containing medium mounting media (Vector, H-1200) and allowed to air dry. This staining protocol, of muscle fiber-type specific identification resulted in DAPI positive nuclei (staining blue), Pax7+ cells (staining yellow), MHC I (staining purple), MHC II (Black - negative staining) and laminin basement membrane (staining red) (Figure 2).
Figure 2.
Vastus lateralis fiber-type specific satellite cell content, myonuclei and myonuclear domain by treatment and representative immunohistochemical image for fiber-type specific identification of Pax7 positive satellite cells and myonuclei. DAPI positive nuclei stained blue (top left), Pax7+ cells stained yellow (top right), laminin basement membrane stained red (middle left), MHC I stained purple and MHC II black - negative staining (middle right) and merged image with arrows highlighting Pax7+ satellite cells (bottom). Protein blend (PB), whey protein (WP) or maltodextrin placebo (MDP). Data are mean ± SEM, n=15 (WP), 22 (PB), 17 (MDP) & 37 (PRO). Units are µ2/myonucleus. * (p<0.05), # (p<0.10) vs pre within that group, main effect of exercise is denoted as a bar across both treatments. PRO (p<0.05) for change in pooled protein group vs pre. PRO > MDP & (p=0.073) via ANCOVA.
Myonuclei were manually counted in images captured at 100x magnification using AxioVision 4.8.2 software to determine the number of myonuclei per fiber. A nucleus was identified as a myonucleus (DAPI+/Pax7-) if it met one of the following criteria: 1) it was clearly located within the laminin boundary; 2) it was on the boundary facing inside the fiber; or 3) greater than 50% of the area fell inside the laminin boundary. Rapid, repeated manual switching back-and-forth between single channel laminin images and merged laminin/DAPI images was used to determine the location of a nucleus as inside or outside of the laminin boundary. Following counting of myonuclei within an image, fiber number was quantified manually to express the number of myonuclei per fiber specific to each fiber type (MHC I or II). Pax7+ nuclei/myofiber, % SC, myonuclei per fiber, and myonuclear domain (fiber area per myonucleus) were determined from >200 cross sectional muscle fibers at each time point.
Statistical Analysis
Values are the raw values or change scores expressed as Mean ± SEM or Mean ± 95% CI as indicated. Primary outcome data were evaluated for equal variances and normality, and no major violations of model assumptions were found. For each outcome, a mixed model ANOVA with fixed effects of treatment, time, and a treatment-by-time interaction was conducted. Subject was treated as a random effect, and time points (e.g., pre and post) were incorporated into each outcome’s mixed model. ANCOVA was conducted on post with treatment as the main effect and pre as the covariate. To test the effect of protein supplementation we pooled the protein treatments WP and PB as PRO. An additional model was conducted with treatment effects of PRO and MDP only. Alpha was set to 0.05 for significance, but p-values between 0.05 and 0.1 were considered indicative of a trend. When interactions were found to be significant (p<0.05) or a trend (p<0.1), Tukey HSD pairwise comparisons were conducted to compare time points and treatments broken down by treatment level and time point, respectively. All analyses were conducted with R 3.1.1. (Vienna, Austria), with the exception of Pearson correlations, which were calculated with Graph Pad Prizm 6.0f for Mac (La Jolla, CA). Effect size and sample size estimations for significant effects of PRO supplementation were calculated as previously described (39). All figures were generated with the same program.
Results
Lean Mass and Strength
Muscle hypertrophy was observed at the whole muscle level. Percent change in DXA whole-body lean mass was increased with all treatments (PB: 5.23±0.52%, WP: 4.08±0.52%; MDP: 3.40±0.80%) (p<0.05) and there was a trend with PB supplementation showing a greater increase in lean mass than MDP (p=0.057). Combined results from pooling treatments with the two protein supplements (PRO) also showed a significant treatment effect (p=0.050) compared to MDP. Percent change in DXA leg lean mass significantly increased across all treatments (PB: 6.03±0.95, WP: 4.67±0.89; MDP: 4.64±0.88) (p<0.05) and was not different by treatment (p=0.444). Thigh circumference (cm) in PB (pre: 50.15±0.78; post: 52.43±0.66) WP (pre: 51.1±1.15; post: 52.60±1.07) and MDP (pre: 49.43±1.09; post: 51.26±1.29) were increased (p<0.05) and were not different across treatment (p=0.454).
At the pre time point, isometric and isokinetic peak torque (relative to body weight) and power for flexion and extension were not different (p>0.10) between treatments (data not shown). Isometric knee extension torque (newton-meters) increased (PB: 34.8±9.8, WP: 44.9±13.6; MDP: 34.0±10.3) across time (p<0.001) but there were no treatment effects (p>0.10) or interactions (p>0.10). Isometric (PB: 8.9±7.0, WP: 9.4±7.6; MDP: 14.6±6.0) and isokinetic (PB: 3.8±6.0, WP: 13.5±9.6; MDP: 7.4±6.6) knee flexion torque did not change across time or demonstrate a treatment effect or an interaction (p>0.10). Isokinetic knee extension torque differed over time (p<0.05) and demonstrated an interaction and a treatment effect (p<0.05). Thus, isokinetic knee extension torque did not change in subjects treated with MDP (1.0±5.8), but treatment with PB (15.9±6.5) and WP (28.8±7.5) similarly resulted in an effect of protein (PRO) that was present compared to MDP for torque (p<0.05). Also, for isokinetic knee extension torque, the change in subjects treated with WP was greater than the change after treatment with MDP (p<0.05).
Muscle RNA concentration
A proxy for translational capacity, vastus lateralis RNA concentration was not different at baseline (PB: 0.559±0.013, WP: 0.570 ± 0.015; MDP: 0.592 ± 0.014 µg RNA/mg muscle) and was increased (p<0.05) with resistance exercise training in each treatment (PB: 0.055 ± 0.018, WP: 0.059 ± 0.019; MDP: 0.072 ± 0.019 µg RNA/mg muscle), but did not differ by treatment or demonstrate an interaction (p>0.10).
Vastus Lateralis MHC Fiber Type Composition
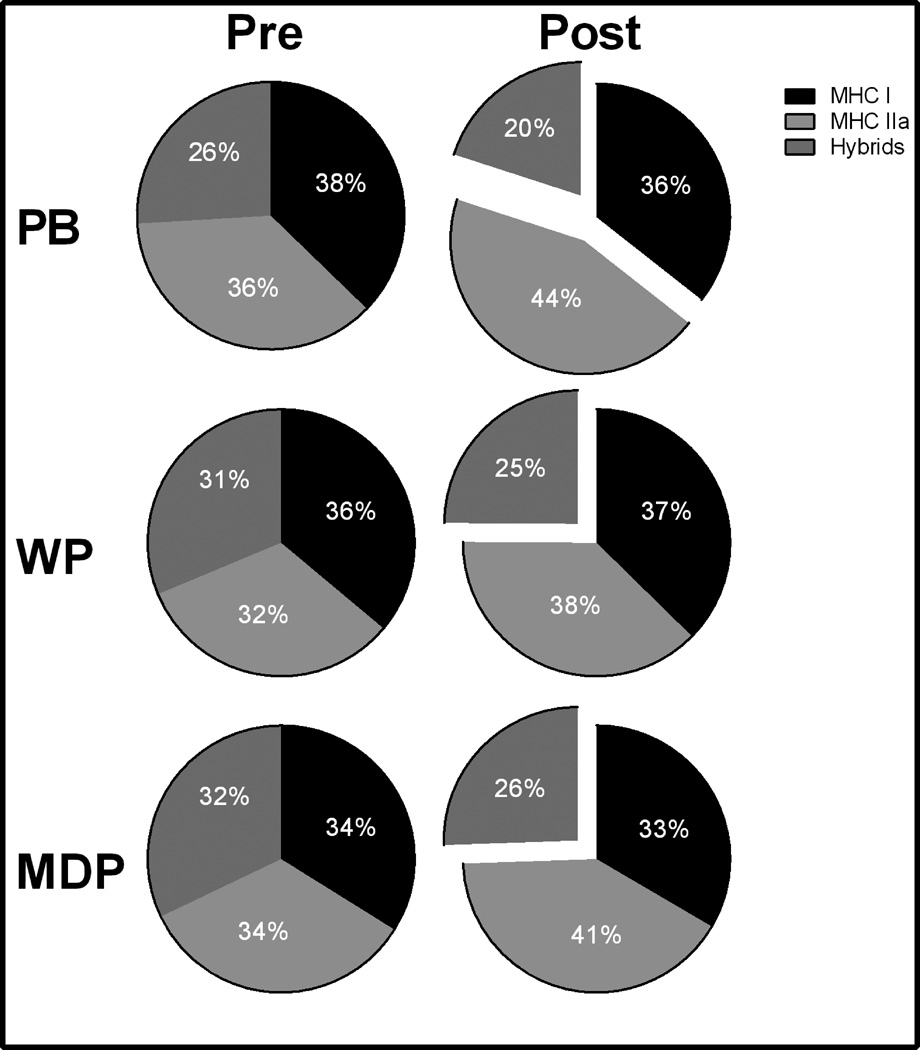
MHC II and MHC hybrid fiber-type compositions were different by time (p<0.05) with no effect of treatment or an interaction (p>0.10). The pre- and post-training MHC fiber-type composition (Figure 3, Table 1) changes showed reduction in hybrid fibers, in all treatments (p<0.05). The reduction in hybrid fibers resulted in a shift toward more pure MHC IIa fibers that was significant for PB and PRO treatments (p<0.05). Myofiber MHC type I frequency remained unchanged (p>0.10) and did not differ by treatment (p>0.10).
Figure 3.
Myosin heavy chain composition (MHC) in the vastus lateralis expressed as relative frequency. PRO indicates an effect of the pooled protein treatments to increase over pre. Significant change vs. pre * (p<0.05). Bar indicates an exercise effect.
Table 1.
Pre- to post-training absolute change for MHC fiber-typing and myofiber CSA immunohistochemical analysis1
| Change | ||||||
|---|---|---|---|---|---|---|
| TRT | PB | WP | PRO | MDP | PRO vs MDP | PRO vs MDP |
| MHC (relative frequency) |
Mixed Model Linear Contrast Estimates | ANCOVA Estimates |
||||
| I | −1.6 (−8.3,5.1) | 1.3 (−6.9,9.4) | −0.2 (−5.3,4.9) | −0.5 (−8.1,7.2) | 0.2 (−4.3,4.6) | 1.8 (−4.0,7.7) |
| IIa | 7.6 (0.3,14.9) | 5.1 (−3.7,14.0) | 6.4 (0.8,11.9) | 6.9 (−1.4,15.3) | −0.3 (−5.2,4.6) | −0.3 (−7.9,7.4) |
| Hybrid | −5.9 (−12.2,0.36) | −6.4 (−14.0,1.2) | −6.2 (−10.9,−1.4) | −6.5 (−13.7,0.55) | 0.22 (−4.0,4.4) | −0.8 (−7.5,5.9) |
| CSA, microns2 | ||||||
| I | 527 (55,998) | 519 (−46,1084) | 523 (168,878) | 849 (321,1378) | −163 (−474,147) | −206 (−721,308) |
| IIa | 963 (409,1518) | 941 (278,1606) | 952 (536,1369) | 936 (315,1558) | 8 (−357,373) | 125 (−506,755) |
| Hybrid | 945 (297,1593) | 1121 (368,1784) | 1033 (554,1513) | 1207 (484,1931) | −87 (−511,337) | 67 (−636,769) |
| All | 857 (343,1372) | 759 (142,1375) | 808 (421,1196) | 1000 (423,1578) | −96 (−435,243) | −33 (−617,551) |
Data are mean ± 95%CI or SEM, n=15 (WP), 22 (PB) & 17 (MDP). Boldface p<0.05, underlined p<0.10 vs pre for that treatment or comparison. MHC, Myosin Heavy Chain. TRT, treatment.
Vastus Lateralis Myofiber Cross-sectional Area
Vastus Lateralis myofiber cross-sectional area (CSA) means (Figure 1, Table 1) were increased following resistance exercise training as revealed by difference by time (p<0.05) with no treatment or interaction effects (p>0.10). Mean fiber area of all fiber types was increased ∼800–900 um2 following resistance exercise training (p<0.05). However, there was no effect of treatment (p=0.967). Mean MHC I CSA was increased ∼500 um2 after WP and PB treatment and ∼750 um2 after supplementation of MDP following resistance exercise training (p<0.05). There was also no effect of treatment (p=0.721). Individual treatment changes revealed significant increases after treatment with PB and MDP (p<0.05) and a trend for an increase (p=0.083) following treatment with WP. Mean MHC IIa CSA was increased ∼900–1000 um2 following resistance exercise training (p<0.05) with no ANCOVA effect of treatment (p=0.921). Mean fiber area (MFA) of all hybrid fiber types increased ∼1000–1100 um2 following resistance exercise training (p<0.05) with no effect of treatment (p=0.906). PRO (PB+WP) treatment displayed significant increases in all fiber types (p<0.05). No significant effect of PRO vs MDP treatment was observed in any fiber type (p>0.423).
Analysis of CSA relative frequency distribution demonstrated that all treatments displayed myofiber growth (rightward shift) (see figure, Supplemental Digital Content 2, Relative frequency of vastus lateralis MHC I, II, and all myofibers pooled by select cross-sectional area bins). However, a few papers report MHC II fiber types are responsive to protein supplementation (12, 19) (see table, Supplemental Digital Content 3, Summary of all protein supplement studies with a placebo group directly assessing muscle mass during RET in young adults). Thus we explored the frequency distributions of MHC II myofibers to determine if slight patterns for differences between treatments not observed with CSA means were evident. CSA bins were expanded to reflect changes in large myofibers (CSA bins in myofbers larger than 6000, 7000, 7500 and 8000 µm2) and also smaller fibers (CSA bins in myofibers sized 1000 to 5000 µm2) following RET (see figure, Supplemental Digital Content 4, Change in the relative frequency of larger vastus lateralis MHC II myofibers by select cross-sectional area bins). Similar to CSA means, this analysis revealed time differences (p<0.001) with no treatment or interaction effects (p>0.10), with the exception of the smaller bin (myofibers sized 1000 to 5000 µm2), which had a treatment effect (p=0.031). This treatment difference was shown as a greater absolute frequency of smaller myofibers at pre (p=0.020) and post (p=0.095) for MDP vs PRO treatment. All treatments demonstrated clear decreases (p<0.001) in the frequency of smaller MHC IIa myofibers (p<0.007). Treatment with (PRO: pooled PB and WP), resulted in clear increases (p<0.001) in the frequency of larger MHC IIa myofibers, whereas increases following treatment with MDP were less evident (p=0.064 to p=0.535). When examining these larger myofiber CSA bins, only tendencies approaching statistical significance (p=0.098–0.194) were observed for an effect of protein (PRO) treatments vs MDP treatment.
Effect size and sample size estimations for significant effects of PRO supplementation during RET on body composition, strength and muscle mass are shown in Supplemental Digital Content (see table, Supplemental Digital Content 5, Effect size and sample size estimations for significant effects of PRO supplementation).
Vastus Lateralis Satellite Cell Content
Vastus Lateralis myofiber Pax7+ satellite cell content displayed a main effect of time (p<0.05), which was evident by a ∼50% increase in abundance following resistance exercise training (Figure 2, Table 2). There were no interactions or effects of treatment with any of the satellite cell outcomes. Since the responses in the PB and WP treatments were identical, the treatments were pooled as PRO and tested against changes in MDP. Mean fiber satellite cell content (SC/fiber), proportion (% SC/myonuclei) and domain (SC/mm2) increased following resistance exercise training (p<0.05) with no effect of treatment (p>0.588). This increase was driven primarily by changes in MHC II myofibers. MHC II satellite cell content (SC/fiber), proportion (% SC/myonuclei) and domain (SC/mm2) increased following resistance exercise training (p<0.05) and there was no effect of treatment (p>0.575). MHC I satellite cell content (SC/fiber) was not globally altered across time (p<0.05), but, there was a trend for an effect of PRO treatment vs MDP treatment (p=0.073). MHC I satellite cell proportion (% SC/myonuclei) and domain (SC/mm2) were unchanged following resistance exercise training (p>0.10). SC domain (SC/mm2) displayed a trend for an effect of PRO treatment vs MDP treatment (p=0.072). MHC I satellite cell proportion (% SC/myonuclei) displayed a weak trend for an effect of PRO treatment vs MDP treatment (p=0.099).
Table 2.
Pre- to post-training absolute change for PAX7 satellite cell immunohistochemical analysis1
| Change | ||||
|---|---|---|---|---|
| TRT | PRO | MDP | PRO vs MDP | PRO vs MDP |
| PAX7+ Satellite Cells/Fiber | Mixed Model Linear Contrast Estimates | ANCOVA Estimates | ||
| I | 0.023 (−0.006,0.053) | 0.001 (−0.043,0.044) | 0.012 (−0.0014,0.037) | 0.032 (−0.003,0.067) |
| II T | 0.078 (0.051,0.106) | 0.082 (0.042,0.123) | −0.002 (−0.026,0.022) | −0.000 (−0.040,0.039) |
| All | 0.053 (0.027,0.079) | 0.048 (0.010,0.086) | 0.002 (−0.020,0.025) | 0.010 (−0.026,0.047) |
| % PAX7+ Satellite Cells/Myonuclei | ||||
| I | 0.8 (−0.3,1.8) | −0.4 (−2.0,1.1) | 0.6 (−0.3,1.5) | 1.1 (−0.2,2.5) |
| II T | 2.4 (1.4,3.4) | 2.7 (1.2,4.2) | −0.2 (−1.1,0.7) | −0.2 (−1.7,1.2) |
| All | 1.6 (0.8,2.5) | 1.4 (0.2,2.7) | 0.1 (−0.6,0.9) | 0.3 (−0.9,1.5) |
| PAX7+ Satellite Cells/mm2 | ||||
| I | 2.3 (−3.1,7.8) | −3.6 (−11.7,4.4) | 3.0 (−1.7,7.7) | 6.2 (−0.6,13.0) |
| II | 8.6 (3.6,13.6) | 10.2 (3.0,17.5) | −0.8 (−5.1,3.5) | −1.9 (−8.9,5.0) |
| All | 5.8 (1.5,10.1) | 4.8 (−1.5,11.1) | 0.5 (−3.2,4.2) | 0.7 (−5.3,6.8 |
Data are mean ± 95%CI, n=15 (WP), 22 (PB) & 17 (MDP). Boldface p<0.05, underlined p<0.10 vs pre for that treatment or comparison. T: P<0.05 for an overall change over time. TRT, Treatment.
Vastus Lateralis Myonuclei
Vastus Lateralis myofiber myonuclear content and domain (Figure 2, Table 3) were altered by resistance exercise training. Pre to post changes in all treatments pooled together demonstrated an increase (p<0.05) with RET. Thus a main effect of time was seen for MHC I, MHC IIa and mean myonuclei content (p<0.05). There were no interactions or effects of treatment with any of the myonuclei outcomes (p>0.10). Since the responses in the PB and WP treatments were identical, the treatments were pooled as PRO and tested against changes in MDP Mean myonuclei content (MyoN/fiber) increased following resistance exercise training (p<0.05) with no effect of PRO vs MDP treatment (p=0.602). The changes in MHC II fibers exerted the greatest influence on the mean myofiber MyoN response. The effect of time was observed as a trend for an overall increase from pre to post (p=0.096) in MHC II fibers, which was seen as an increased change score (p<0.05) with no PRO vs MDP treatment effect (p=0.378). MHC I myonuclei content did not show changes from pre to post, except with PRO (p<0.05) and a PRO treatment effect vs MDP was not evident (p=0.143).
Table 3.
Pre- to post-training absolute change for myonuclei immunohistochemical analysis1
| Change | ||||
|---|---|---|---|---|
| TRT | PRO | MDP | PRO vs MDP | PRO vs MDP |
| MyoN/Fiber | Mixed Model Linear Contrast Estimates | ANCOVA Estimates | ||
| I | 0.12 (−0.08,0.32) | 0.22 (−0.06,0.51) | −0.05 (−0.22,0.12) | 0.01 (−0.24,0.26) |
| II | 0.21 (0.02,0.40) | 0.20 (−0.08,0.48) | 0.01 (−0.16,0.17) | 0.11 (−0.14,0.38) |
| All | 0.18 (0.01,0.36) | 0.22 (−0.04,0.48) | −0.02 (−0.18,0.13) | 0.06 (−0.18,0.30) |
| Myonuclear Domain | ||||
| I | 107 (−63,276) | 54 (−193,301) | 26 (−120,173) | −10 (−146,125) |
| II | 187 (45,329) | 226 (18,434) | −20 (−143,103) | 4 (−178,186) |
| All | 157 (22,293) | 182 (−16,380) | −12 (−129,105) | 23 (−116,161) |
Data are mean ± 95% CI, n=15 (WP), 22 (PB), 17 (MDP) & 37 (PRO). Boldface p<0.05, underlined p<0.10 vs pre for that treatment or comparison. TRT, Treatment.
Myonuclear domain (Figure 2, Table 3) (MHC II myofibers and all myofibers pooled) demonstrated a slight increase following resistance exercise training (p<0.05), while MHC I myofibers were not significant (p>0.10). There were no interactions or treatment effects (p>0.10). Overall increases from pre to post were evident in MHC II myofibers (p<0.05) and demonstrated as a trend (p=0.086) when all myofiber types were pooled. Both PRO and MDP treatments increased myonuclear domain (p<0.05) in MHC II myofibers. When pooling all fiber types, a significant increase was indicated in treatment with PRO (p<0.05) and a trend following treatment with MDP (p=0.084). This increase was likely due to greater statistical power by grouping a greater number of myofibers with PRO. No changes were observed in MHC I fiber myonuclear domain (p>0.10). There was no significant effect in the change of PRO over MDP in all fibers pooled (p=0.746), MHC I (p=0.880) or MHC II (p=0.379) myofibers.
Correlational Analysis
Associations and statistical results between measures of muscle hypertrophy (lean mass, myofiber CSA, muscle thickness) and satellite cells, myonuclei and myonuclear domain are shown in Supplemental Digital Content (see tables, Supplemental Digital Content 6, Correlation analysis between myofiber cross-sectional area and myonuclear number; and Supplemental Digital Content 7, secondary correlation analysis). Myonuclei number per fiber was highly correlated with fiber size at each time point and in all fiber types and with the increase in myofiber size. Myofiber number per fiber change was well correlated to CSA change and also to the change in satellite cells per fiber. Those who had smaller myonuclear domains at Pre experienced expansion of their myonuclear domain at post. Also, those particitants with the largest CSA changes also experienced the greatest expansion of their myonuclear domain. Absolute vales of lean mass, but not change values correlated with myofiber size. The was a moderate association for MFA change to positively correlate with all satellite cells per fiber change, which was prominent in MHC I, but not MHC II fibers.
Discussion
This is the first study reporting the role of protein supplementation and protein supplementation type on fiber-type specific adaptations of myofiber growth, satellite cells and myonuclei during traditional progressive resistance training using shortening and lengthening contractions. We demonstrated a similar increase in myofiber CSA, satellite cell content and myonuclear addition for all three treatment groups. This study was a follow-up analysis to our initial clinical trial (38), where we demonstrate minimal trends for group differences in whole body and arm specific lean mass (PB >MDP), yet no differences in the increases in leg lean mass or vastus lateralis muscle thickness,. Collectively, these data suggest that the additional lean mass in the PB group was accrued in other locations (arms or trunk) and/or that leg hypertrophy had peaked for all treatments after 3 months of RET with our protocol. This indicates that protein supplementation during resistance exercise training did not enhance muscle specific adaptions in the lower limb.
Unfortunately, most of the studies claiming an effect of protein to enhance muscle adaptations to RET rely on whole body lean mass. Indeed, in examination of the literature, we have previously highlighted that ∼62 to ∼140 participants (depending on the clinical trial, Effect Size 0.24–0.67) would be needed to find a statistical effect of protein supplementation on whole body lean mass or fat-free mass (39). Similar to these studies we report an effect for protein supplementation to increases whole body lean mass as compared to placebo (effect size 0.565), yet we found similar changes in more direct measures of muscle hypertrophy (ultrasound muscle thickness (38), leg lean mass and myofiber type specific CSA) following RET. As we previously highlighted (38, 39), the use and interpretation of whole body lean mass DXA data to act as the sole measure of muscle hypertrophy is suspect and would likely have limited functional relevance on force production.
Our whey protein treatment demonstrated similar adaptations when compared to maltodextrin placebo. Contrary to popular dogma, it is not unusual to observe no effect of protein supplementation, in particular whey protein, over placebo on lean mass or myofiber CSA (39). A meta-analysis determined that protein supplementation during resistance exercise training in young adults will produce greater increases in vastus lateralis CSA, ∼250 µm2, yet that analysis only included data from 4 studies and is in conflict with results from another meta-analysis (41). We are aware of only 3 studies demonstrating greater changes in vastus lateralis myofiber CSA (2, 12, 19) and 2 studies with magnetic resonance imaging (MRI) (13, 21) comparing protein versus carbohydrate placebo supplementation during RET. In one of the vastus lateralis myofiber CSA studies, the placebo group started with higher CSA and did not experience hypertrophy following resistance exercise training (2), while the other two studies demonstrated this effect only in MHC II fibers (12, 19). In comparison, 5 other studies demonstrated equivalent increases in vastus lateralis myofiber CSA in protein supplemented treatments [whey protein (n=3), milk (n=1) or EAA (n=1)] and carbohydrate placebo treatments (6, 9, 23, 31, 32). In addition, studies utilizing MRI of the biceps (10) or latissimus dorsi (33) and ultrasound (3, 4, 22, 23, 48) of the thigh muscles have clearly shown the same pattern; no effect of protein supplementation (whey) to enhance vastus lateralis muscle hypertrophy. Given these findings, it is no surprise that only one study on protein supplementation showed an enhancement of strength, even though myofiber CSA was not different with protein supplementation (9). The remainder of the studies demonstrate identical increases in strength with protein supplementation compared to carbohydrate placebo (2, 6, 10, 12, 13, 19, 21–23, 31–33), similar to our observations, in part. Indeed, in examination of the literature, we have previously highlighted that ∼40 to 500 participants (depending on the clinical trial) would be needed to find a statistical effect of protein supplementation on myofiber CSA (39), we show in this trial that (see figure, Supplemental Digital Content 4, Change in the relative frequency of larger vastus lateralis MHC II myofibers by select cross-sectional area bins) 115 to >1000 participants (Effect Size: Mean fiber Area −0.174, MHC I −0.375, MHC II 0.052) would be required. Also, 550 participants would be needed to find a statistical effect (Effect Size: −0.174) of protein supplementation on vastus lateralis muscle thickness. These data further illustrate the minimal effect of protein supplementation to enhance thigh, in particular, vastus lateralis muscle strength and hypertrophy during resistance exercise training.
Analysis of mean CSA, the predominant method utilized in these types of clinical trials, can obscure subtle changes in myofiber hypertrophy. Recently, Farup et al. completed an elegant study comparing the effect of whey protein supplementation on isolated lengthening or shortening contractions of skeletal muscle (12). They demonstrated that myofiber CSA was enhanced in MHC II fibers with whey protein supplementation during shortening, but not lengthening contractions. They conducted a follow up analysis by demonstrating a tendency (p<0.10) for protein supplementation to result in a shift toward a greater frequency of larger myofibers (>8000 µm2) and a lower frequency of smaller fibers (>1000 <5000 µm2) post-training, compared to post-training whey-supplemented eccentric training. Although we did not observe a difference in the CSA means between the protein-supplemented and carbohydrate placebo treatments, we similarly demonstrated that protein supplementation displayed a pattern for a slightly greater improvement (non-significant) in the frequency of MHC II bins with larger fibers vs the maltodextrin placebo. This suggests that protein supplementation may play a very limited role in expanding MHC II size during resistance exercise training. However, we stress that this effect is minimal, and given the low statistical confidence seen in these examples, we believe this effect is limited to a sub-population of myofibers/individuals that is likely an example of responder/non-responder clustering. The functional relevance of this finding is unknown. However, a minimal effect of protein supplementation to increase whole body lean mass (not limb/appendicular lean mass) following RET does exist, which we have speculated (39) may likely include non-force producing lean mass (e.g., trunk muscle). This would result in increased body weight with similar changes in muscle mass. Thus, to maintain the mass to strength ratio – this pattern with MHC II myofibers may provide support for increased muscle force to serve as a compensatory mechanism and offset the increased weight. In partial support of this concept, we demonstrated improved isokinetic torque in the protein-supplemented treatments only, suggesting a possible role for the changes in these MHC II fibers with protein supplementation.
Although, satellite cells are not necessary to support hypertrophy through myonuclear addition (14), they are involved in the magnitude of muscle growth (5, 14, 37). Given that protein supplementation was also thought to influence the magnitude of muscle growth we sought to examine the role between satellite cells and protein supplementation. Very little research has examined the acute effects of protein/amino acids on the enhancement of satellite cells content following RE. We were aware of one study that employed a severe 4-day protein restriction protocol to compare normal (∼90g) vs very low (∼11g) of protein per day, to find no effect on skeletal muscle SC content during the 3 day recovery period following RE (43). It is hard to find relevancy in that study design to our findings other than an overall lack of effect of protein to enhance myogenic adaptations. Olsen et al. first demonstrated that chronic resistance exercise training (RET) with protein supplementation may provide a slight enhancement of the satellite cell pool compared to RET alone (32). Based on basic science and pre-clinical findings, we anticipated that protein supplementation would enhance satellite cell activity and content through mTORC1 (17, 40) and particularly on MHC II fibers (1, 11). Instead, we demonstrated similar increases in satellite cell content between treatments, which were driven primarily through increases in MHC II fibers. However, we did demonstrate a significant increase in satellite cell number per fiber for MHC I fibers with protein supplementation but not with a maltodextrin placebo. This resulted in a trend for an effect of protein (p=0.073) over maltodextrin placebo, which was also seen when expressed as SC/mm2 and proportion of SC/MyoN. Interestingly, MHC I, but not MHC II, satellite cell number per myofiber change was correlated with CSA change. Farup and colleagues demonstrated similar findings, to ours, after 3 months of RET with protein supplementation in MHC I, but not MHC II fibers, suggesting that protein supplementation may provide greater expansion of the SC pool in this fiber type to regulate myofiber growth. Taken together, these findings are somewhat contradictory, although they may possibly be explained as differences between pre-clinical and clinical research. MHC II fibers are thought to be most responsive to heavy strength training (47), yet the training program we utilized was whole body, high-intensity training, which likely recruited all fiber types. We also discovered that those who had lower initial satellite cell content in MHC I fibers, experienced the greatest change in MHC I satellite cells per fiber (r=-0.529, p<0.001) and MHC I myonuclei per fiber (r=-0.383, p=0.006). However this effect was absent in MHC II fibers. These data suggest that myonuclear addition was a primary fate of satellite cells in MHC I fibers. Our data is in agreement with Bellany et al. (5), but is in contrast with a previous report (37) in the literature suggesting that a higher pre-training satellite cell content is a characteristic of high-responders to RET. We are unsure as to why this difference exists in the literature, but we suspect that the differences could possibly be explained by the use of different markers of satellite cells (NCAM for Petrella et al. and Pax7 in this manuscript and with Bellany et al.)
Myonuclear accretion occurred with RET, as has been previously demonstrated (36), but was not different by treatment as has been demonstrated elsewhere (33, 44). A significant increase was seen with PB and WP but not MDP treatment. Others have suggested that CSA changes greater than ∼15% are needed before changes in myonuclear number occur (25, 36). Here we demonstrated 15–20%, ∼20% and 20–30% increases in CSA of MHC I, II and hybrid fibers, respectively, suggesting that our larger sample size included enough participants with substantial changes in CSA to detect changes in myonuclear number with RET. Myonuclear number was highly correlated with fiber size at each time point and in all fiber types (r=0.724–0.826, p<0.001), illustrating remarkable control of the myonuclear domain, as others have shown (20, 24, 26, 29, 30).
Even with such tight coupling of myonuclear number to myofiber size we observed a slight but significant expansion of the myonuclear domain, ∼150 µm2/myonucleus, after 3 months of RET. In fact, a significant, inverse relationship (r=-0.634, p<0.001) was demonstrated, indicating that those with smaller initial myonuclear domains experienced the greatest change in myonuclear domain over the course of the training. This effect was most evident in MHC II fibers, highlighting their remarkable plasticity to this contractile stimulus. Maintenance of this expanded domain was likely assisted by increased total RNA content (translational capacity), and through increases in myonuclear size, as demonstrated by Cabric et al. in human skeletal muscle following 3 weeks of electrical stimulation (7). This would suggest enhanced transcriptional capacity per myonucleus.
Certainly, many studies, including those from our laboratory, have clearly demonstrated a robust effect of protein/amino acids to stimulate the early metabolic response of muscle growth (i.e. muscle protein synthesis) (39). The question persists as to why these effects are not as readily discovered in physiological outcomes following chronic exposure to such a stimulus (35). Our hypothesis is that physiological adaptation may best explain the insensitivity to protein supplementation typically seen in chronic exercise studies. Farup and colleagues demonstrated that whey protein supplementation following eccentric exercise accelerated the satellite cell pool expansion compared to consumption of carbohydrate placebo (11). However, by 168 hr post exercise (11) and after 12 weeks of training (12) the satellite cell pool was identical between treatments. For novice exercisers, peak satellite cell activity occurs after 2 weeks of RET (18). Also, some evidence suggests that the majority of the satellite cell pool expansion occurs early, 1–4 wk into RET, during dietary supplementation (32). These data suggest that protein supplementation may provide an enhancement early during exercise training, but additional protein is unlikely to confer added benefit to further promote muscle growth as adaptation occurs. Interestingly, this time frame is also when most myofiber damage and remodeling is likely to occur. Although attractive, this hypothesis has not yet been clearly proven (34, 35). Protein metabolism also becomes more efficient after resistance training (80, 81), which provides further support that in the presence of a well-balanced diet, muscle hypertrophy and strength are not further augmented by protein supplementation (35).
Limitations
A limitation to this study is that several samples from the WP group were not suitable for immunohistochemical analysis and as a result the sample size of that group was smaller than the size of the other treatments. It is possible that we were slightly underpowered in our ability to determine certain exercise effects (myonuclear domain or number); however, statistical analysis clearly demonstrated an absence of treatment differences in most outcomes suggesting that sample size was not an issue in delineating treatment effects. It was not feasible for us to sample at earlier time points throughout the training, although this may have provided greater insight regarding the effect of protein supplementation. This would have allowed a preferential examination of satellite cell content, myonuclear domain and myonuclear addition during resistance exercise training. Also, although many of the inferences were made using correlational analyses, a major strength of this study is that a cohort of this size makes correlational analysis possible and generates additional research questions.
The majority of similar studies collected post biopsies at 24–48 hours following the last exercise session, however, we took our samples at 72 hours post-exercise. It could be hypothesized that this 72 hour time point was examining the acute effects of the last exercise session. We found only one paper which examined the acute response (in the trained state) demonstrating an increase in satellite cell content at 72 h following exercise and a return to basal –pre-training values at 96 h (4 days) post training. This recent study may suggest that our post biopsy effects could be due to the acute exercise response. However, there are several studies in the literature with conflicting results showing that increases in satellite cell content are detected 4 days post RE training (28, 42, 46) and after 10 days of detraining (25). These conflicting data suggest that the timing of sampling for satellite cell studies should be an important considerations in designing these studies.
Conclusions
Daily supplementation of protein during resistance exercise training did not enhance muscle adaptations in the vastus lateralis as demonstrated by the nearly identical increases in muscle strength, hypertrophy (whole muscle and myofiber-type specific), MHC II satellite cell content, and overall myonuclear addition. When results from the soy-dairy protein blend and whey protein treatments were pooled, very modest effects of protein supplementation existed to enhance MHC I satellite cell content, isokinetic torque and a slight expansion of a greater proportion of larger MHC II fibers over placebo after resistance exercise training. We conclude that protein supplementation during resistance exercise training has a modest effect on promoting a larger gain in whole body lean mass as compared to exercise training without protein supplementation. However, protein supplementation does not enhance resistance exercise induced increases in myofiber hypertrophy, satellite cell content or myonuclear addition in young healthy men. We propose that as long as protein intake is adequate during muscle overload the adaptations in muscle growth and function will not be influenced by protein supplementation. Future work should focus on the effectiveness of protein supplementation or increasing daily protein intake in clinical populations undergoing significant muscle atrophy.
Supplementary Material
Acknowledgments
The (PAX7 and BA.D5) developed by [Kawakami, A. and Schiaffino, S.] were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. We wish to thank the Clinical Research staff of the Institute for Translational Science Clinical Research Center at UTMB for assisting in screening and consenting patients and participants and for assisting in data collection. We wish to thank DPT Samantha Dillon, DPT Matthew Nguyen, SPT Benjamin Brightwell, Camille Brightwell and SPT Jennifer Thedinga for their assistance in supervising the exercise training of research participants and Michael Borack, Jared Dickinson, Melissa Markofski, and Syed Husaini for assistance during the clinical portion of the study. We would also like to thank Dr. Marinel Ammenheuser for editing the manuscript.
This project is supported by a grant from DuPont Protein Solutions with assistance from NIH R01 AR49877, T32-HD07539, NIDRR H133P110012, in part by a NIH Clinical and Translational Science Award UL1TR000071 from the National Center for Advancing Translational Sciences and from NIH/NIA grant P30 AG024832.
Footnotes
This was a subset of the trial registered at clinicaltrials.gov as NCT01749189
Authors’ contributions
BBR, EV, PTR, RRD, MBC and RM designed the research; PTR, CSF, BBR, SI, MSB and RRD conducted research; BBR, EV, PTR, SHH, RRD, CSF, KJ, MBC and RM, reviewed the manuscript; PTR, RRD, MSB, KJ, and BBR analyzed data; and PTR and BBR wrote the manuscript and had primary responsibility for final content. MBC and RM were not involved with conducted research or laboratory analysis. All authors read and approved the final manuscript.
Conflicts of Interest
PTR, MSB, RRD, SI, MBC, RM, KJ, EV, BBR have no conflicts of interest.
The authors declare that this study was funded by Dupont Nutrition & Health. Representatives from Dupont Nutrition & Health were not involved with data collection and laboratory analysis
The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by ACSM.
Supplemental Digital Content (SDC)
SDC 1: List of Abbreviations
SDC 2: Relative frequency of vastus lateralis MHC I, II, and all myofibers pooled by select cross-sectional area bins
SDC 3: Summary of all protein supplement studies with a placebo group directly assessing muscle mass during RET in young adults
SDC 4: Change in the relative frequency of larger vastus lateralis MHC II myofibers by select cross-sectional area bins.
SDC 5: Effect Size and Sample Size Estimations for significant effects of PRO supplementation during RET on body composition, strength and muscle mass1
SDC 6: Correlation analysis between myofiber cross-sectional area and myonuclear number
SDC 7: Secondary correlation analysis
References
- 1.Alway SE, Pereira SL, Edens NK, Hao Y, Bennett BT. beta-Hydroxy-beta-methylbutyrate (HMB) enhances the proliferation of satellite cells in fast muscles of aged rats during recovery from disuse atrophy. Exp Gerontol. 2013;48(9):973–984. doi: 10.1016/j.exger.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Andersen LL, Tufekovic G, Zebis MK, et al. The effect of resistance training combined with timed ingestion of protein on muscle fiber size and muscle strength. Metabolism. 2005;54(2):151–156. doi: 10.1016/j.metabol.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Babault N, Deley G, Le Ruyet P, Morgan F, Allaert FA. Effects of soluble milk protein or casein supplementation on muscle fatigue following resistance training program: a randomized, double-blind, and placebo-controlled study. Journal of the International Society of Sports Nutrition. 2014;11:36. doi: 10.1186/1550-2783-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babault N, Paizis C, Deley G, et al. Pea proteins oral supplementation promotes muscle thickness gains during resistance training: a double-blind, randomized, Placebo-controlled clinical trial vs. Whey protein. Journal of the International Society of Sports Nutrition. 2015;12(1):3. doi: 10.1186/s12970-014-0064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellamy LM, Joanisse S, Grubb A, et al. The Acute Satellite Cell Response and Skeletal Muscle Hypertrophy following Resistance Training. PLOS ONE. 2014;9(10):e109739. doi: 10.1371/journal.pone.0109739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bird SP, Tarpenning KM, Marino FE. Independent and combined effects of liquid carbohydrate/essential amino acid ingestion on hormonal and muscular adaptations following resistance training in untrained men. Eur J Appl Physiol. 2006;97(2):225–238. doi: 10.1007/s00421-005-0127-z. [DOI] [PubMed] [Google Scholar]
- 7.Cabric M, Appell HJ, Resic A. Effects of electrical stimulation of different frequencies on the myonuclei and fiber size in human muscle. International journal of sports medicine. 1987;8(5):323–326. doi: 10.1055/s-2008-1025677. [DOI] [PubMed] [Google Scholar]
- 8.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. The American journal of clinical nutrition. 2012;96(6):1454–1464. doi: 10.3945/ajcn.112.037556. [DOI] [PubMed] [Google Scholar]
- 9.Cribb PJ, Williams AD, Stathis CG, Carey MF, Hayes A. Effects of whey isolate, creatine, and resistance training on muscle hypertrophy. Med Sci Sports Exerc. 2007;39(2):298–307. doi: 10.1249/01.mss.0000247002.32589.ef. [DOI] [PubMed] [Google Scholar]
- 10.Erskine RM, Fletcher G, Hanson B, Folland JP. Whey protein does not enhance the adaptations to elbow flexor resistance training. Med Sci Sports Exerc. 2012;44(9):1791–1800. doi: 10.1249/MSS.0b013e318256c48d. [DOI] [PubMed] [Google Scholar]
- 11.Farup J, Rahbek SK, Knudsen IS, de Paoli F, Mackey AL, Vissing K. Whey protein supplementation accelerates satellite cell proliferation during recovery from eccentric exercise. Amino Acids. 2014;46(11):2503–2516. doi: 10.1007/s00726-014-1810-3. [DOI] [PubMed] [Google Scholar]
- 12.Farup J, Rahbek SK, Riis S, Vendelbo MH, Paoli F, Vissing K. Influence of exercise contraction mode and protein supplementation on human skeletal muscle satellite cell content and muscle fiber growth. J Appl Physiol (1985) 2014;117(8):898–909. doi: 10.1152/japplphysiol.00261.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farup J, Rahbek SK, Vendelbo MH, et al. Whey protein hydrolysate augments tendon and muscle hypertrophy independent of resistance exercise contraction mode. Scand J Med Sci Sports. 2014;24(5):788–798. doi: 10.1111/sms.12083. [DOI] [PubMed] [Google Scholar]
- 14.Fry CS, Lee JD, Jackson JR, et al. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28(4):1654–1665. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fry CS, Noehren B, Mula J, et al. Fibre type-specific satellite cell response to aerobic training in sedentary adults. J Physiol. 2014;592(Pt 12):2625–2635. doi: 10.1113/jphysiol.2014.271288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gundersen K. Muscle memory and a new cellular model for muscle atrophy and hypertrophy. The Journal of Experimental Biology. 2016;219(2):235–242. doi: 10.1242/jeb.124495. [DOI] [PubMed] [Google Scholar]
- 17.Han B, Tong J, Zhu MJ, Ma C, Du M. Insulin-like growth factor-1 (IGF-1) and leucine activate pig myogenic satellite cells through mammalian target of rapamycin (mTOR) pathway. Molecular reproduction and development. 2008;75(5):810–817. doi: 10.1002/mrd.20832. [DOI] [PubMed] [Google Scholar]
- 18.Hanssen KE, Kvamme NH, Nilsen TS, et al. The effect of strength training volume on satellite cells, myogenic regulatory factors, and growth factors. Scand J Med Sci Sports. 2013;23(6):728–739. doi: 10.1111/j.1600-0838.2012.01452.x. [DOI] [PubMed] [Google Scholar]
- 19.Hartman JW, Tang JE, Wilkinson SB, et al. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. The American journal of clinical nutrition. 2007;86(2):373–381. doi: 10.1093/ajcn/86.2.373. [DOI] [PubMed] [Google Scholar]
- 20.Hikida RS, Staron RS, Hagerman FC, et al. Effects of high-intensity resistance training on untrained older men. II. Muscle fiber characteristics and nucleo-cytoplasmic relationships. The journals of gerontology. Series A, Biological sciences and medical sciences. 2000;55(7):B347–B354. doi: 10.1093/gerona/55.7.b347. [DOI] [PubMed] [Google Scholar]
- 21.Hulmi JJ, Kovanen V, Selanne H, Kraemer WJ, Hakkinen K, Mero AA. Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids. 2009;37(2):297–308. doi: 10.1007/s00726-008-0150-6. [DOI] [PubMed] [Google Scholar]
- 22.Hulmi JJ, Laakso M, Mero AA, Häkkinen K, Ahtiainen JP, Peltonen H. The effects of whey protein with or without carbohydrates on resistance training adaptations. Journal of the International Society of Sports Nutrition. 2015;12(1):48. doi: 10.1186/s12970-015-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hulmi JJ, Tannerstedt J, Selanne H, Kainulainen H, Kovanen V, Mero AA. Resistance exercise with whey protein ingestion affects mTOR signaling pathway and myostatin in men. J Appl Physiol. 2009;106(5):1720–1729. doi: 10.1152/japplphysiol.00087.2009. [DOI] [PubMed] [Google Scholar]
- 24.Kadi F, Eriksson A, Holmner S, Thornell LE. Effects of anabolic steroids on the muscle cells of strength-trained athletes. Med Sci Sports Exerc. 1999;31(11):1528–1534. doi: 10.1097/00005768-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Kadi F, Schjerling P, Andersen LL, et al. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol. 2004;558(Pt 3):1005–1012. doi: 10.1113/jphysiol.2004.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadi F, Thornell LE. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochemistry and cell biology. 2000;113(2):99–103. doi: 10.1007/s004180050012. [DOI] [PubMed] [Google Scholar]
- 27.Kornasio R, Riederer I, Butler-Browne G, Mouly V, Uni Z, Halevy O. Beta-hydroxy-beta-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways. Biochimica et biophysica acta. 2009;1793(5):755–763. doi: 10.1016/j.bbamcr.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Nilwik R, van Loon LJC. Elderly Men and Women Benefit Equally From Prolonged Resistance-Type Exercise Training. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2013;68(7):769–779. doi: 10.1093/gerona/gls241. [DOI] [PubMed] [Google Scholar]
- 29.Lindstrom M, Pedrosa-Domellof F, Thornell LE. Satellite cell heterogeneity with respect to expression of MyoD, myogenin, Dlk1 and c-Met in human skeletal muscle: application to a cohort of power lifters and sedentary men. Histochemistry and cell biology. 2010;134(4):371–385. doi: 10.1007/s00418-010-0743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindstrom M, Thornell LE. New multiple labelling method for improved satellite cell identification in human muscle: application to a cohort of power-lifters and sedentary men. Histochemistry and cell biology. 2009;132(2):141–157. doi: 10.1007/s00418-009-0606-0. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell CJ, Oikawa SY, Ogborn DI, et al. Daily chocolate milk consumption does not enhance the effect of resistance training in young and old men: a randomized controlled trial. Appl Physiol Nutr Metab. 2015;40(2):199–202. doi: 10.1139/apnm-2014-0329. [DOI] [PubMed] [Google Scholar]
- 32.Olsen S, Aagaard P, Kadi F, et al. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol. 2006;573(Pt 2):525–534. doi: 10.1113/jphysiol.2006.107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paoli A, Pacelli Q, Cancellara P, et al. Protein Supplementation Does Not Further Increase Latissimus Dorsi Muscle Fiber Hypertrophy after Eight Weeks of Resistance Training in Novice Subjects, but Partially Counteracts the Fast-to-Slow Muscle Fiber Transition. Nutrients. 2016;8(6):331. doi: 10.3390/nu8060331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasiakos SM, Lieberman HR, McLellan TM. Effects of protein supplements on muscle damage, soreness and recovery of muscle function and physical performance: a systematic review. Sports Med. 2014;44(5):655–670. doi: 10.1007/s40279-013-0137-7. [DOI] [PubMed] [Google Scholar]
- 35.Pasiakos SM, McLellan TM, Lieberman HR. The effects of protein supplements on muscle mass, strength, and aerobic and anaerobic power in healthy adults: a systematic review. Sports Med. 2015;45(1):111–131. doi: 10.1007/s40279-014-0242-2. [DOI] [PubMed] [Google Scholar]
- 36.Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab. 2006;291(5):E937–E946. doi: 10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- 37.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol (1985) 2008;104(6):1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- 38.Reidy PT, Borack MS, Markofski MM, et al. Protein Supplementation Has Minimal Effects on Muscle Adaptations during Resistance Exercise Training in Young Men: A Double-Blind Randomized Clinical Trial. J Nutr. 2016;146(9):1660–1669. doi: 10.3945/jn.116.231803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reidy PT, Rasmussen BB. Role of Ingested Amino Acids and Protein in the Promotion of Resistance Exercise-Induced Muscle Protein Anabolism. J Nutr. 2016;146(2):155–183. doi: 10.3945/jn.114.203208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodgers JT, King KY, Brett JO, et al. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert) Nature. 2014;510(7505):393–396. doi: 10.1038/nature13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoenfeld BJ, Aragon AA, Krieger JW. The effect of protein timing on muscle strength and hypertrophy: a meta-analysis. Journal of the International Society of Sports Nutrition. 2013;10(1):53. doi: 10.1186/1550-2783-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snijders T, Nederveen JP, Joanisse S, et al. Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. Journal of Cachexia, Sarcopenia and Muscle. 2016 Aug 4; doi: 10.1002/jcsm.12137. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snijders T, Verdijk LB, McKay BR, et al. Acute Dietary Protein Intake Restriction Is Associated with Changes in Myostatin Expression after a Single Bout of Resistance Exercise in Healthy Young Men. The Journal of Nutrition. 2014;144(2):137–145. doi: 10.3945/jn.113.183996. [DOI] [PubMed] [Google Scholar]
- 44.Spillane M, Willoughby DS. Daily Overfeeding from Protein and/or Carbohydrate Supplementation for Eight Weeks in Conjunction with Resistance Training Does not Improve Body Composition and Muscle Strength or Increase Markers Indicative of Muscle Protein Synthesis and Myogenesis in Resistance-Trained Males. Journal of Sports Science & Medicine. 2016;15(1):17–25. [PMC free article] [PubMed] [Google Scholar]
- 45.Thalacker-Mercer AE, Petrella JK, Bamman MM. Does habitual dietary intake influence myofiber hypertrophy in response to resistance training? A cluster analysis. Appl Physiol Nutr Metab. 2009;34(4):632–639. doi: 10.1139/H09-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verdijk LB, Gleeson BG, Jonkers RAM, et al. Skeletal Muscle Hypertrophy Following Resistance Training Is Accompanied by a Fiber Type–Specific Increase in Satellite Cell Content in Elderly Men. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2009;64A(3):332–339. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle; from birth to old age. Age. 2014;36(2):545–547. doi: 10.1007/s11357-013-9583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vieillevoye S, Poortmans JR, Duchateau J, Carpentier A. Effects of a combined essential amino acids/carbohydrate supplementation on muscle mass, architecture and maximal strength following heavy-load training. Eur J Appl Physiol. 2010;110(3):479–488. doi: 10.1007/s00421-010-1520-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.