Abstract
Aim
We have previously shown that the secreted glycoprotein MFG-E8 has anti-inflammatory and anti-osteoclastogenic properties. Our objective was to investigate the potential of MFG-E8 as a diagnostic or therapeutic agent in periodontitis.
Materials and Methods
Periodontitis was induced in non-human primates (NHPs) by placing ligatures around posterior teeth on both halves of the mandible for a split-mouth design: one side was treated with MFG-E8−Fc and the other with Fc control. Disease was assessed by clinical periodontal examinations, radiographic analysis of bone loss, and analysis of cytokine mRNA expression in gingival biopsy samples. Gingival crevicular fluid (GCF) was collected from human healthy volunteers or subjects with gingivitis, chronic moderate periodontitis, or chronic severe periodontitis. Additionally, GCF was collected from a subset of severe periodontitis patients following scaling and root planing (SRP) and after pocket reduction surgery. GCF was analyzed to quantify MFG-E8 and periodontitis-relevant cytokines using multiplex assays.
Results
In NHPs, sites treated with MFG-E8-Fc exhibited significantly less ligature-induced periodontal inflammation and bone loss than Fc control-treated sites. In humans, the GCF levels of MFG-E8 were significantly higher in health than in periodontitis, whereas the reverse was true for the proinflammatory cytokines tested. Consistently, MFG-E8 was elevated in GCF after both non-surgical (SRP) and surgical periodontal treatment of periodontitis patients.
Conclusion
MFG-E8 is, in principle, a novel therapeutic agent and biomarker of periodontitis.
Keywords: periodontitis, inflammation, MFG-E8, cytokines, non-human primates
Introduction
Recent human microbiome analyses and mechanistic studies in relevant preclinical models indicate that chronic periodontitis is not, strictly speaking, a bacterial infection but rather a dysbiotic disease (Diaz et al., 2016, Lamont and Hajishengallis, 2015). In susceptible individuals, dysbiosis leads to dysregulated host-microbial interactions and destructive periodontal inflammation, which in turn exacerbates dysbiosis. In this regard, inflammatory breakdown products of connective tissue are released into the gingival crevicular fluid (GCF) and can be used as nutrients by pathobiont species in the subgingival microbial community, which can thereby flourish and persist (Diaz et al., 2016, Hajishengallis, 2014, Marsh, 2003). This feed-forward loop between inflammation and dysbiosis may contribute to the chronicity of periodontitis and implies that host-response modulation approaches may have a great potential as adjunctive treatments in conventional periodontal therapy, which is only partially effective for the majority of the patients (Tonetti et al., 2011). Moreover, host-derived molecules that can be quantified non-invasively in the GCF and accurately reflect periodontal inflammation and disease activity may have utility as biomarkers of periodontitis (Baeza et al., 2016). In this study, we have investigated a homeostatic molecule, termed milk fat globule epidermal growth factor 8 (MFG-E8), for its potential as a therapeutic or diagnostic agent in periodontitis.
MFG-E8 (also known as lactadherin) is a 55–66–kDa secreted glycoprotein that was originally identified in mice, where it is readily detectable in the mammary tissue and especially in the lactating gland (Stubbs et al., 1990). Subsequent studies in both mice and humans showed that MFG-E8 is widely expressed in various organs and tissues (e.g., spleen, lungs, liver, kidneys, and intestine, in addition to mammary glands) by macrophages, fibroblasts, dendritic and epithelial cells (Aziz et al., 2011). The protein was named MFG-E8 owing to its abundance in milk fat globules and its amino-acid sequence similarities with epidermal growth factor and the discoidin domains of blood coagulation factor VIII. Indeed, in mice, the secreted molecule consists of two N-terminal epidermal growth factor (EGF)-like domains and two C-terminal discoidin-like domains (Stubbs et al., 1990). Similarly to its mouse counterpart, human MFG-E8 contains two discoidin-like domains; however, it retained only the second EGF-like domain, which includes an integrin-binding RGD motif (Yi, 2016).
Despite their structural similarity, mouse and human MFG-E8 share only 57% amino-acid sequence identity, suggesting that the evolved gene may have, in part, distinct functions in primates (the human protein is 96–99% identical with its counterparts in non-human primate species) (Podlaha et al., 2006). Nevertheless, certain important functions appear to be shared between mouse and human MFG-E8, such as the ability to contribute to inflammation resolution by promoting efferocytosis, i.e., the phagocytosis of apoptotic cells. In this regard, MFG-E8 acts as a bridge between the “eat-me” signal phosphatidylserine on apoptotic cells (recognized by the C-terminal discoidin-like domains of MFG-E8) and the αvβ3 integrin on phagocytes (bound by the RGD motif in the N-terminal region of MFG-E8) (Hanayama et al., 2002). Moreover, another conserved anti-inflammatory function of MFG-E8 involves its ability to bind αvβ3/5-integrins and induce STAT3-mediated activation of suppressor of cytokine signaling-3 in macrophages (Aziz et al., 2011). Accordingly, recombinant human MFG-E8 was shown to inhibit inflammation in animal models of sepsis or cerebral ischemic injury (Cheyuo et al., 2012, Shah et al., 2012).
Our group has recently described a novel function of MFG-E8. Specifically, we showed that MFG-E8 is expressed by and regulates the function of human and mouse osteoclasts in ways that prevent excessive resorptive activity (Abe et al., 2014). Consistent with its anti-osteoclastogenic and anti-inflammatory properties, recombinant mouse MFG-E8 inhibited periodontal inflammation and bone loss in a mouse model of ligature-induced periodontitis. Moreover, in the same model, mice genetically deficient in MFG-E8 exhibited increased susceptibility to periodontal inflammation and bone loss (Abe et al., 2014). Subsequent independent studies confirmed the expression of MFG-E8 by osteoclasts and additionally showed that MFG-E8–deficient mice are also more susceptible to ovariectomy-induced osteoporosis and to serum transfer-induced arthritis as compared to MFG-E8–proficient controls (Sinningen et al., 2015, Albus et al., 2016).
The main objective of the present study was to determine the relevance of MFG-E8 in human periodontitis. To this end, we followed two distinct approaches. We first tested the ability of recombinant human MFG-E8 (expressed as an Fc fusion protein; MFG-E8–Fc) to inhibit ligature-induced periodontitis in non-human primates (NHPs), a highly relevant preclinical model of the human disease (Brecx et al., 1985, Page and Schroeder, 1982, Maekawa et al., 2016). Moreover, we investigated possible association of MFG-E8 GCF levels with human periodontitis. In this context, the expression of MFG-E8 in humans and animal models is strongly regulated in various conditions: It declines considerably in inflammatory conditions, including sepsis, colitis, acute lung injury, ischemia/reperfusion injury, atherosclerosis, and Alzheimer’s disease (Aziz et al., 2011, Boddaert et al., 2007, Ait-Oufella et al., 2007), whereas it is upregulated in certain other pathological conditions, such as systemic lupus erythematosus, lung fibrosis, melanoma, and breast cancer (Aziz et al., 2011, Yi, 2016). We reasoned that if MFG-E8 is strongly up- or down-regulated in human periodontitis (compared to health and/or gingivitis) as reflected by quantitative changes in the GCF, then the monitoring of MFG-E8 might have predictive or diagnostic value. Our collective findings from both approaches (i.e., in NHPs and humans) suggest that MFG-E8 can potentially find application as a novel host-modulation therapeutic in periodontitis and/or as a potential indicator of this oral inflammatory disease.
Materials and Methods
Expression and purification of human MFG-E8-Fc
Human MFG-E8−Fc was expressed and purified in our laboratory, as follows. The cDNA for human MFGE8 (encoding MFG-E8) was obtained from OriGene (Rockville, MD, USA). The gene encoding full-length MFG-E8 protein sequence was amplified by PCR followed by cloning into the HindIII and KpnI sites of the mammalian expression vector pSecTag2 (InVitrogen/ThermoFischer Scientific, Waltham, MA, USA), which contains an N-terminal secretory tag (murine Igk leader sequence) and a C-terminal polyhistidine tag (His-tag). The human IgG Fc gene (synthesized by Integrated DNA Technologies; Coralville, IA, USA) was cloned between MFGE8 and His-tag at EcoRI and XhoI sites and in frame with both sequences. MFG-E8 was therefore expressed as a soluble Fc-fusion protein (MFG-E8-Fc; 72.9kDa) secreted into the culture medium of transfected HEK-293F suspension cells (InVitrogen/ThermoFischer Scientific). The protein was purified by Ni-affinity chromatography after loading concentrated culture supernatants onto a His-Trap column connected to an ÄKTA-FPLC system (GE Healthcare, Madison, Wisconsin, USA). The eluted MFG-E8-Fc was pooled, dialyzed, and the identity and purity of the protein were confirmed by immunoblotting using anti-MFG-E8 and anti-Fc antibodies from R&D Systems (Minneapolis, MN, USA) and Southern Biotech (Birmingham, AL, USA), respectively, and SDS-PAGE. Fc protein control was purchased from R&D Systems.
Non-human primates: Clinical examinations, periodontitis, and sample collection
All animal procedures were performed according to protocols reviewed and approved by the Institutional Animal Care and Use Committees of the University of Pennsylvania and the Covance Research Products, where the work was performed. All animals enrolled in the study were systemically healthy and maintained good systemic health during the observation period. No adverse effects were observed during the course of the study. Three adult female cynomolgus monkeys (Macaca fascicularis) (5–8 years old; 3.3–4.8 kg) were purchased from an approved vendor from stocks that are bred in captivity and were used in the study after a seven-day acclimation period. The animals were socially housed in steel cages elevated off the floor, in a controlled environment with a temperature of 64° to 84° F and a light/dark cycle of 12:12 hours. Environmental enrichment was provided through daily handling by animal care technicians, environmental enrichment items, visual contact with other study animals, and appropriate background music in the animal facility. Each animal was offered a measured amount of an approved feed mixture. Fresh, potable drinking water was available to the animals ad libitum. Clinical periodontal examinations, dental X-rays, and periodontal tissue biopsies were performed in a manner similar to a human clinical study. The animals were not euthanized at the completion of the study.
The monkeys were used for local (intragingival) administration of MFG-E8−Fc or Fc control. All treatments and clinical examinations were performed on anesthetized animals. Experimental periodontitis was induced by tying size-2.0 silk ligatures around posterior mandibular teeth (second premolars and first molars) as previously described (Shin et al., 2015). To reduce the number of animals required, ligatures were placed on both halves of the mandible for a split-mouth experimental design: one side was treated with MFG-E8−Fc and the other with Fc control.
Clinical examinations were performed and diagnosis was established according to the criteria of the American Academy of Periodontology for human periodontal diseases (Armitage, 1999). Examinations using a periodontal probe were performed at baseline and throughout the study (weeks 1, 2, 4, and 6) to monitor the progression of the disease and the effects of MFG-E8−Fc treatment. The examinations included determination of probing pocket depth (PPD), clinical attachment level (CAL), gingival index (GI), bleeding on probing (BOP), tooth mobility index (Mob), and plaque index (PI). PPD, CAL, and BOP were measured at six sites: mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, and disto-lingual aspects of each of the premolar and molar maxillary teeth. GI and PI were assessed at four sites (buccal, lingual, mesial and distal). GI and BOP are measures of periodontal inflammation, CAL and PPD assess tissue destruction, and the tooth mobility index is often associated with bone loss. At the beginning of the study, the gingival margins in all animals were at the cement-enamel junction and there was no recession of the gingival margin during the study, hence the PPD readings equaled CAL (CAL therefore was not shown in Results).
Therapeutic treatments were performed three times per week, starting three days after study initiation. Using a 30-G short needle, MFG-E8−Fc was injected into the interdental papillae from the first premolar to the second molar (i.e., three sites; 50 μg/site in a volume of 50 μL) on one side of the mouth. On the contralateral side, an equal amount and volume of Fc control was injected in a similar manner. At the completion of the study, the ligatures were removed, and biopsies of gingiva and bone were removed en bloc corresponding to the first molar tooth at the ligated sides.
At baseline and at the completion of the study (6 weeks), bitewing dental X-ray images were taken using a digital X-ray dental system (Vatech, Fort Lee, NJ, USA) to evaluate bone loss. A paralleling technique was used to standardize the radiographs. Specifically, the angle of the X-ray beam and the distance between the film and the X-ray unit were kept constant by means of a bite-wing holder. The bone heights were determined using the digital X-ray images and Nikon Imaging System software. Specifically, the distances from the cement–enamel junction (CEJ) to the alveolar bone crest (ABC) were measured by using a 5-mm metal wire (attached on the tooth surface at the time the X-rays were taken) for digital unit calibration. The CEJ-ABC distances were determined at six points (first premolar, distal; second premolar, mesial and distal; first molar, mesial and distal; second molar, mesial) and the data shown in figure 2 reflect the 6-site total. To calculate bone loss, the 6-site total CEJ-ABC distance at 6 week was subtracted from the 6-site total CEJ-ABC distance at baseline and the results were presented in mm (negative values indicated bone loss relative to the baseline).
Figure 2. Inhibition of periodontal bone loss after treatment of NHP periodontitis with MFG-E8-Fc.
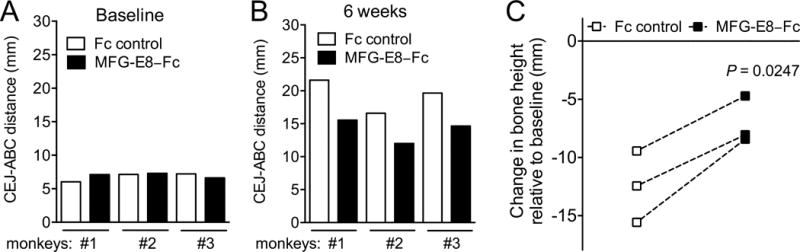
Three monkeys were treated as described in the legend to figure 1, and their mandibular bone heights (CEJ-ABC distance) were measured using Nikon Imaging System software and standardized X-ray images (taken at baseline and at week 6). Measurements were made at six points (first premolar, distal; second premolar, mesial and distal; first molar, mesial and distal; second molar, mesial) and the data in A and B reflect the 6-site total at baseline and at week 6, respectively. For each pair of Fc control and MFG-E8-Fc treatments, bone loss was calculated as bone height at baseline minus bone height at 6 week (C); the difference between Fc control and MFG-E8-Fc treatments was significant (P < 0.05; paired t test).
Quantitative real-time PCR
Gingival tissue from non-human primate biopsies was used to extract total RNA (using RNeasy Mini Kit (Qiagen, Hilden, Germany), which was quantified by spectrophotometry at 260 and 280 nm. The RNA was reverse-transcribed using the High Capacity RNA-to-cDNA Kit (Life Technologies, Gaithersburg, MD) and real-time PCR with cDNA was performed using the Applied Biosystems 7500 Fast Real-Time PCR System according to the manufacturer’s protocol (Life Technologies). Data were analyzed using the comparative (ΔΔCt) method. TaqMan probes, sense primers, and antisense primers for detection and quantification of genes investigated in this paper were purchased from Life Technologies. The primers were specific for human genes and cross-reacted with the non-human primate genes investigated in this study.
Human subjects
Research was performed under an Institutional Review Board–approved protocol. Signed informed consent was obtained to procure samples of GCF from individuals treated in the postgraduate clinic of the Department of Periodontics, University of Pennsylvania School of Dental Medicine. The subjects enrolled were grouped as healthy, gingivitis, moderate periodontitis, or severe periodontitis. The main inclusion criteria were adult subjects with good general health, at least 10 teeth in the functional dentition (excluding third molars), and who had never received periodontal treatment at the time of initial clinical examination. For patients with periodontitis, at least two quadrants had to involve deep probing pocket depths, clinical attachment loss and radiographically detectable bone loss. PPD and CAL were measured at 6 sites for every tooth using a manual probe (UNC 15, Hu-Friedy, Chicago, IL, USA). Subjects with the following conditions were excluded: Smoking, drug or alcohol abuse; uncontrolled diabetes; previous head and neck radiotherapy; history of chemotherapy in the previous 12 months; immunocompromised subjects or subjects suffering from systemic diseases that significantly affect the periodontium (e.g, neutropenia); subjects taking medications known to affect the periodontium (e.g., phenytoin, calcium channel blockers, and cyclosporine); pregnant or lactating females; subjects requiring prophylactic antibiotics or who have used systemic antibiotics within three months prior to enrollment; subjects taking steroid medications except for acute topical treatment; subjects who currently have, or with history of (within three months) the following diseases: severe cardiovascular, pulmonary or liver diseases, end-stage renal disease, active malignancy, cerebral vascular disease, HIV, tuberculosis, hepatitis or other active infectious diseases.
All subjects who volunteered to participate underwent routine periodontal examination and were classified into four groups based predominantly on the clinical criteria established by the 1999 International Workshop for a Classification of Periodontal Diseases and Conditions with slight modifications (Armitage, 1999, Armitage, 2004). Specifically, the subjects were classified according to the following definitions:
Periodontally healthy (n = 7): subjects with PPD < 3 mm, no signs of gingival inflammation, attachment loss, BOP or radiographic evidence of bone loss.
Plaque-induced gingivitis (n = 7): subjects with PPD < 3 mm and signs of gingival inflammation or BOP but without attachment loss or radiographic bone loss
Chronic moderate periodontitis (n = 12): subjects with CAL of 3 to 4 mm and moderate radiographic bone loss.
Chronic severe periodontitis (n = 14): subjects with CAL of ≥ 5 mm and severe radiographic bone loss.
The study had no dropouts prior or during the treatment.
GCF sample collection and analysis
All samples were collected by one of the investigators (F.M.) after reviewing the medical history and performing clinical and radiographic periodontal examination and establishing a periodontal diagnosis, as described above. Five teeth were selected from each subject with at least one anterior tooth and two posterior teeth from two different quadrants. The teeth were dried and isolated with cotton rolls and the samples were obtained from the surface with the deepest PD. Samples were collected using PerioPaper strips (OraFlow Inc., Plainview, N.Y., USA), which were placed in the selected sites until mild resistance was felt and kept in place for 30 s, as previously described (Griffiths, 2003, Bostanci et al., 2007). Samples with blood or saliva contamination were discarded. The individual paper strips were subsequently transferred into polypropelene tubes and were stored at −80°C until analysis. At the time of analysis, the contents of the GCF samples were eluted from the paper strips using phosphate-buffered saline (200 μl per sample).
The levels of MFG-E8 and various cytokines in collected GCF samples were measured using bead-based multiplex assays on a Bio-Plex system following the instructions of the manufacturers (R&D Systems, Minneapolis, MN, USA, for MFG-E8; EMD Millipore, Darmstadt, Germany, for cytokines). Twenty-five μl of each eluted sample were used without further dilution in the assays. MFG-E8 and cytokine levels were calculated from standard curves and data were presented as picograms (pg) per site for total levels by taking under consideration the volume of the GCF samples. Sites with cytokine levels below the detection limits of the assays were reported as 0 pg/site. The detection limits of the assays were: MFG-E8, 5.6 pg/ml; IL-1β, 0.8 pg/ml; IL-6, 0.9 pg/ml; IL-17A, 0.7 pg/ml; RANKL, 5.0 pg/ml; OPG, 7.0 pg/ml).
Statistical analysis
The preclinical study in NHPs involved a split-mouth experimental design (one side was treated with MFG-E8−Fc and the other with Fc control). Therefore, each animal served as its own control, which allowed two-tailed paired t test analysis and the potential to obtain statistical significant differences with n = 3, according to our previous publication using the same model (Maekawa et al., 2014, Shin et al., 2015). Statistical analysis was performed using the GraphPad Prism software (La Jolla, CA, USA). For the human GCF analysis, the comparison of values among health and disease groups was performed using generalized estimating equations (GEE) model and repeated measures analysis as implemented in SAS Proc GENMOD (SAS Institute Inc, Cary, NC). P < 0.05 was set as the level of significance.
Results
Inhibition of periodontitis in NHPs by locally administered MFG-E8−Fc
To evaluate MFG-E8 as a potential therapeutic agent in human periodontitis, we tested whether human MFG-E8−Fc can inhibit inflammatory bone loss in a highly relevant preclinical model of periodontitis. To this end, we induced ligature-induced periodontitis in the posterior mandibular teeth of cynomolgus monkeys and treated them using a split-mouth experimental design; i.e., one side of the mandible was locally injected in the gingiva with MFG-E8−Fc and the other side with Fc control. Each animal therefore served as its own control. Treatments started three days after placing the ligatures and continued three times weekly throughout the 6-week-long study. As expected, the placement of ligatures caused a gradual increase in clinical indices that measure periodontal inflammation and tissue destruction, as well as in the plaque index (PI), a clinical measure of biofilm accumulation on tooth surfaces (Fig. 1). Importantly, however, the sites treated with MFG-E8−Fc displayed significantly reduced pocket depths (PPD), clinical inflammation (GI and BOP) and mobility of the associated teeth (Mob), as compared to sites treated with Fc control (P < 0.05; Fig. 1, A–D). On the other hand, differences in PI did not reach statistical significance (Fig. 1E). Consistent with the observed decrease in tooth mobility, radiographic analysis showed that MFG-E8−Fc caused a significant inhibition of bone loss (Fig. 2). At baseline, experimental and control sites had comparable bone heights (CEJ-ABC distances) (Fig. 2A); however, at the end of the 6-week experimental period, the sites injected with MFG-E8−Fc exhibited lower bone heights than their corresponding contralateral sites that were injected with Fc control (Fig. 2B). These differences reached statistical significance (P < 0.05; Fig. 2C). In line with the clinical data, quantitative real-time PCR revealed that the MFG-E8-Fc treatment resulted in decreased expression of proinflammatory and osteoclastogenic cytokines (Fig. 3). Specifically, IL-17A and RANKL mRNA expression was significantly inhibited by >90% (Fig. 3C and 3D; P < 0.01 and P < 0.05, respectively). Although MFG-E8-Fc appeared to reduce the mean expression of IL-1β and IL-6, these differences did not reach statistical significance (Fig. 3A and 3B; P > 0.05 relative to Fc control). In contrast to RANKL, the expression of its natural inhibitor OPG (Miossec and Kolls, 2012) was comparable in MFG-E8−Fc–treated and Fc control–treated sites (Fig. 3E). In summary, MFG-E8−Fc treatment resulted in decreased clinical bone loss and inflammation parameters, consistent with a decrease in proinflammatory cytokines in the periodontal tissue of NHPs subjected to ligature-induced periodontitis.
Figure 1. MFG-E8-Fc decreases inflammatory clinical parameters of NHP periodontitis.
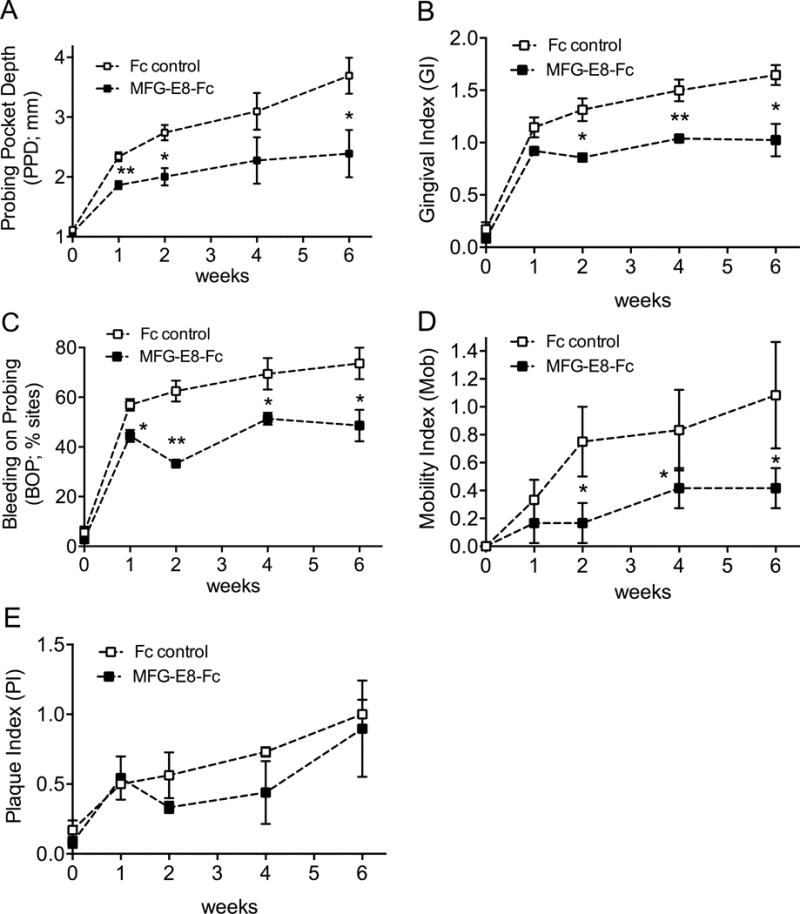
Starting 3 days after initiation of ligature-induced periodontitis, MFG-E8-Fc or Fc control (both at 50 μg) were injected locally into the mandibular interdental papillae from the first premolar to the second molar, three times weekly, in opposite sides of the mouth (split-mouth design). The animals were clinically examined at the indicated timepoints and the effects of MFG-E8-Fc on the indicated inflammatory clinical parameters were recorded: (A) probing pocket depth (PPD), (B) gingival index (GI), (C) bleeding on probing (BOP), (D) mobility index (Mob), and (E) plaque index (PI). Data are means ± SD (n = 3 monkeys). *P < 0.05; **P < 0.01 compared with time-matched control (paired t test).
Figure 3. Effect of MFG-E8-Fc treatment on cytokine expression in the NHP periodontal tissue.
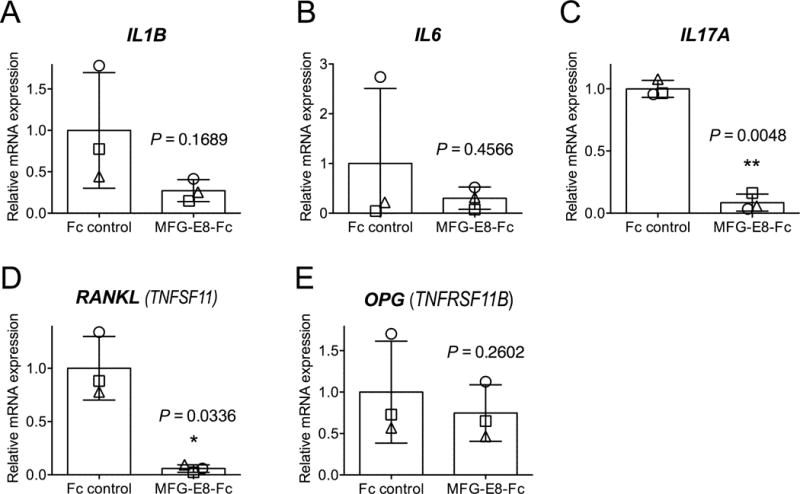
Dissected gingiva were processed for real-time PCR to determine mRNA expression of the indicated cytokines. Results were normalized to those of GAPDH mRNA and were presented as fold change relative to the mRNA transcript levels of the Fc control group, which were assigned an average value of 1. The bar graphs indicate the means ± SD (n = 3 animals, each of which is represented with a distinct symbol). *P < 0.05; **P < 0.01 versus Fc control (paired t test).
Decreased GCF levels of MFG-E8 in periodontitis as compared to gingivitis and health
The ability of exogenously administered MFG-E8 to protect against periodontal inflammation and bone loss in NHPs prompted us to examine the levels of endogenous MFG-E8 in human periodontal health vs. disease. To this end, we sought to assess MFG-E8 in the GCF, which provides a ‘window’ for non-invasive analysis of periodontal conditions (Uitto, 2003). Table S1 presents the mean demographic (age and gender) and PPD data of each of the groups of subjects, classified as periodontally healthy, gingivitis, moderate or severe periodontitis. A total of 40 subjects (mean age of 53.2 ± 4.3; 45.7% males and 54.3% females) were included in GCF analysis with a total of 200 sites (5 distinct sites per individual). The site distribution was as follows: 35 sites (7 subjects) in the healthy group, 35 sites (7 subjects) in the gingivitis group, 60 sites (12 subjects) in the moderate periodontitis group, and 70 sites (14 subjects) in the severe periodontitis group (Fig. 4).
Figure 4. Flowchart showing distribution of human subjects in health, disease and treatment groups.
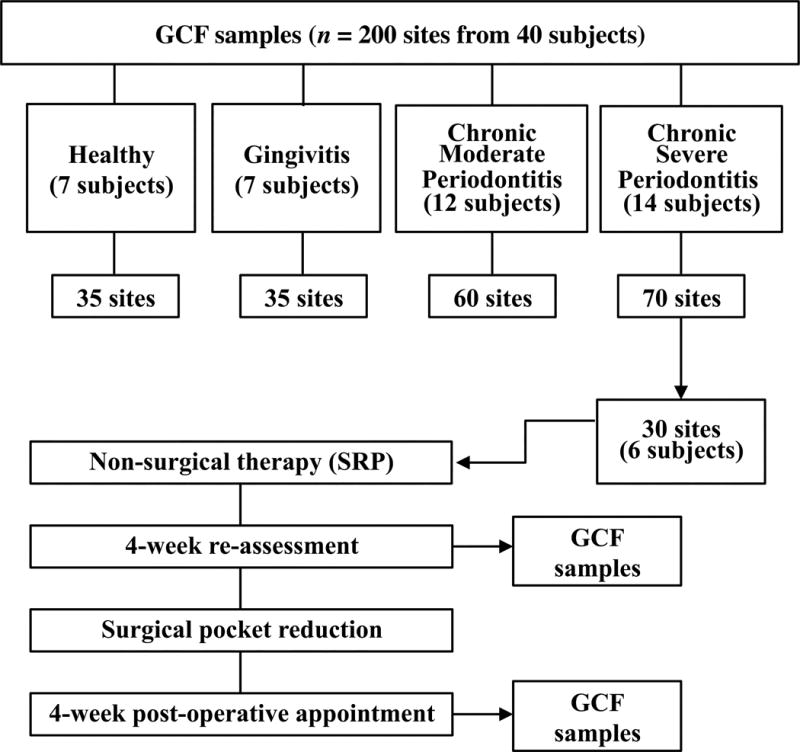
GCF was collected at baseline from all subjects. GCF collection was repeated for a subset of chronic severe periodontitis patients following non-surgical treatment (SRP) and after surgical treatment for pocket reduction.
Analysis of the GCF samples readily detected the presence of MFG-E8 in healthy subjects and patients (Fig. 5A). To our knowledge, this is the first time that the presence of MFG-E8 is demonstrated in human GCF. No significant difference was found in the levels of MFG-E8 in health as compared to gingivitis. In both of these two groups, however, the MFG-E8 levels were significantly higher than those of the periodontitis groups (P < 0.0001; Fig. 5A). Moreover, the moderate periodontitis group showed significantly higher levels of MFG-E8 than the severe periodontitis group (P < 0.0001; Fig. 5A). Therefore, the levels of MFG-E8 appeared to decrease with increasing disease severity. For comparative purposes and to control the validity of our approach, we also analyzed the levels of several cytokines that have been previously analyzed in the GCF. In agreement with earlier reports (Bostanci et al., 2007, Vernal et al., 2005, Mogi et al., 1999), we found that periodontitis is associated with significantly increased levels of IL-1β, IL-6, IL-17A, and RANKL but decreased levels of OPG, as compared to health or gingivitis (Fig. 5, B–F). In other words, from health to severe periodontitis, the GCF levels of pro-inflammatory/pro-osteoclastogenic cytokines followed a pattern that was opposite of that of MFG-E8, whereas OPG, a natural inhibitor of RANKL and hence osteoclastogenesis, displayed a similar pattern with MFG-E8 (Fig. 5).
Figure 5. GCF levels of MFG-E8 and indicated cytokines in periodontal health and disease.
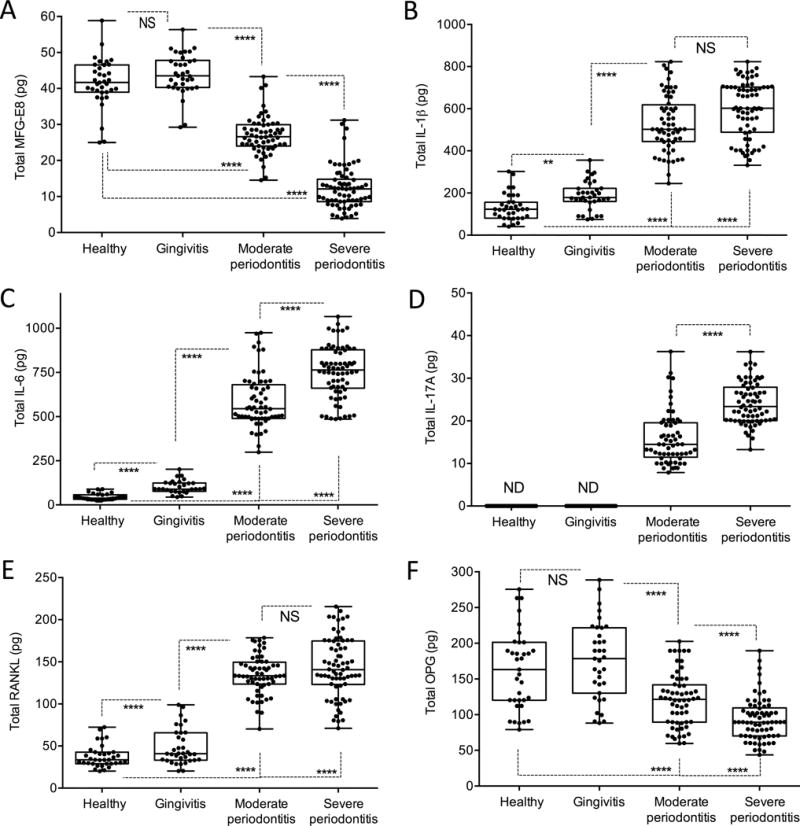
GCF was collected from 40 subjects classified as either periodontally healthy or as having plaque-induced gingivitis, chronic moderate periodontitis, or chronic severe periodontitis. GCF was sampled from a total of 200 distinct sites (5 sites per individual; see Fig. 4 for site distribution among groups). Bead-based multiplex assays on a Bio-Plex system were used to assay the following molecules: (A) MFG-E8, (B) IL-1β, (C) IL-6, (D) IL-17A, (E) RANKL and (F) OPG. Dots represent individual values from distinct sampled sites and each box in the box-and-whisker plots extends from the 25th to the 75th percentiles. **P < 0.01; ****P < 0.0001 between indicated groups (Repeated measures analysis as implemented in SAS Proc GENMOD). ND, not detectable; NS, not significant.
Increased levels of MFG-E8 in the GCF following periodontal treatment
A subset of six patients from the severe periodontitis group were re-examined and sampled for GCF (5 sites per individual) at a re-assessment appointment following non-surgical periodontal treatment (consisting of scaling and root planing [SRP] and home care oral hygiene instructions) as well as after pocket reduction surgery (Fig. 4). In comparison to the baseline, the levels of MFG-E8 were significantly (P < 0.01) increased after SRP and were further elevated significantly (P < 0.0001) after surgical therapy for pocket reduction (Fig. 6A). The opposite pattern was observed for pro-inflammatory/pro-osteoclastogenic cytokines, as the levels of IL-6, IL-17A and RANKL significantly declined following SRP and surgery for pocket reduction (Fig. 6, B–D). The success of the therapy was confirmed clinically by the significantly reduced probing depths after both non-surgical and surgical therapy (Fig. 6E).
Figure 6. GCF levels of MFG-E8 and indicated cytokines after treatment of severe periodontitis.
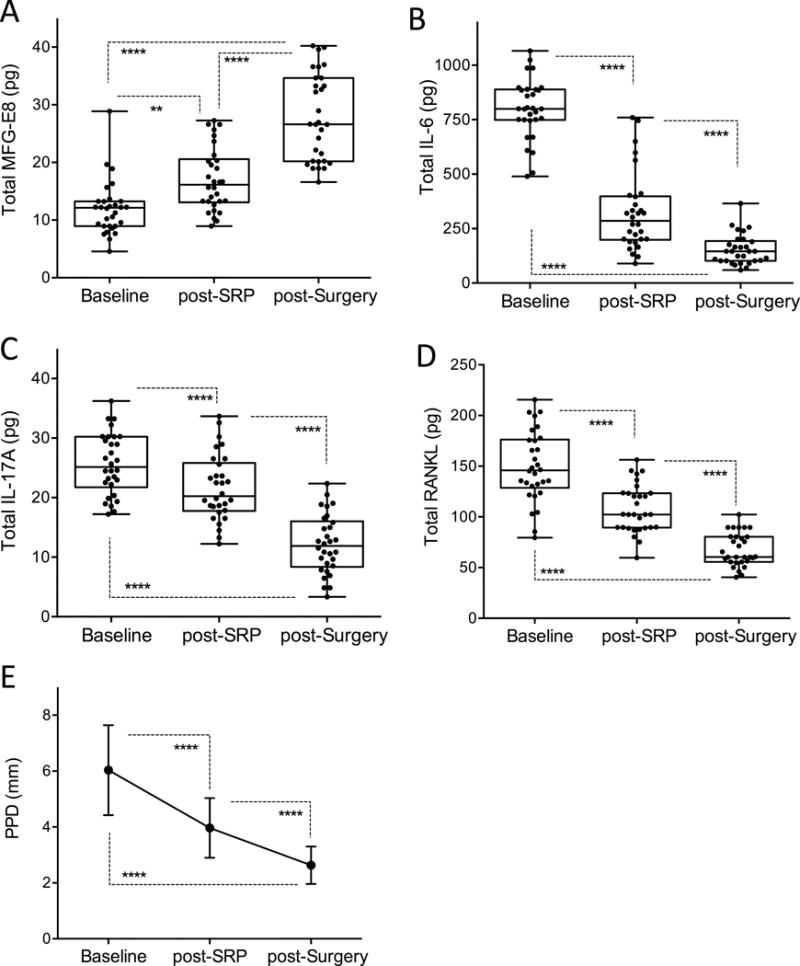
GCF was collected from 5 subjects classified as having chronic severe periodontitis at baseline, at a re-assessment appointment following non-surgical periodontal treatment (SRP) and after pocket reduction surgery. GCF was sampled from a total of 30 distinct sites (6 sites per individual). Bead-based multiplex assays on a Bio-Plex system were used to assay the following molecules: (A) MFG-E8, (B) IL-6, (C) IL-17A, and (D) RANKL. (E) Measured deep probing pocket depths (PPD) before and after treatments. In A-D, dots represent individual values from distinct sampled sites and each box in the box-and-whisker plots extends from the 25th to the 75th percentiles. In E, data are means ± SD (n = 30 sites). **P < 0.01; ****P < 0.0001 between indicated groups (Repeated measures analysis as implemented in SAS Proc GENMOD).
Discussion
Our human and NHP studies collectively indicate that the secreted glycoprotein MFG-E8 is associated with and contributes to periodontal health. Our clinical investigation has shown for the first time the presence of MFG-E8 in the human GCF. Importantly, the GCF levels of MFG-E8 were significantly higher in health and gingivitis than in periodontitis and were inversely associated with inflammation and the severity of the disease (as determined by pocket depths). In sites with severe periodontitis that were successfully subjected to non-surgical and surgical periodontal treatments, the GCF levels of MFG-E8 were shown to progressively increase at the re-assessment visits. In the treated patients, moreover, the changes in the levels of MFG-E8 followed a pattern that was opposite to that of pro-inflammatory/pro-osteoclastogenic cytokines (IL-6, IL-17A, and RANKL), the levels of which progressively declined. Therefore, MFG-E8 might be a reliable indicator of inflammation resolution.
In fact, MFG-E8 may proactively be involved in inflammation resolution by promoting efferocytosis. Efferocytosis not only prevents the release of potentially harmful intracellular contents from dying cells but also reprograms the transcriptional profile of the efferocytic macrophage to reduce the expression of proinflammatory cytokines and increase the expression of pro-resolving cytokines, such as transforming growth factor-β and IL-10 (Ortega-Gomez et al., 2013, Ravichandran and Lorenz, 2007, Szondy et al., 2014). Moreover, efferocytosis promotes the expression of MFG-E8, thus generating a feed-forward loop that further enhances efferocytosis (Ortega-Gomez et al., 2013, Ravichandran and Lorenz, 2007, Szondy et al., 2014).
It was previously shown that MFG-E8 can inhibit IL-17A expression by down-regulating the phosphorylation of the signal transducer and activator of transcription-3 (STAT3) (Cen et al., 2016). This property of MFG-E8 could account for the pronounced inhibition of IL-17A mRNA expression seen in the treated gingival sites of NHPs. The diminished IL-17A levels may, in turn, explain (at least in part) the remarkable inhibition of RANKL mRNA expression in MFG-E8–treated sites, since IL-17A is a potent inducer of RANKL (Zenobia and Hajishengallis, 2015). The fact that MFG-E8-Fc was able to diminish RANKL expression without significantly affecting OPG expression suggests that the RANKL/OPG ratio was reduced in treated sites. This notion is important given that the RANKL/OPG ratio is thought to represent a potential indicator of periodontitis (Belibasakis and Bostanci, 2012).
In contrast to MFG-E8 upregulation by efferocytosis, certain pathological conditions cause a reduction in the expression of MFG-E8 (Aziz et al., 2011, Boddaert et al., 2007, Ait-Oufella et al., 2007, Albus et al., 2016). In this regard, it has been shown that lipopolysaccharide suppresses the expression of MFG-E8 in certain cell types, including macrophages and osteoblasts (Komura et al., 2009, Albus et al., 2016, Miksa et al., 2008). In periodontitis, bacterial lipopolysaccharide readily translocates from the gingival crevice/periodontal pocket into the underlying connective tissue (gingival corium) (Moutsopoulos et al., 2015), where it may inhibit the production of MFG-E8 by macrophages (or other cell types). This would reduce the amount of available MFG-E8 that could reach the GCF via the gingival plexus of blood vessels in the gingival corium. This notion is consistent with and provides an explanation for the GCF data of this study.
Inflammatory disease biomarkers reflect current inflammatory activity as opposed to clinical examination and radiographic assessment of bone loss, which may represent accumulated damage resulting from earlier episodes of inflammatory tissue destruction (thus, not necessarily reflecting current disease activity). Moreover, reliable biomarkers may have increased sensitivity to identify disease-susceptible and treatment–non-responsive individuals (Buduneli and Kinane, 2011, Offenbacher et al., 2008, Baeza et al., 2016, Teles et al., 2010). The perception of periodontitis as being site-specific has resulted in site-based approaches to diagnosis and therapeutic decisions in clinical settings (Persson, 2005). Accordingly, in our GCF analysis study we considered the site as the unit of observation. In this regard, highly site-specific patterns of potential biomarkers have been detected in the GCF including matrix metalloproteinase-8, elastase, prostaglandin E2, and IL-1β (Persson, 2005). Whether MFG-E8 can serve as a useful biomarker in periodontitis is uncertain at present and requires further and larger clinical studies. Nevertheless, our current findings are encouraging as they indicate that the GCF levels of MFG-E8 can potentially reflect the severity of the disease and the response to periodontal treatment.
After our discovery that MFG-E8 is expressed by osteoclasts and down-regulates their differentiation and function (Abe et al., 2014), another study showed that MFG-E8 is additionally expressed by and regulates osteoblasts (Albus et al., 2016). Given that MFG-E8 is produced also by innate immune and epithelial cells and has anti-inflammatory and pro-resolution functions (Aziz et al., 2011, Akhtar et al., 2014, Das et al., 2016, Szondy et al., 2014), MFG-E8 may play an important role in the homeostasis of the periodontium and other barrier sites, which are constantly challenged by microbial or inflammatory stimuli. A unique feature of the periodontium compared with other mucosal sites (e.g., the gastrointestinal and respiratory tracts) is that it consists of both mucosal and bone tissue. The ability of MFG-E8 to regulate the function of both immune and bone cells suggests that it could be a potentially important target for host-modulation in periodontitis. Proof-of-concept for this notion was obtained in the mouse model, where our previous work showed that recombinant mouse MFG-E8 blocked experimental periodontitis in C57BL/6J mice (Abe et al., 2014).
Laboratory mice have been quite useful as a tool for basic immunology, but less so as models of human disease and therapeutic interventions (Brodin and Davis, 2017). Regarding the predictive value of interventional studies in mice, a recent systematic study has questioned the reliability of this species as models of human inflammatory diseases. Specifically, in endotoxemia, gene expression profiling of C57BL/6J mice and humans revealed poor correlation between the human genes and mouse orthologues and vice versa (Seok et al., 2013). Moreover, mouse and human MFG-E8 molecules share only 57% amino-acid sequence identity, suggesting that the two molecules may not share all their functions, unless empirically tested. Therefore, it was important to test human MFG-E8 in an appropriate preclinical model that is close to humans. Importantly, our previous study in mice was validated in the present study, which employed a NHP model of periodontitis and demonstrated that locally administered recombinant human MFG-E8 protected the animals from disease. Therapeutic studies in NHPs should be highly predictive of drug efficacy in humans since periodontitis in these animals shares key clinical, microbiological, and immunohistological features with the human disease (Brecx et al., 1985, Kornman et al., 1981, Assuma et al., 1998, Page and Schroeder, 1982, Maekawa et al., 2016).
Given that MFG-E8 is an endogenously produced molecule, the expression of which declines in periodontitis, its therapeutic administration should re-instate sufficient levels of this factor, thereby potentially controlling inflammation and restoring tissue homeostasis. Especially when administered locally, as performed in this periodontal NHP study, MFG-E8 therapies are likely to involve only minor, if any, safety issues. In recent years, there is an increase in approved therapies involving endogenous molecule administration. In this regard, protein replacement therapies have a higher probability of regulatory approval as compared to biologics or small molecules (Gorzelany and de Souza, 2013). There are many examples that endogenous molecule replacement is well tolerated, such as digestive enzyme replacement therapy or replacement of α1-antitrypsin in patients with α1-antitrypsin deficiency (Stoller and Aboussouan, 2012, Fieker et al., 2011).
Being a functionally versatile molecule that regulates both upstream inflammatory events and downstream processes that control bone metabolism, MFG-E8 could be a promising therapeutic agent for treating periodontitis. The high prevalence of periodontitis which affects almost half of adults (Eke et al., 2012, White et al., 2012), its association with the systemic health of the patients (Hajishengallis, 2015), and its considerable economic burden (Brown et al., 2002, Beikler and Flemmig, 2011) underscore the importance of implementing innovative adjunctive therapeutic approaches, especially since many patients do not respond favorably to conventional treatment (Tonetti et al., 2011, Colombo et al., 2012). Besides periodontitis, MFG-E8 could prove useful for the treatment of other disorders associated with inflammatory bone loss, such as rheumatoid arthritis, ankylosing spondylitis, and osteoporosis. Moreover, our findings that periodontitis results in decreased GCF levels of MFG-E8, whereas periodontal health or successful treatment of periodontitis are associated with elevated MFG-E8, suggest that MFG-E8 is potentially a novel indicator of periodontal disease activity. Future clinical studies are warranted to determine the utility of MFG-E8 either as a biomarker or therapeutic agent in periodontitis.
Supplementary Material
Clinical Relevance.
Scientific rationale for study
As MFG-E8 can regulate inflammation and osteoclastogenesis, we investigated its therapeutic potential in a highly relevant preclinical model (non-human primates) of periodontitis and quantified its levels in human gingival crevicular fluid in relation to periodontal disease activity.
Principal findings
MFG-E8 inhibited ligature-induced periodontitis in non-human primates and its levels in human gingival crevicular fluid were significantly lower in periodontitis as compared to health or post-treatment.
Practical implications
These findings support the utility of MFG-E8 as a potential biomarker or therapeutic agent in periodontitis, a notion that can be confirmed in future clinical studies.
Acknowledgments
We thank Dr. Ricardo Teles for valuable advice and comments.
Source of Funding Statement
G.H. and T.A. have a joint patent application that describes the use of MFG-E8 for therapeutic purposes in periodontitis. This work was supported by grants from the National Institutes of Health (AI068730, DE024153, DE024716 and DE015254 to GH and DE026152 to GH and TC). TC was also supported by the European Community’s Seventh Framework Program under grant agreement No. 602699 (DIREKT).
Footnotes
Conflict of Interest
The other authors have no conflict of interest to declare.
References
- Abe T, Shin J, Hosur K, Udey MC, Chavakis T, Hajishengallis G. Regulation of osteoclast homeostasis and inflammatory bone loss by MFG-E8. J Immunol. 2014;193:1383–1391. doi: 10.4049/jimmunol.1400970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R, Esposito B, Teissier E, Daemen MJ, Leseche G, Boulanger C, Tedgui A, Mallat Z. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–2177. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- Akhtar S, Wang X, Bu HF, Tan XD. Role of MFG-E8 in Protection of Intestinal Epithelial Barrier Function and Attenuation of Intestinal Inflammation. In: Wang P, editor. MFG-E8 and Inflammation. New York: Springer; 2014. pp. 55–64. [Google Scholar]
- Albus E, Sinningen K, Winzer M, Thiele S, Baschant U, Hannemann A, Fantana J, Tausche AK, Wallaschofski H, Nauck M, Volzke H, Grossklaus S, Chavakis T, Udey MC, Hofbauer LC, Rauner M. Milk Fat Globule-Epidermal Growth Factor 8 (MFG-E8) Is a Novel Anti-inflammatory Factor in Rheumatoid Arthritis in Mice and Humans. J Bone Miner Res. 2016;31:596–605. doi: 10.1002/jbmr.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- Aziz M, Jacob A, Matsuda A, Wang P. Review: milk fat globule-EGF factor 8 expression, function and plausible signal transduction in resolving inflammation. Apoptosis. 2011;16:1077–1086. doi: 10.1007/s10495-011-0630-0. [DOI] [PubMed] [Google Scholar]
- Baeza M, Garrido M, Hernandez-Rios P, Dezerega A, Garcia-Sesnich J, Strauss F, Aitken JP, Lesaffre E, Vanbelle S, Gamonal J, Brignardello-Petersen R, Tervahartiala T, Sorsa T, Hernandez M. Diagnostic accuracy for apical and chronic periodontitis biomarkers in gingival crevicular fluid: an exploratory study. J Clin Periodontol. 2016;43:34–45. doi: 10.1111/jcpe.12479. [DOI] [PubMed] [Google Scholar]
- Beikler T, Flemmig TF. Oral biofilm-associated diseases: trends and implications for quality of life, systemic health and expenditures. Periodontol 2000. 2011;55:87–103. doi: 10.1111/j.1600-0757.2010.00360.x. [DOI] [PubMed] [Google Scholar]
- Belibasakis GN, Bostanci N. The RANKL-OPG system in clinical periodontology. J Clin Periodontol. 2012;39:239–248. doi: 10.1111/j.1600-051X.2011.01810.x. [DOI] [PubMed] [Google Scholar]
- Boddaert J, Kinugawa K, Lambert JC, Boukhtouche F, Zoll J, Merval R, Blanc-Brude O, Mann D, Berr C, Vilar J, Garabedian B, Journiac N, Charue D, Silvestre JS, Duyckaerts C, Amouyel P, Mariani J, Tedgui A, Mallat Z. Evidence of a role for lactadherin in Alzheimer’s disease. Am J Pathol. 2007;170:921–929. doi: 10.2353/ajpath.2007.060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostanci N, Ilgenli T, Emingil G, Afacan B, Han B, Toz H, Atilla G, Hughes FJ, Belibasakis GN. Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: implications of their relative ratio. J Clin Periodontol. 2007;34:370–376. doi: 10.1111/j.1600-051X.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- Brecx MC, Nalbandian J, Ooya K, Kornman KS, Robertson PB. Morphological studies on periodontal disease in the cynomolgus monkey. II. Light microscopic observations on ligature-induced periodontitis. J Periodontal Res. 1985;20:165–175. doi: 10.1111/j.1600-0765.1985.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Brodin P, Davis MM. Human immune system variation. Nat Rev Immunol. 2017;17:21–29. doi: 10.1038/nri.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LJ, Johns BA, Wall TP. The economics of periodontal diseases. Periodontol 2000. 2002;29:223–234. doi: 10.1034/j.1600-0757.2002.290111.x. [DOI] [PubMed] [Google Scholar]
- Buduneli N, Kinane DF. Host-derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. J Clin Periodontol. 2011;38(Suppl 11):85–105. doi: 10.1111/j.1600-051X.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- Cen C, Aziz M, Yang WL, Nicastro J, Coppa GF, Wang P. Milk fat globule-epidermal growth factor-factor VIII downregulates interleukin-17 expression in sepsis by modulating STAT3 activation. Surgery. 2016;159:560–569. doi: 10.1016/j.surg.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyuo C, Jacob A, Wu R, Zhou M, Qi L, Dong W, Ji Y, Chaung WW, Wang H, Nicastro J, Coppa GF, Wang P. Recombinant human MFG-E8 attenuates cerebral ischemic injury: its role in anti-inflammation and anti-apoptosis. Neuropharmacology. 2012;62:890–900. doi: 10.1016/j.neuropharm.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo A, Bennet S, Cotton S, Goodson J, Kent R, Haffajee A, Socransky S, Hasturk H, Van Dyke TE, Dewhirst F, Paster B. Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: comparison between good responders and individuals with refractory periodontitis using the human oral microbe identification microarray. J Periodontol. 2012;83:1279–1287. doi: 10.1902/jop.2012.110566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Ghatak S, Sinha M, Chaffee S, Ahmed N, Parinandi N, Wohleb E, Sheridan J, Sen C, Roy S. Correction of MFG-E8 Resolves Inflammation and Promotes Cutaneous Wound Healing in Diabetes. J Immunol. 2016;196:5089–5100. doi: 10.4049/jimmunol.1502270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz P, Hoare A, Hong B. Subgingival Microbiome Shifts and Community Dynamics in Periodontal Diseases. J Calif Dent Assoc. 2016;44:421–435. [PubMed] [Google Scholar]
- Eke P, Dye B, Wei L, Thornton-Evans GO, Genco R. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Fieker A, Philpott J, Armand M. Enzyme replacement therapy for pancreatic insufficiency: present and future. Clin Exp Gastroenterol. 2011;4:55–73. doi: 10.2147/CEG.S17634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzelany J, de Souza MP. Protein replacement therapies for rare diseases: a breeze for regulatory approval? Sci Transl Med. 2013;5:178fs110. doi: 10.1126/scitranslmed.3005007. [DOI] [PubMed] [Google Scholar]
- Griffiths G. Formation, collection and significance of gingival crevice fluid. Periodontol 2000. 2003;31:32–42. doi: 10.1034/j.1600-0757.2003.03103.x. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 2014;29:248–257. doi: 10.1111/omi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- Komura H, Miksa M, Wu R, Goyert S, Wang P. Milk Fat Globule Epidermal Growth Factor-Factor VIII Is Down-Regulated in Sepsis via the Lipopolysaccharide-CD14 Pathway. The Journal of Immunology. 2009;182:581–587. doi: 10.4049/jimmunol.182.1.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornman K, Holt S, Robertson P. The microbiology of ligature-induced periodontitis in the cynomolgus monkey. J Periodontal Res. 1981;16:363–371. doi: 10.1111/j.1600-0765.1981.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Lamont R, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21:172–183. doi: 10.1016/j.molmed.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T, Abe T, Hajishengallis E, Hosur KB, DeAngelis RA, Ricklin D, Lambris JD, Hajishengallis G. Genetic and intervention studies implicating complement C3 as a major target for the treatment of periodontitis. J Immunol. 2014;192:6020–6027. doi: 10.4049/jimmunol.1400569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T, Briones R, Resuello R, Tuplano J, Hajishengallis E, Kajikawa T, Koutsogiannaki S, Garcia C, Ricklin D, Lambris J, Hajishengallis G. Inhibition of pre-existing natural periodontitis in non-human primates by a locally administered peptide inhibitor of complement C3. J Clin Periodontol. 2016;43:238–249. doi: 10.1111/jcpe.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh P. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149:279–294. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- Miksa M, Amin D, Wu R, Jacob A, Zhou M, Dong W, Yang W, Ravikumar T, Wang P. Maturation-induced down-regulation of MFG-E8 impairs apoptotic cell clearance and enhances endotoxin response. Int J Mol Med. 2008;22:743–748. [PMC free article] [PubMed] [Google Scholar]
- Miossec P, Kolls J. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- Mogi M, Otogoto J, Ota N, Inagaki H, Minami M, Kojima K. Interleukin 1 beta, interleukin 6, beta 2-microglobulin, and transforming growth factor-alpha in gingival crevicular fluid from human periodontal disease. Arch Oral Biol. 1999;44:535–539. doi: 10.1016/s0003-9969(99)00020-5. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos N, Chalmers N, Barb J, Abusleme L, Greenwell-Wild T, Dutzan N, Paster B, Munson P, Fine D, Uzel G, Holland S. Subgingival microbial communities in leukocyte adhesion deficiency and their relationship with local immunopathology. PLoS Pathog. 2015;11:e1004698. doi: 10.1371/journal.ppat.1004698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher S, Barros S, Beck J. Rethinking periodontal inflammation. J Periodontol. 2008;79:1577–1584. doi: 10.1902/jop.2008.080220. [DOI] [PubMed] [Google Scholar]
- Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. 2013;5:661–674. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RC, Schroeder HE. Periodontitis in man and other animals- A comparative review. Basel, Switzerland: Karger; 1982. [Google Scholar]
- Persson G. Site-based versus subject-based periodontal diagnosis. Periodontol 2000. 2005;39:145–163. doi: 10.1111/j.1600-0757.2005.00130.x. [DOI] [PubMed] [Google Scholar]
- Podlaha O, Webb D, Zhang J. Accelerated evolution and loss of a domain of the sperm-egg-binding protein SED1 in ancestral primates. Mol Biol Evol. 2006;23:1828–1831. doi: 10.1093/molbev/msl066. [DOI] [PubMed] [Google Scholar]
- Ravichandran K, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- Seok J, Warren H, Cuenca A, Mindrinos M, Baker H, Xu W, Richards D, McDonald-Smith GP, Gao H, Hennessy L, Finnerty C, Lopez C, Honari S, Moore E, Minei J, Cuschieri J, Bankey P, Johnson J, Sperry J, Nathens A, Billiar T, West M, Jeschke M, Klein M, Gamelli R, Gibran N, Brownstein B, Miller-Graziano C, Calvano S, Mason P, Cobb J, Rahme L, Lowry S, Maier R, Moldawer L, Herndon D, Davis R, Xiao W, Tompkins R. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Wu R, Jacob A, Molmenti E, Nicastro J, Coppa G, Wang P. Recombinant human milk fat globule-EGF factor 8 produces dose-dependent benefits in sepsis. Intensive Care Med. 2012;38:128–136. doi: 10.1007/s00134-011-2353-7. [DOI] [PubMed] [Google Scholar]
- Shin J, Maekawa T, Abe T, Hajishengallis E, Hosur K, Pyaram K, Mitroulis I, Chavakis T, Hajishengallis G. DEL-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates. Sci Transl Med. 2015;7:307ra155. doi: 10.1126/scitranslmed.aac5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinningen K, Albus E, Thiele S, Grossklaus S, Kurth T, Udey M, Chavakis T, Hofbauer L, Rauner M. Loss of milk fat globule-epidermal growth factor 8 (MFG-E8) in mice leads to low bone mass and accelerates ovariectomy-associated bone loss by increasing osteoclastogenesis. Bone. 2015;76:107–114. doi: 10.1016/j.bone.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Stoller J, Aboussouan L. A review of alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185:246–259. doi: 10.1164/rccm.201108-1428CI. [DOI] [PubMed] [Google Scholar]
- Stubbs J, Lekutis C, Singer K, Bui A, Yuzuki D, Srinivasan U, Parry G. cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor-like domains linked to factor VIII-like sequences. Proc Natl Acad Sci U S A. 1990;87:8417–8421. doi: 10.1073/pnas.87.21.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szondy Z, Garabuczi E, Joos G, Tsay G, Sarang Z. Impaired clearance of apoptotic cells in chronic inflammatory diseases: therapeutic implications. Front Immunol. 2014;5:354. doi: 10.3389/fimmu.2014.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles R, Sakellari D, Teles F, Konstantinidis A, Kent R, Socransky S, Haffajee A. Relationships among gingival crevicular fluid biomarkers, clinical parameters of periodontal disease, and the subgingival microbiota. J Periodontol. 2010;81:89–98. doi: 10.1902/jop.2009.090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti M, Chapple I, Working Group 3 of Seventh European Workshop on, P Biological approaches to the development of novel periodontal therapies–consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol 38 Suppl. 2011;11:114–118. doi: 10.1111/j.1600-051X.2010.01675.x. [DOI] [PubMed] [Google Scholar]
- Uitto V. Gingival crevice fluid–an introduction. Periodontol 2000. 2003;31:9–11. doi: 10.1034/j.1600-0757.2003.03101.x. [DOI] [PubMed] [Google Scholar]
- Vernal R, Dutzan N, Chaparro A, Puente J, Antonieta Valenzuela M, Gamonal J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol. 2005;32:383–389. doi: 10.1111/j.1600-051X.2005.00684.x. [DOI] [PubMed] [Google Scholar]
- White D, Tsakos G, Pitts N, Fuller E, Douglas G, Murray J, Steele J. Adult Dental Health Survey 2009: common oral health conditions and their impact on the population. Br Dent J. 2012;213:567–572. doi: 10.1038/sj.bdj.2012.1088. [DOI] [PubMed] [Google Scholar]
- Yi Y. Functional Role of Milk Fat Globule-Epidermal Growth Factor VIII in Macrophage-Mediated Inflammatory Responses and Inflammatory/Autoimmune Diseases. Mediators Inflamm. 2016;2016:5628486. doi: 10.1155/2016/5628486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenobia C, Hajishengallis G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol 2000. 2015;69:142–159. doi: 10.1111/prd.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


