Abstract
Background
Aerobic capacity, as measured by peak oxygen uptake (V̇O2), is one of the most powerful predictors of prognosis in heart failure (HF). Inflammation is a key factor contributing to alterations in aerobic capacity, and interleukin (IL)-1 cytokines are implicated in this process. The adaptor protein ASC is necessary for inflammasome activation of IL-1β and IL-18. ASC expression is controlled through epigenetic modification; lower ASC methylation is associated with worse outcomes in HF. The purpose of this study is to examine the relationships between ASC methylation, IL-1β, and IL-18 with peak V̇O2 in persons with HF.
Methods
This study examined the relationship between ASC methylation, IL-1β, and IL-18 with peak V̇O2 in 54 stable outpatients with HF. All participants were NYHA class II or III, not engaged in an exercise program, and physically able to complete an exercise treadmill test.
Results
Mean peak V̇O2 was 16.68 ± 4.7 ml/kg/min. Peak V̇O2was positively associated with mean percent ASC methylation (r=.47, p=.001) and negatively associated with IL-1β (r=-.38, p=.007). Multiple linear regression models demonstrated that peak V̇O2 increased by 2.30 ml/kg/min for every 1% increase in ASC methylation and decreased by 1.91 ml/kg/min for every 1 pg/mL increase in plasma IL-1β.
Conclusions
Mean percent ASC methylation and plasma IL-1β levels are associated with clinically meaningful differences in peak V̇O2 in persons with HF. Inflammasome activation may play a mechanistic role in determining aerobic capacity. ASC methylation is a potentially modifiable mechanism for reducing the inflammatory response, thereby improving aerobic capacity in HF.
Keywords: inflammation, inflammasome, exercise testing, cytokines
Introduction
Reduced exercise capacity is a characteristic symptom of heart failure (HF), and is accompanied by dyspnea, fatigue, and muscle weakness, even during low-intensity exercise (2, 39). This exercise intolerance is associated with reduced aerobic capacity and low ventilatory efficiency, leading to decreased functional capacity and poor quality of life (2, 3). Clinical evaluation of aerobic capacity in HF is measured by peak oxygen uptake (V̇O2), a measure of oxygen consumption during a maximum effort treadmill test, and is a strong prognostic indicator of decompensation and mortality (7).
Aerobic capacity is determined by the integrity of the cardiovascular, respiratory, and skeletal muscle systems, all of which are diminished in HF (15, 24, 39). Inflammation negatively affects cardiac, respiratory, and skeletal muscle pathophysiology (1, 15, 16, 37). Chronic inflammation and increased circulating cytokines contribute to the pathophysiology and worsening of HF by altering cardiac structure and function (22). These inflammatory cytokines also play a role in altering skeletal muscle function due to their catabolic effects (1). Chronic inflammation in HF leads to altered breathing patterns, such as inspiratory muscle weakness (21). Thus, inflammation is a key component of pathophysiological changes that lead to decreased aerobic capacity in persons with HF.
Inflammation and Peak V̇O2
Aerobic capacity is negatively associated with inflammatory cytokines, in both healthy populations and in disease states, such as HF (34). Further, changes in peak V̇O2 in response to an intervention are accompanied by changes in systemic inflammatory cytokines, such as creactive protein (30, 34). A two-week treatment with the IL-1 cytokine blocker anakinra demonstrated a reduction in systemic inflammation and improvement in peak V̇O2 in persons with HF in the absence of an exercise intervention (35, 36). Thus, aerobic capacity in HF may be related to increased circulating IL-1 inflammatory cytokines.
The IL-1 cytokines, IL-1β and IL-18, are activated by the inflammasome, a complex of intracellular interaction proteins that triggers maturation of cytokines to initiate and amplify the inflammatory response (9, 18). The inflammasome is composed of a NOD (nucleotide-binding oligomerization domain)-like receptor, ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), and pro-caspase-1 (9). The activated inflammasome cleaves pro-caspase-1 into the active enzyme caspase-1. Caspase-1, in turn, activates IL-1 cytokines IL-1β and IL-18, by cleavage of pro-IL-1β and pro-IL-18 into active forms (8, 18).
The adaptor protein ASC recruits pro-caspase-1 to the inflammasome complex and is necessary for caspase-1 mediated activation of IL-1β and IL-18 (8). ASC expression is controlled by epigenetic modification via methylation of a CpG island in the promoter region of exon 1 (31). Increased methylation of ASC is associated with decreased plasma IL-1β in persons with HF (10).
Higher percent ASC methylation and lower ASC mRNA expression are positively associated with the six-minute walk test (6MWT) in persons with HF (10). Although the 6MWT has been shown to be a reliable submaximal measure of functional capacity (5), a measure of maximal aerobic capacity can provide better insight into the functional capacity of an individual with HF. The purpose of this study, therefore, was to examine the relationships between ASC methylation, IL-1β, and IL-18 with peak V̇O2 in persons with HF.
Methods
Study Design
This cross-sectional study examined the relationship between ASC methylation and peak V̇O2 in stable outpatients with HF. Fifty-four participants were enrolled from multidisciplinary outpatient HF clinics.
Study Sample
Inclusion criteria included: documented medical diagnosis of NYHA class II or III, aged 40-75 years; left ventricular ejection fraction (LVEF) ≥10% documented within the last year by echocardiogram, cardiac catheterization ventriculography, or radionuclide ventriculography; and receiving medication therapy for HF according to the American College of Cardiology/American Heart Association recommendation guidelines, including angiotensin-converting-enzyme inhibitors or angiotensin receptor blockers, beta blockers, and diuretics for participants with reduced ejection fraction (LVEF ≤ 40%) (40), for at least 8 weeks prior to study enrollment. Exclusion criteria included: medical diagnosis of NYHA class I or IV; change in HF therapy within the previous 8 weeks; worsening HF symptoms within the last 5 days; unstable angina; renal insufficiency (serum creatinine > 3.0 mg/dL); fixed rate pacemaker; uncontrolled hypertension, involved in any structured exercise program or exercising 3 or more times per week for a minimum of 30 minutes; hospitalization within the previous 30 days; and any disorder precluding an exercise treadmill test.
Study Protocol
All studies were performed under research protocols approved by the Institutional Review Boards of Emory University and participating institutions. Subjects were informed of testing protocols and the potential risks and benefits of participation. All participants provided written consent before participation. Blood samples were collected in the morning after an overnight fast. Blood was collected in a vacutainer with EDTA, separated into plasma and buffy coat, and stored at -80°C until analysis.
Measurements
Demographic and Clinical Data
Sociodemographic and clinical variables were obtained from medical records and a self-report questionnaire. Height was measured with a standard stadiometer, without shoes. Weight was measured using a calibrated scale with the participant in light clothing, without shoes. Body mass index (BMI) was calculated by the formula: BMI = (weight in kg)/(height in cm)2.
Cardiopulmonary Exercise Stress Test
Aerobic capacity was assessed using the modified Balke maximal symptom-limited treadmill test (4, 14) to determine peak oxygen consumption (peak V̇O2). Continuous gas exchange (VMAX Spectra 29 CPET Instrument, Yorba Linda, CA), telemetry, blood pressure, rating of perceived exertion, and oxygen saturation were assessed for each patient 1 minute before, continuously during exercise, and 4 minutes after the exercise test according to American Heart Association guidelines (3). The test protocol was as follows: 0% incline at 2.0 mph on motorized treadmill for 3 minutes with an increase in incline of 3.5% every 3 minutes for 18 minutes. At 18 minutes, speed increased to 3.0 mph and incline decreased to 12.5%. No participants progressed beyond this point.
ASC Methylation
Percent methylation of 7 CpG sites in the intron region of ASC was measured as previously reported (10). In brief, genomic DNA from peripheral blood mononuclear cells (PBMCs) was bisulfite treated and amplified by PCR followed by pyrosequencing for methylation quantification.(25) Methylation was analyzed as mean percent methylation.
Cytokines
IL-1β and IL-18 were analyzed from plasma that has been separated from collected whole blood and stored at -80°C immediately after collection. Plasma cytokines were measured in duplicate using commercially available ELISA kits (eBioscience). Plates were read on a BioTek microplate reader and analyzed using Gen5 software. Curve fitting was selected among linear, quadratic and 4-point based on the best regression coefficient.
Data Analysis
All data were reviewed for data entry errors, potential outliers, and missing data. Clinical and demographic data were complete, and only participants who completed the study protocol (blood draw, 6MWT, treadmill test, and physical examination) were included in the analysis. Distributions were assessed for deviations from normality. IL-18 did not meet criteria for normality and was log transformed (LN) for statistical analysis. However, non-transformed IL-18 values are presented in descriptive data. Pearson correlation analysis was performed to identify linear associations between variables and identify covariates for further analysis. An unpaired Student's t test was used to determine differences in measures between demographic groups. Linear regression analysis was performed to examine linear relationships between the dependent variable, aerobic capacity (as measured by peak V̇O2), and the independent variables, ASC methylation and cytokines, controlling for covariates. Linear regression analysis was performed using 2 models. Model 1 consisted of the independent and dependent variables, without adjusting for covariates. Model 2 also included covariates (age, gender, LVEF, 6MWT, BMI and medications) based on previous documentation and performance of the data and relationships within this study. The variables included in the final model were selected using stepwise selection with an alpha set at 0.10 to minimize multicollinearity and choose the most parsimonious final model. All analysis was performed using SPSS version 23.
Results
Patient Characteristics
Demographic and clinical data are presented in Table 1. Sixty-three percent of the participants had LVEF <40%. The most common comorbidities were hypertension (N=36, 66.7%), dyslipidemia (N=30, 55.6%), depression (N=20, 37.0%), and diabetes (N=19, 35.2%). One-third of participants (N=16, 29.6%) had a previous MI. Age was positively associated with LVEF among females (r=.36, p=.05) but not among males (r=.25, p=.22). Further, females had higher LVEF as compared to males (38.23 ± 14.9 vs. 29.44 ± 13.6, respectively; t=2.26, p=.03).
Table 1. Demographic and Clinical Characteristics.
| Characteristic N=54 | Mean ± SD | Range |
|---|---|---|
| Age (years) | 59.46 ± 9.7 | 40 – 75 |
| Ejection Fraction (%) | 34. ± 14.8 | 15 – 65 |
| BMI (kg/m2) | 31.37 ± 6.8 | 19.1 – 49.7 |
|
| ||
| N | % | |
|
| ||
| Female | 28 | 51.9% |
| African American | 32 | 59.3% |
| β-Blocker use | 46 | 86.8 % |
| ACE inhibitor use | 23 | 45.6% |
| ARB use | 13 | 24.1% |
| Diuretic use | 45 | 83.3% |
| Pacer/ICD Device | 30 | 55.6% |
| College Education | 27 | 50.0% |
HFrEF = Heart failure with reduced ejection fraction
BMI = Body mass index
ACE = Angiotensin converting enzyme
ARB = Angiotensin receptor blocker
Physical Measures
Total treadmill time ranged from 1.8 – 18.3 minutes and peak V̇O2 ranged from 7.6 – 29.9 ml/kg/min (Table 2). Males had higher treadmill duration and peak V̇O2 as compared to females (Table 2). Peak V̇O2 was positively associated with total treadmill time (r=.58, p=<.001) and negatively associated with LVEF (r=-.32, p=.02) and BMI (r=-.31, p=.03).
Table 2. Physical Measures by Gender (Mean ± SD).
| Measure | Total N=54 | Females n=28 | Males n=26 | p-value |
|---|---|---|---|---|
| Peak V̇O2 (ml/kg/min) | 16.68 ± 4.7 | 14.59 ± 3.6 | 18.85 ± 4.8 | .001 |
| Total Treadmill Time (minutes) | 8.27 ± 4.4 | 6.16 ± 3.0 | 10.58 ± 4.5 | <.001 |
| Six Minute Walk Test (meters) | 348.9 ± 78.2 | 338.77 ± 76.7 | 359.81 ± 79.9 | .33 |
ASC and Cytokines
ASC and cytokine data are presented in Table 3. Mean percent ASC methylation and IL-1β were not associated with age, gender, or race (Table 4). ASC methylation was negatively associated with IL-1β (r=-.44, p=.001), but not IL-18 (r=-.07, p=.64). Peak V̇O2 (Figure 1) was positively associated with mean percent ASC methylation (r=.47, p=.001) and negatively associated with IL-1β (r=-.38, p=.007). No association with aerobic capacity and IL-18 was found.
Table 3. ASC and Cytokines.
| Measure | Mean ± SD | Range |
|---|---|---|
| ASC Methylation (%) | 5.84 ± 0.8 | 1.33 – 6.82 |
| IL-1β (pg/mL) | 1.72 ± 0.8 | 0 – 5.18 |
| IL-18 (pg/mL) | 158.49 ± 56.2 | 96.2 – 333.1 |
ASC = apoptosis associated speck-like protein with a caspase recruitment domain
IL = interleukin
Table 4. Linear Associations Between Clinical/Demographic Characteristics and ASC and Interleukin-1β.
| N=54 | ASC | IL-1β | |
|---|---|---|---|
|
| |||
| ASC | Pearson's r | 1 | -.443** |
| Sig. (2-tailed) | - | .001 | |
|
| |||
| IL-1β | Pearson's r | -.443** | 1 |
| Sig. (2-tailed) | .001 | - | |
|
| |||
| Age | Pearson's r | .059 | -.026 |
| Sig. (2-tailed) | .691 | .849 | |
|
| |||
| Female | Pearson's r | -.042 | .240 |
| Sig. (2-tailed) | .813 | .081 | |
|
| |||
| AA/Black | Pearson's r | -.188 | .218 |
| Sig. (2-tailed) | .213 | .114 | |
|
| |||
| BMI | Pearson's r | -.048 | .111 |
| Sig. (2-tailed) | .732 | .425 | |
|
| |||
| β-blocker | Pearson's r | -.03 | .006 |
| Sig. (2-tailed) | .841 | .966 | |
|
| |||
| ACEi | Pearson's r | -.227 | .031 |
| Sig. (2-tailed) | .138 | .843 | |
|
| |||
| ARB | Pearson's r | -.038 | -.066 |
| Sig. (2-tailed) | .811 | .679 | |
|
| |||
| Diuretic | Pearson's r | -.160 | .096 |
| Sig. (2-tailed) | .273 | .514 | |
|
| |||
| Pacer/ICD | Pearson's r | -.131 | .028 |
| Sig. (2-tailed) | .322 | .844 | |
|
| |||
| Hypertension | Pearson's r | -.222 | .156 |
| Sig. (2-tailed) | .107 | .260 | |
|
| |||
| Dyslipidemia | Pearson's r | .123 | -.156 |
| Sig. (2-tailed) | .374 | .259 | |
|
| |||
| Depression | Pearson's r | .098 | -.161 |
| Sig. (2-tailed) | .479 | .245 | |
|
| |||
| Diabetes Mellitus | Pearson's r | -.200 | .028 |
| Sig. (2-tailed) | .147 | .839 | |
|
| |||
| Previous MI | Pearson's r | -.063 | -.204 |
| Sig. (2-tailed) | .648 | .140 | |
ASC = apoptosis associated speck-like protein with a caspase recruitment domain
IL = interleukin
AA = African American
BMI = Body mass index
ACEi = Angiotensin converting enzyme inhibitor
ARB = Angiotensin receptor blocker
ICD = Implantable cardioverter defibrillator
MI = Myocardial Infarction
Significant (sig.) at p<.05 using 2-tailed bivariate Pearson correlation analysis.
Figure 1. Peak V̇O2 is Associated with ASC Methylation and Interleukin-1β.
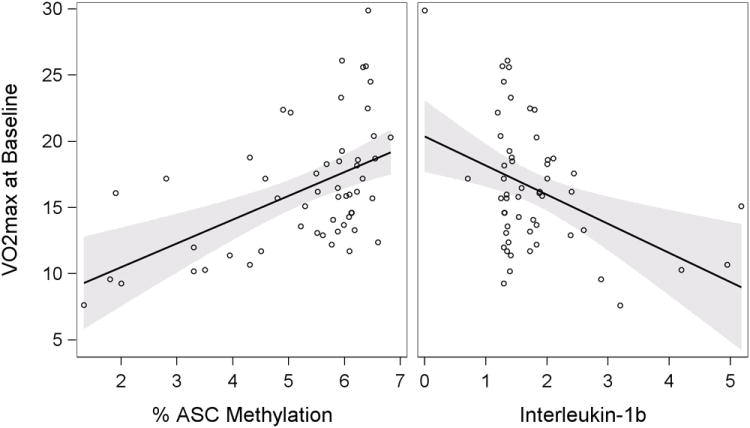
Peak V̇O2 was positively associated with mean percent ASC methylation (left, r=.47, p=.001).
Peak V̇O2 was negatively associated with IL-1β (right, r=-.38, p=.007).
A multiple linear regression was performed to predict peak V̇O2 based on mean percent ASC methylation and plasma IL-1β levels, controlling for covariates (Table 4). The models demonstrated that peak V̇O2 increased by 2.30 ml/kg/min for every 1% increase in mean ASC methylation and decreased by 1.91 ml/kg/min for every 1 pg/mL increase in plasma IL-1β, adjusting for LVEF and gender. Gender remained a significant predictor of aerobic capacity in both models, such that male gender contributed to 2.42 ml/kg/min and 2.62 ml/kg/min in the ASC and IL-1β models, respectively.
Discussion
This study demonstrated that the inflammasome pathway may impact aerobic capacity in HF patients and serves to broaden our understanding of the biological determinants of aerobic exercise capacity. Previously, we reported a positive linear association between ASC methylation and 6MWT total distance in a different population of persons with HF (10). Here, we show that ASC methylation is positively related to peak V̇O2. In addition, IL-1β had a negative relationship with peak V̇O2, suggesting the inflammasome may play a mechanistic role in aerobic capacity in HF. Studies of short-term administration of IL-1β blockade (anakinra) were associated with an increase in peak V̇O2 in persons with both HFrEF (36) and HFpEF (35). Analysis of a small subset in the HFrEF study (n=3) demonstrated a decrease in IL-1β with anakinra treatment (36). However, this analysis was too small to determine statistical significance and was not compared to changes in peak V̇O2. Nonetheless, the changes in peak V̇O2 after IL-1β blockade implicate this interleukin-1 family cytokine in the pathophysiology of decreased aerobic capacity in HF.
Mean percent ASC methylation was 5.84 ± 0.78, which is similar to our previous results in a different HF population (10). The positive association of ASC methylation and peak V̇O2 in this study adds a putative mechanism of epigenetic control of inflammatory gene expression contributing to decreased aerobic capacity in HF. Increased ASC methylation is associated with decreased ASC protein and mRNA expression in HF (10). Further, decreased ASC protein expression is associated with decreased IL-1β in HF (10). In this study, linear regression analysis revealed that mean percent ASC methylation and plasma IL-1β levels are associated with clinically meaningful changes in peak V̇O2 in persons with HF. Together, these data suggest inflammasome activation of IL-1β plays a mechanistic role in physiological processes in HF.
Increased ASC methylation was associated with decreased plasma IL-1β. No association was found with ASC methylation and plasma IL-18. These findings are similar to our findings in a previous study (10), and are likely indicative of redundancies in function and activation of IL-18 (6). Unlike IL-1β, IL-18 is not involved in the acute phase response and does not induce systemic inflammatory events, such as neutropenia and fever (32, 33). Monocytes can differentially secrete IL-18 or IL-1β, in response to specific signaling, such as leptin (20). Further, while IL-1β activation is tightly regulated by the inflammasome (19), IL-18 can be activated by non-canonical pathways (caspase-3 and Fas-induced caspase-8) and is regulated by the endogenous inhibitor, IL-18BP (6, 28, 38). Thus, IL-18 may represent a common signal downstream of different inflammatory pathways, and may occasionally function independent of IL-1β activity. Mechanistic and tissue-level studies may provide more insight into this relationship.
Gender differences in HF presentation have been previously reported (11). In particular, females with HF, tend to have higher LVEF and lower peak V̇O2 than males (11). Our inclusion criteria for LVEF was ≥10% without an upper limit, leading to the inclusion of persons with preserved ejection fraction. This accounts for the positive association with age among females only, as persons with HF and preserved ejection fraction are largely older women.(17) In this study, the effects of gender and LVEF on peak V̇O2 were accounted for in the regression analysis. The relationships between ASC methylation and IL-1β with peak V̇O2 remained significant when controlling for both gender and LVEF. Thus, ASC methylation and IL-1β appear to influence peak V̇O2 independent of gender and LVEF.
No association with ASC methylation and age were found, which is similar to our previous study in persons with HF (10). This is in contrast to a previous study by Nakajima et al (25), who found a difference in levels of ASC methylation between younger and older groups of healthy adults. The age-related changes in DNA methylation in healthy adults may not be relevant in HF, which is characterized by a premature cellular aging due to the physiological and psychological stressors of this chronic disease (13). Thus, while among the general adult population there is a decrease in global DNA methylation with age (23, 29), further studies are needed to determine the relationships between epigenetic control of gene expression and age in HF.
Nakajima et al. (25) found no relationship between ASC methylation and V̇O2max in their study of healthy adults. This difference in findings reported in the current study may be related to the pathophysiological and structural changes that limit aerobic capacity in HF. In HF, aerobic capacity is measured as peak V̇O2, as opposed to the V̇O2max measurement in the healthy population. The measurement of V̇O2max requires an individual to reach a plateau of maximum volume of oxygen used; persons with HF are rarely able to reach and sustain this plateau. Peak V̇O2 is reduced primarily by impaired cardiac output in both heart failure with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF) (2). Other factors such as endothelial dysfunction leading to vasoconstriction and decreased capacity for aerobic metabolism contribute to the reduction in maximum oxygen uptake in HF (2). These physiological processes are amplified by a positive feedback loop of inflammation and may explain, at least in part, the association between peak V̇O2 and the inflammasome-related measures. Further investigation comparing these relationships to age and gender matched healthy controls is needed.
Increased cytokines in HF may contribute to the pathophysiology and disease progression by altering cardiac structure and function. Inflammatory cytokines may also contribute to peripheral alterations in vascular and skeletal muscle function. The cardiovascular, respiratory, and skeletal muscle systems all influence aerobic capacity (2), and the individual contributions of pathophysiological changes in each system to impaired aerobic capacity are difficult to discern. Here we show the relationship between IL-1β and peak V̇O2 in a systemic source (plasma), but further investigation into the relationships and mechanisms of inflammatory cytokines in cardiovascular and skeletal muscle tissue is warranted.
In this study, we used a linear regression model to predict peak V̇O2 based on markers of inflammation, IL-1β and ASC methylation. However, the linear relationship would be the same had we used peak V̇O2 to predict inflammation. While we cannot determine if inflammation is a driver of decreased aerobic capacity, if decreased aerobic capacity is a driver of increased inflammation, or if there is a causal relationship at all, the linear association between peak V̇O2 and IL-1β suggests that changes in these physiological measures occur together. Low aerobic capacity is a powerful predictor of premature morbidity and higher mortality in HF (7), and adding IL-1β levels may provide better prediction of adverse outcomes in HF. Previously we demonstrated that ASC methylation, protein, and mRNA expression are predictors of clinical events in HF (10). Taken together, this further implicates the inflammasome pathway in the pathophysiological processes of HF. Modification of inflammation via epigenetic modulation may be a novel target for intervention in HF.
Moderate intensity aerobic exercise, such as a walking program, has been shown to increase ASC methylation (25), however these exercise-induced epigenetic changes have not been correlated with circulating cytokines, such as IL-1β or IL-18, or assessed in persons with HF. Aerobic exercise has been shown to be beneficial for most HF patients by altering the deleterious peripheral and central mechanisms, such as inflammatory cytokines, that contribute to HF exacerbations, worsened symptom severity, and poor clinical outcomes (12, 26). In addition, aerobic exercise reduces vascular resistance and improves endothelial function as well as the oxidative capacity of peripheral muscles, without worsening left ventricular remodeling (12, 26). The HF-ACTION trial (27) established the safety and efficacy of moderate-intensity aerobic exercise in patients with stable HF. Aerobic exercise has been shown to reduce inflammatory cytokines, but further studies are needed to establish the effect of aerobic exercise on ASC methylation and IL-1β in persons with HF.
Limitations
This study was cross-sectional with a relatively small sample size of 54 participants. A larger study following changes in V̇O2 and ASC methylation over time may shed more light on the dynamic relationship between these measures. ASC methylation and cytokine analysis was performed using collected whole blood. While peripheral blood mononuclear cells (PBMCs) and serum cytokine levels are indicative of systemic inflammation, including cardiac and/or skeletal muscle biopsy samples would provide more insight into localized inflammatory changes affecting aerobic capacity. In addition, percent DNA methylation of ASC was measured in pooled PBMCs; differences in ASC methylation among participants may reflect varying proportions of cell subsets or epigenetic changes in varying proportions of cells. Further, healthy controls were not included in the study for comparison.
Conclusion
Mean percent ASC methylation and plasma IL-1β levels are associated with peak V̇O2 in persons with HF. This suggests inflammasome activation may play a role in determining aerobic capacity in persons with HF. ASC methylation is a potentially modifiable mechanism of reducing inflammation and improving functional capacity in HF. Future research examining modification of ASC expression, such as exercise interventions that increase ASC methylation, may improve aerobic capacity in persons with HF.
Table 5. Multivariate Analysis of Predictors of Aerobic Capacity.
| Aerobic Capacity (Peak V̇O2 ml/kg/min) | |||
|---|---|---|---|
|
|
|||
| N=54 | Model 1a | Model 2b | |
|
|
|||
| Variable | β | β | 95% CI |
| Constant | 0.44 | 4.35 | [-4.41, 13.10] |
| % ASC Methylation | 2.73** | 2.30** | [0.91, 3.70] |
| LVEF | -0.78* | [-0.15, -0.002] | |
| Gender† | 2.42* | [0.07, 4.76] | |
|
| |||
| R2 | .22 | .40 | |
| F | 12.90** | 9.72*** | |
| ΔR2 | .18 | ||
| ΔF | 6.54** | ||
|
| |||
| Aerobic Capacity (Peak V̇O2 ml/kg/min) | |||
|
|
|||
| N=54 | Model 1c | Model 2d | |
|
|
|||
| Variable | β | β | 95% CI |
|
| |||
| Constant | 20.38*** | 21.51*** | [16.73, 26.29] |
| IL-1β | -2.15** | -1.91** | [-3.32, -0.51] |
| LVEF | -0.085** | [-0.17, -.004] | |
| Gender† | 2.62* | [0.12, 5.12] | |
|
| |||
| R2 | .15 | .35 | |
| F | 8.47** | 8.20*** | |
| ΔR2 | .20 | ||
| ΔF | 6.99** | ||
ASC = apoptosis associated speck-like protein containing a caspase recruitment domain.
LVEF = left ventricular ejection fraction. CI = confidence interval.
p<.05.
p<.01
p<.001
Female is coded as 0.
Model 1 is the direct effect of percent ASC Methylation not adjusted for covariates.
Model 2 considered age, gender, six-minute walk test, and BMI covariates for inclusion in the final model in addition to percent ASC Methylation: stepwise variable selection used.
Model 1 is the direct effect of IL-1β not adjusted for covariates.
Model 2 considered age, gender, six-minute walk test, and BMI covariates for inclusion in the final model in addition to IL-1β: stepwise variable selection used.
Acknowledgments
Supported in part by National Institutes of Health National Institute of Nursing Research grant number 1P30NR014134-01 (Co-investigator – R. Gary), the Heart Failure Society of America Nursing Research Grant (PI – B. Butts), and by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award number UL1TR000454 (PI – D. Stephens).
Effort for B. Butts was funded in part by the National Institutes of Health National Institute of Nursing Research grant numbers T32NR012715 (PI – S. Dunbar) and 1F31NR015180-01 (PI – B. Butts).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: Relation with Industry: None for BB, RG, EC, and SD. JB is a consultant to Amgen, Bayer, Cardiocell, Celladon, Novartis, Stealth Peptide, Relypsa, Z Pharma, Trevena, and Zensun.
Results of the present study do not constitute endorsement by ACSM. The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Anker SD, Ponikowski PP, Clark AL, et al. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J. 1999;20(9):683–93. doi: 10.1053/euhj.1998.1446. [DOI] [PubMed] [Google Scholar]
- 2.Arena R, Cahalin LP, Borghi-Silva A, Phillips SA. Improving functional capacity in heart failure: the need for a multifaceted approach. Curr Opin Cardiol. 2014;29(5):467–74. doi: 10.1097/hco.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 3.Balady GJ, Arena R, Sietsema K, et al. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 4.Balke B, Ware RW. An experimental study of fitness of Air Force personnel. US Armed Forces Medical Journal. 1959;10:678–88. [PubMed] [Google Scholar]
- 5.Bittner V, Weiner DH, Yusuf S, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA. 1993;270(14):1702–7. [PubMed] [Google Scholar]
- 6.Bossaller L, Chiang PI, Schmidt-Lauber C, et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. J Immunol. 2012;189(12):5508–12. doi: 10.4049/jimmunol.1202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowen TS, Eisenkolb S, Werner S, Schwarzer M, Schuler G, Adams V. Inheriting a high aerobic fitness predisposes to skeletal muscle and endothelial dysfunction in chronic heart failure. Int J Cardiol. 2016;203:353–6. doi: 10.1016/j.ijcard.2015.10.125. [DOI] [PubMed] [Google Scholar]
- 8.Bracey NA, Beck PL, Muruve DA, et al. The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1beta. Exp Physiol. 2013;98(2):462–72. doi: 10.1113/expphysiol.2012.068338. [DOI] [PubMed] [Google Scholar]
- 9.Butts B, Gary RA, Dunbar SB, Butler J. The Importance of NLRP3 Inflammasome in Heart Failure. J Card Fail. 2015;21(7):586–93. doi: 10.1016/j.cardfail.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butts B, Gary RA, Dunbar SB, Butler J. Methylation of Apoptosis-Associated Speck-Like Protein With a Caspase Recruitment Domain and Outcomes in Heart Failure. J Card Fail. 2016;22(5):340–6. doi: 10.1016/j.cardfail.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corra U, Mezzani A, Giordano A, et al. Peak oxygen consumption and prognosis in heart failure: 14 mL/kg/min is not a “gender-neutral” reference. Int J Cardiol. 2013;167(1):157–61. doi: 10.1016/j.ijcard.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 12.Feiereisen P, Vaillant M, Gilson G, Delagardelle C. Effects of different training modalities on circulating anabolic/catabolic markers in chronic heart failure. Journal of Cardiopulmonary Rehabilitation and Prevention. 2013;33:303–8. doi: 10.1097/HCR.0b013e3182a1e4e5. [DOI] [PubMed] [Google Scholar]
- 13.Fyhrquist F, Saijonmaa O, Strandberg T. The roles of senescence and telomere shortening in cardiovascular disease. Nat Rev Cardiol. 2013;10(5):274–83. doi: 10.1038/nrcardio.2013.30. [DOI] [PubMed] [Google Scholar]
- 14.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2002;106(14):1883–92. doi: 10.1161/01.cir.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 15.Gielen S, Adams V, Mobius-Winkler S, et al. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42(5):861–8. doi: 10.1016/s0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- 16.Gosselin LE, Barkley JE, Spencer MJ, McCormick KM, Farkas GA. Ventilatory dysfunction in mdx mice: impact of tumor necrosis factor-alpha deletion. Muscle Nerve. 2003;28(3):336–43. doi: 10.1002/mus.10431. [DOI] [PubMed] [Google Scholar]
- 17.Goyal P, Almarzooq ZI, Horn EM, et al. Characteristics of Hospitalizations for Heart Failure with Preserved Ejection Fraction. Am J Med. 2016;129(6):635.e15–26. doi: 10.1016/j.amjmed.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–87. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horsburgh S, Robson-Ansley P, Adams R, Smith C. Exercise and inflammation-related epigenetic modifications: focus on DNA methylation. Exerc Immunol Rev. 2015;21:26–41. [PubMed] [Google Scholar]
- 20.Jitprasertwong P, Jaedicke KM, Nile CJ, Preshaw PM, Taylor JJ. Leptin enhances the secretion of interleukin (IL)-18, but not IL-1beta, from human monocytes via activation of caspase-1. Cytokine. 2014;65(2):222–30. doi: 10.1016/j.cyto.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Kusunose K, Mehra R. Targeting Sleep Disordered Breathing to Prevent Heart Failure: What is the Evidence? Current cardiovascular risk reports. 2014;8(10):403. doi: 10.1007/s12170-014-0403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116(7):1254–68. doi: 10.1161/circresaha.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClay JL, Aberg KA, Clark SL, et al. A methylome-wide study of aging using massively parallel sequencing of the methyl-CpG-enriched genomic fraction from blood in over 700 subjects. Hum Mol Genet. 2014;23(5):1175–85. doi: 10.1093/hmg/ddt511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myers J, Gujja P, Neelagaru S, Burkhoff D. Cardiac output and cardiopulmonary responses to exercise in heart failure: application of a new bio-reactance device. J Card Fail. 2007;13(8):629–36. doi: 10.1016/j.cardfail.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima K, Takeoka M, Mori M, et al. Exercise effects on methylation of ASC gene. Int J Sports Med. 2010;31:671–375. doi: 10.1055/s-0029-1246140. [DOI] [PubMed] [Google Scholar]
- 26.Nunes RB, Alves JP, Kessler LP, Lago PD. Aerobic exercise improves the inflammatory profile correlated with cardiac remodeling and function in chronic heart failure rats. Clin Chest Med. 2013;68(6):876–82. doi: 10.6061/clinics/2013(06)24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouzounidis N, Giakoustidis A, Poutahidis T, et al. Interleukin 18 binding protein ameliorates ischemia/reperfusion-induced hepatic injury in mice. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2016;22(2):237–46. doi: 10.1002/lt.24359. [DOI] [PubMed] [Google Scholar]
- 29.Rang FJ, Boonstra J. Causes and Consequences of Age-Related Changes in DNA Methylation: A Role for ROS? Biology (Basel) 2014;3(2):403–25. doi: 10.3390/biology3020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos RV, Viana VA, Boscolo RA, et al. Moderate exercise training modulates cytokine profile and sleep in elderly people. Cytokine. 2012;60(3):731–5. doi: 10.1016/j.cyto.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 31.Stone AR, Bobo W, Brat DJ, Devi NS, Van Meir EG, Vertino PM. Aberrant methylation and down-regulation of TMS1/ASC in human glioblastoma. Am J Pathol. 2004;165(4):1151–61. doi: 10.1016/s0002-9440(10)63376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stuyt RJ, Netea MG, Verschueren I, Dinarello CA, Kullberg BJ, van der Meer JW. Interleukin-18 does not modulate the acute-phase response. Journal of endotoxin research. 2005;11(2):85–8. doi: 10.1179/096805105x35170. [DOI] [PubMed] [Google Scholar]
- 33.Toldo S, Mezzaroma E, O'Brien L, et al. Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. American journal of physiology Heart and circulatory physiology. 2014;306(7):H1025–31. doi: 10.1152/ajpheart.00795.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toth MJ, Ades PA, Tischler MD, Tracy RP, LeWinter MM. Immune activation is associated with reduced skeletal muscle mass and physical function in chronic heart failure. Int J Cardiol. 2006;109(2):179–87. doi: 10.1016/j.ijcard.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Van Tassell BW, Arena R, Biondi-Zoccai G, et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study) Am J Cardiol. 2014;113(2):321–7. doi: 10.1016/j.amjcard.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Tassell BW, Arena RA, Toldo S, et al. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS One. 2012;7(3):e33438. doi: 10.1371/journal.pone.0033438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Tassell BW, Raleigh JM, Abbate A. Targeting interleukin-1 in heart failure and inflammatory heart disease. Current heart failure reports. 2015;12(1):33–41. doi: 10.1007/s11897-014-0231-7. [DOI] [PubMed] [Google Scholar]
- 38.Wawrocki S, Druszczynska M, Kowalewicz-Kulbat M, Rudnicka W. Interleukin 18 (IL-18) as a target for immune intervention. Acta Biochim Pol. 2016 doi: 10.18388/abp.2015_1153. [DOI] [PubMed] [Google Scholar]
- 39.Wong E, Selig S, Hare DL. Respiratory muscle dysfunction and training in chronic heart failure. Heart Lung Circ. 2011;20(5):289–94. doi: 10.1016/j.hlc.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]


