Abstract
Background
Anastomotic leak (AL) is a major source of morbidity in colorectal surgery and has become an area of interest in performance metrics. It is unclear whether AL is associated primarily with surgeons’ technical performance or explained better by patient characteristics and institutional factors. We sought to establish if AL could serve as a valid quality metric in colorectal surgery by evaluating provider variation after adjusting for patient factors.
Methods
We performed a retrospective cohort study of colorectal resection patients in the Michigan Surgical Quality Collaborative. Clinically relevant patient and operative factors were tested for association with AL. Hierarchical logistic regression was used to derive risk-adjusted rates of AL.
Results
Of 9,192 colorectal resections, 244 (2.7%) had a documented AL. The incidence of AL was 3.0% for patients with pelvic anastomoses and 2.5% for those with intra-abdominal anastomoses. Multivariable analysis showed that a greater operative duration, male sex, body mass index > 30 kg/m2, tobacco use, chronic immunosuppressive medications, thrombocytosis (platelet count > 400×109/L), and urgent/emergent surgery were independently associated with AL (C-statistic = 0.75). After accounting for patient and procedural risk factors, there were five hospitals with a significantly greater incidence of postoperative AL.
Conclusions
This population-based study shows that risk factors for AL include male sex, obesity, tobacco use, immunosuppression, thrombocytosis, greater operative duration, and urgent/emergent surgery; models including these factors predict most of the variation in AL rates. This study suggests that AL can serve as a valid metric that can identify opportunities for quality improvement.
Introduction
Anastomotic leak (AL) is a major source of morbidity and mortality after colorectal resection, occurring in 2–19% of patients.1–6 Prolonged duration of hospital stay, poor oncologic outcomes, and increased mortality have all been associated with AL.7, 8 In recognition of the considerable impact of AL on patients undergoing colorectal resection, it has been identified as “the most important quality indicator”9 after colorectal operations.
Nevertheless, it is unclear whether AL represents an unavoidable outcome determined by patient factors or a modifiable outcome influenced by providers. There are essentially no data providing risk-adjusted comparisons between providers on this important outcome, due in part to a lack of consensus on risk factors for AL. Many single-center and small multicenter studies have been performed to attempt to identify risk factors but there have been varying results.10–13 Until more generalizable studies are available which enumerate the most important risk-factors,8 performing risk-adjusted comparisons between providers is not possible.14 In addition, many studies utilize administrative data which rely on surrogates that may not capture ALs effectively.15 The demonstration of variation in risk-adjusted rates of clinically defined AL between providers would be an important finding, indicating the potential for performance improvement.16
In this context, we conducted a population-based, retrospective cohort study of risk factors for AL using data from a validated clinical registry with 30-day follow-up. We then used a model developed from statistically-significant risk factors to determine whether there was hospital variation in adjusted AL rates. We hypothesized that substantial hospital variation would exist, as with other postoperative complications of surgery.17, 18
Materials and Methods
Study Population and Setting
This study analyzes data from the Michigan Surgical Quality Collaborative (MSQC), a statewide organization of community and academic hospitals with a validated surgical registry focused on quality assessment and improvement in general and vascular surgery.19, 20 The MSQC is a provider-led, quality improvement organization funded by Blue Cross and Blue Shield of Michigan. Participating hospitals vary in size and teaching status, with a predominance of community hospitals. At every hospital, trained, dedicated nurse abstractors collect patient characteristics, perioperative and intraoperative processes of care, and 30-day postoperative outcomes for general and vascular operations. Routine validation of the data collection is performed with regular training sessions, conference calls, and internal data audits.
All patients > 18 years of age who underwent colon or rectal resection with anastomosis between July 2012 and June 2015 were included in the study. These procedures were identified and classified according to Current Procedural Terminology (CPT) codes, including the following: (1) Colorectal resections with pelvic anastomoses21–24 (44145, 44146, 44157, 44158, 44207, 44208, 44210, 44211, 45111, 45112, 45113, 45114, 45119, 45135, 45397) and (2) colorectal resections with abdominal anastomoses (44140, 44147, 44160, 44204, 44205, 45402, 45550). (Supplement Table 1) The primary analysis included all patients; however, a sensitivity analysis was performed from which patients who likely had a proximal diversion were excluded. For the sensitivity analysis, “operations with proximal diversion” were defined as those with the following CPT codes: 44146, 44157, 44208, 44210, 44211, 45113, 45119, 45397. (Supplemental Table 1-online only)
Outcomes
The primary outcome was anastomotic leak within 30 days. This was abstracted as a categorical variable, with categories similar to the International Study Group of Rectal Cancer, which grades AL based on the impact on clinical management.25 Major leaks included those resulting in reoperations with new anastomosis, reoperations with proximal diversion, or reoperation with formation of an end stoma. Minor leaks included anastomoses requiring only antibiotics or percutaneous drainage. Secondary outcomes included length of stay, superficial, deep, and organ/space surgical site infection (SSI), postoperative sepsis; readmission, reoperation, renal insufficiency, and death.
Covariates
Demographic characteristics, comorbidities, and procedural characteristics were tested for associations with AL. Demographic characteristics included age, sex, race (white, non-white), and insurance type (Medicaid, Medicare, private, uninsured, other). Measures of functional status included the American Society of Anesthesiologists (ASA) physical status classification scores, dyspnea on exertion or at rest, and functional status (independent, partially dependent, dependent). Comorbidities included body mass index (BMI) > 30 kg/m2, coronary artery disease (CAD), congestive heart failure, hypertension, peripheral vascular disease, current tobacco use, chronic obstructive pulmonary disease (COPD), renal dialysis dependence, bleeding disorders, preoperative transfusion requirements, history of deep venous thrombosis, diabetes, use of immunosuppressive medications (defined in MSQC as: “steroids/immunosuppressive drugs for chronic conditions”), and platelet count. Operative indications were categorized as colorectal cancer, other neoplasms, diverticulitis, inflammatory bowel disease (IBD), bowel obstruction or volvulus, vascular insufficiency, and other. Operative factors analyzed include urgent/emergent case priority, operative duration (hours), open vs. laparoscopic approach, the formation of a proximal intestinal diversion, and wound classification (clean, clean/contaminated, contaminated, dirty/infected).
Statistical Analysis
Continuous variables were assessed for association with AL using Student’s t-test if normally distributed or Wilcoxon Rank-Sum nonparametric tests if non-normally distributed. Categorical variables were tested using Chi-Square or Fisher’s Exact Test in the case of small cell sizes. Clinically relevant and statistically significant variables from bivariate analyses were considered for inclusion in multivariable, hierarchical logistic regression models. Stepwise logistic regression was performed for initial variable selection (significance level of p < 0.05); however, several particularly important variables were “forced into” final models to adjust for clinically relevant risk factors (e.g. abdominal vs. pelvic anastomosis). Collinearity was measured amongst candidate variables in the final model using Spearman’s or Pearson’s correlation matrices, and variables assessed to be collinear with other variables in the model were excluded. A final hierarchical model was established with case-mix as fixed effects and hospital as random effects to account for clustering of patients within hospitals. This approach of reliability adjustment resulted in shrinkage of hospitals’ adjusted rates toward the overall adjusted MSQC AL rate and accounted for low hospital-specific case volume of colectomies. Model fit was assessed using quartile and decile analyses of observed and predicted leak rates, measures of concordance (C-statistic), and evaluation of Pearson’s Chi-Square residuals to identify any over-dispersion. Validation of the model was done by selecting randomly a 50% subset of the original cohort and fitting a hierarchical logistic regression using the same covariates; the results were similar to the primary analysis that modeled all cases analyzed, other than the expected loss of power due to the smaller sample size. For the validation exercise, the C-statistic was 0.75. The Type III overall fixed effects for the validation analysis indicating significant association with AL are supplied in Supplement Table 2 (online only).
Final models were used to calculate case-mix-adjusted rates of AL, and hospitals were ranked by their adjusted rates of AL with 95% confidence intervals. Hospitals with fewer than 20 colectomies were excluded from hospital comparisons due to small sample size; therefore, 59 of the 64 hospitals within the collaborative were compared on their adjusted leak rates. The 5 excluded hospitals reported a total of 41 cases, in which 2 ALs were identified (4.9%). Statistical outliers included hospitals whose 95% confidence interval for their hospital-specific adjusted AL rate did not cross the overall adjusted AL rate.
All statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC). This study was reviewed and deemed “not regulated” by the University of Michigan Institutional Review Board. Data are presented as means±SD or median (interquartile range) as appropriate.
Results
Study Population
We studied 9,192 patients who underwent colorectal resection with anastomosis at 64 Michigan hospitals. The mean number of cases per hospital was 144±94. There were 244 (2.7%) ALs identified. Among the 82 minor leaks (34%), there were 42 (17%) managed with antibiotics alone and 40 (16%) who underwent percutaneous drainage. The 162 (66%) major leaks managed with reoperation included 46 exploration and re-anastomosis (18.9%), 33 exploration with creation of a defunctioning stoma (13.5%), and 83 exploration with creation of an end stoma (34.0%).
Clinical and demographic characteristics of patients with and without AL are compared in Table 1. Overall, patients who developed AL were significantly older, more likely to be of male sex, have poorer functional status (partially or totally dependent), higher ASA classification scores, and greater rates of certain comorbid conditions (COPD, preoperative tobacco use, obesity, use of immunosuppressive medications).
Table 1.
Patient Population
| Characteristic | No Leak n=8948 | Leak n= 244 | P-value |
|---|---|---|---|
| Patient Preoperative (%) | |||
| Age, years, (SD) | 63.3 (14.7) | 61.5 (14.0) | 0.05 |
| Male sex, n (%) | 3944 (44.08) | 146 (59.84) | <0.0001 |
| Body mass index > 30 kg/m2 | 3210 (35.87) | 113 (46.31) | 0.0008 |
| Race, n (%) | |||
| White | 7522 (87.11) | 204 (86.81) | 0.8916 |
| Non-white | 1113 (12.89) | 31 (13.19) | |
| American Society of Anesthesiologists Class, n (%) | |||
| 1 | 156 (1.74) | 0 (0.00) | 0.0012 |
| 2 | 3690 (41.24) | 82 (33.61) | |
| 3 | 4374 (48.88) | 133 (54.51) | |
| 4 | 698 (7.80) | 27 (11.07) | |
| 5 | 27 (0.30) | 1 (0.41) | |
| Functional Status, n (%) | |||
| Independent | 8462 (94.57) | 217 (88.93) | 0.0001 |
| Partially dependent | 344 (3.84) | 20 (8.20) | |
| Dependent | 106 (1.18) | 3 (1.23) | |
| Insurance, n (%) | |||
| Medicaid | 541 (6.05) | 27 (11.07) | 0.0365 |
| Medicare | 4356 (48.68) | 118 (48.36) | |
| Private | 3574 (39.94) | 89 (36.48) | |
| Uninsured | 108 (1.21) | 1 (0.41) | |
| All Others | 365 (4.08) | 9 (3.69) | |
| Patient Comorbidities, n (%) | |||
| Cardiovascular | |||
| Coronary artery disease | 1520 (16.99) | 50 (20.49) | 0.1512 |
| Congestive heart failure | 102 (1.14) | 3 (1.23) | 0.8966 |
| Hypertension | 5021 (56.11) | 139 (56.97) | 0.7908 |
| Peripheral vascular disease | 276 (3.08) | 9 (3.69) | 0.5912 |
| Pulmonary | |||
| Dyspnea Upon Exertion | 945 (10.56) | 32 (13.11) | 0.1276 |
| Dyspnea at rest | 68 (0.76) | 4 (1.64) | |
| Tobacco use | 1910 (21.35) | 76 (31.15) | 0.0002 |
| Chronic obstructive pulmonary disease | 925 (10.34) | 38 (15.57) | 0.0084 |
| Pneumonia | 56 (0.63) | 2 (0.81) | 0.7060 |
| Dialysis Dependent | 85 (0.95) | 4 (1.64) | 0.3012 |
| Hematologic | |||
| Bleeding Disorder | 432 (4.83) | 17 (6.97) | 0.1261 |
| Preoperative Transfusion | 375 (4.19) | 12 (4.92) | 0.5768 |
| History of deep venous thrombosis | 554 (6.19) | 19 (7.79) | 0.3091 |
| Platelet count > 400 × 109/L | 614 (7.05) | 29 (12.29) | 0.0021 |
| Endocrine | |||
| Diabetes | 1783 (19.93) | 51 (20.90) | 0.7068 |
| Chronic Immunosuppression | 485 (5.42) | 33 (13.52) | <0.0001 |
| Indication for Operation, n (%) | |||
| Colorectal Cancer | 3252 (36.34) | 75 (30.74) | 0.0722 |
| Diverticulitis | 2662 (29.75) | 77 (31.56) | 0.5424 |
| Inflammatory Bowel Disease | 303 (3.39) | 16 (6.56) | 0.0076 |
| Obstruction/volvulus | 616 (6.88) | 20 (8.20) | 0.4254 |
| Other Benign/Malignant Neoplasms | 1449 (16.19) | 35 (14.34) | 0.4386 |
| Vascular insufficiency | 189 (2.11) | 8 (3.28) | 0.2145 |
| Other | 477 (5.33) | 13 (5.33) | 0.9984 |
| Operative Factors, n (%) | |||
| Urgent/Emergent Operation | 2427 (27.12) | 93 (38.11) | 0.0001 |
| Median operative time, hours (IQR) | 2.87 (2.23–3.77) | 3.19 (2.46–4.22) | 0.0001 |
| Colorectal Procedure Type | |||
| Open abdominal anastomosis | 3662 (40.93) | 116 (47.54) | 0.0382 |
| Open pelvic anastomosis | 1284 (14.35) | 44 (18.03) | 0.1064 |
| Laparoscopic abdominal anastomosis | 3054 (34.13) | 58 (23.77) | 0.0007 |
| Laparoscopic pelvic anastomosis | 948 (10.59) | 26 (10.66) | 0.9756 |
| Proximal intestinal diversion | 405 (4.53) | 11 (4.51) | 0.9894 |
| Wound Classification | |||
| Clean | 68 (0.75) | 0 (0.00) | 0.2656 |
| Clean/Contaminated | 6972 (77.92) | 173 (70.9) | 0.0094 |
| Contaminated | 1014 (11.33) | 34 (13.93) | 0.207 |
| Dirty/Infected | 894 (9.99) | 37 (15.96) | 0.0082 |
| Intraoperative Endoscopy | 608 (6.79) | 19 (7.79) | 0.5442 |
ASA, American Society of Anesthesiologists
Operative factors associated with AL included urgent/emergent case designations, increased operative times, and class 3 or 4 wound classifications. Colorectal cancer and diverticulitis represented the most common indications for operation. Patients with pelvic anastomoses (n = 2302) developed leak in 3.0% (n = 70) of cases compared to 2.5% (n = 174) of patients who had intra-abdominal anastomoses (n = 6714, p = 0.18). As expected, patients diagnosed with AL had significantly greater durations of hospitalizations, increased reoperation rates, increased readmission rates, renal insufficiency, and greater mortality rates (Table 2).
Table 2.
Resource utilization
| Post operative outcomes, n (%) | No Anastomotic Leak | Anastomotic Leak | P- value |
|---|---|---|---|
| Median duration of stay (days, IQR) | 5 (5) | 13.5 (15) | < 0.0001 |
| Reoperation | 647 (7.40) | 164 (67.77) | < 0.0001 |
| Readmission | 883 (10.09) | 112 (46.47) | < 0.0001 |
| Renal insufficiency | 177 (1.98) | 24 (9.84) | < 0.0001 |
| Death | 229 (2.57) | 19 (8.37) | < 0.0001 |
Analysis of Independent Risk Factors
Multivariable analysis was performed to identify independent risk factors for AL. (Table 3) Seven independent risk factors for AL were identified: male sex (aOR 1.97; 95% CI 1.50–2.58), BMI > 30 kg/m2 (aOR 1.71; 95% CI 1.31–2.24), preoperative immunosuppressive medication use (aOR 2.62; 95% CI 1.76–3.89), surgical duration (aOR 1.16; 95% CI 1.06–1.26), indication as urgent or emergent operation (aOR 1.66; 95% CI 1.23–2.23), tobacco use history (aOR 1.59; 95% CI 1.20–2.10), and platelet count (aOR 1.18; 95% CI 1.18–2.69). While pelvic versus abdominal surgery and laparoscopic versus open surgery were kept in the model for face validity, neither factor was statistically significant after adjusting for the factors above. The model predicted most of the variation in rates of AL (C-statistic = 0.75). (Tables 3)
Table 3.
Multivariable Regression: Factors Associated with Colorectal Anastomotic Leak
| Proximal intestinal diversion included | Proximal intestinal diversion excluded | |||||
|---|---|---|---|---|---|---|
| Variable | Co-efficient | Adjusted OR (95% CI) | P-value | Co-efficient | Adjusted OR (95% CI) | P-value |
| Male sex | 0.6782 | 1.970 (1.504, 2.581) | <0.0001 | 0.6579 | 1.931 (1.466, 2.543) | <0.0001 |
| BMIa > 30 kg/m2 | 0.5384 | 1.713 (1.310, 2.241) | <0.0001 | 0.5775 | 1.782 (1.354, 2.344) | <0.0001 |
| Chronic immunosuppression | 0.9621 | 2.617 (1.762, 3.887) | <0.0001 | 1.0244 | 2.785 (1.841, 4.215) | <0.0001 |
| Surgical time (hours) | 0.1463 | 1.157 (1.062, 1.262) | 0.0009 | 0.1573 | 1.170 (1.072, 1.278) | 0.0005 |
| Urgent/emergent | 0.5055 | 1.658 (1.230, 2.234) | 0.0009 | 0.4982 | 1.646 (1.214, 2.231) | 0.0013 |
| Tobacco use | 0.4642 | 1.591 (1.203, 2.104) | 0.0011 | 0.4259 | 1.531 (1.149, 2.040) | 0.0036 |
| Platelet > 400 × 109/L | 0.5788 | 1.784 (1.183, 2.691) | 0.0058 | 0.5875 | 1.800 (1.177, 2.752) | 0.0067 |
| Open approach | 0.1516 | 1.164 (0.863, 1.570) | 0.3206 | 0.2126 | 1.237 (0.909, 1.682) | 0.1754 |
| Pelvic anastomosis | 0.1303 | 1.139 (0.835, 1.550) | 0.4115 | 0.2257 | 1.253 (0.900, 1.745) | 0.1813 |
BMI, body mass index.
Hospital Variation in Anastomotic Leak
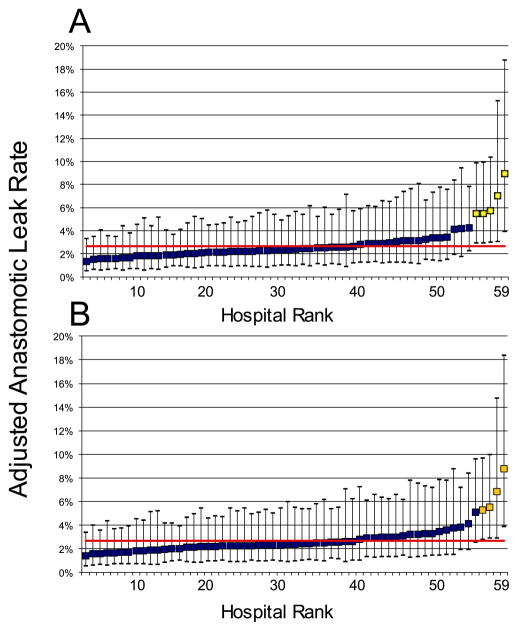
Plots of hospital adjusted leak rates including 95% CIs were generated to identify hospitals for which the performance was significantly different from the overall adjusted MSQC AL rate (Figure 1). There were 5 high-outlier hospitals in the primary analysis which reported between 41 and 224 cases during the study period. For the outlier hospitals, point estimates of adjusted AL rates ranged from 5.5% to 9.0%, while for other hospitals adjusted rates ranged from 1.4% to 4.3%.
Figure 1.
Comparison of case-mix adjusted anastomotic leak (AL) rates for 59 hospitals, including adjusted 95% confidence intervals (CI), in the MSQC. The MSQC adjusted anastomotic leak rate (2.7%) is displayed as a red horizontal line. Outlier hospitals are identified with yellow squares because their 95% CI do not intersect with the MSQC adjusted AL rate. Figure (A) includes cases with proximal diversion and identifies 5 high-outlier hospitals. Figure (B) excluding proximal diversion and identifies 4 outlier hospitals.
Sensitivity Analysis Excluding Proximal Diversion
A sensitivity analysis was performed from which patients who likely had a proximal diversion were excluded. When multivariable analysis was performed to identify independent risk factors for AL, results were the same with adjusted AL rates similar to the primary analysis (Proximal diversion included: 2.65%, 95% exact binomial CI: 2.34–3.00%; Proximal diversion excluded: 2.65%, 95% exact binomial CI: 2.33–3.01%). (Table 3) Again, we plotted hospital adjusted AL rates with confidence intervals and compared them to the overall MSQC rate. (Figure 1). Four high-outlier hospitals were identified, all of which were also outliers in the first model. No low-outlier hospitals with statistically lesser adjusted AL rates were identified.
Discussion
In this population-based study, we found a significant variation in hospital risk-adjusted rates of AL after colorectal resection. In addition, we identified independent risk factors for AL that will be important in performing valid, risk-adjusted comparisons of hospital AL rates. Following a modified Delphi methodology, the American Society of Colon and Rectal Surgeons generated recently a consensus of outcome measures, which identified AL as the “most important” quality indicator after colectomy.9 If AL is to be used as a quality metric for colorectal resections, data such as these are important for valid benchmarking of providers. A better understanding of risk factors for AL may also be valuable in the clinical setting. While factors such as sex of the patient, preoperative platelet count, and surgical priority are not modifiable, our study identifies several potentially modifiable factors, including pre-operative tobacco use, obesity, and immunosuppressive drugs.
This study also highlights the extreme morbidity associated with anastomotic leak. The majority of patients diagnosed with AL underwent reoperative interventions—which we classified as “major leaks”. Downstream complications and resource utilization were also increased with an AL, with high rates of readmission, reoperation, and prolonged duration of hospital stay.26 Our reoperation rate for AL of 67.8% is consistent with currently published data.27 In addition, we are encouraged by the low failure to rescue rate of 8.4%, which compares well with a recent study performed by Tevis et al28 and is less than historic studies which have cited rates between 12.0–18.6%.12
Midura and colleagues published recently a study evaluating risk factors and consequences of AL in a cohort of patients from the American College of Surgeons’ National Surgical Quality Improvement Program (ACS-NSQIP) colectomy procedure-targeted database.8 Our study expands on this prior work by adding to the analysis a broader group of patients undergoing colorectal anastomoses (the prior work included only CPT codes 44140, 44204, 44160, 44205, 44145, 44207), as well as patients with rectal cancer (not included in the NSQIP colectomy database). Both studies used trained data abstractors and standard coding criteria to identify AL in relatively low percentages of patients (NSQIP: 3.7% versus MSQC: 2.7%). Several risk-factors from the Midura study were also identified in the present study (male sex, prolonged surgical times, smoking history, chronic steroid use/immunosuppression).8 In contrast to the prior study,8 we did not find a significant difference in AL rates related to open versus laparoscopic procedures and did not identify a protective effect related to the proximal diversion. Of several laboratory values tested, we found that thrombocytosis was independently associated with AL. We theorize that thrombocytosis is not a risk factor itself but rather a manifestation of high-risk clinical conditions, such as chronic inflammatory states, malignancy, or acute infectious processes.29–32 Therefore, this may be a reasonable marker for patients at risk of AL, and useful in risk-adjustment.
The identified AL rate of 2.7% is low when compared to historic studies that have cited rates as high as 19% for rectal anastomoses.2, 33–35 The the results, however, of this study are consistent with several, recent, randomized trials of rectal cancer surgery, which have shown similarly low rates of AL (0.6–7%)3–6. We hypothesize that selective, proximal diversion is a factor in the low AL rate demonstrated.36 Our sensitivity analysis excluding cases with proximal diversion showed that risk factors and leak rates were remarkably similar to those of cohort in which proximal diversion was included. It is possible that the greatest risk cases are either being diverted (thus decreasing clinically-apparent leaks) or having procedures in which no anastomosis is made (eliminating the potential for an anastomotic leak).
The finding from this study that AL rates vary between hospitals raises the following question: can AL rates be decreased through quality improvement projects? MSQC is addressing AL in several ways. First, MSQC now provides hospitals with data on their AL rates relative to other hospitals, thus informing high-outlier hospitals of their performance status. Audit and feedback reports have been shown to be an effective strategy in improving performance in multiple health care domains; however, the effect size is generally low, and other strategies are also needed.20 For example, in the field of bariatric surgery, coaching of technical skills coaching is being explored as a means to disseminate best technical practices among surgeons. Peer-ratings of technical videos have been shown to correlate with surgical outcomes,37 prompting efforts to institute coaching of technical skills coaching.38 Though this work is in its infancy, the MSQC is embarking on a project of coaching in colectomy technical skills in which surgeons will share intraoperative videos with one another. Finally, the results of this study will be disseminated across the collaborative so that risk factors we have identified can be taken into account by surgeons deciding on whether or not to perform a proximal diversion after anastomosis. These measures, coupled with the technical innovations such as intraoperative leak testing39 and anastomotic perfusion assessment40, may lead to even fewer ALs in the future.
When interpreting these results, several limitations must be considered. Although the MSQC performs a detailed chart review, identification of complications is dependent on provider documentation. For example, if the term “anastomotic leak” is not used in the medical record but rather substituted with terms such as “abscess” or “collection,” minor leaks may not be identified. While relying on provider documentation of anastomotic leak may lead to some underreporting of minor leaks, we believe that this definition of “anastomotic leak” is more valid than the use of surrogate measures for leak as has been done in other studies. In fact, the surrogate “organ space infection” as used in NSQIP and MSQC has been shown to be a poor surrogate for AL.15 Fortunately, the misclassification (as with under-reporting) of rare outcomes such as AL generally results in less bias than misclassification of exposure variables. As such, the associations identified (risk factors) are likely to be true, regardless of this limitation. Although unmeasured differences in case-mix may influence results in observational studies, our study is strengthened by registry data that are collected prospectively and much more granular than administrative data sources. Other studies have used RVU-based adjustment for complexity, which does not adequately capture procedure-specific risk for AL. Thus, we used clinically relevant predictors, such as operative time, laparoscopic approach, and pelvic anastomosis, as surrogates for case complexity. Unfortunately, some clinically important data were not available for this study, including technical details about operations (e.g. type of anastomosis, intraoperative leak-testing, anastomotic distance from anal verge) and patient factors (e.g. nutritional status). Finally, the choice to perform provider comparisons at the hospital level rather than the surgeon level was related to the inability to make statistically valid comparisons at the surgeon level due to (1) our agreement for use of the data, which did not allow for surgeon identifiers, and (2) small case volumes at the surgeon level.14 Nevertheless, future work at the surgeon level may be possible (as the volume of available cases with this outcome increases with additional data collection).
In conclusion, this study finds significant variation in risk-adjusted AL rates and identifies multiple risk factors for AL. These data may allow for more accurate risk-assessment in clinical practice and for appropriate risk-adjustment in audit and feedback programs with AL as an outcome. These data suggest the potential for quality improvement programs targeting technical abilities in hospitals with high rates of AL.
Supplementary Material
Footnotes
Disclaimers: The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article. This study was presented at the annual meeting of the American Society of Colon and Rectal Surgeons in Los Angeles, CA, April 30th – May 4th, 2016.
Contributions: The elements of the study would not be possible without significant contributions from all authors. Conception of design: SH, VCN, NK. Acquisition of data: NK, VCN, SH. Analysis and interpretation of data: VCN, NK, SH, AMM, SER, JCB, PAS, DAC. Drafting article: VCN, NK, SH. Critical revisions: all authors. Final approval of the version to be submitted: all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bertelsen CA, Andreasen AH, Jorgensen T, et al. Anastomotic leakage after curative anterior resection for rectal cancer: short and long-term outcome. Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland. 2010;12:e76–81. doi: 10.1111/j.1463-1318.2009.01935.x. [DOI] [PubMed] [Google Scholar]
- 2.Damen N, Spilsbury K, Levitt M, et al. Anastomotic leaks in colorectal surgery. ANZ journal of surgery. 2014;84:763–8. doi: 10.1111/ans.12494. [DOI] [PubMed] [Google Scholar]
- 3.Fleshman J, Branda M, Sargent DJ, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA. 2015;314:1346–55. doi: 10.1001/jama.2015.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeong SY, Park JW, Nam BH, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15:767–74. doi: 10.1016/S1470-2045(14)70205-0. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson AR, Solomon MJ, Lumley JW, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA. 2015;314:1356–63. doi: 10.1001/jama.2015.12009. [DOI] [PubMed] [Google Scholar]
- 6.Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324–32. doi: 10.1056/NEJMoa1414882. [DOI] [PubMed] [Google Scholar]
- 7.Branagan G, Finnis D Wessex Colorectal Cancer Audit Working G. Prognosis after anastomotic leakage in colorectal surgery. Diseases of the colon and rectum. 2005;48:1021–6. doi: 10.1007/s10350-004-0869-4. [DOI] [PubMed] [Google Scholar]
- 8.Midura EF, Hanseman D, Davis BR, et al. Risk factors and consequences of anastomotic leak after colectomy: a national analysis. Diseases of the colon and rectum. 2015;58:333–8. doi: 10.1097/DCR.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 9.Manwaring ML, Ko CY, Fleshman JW, Jr, et al. Identification of consensus-based quality end points for colorectal surgery. Diseases of the colon and rectum. 2012;55:294–301. doi: 10.1097/DCR.0b013e318241b11f. [DOI] [PubMed] [Google Scholar]
- 10.Tan WP, Hong EY, Phillips B, et al. Anastomotic leaks after colorectal anastomosis occurring more than 30 days postoperatively: a single-institution evaluation. The American surgeon. 2014;80:868–72. [PubMed] [Google Scholar]
- 11.Leahy J, Schoetz D, Marcello P, et al. What is the risk of clinical anastomotic leak in the diverted colorectal anastomosis? Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2014;18:1812–6. doi: 10.1007/s11605-014-2588-z. [DOI] [PubMed] [Google Scholar]
- 12.Alves A, Panis Y, Trancart D, et al. Factors associated with clinically significant anastomotic leakage after large bowel resection: multivariate analysis of 707 patients. World journal of surgery. 2002;26:499–502. doi: 10.1007/s00268-001-0256-4. [DOI] [PubMed] [Google Scholar]
- 13.Marinello FG, Baguena G, Lucas E, et al. Anastomotic leaks after colon cancer resections: Does the individual surgeon matter? Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland. 2015 doi: 10.1111/codi.13212. [DOI] [PubMed] [Google Scholar]
- 14.Shih T, Cole AI, Al-Attar PM, et al. Reliability of surgeon-specific reporting of complications after colectomy. Annals of surgery. 2015;261:920–5. doi: 10.1097/SLA.0000000000001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rickles AS, Iannuzzi JC, Kelly KN, et al. Anastomotic leak or organ space surgical site infection: What are we missing in our quality improvement programs? Surgery. 2013;154:680–7. doi: 10.1016/j.surg.2013.06.035. discussion 7–9. [DOI] [PubMed] [Google Scholar]
- 16.Kao LS, Ghaferi AA, Ko CY, et al. Reliability of superficial surgical site infections as a hospital quality measure. J Am Coll Surg. 2011;213:231–5. doi: 10.1016/j.jamcollsurg.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietz DW Consortium for Optimizing Surgical Treatment of Rectal C. Multidisciplinary management of rectal cancer: the OSTRICH. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2013;17:1863–8. doi: 10.1007/s11605-013-2276-4. [DOI] [PubMed] [Google Scholar]
- 18.Birkmeyer NJ, Finks JF, Carlin AM, et al. Comparative effectiveness of unfractionated and low-molecular-weight heparin for prevention of venous thromboembolism following bariatric surgery. Arch Surg. 2012;147:994–8. doi: 10.1001/archsurg.2012.2298. [DOI] [PubMed] [Google Scholar]
- 19.Waljee JF, Birkmeyer NJ. Collaborative quality improvement in surgery. Hand clinics. 2014;30:335–43. vi. doi: 10.1016/j.hcl.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Share DA, Campbell DA, Birkmeyer N, et al. How a regional collaborative of hospitals and physicians in Michigan cut costs and improved the quality of care. Health Aff (Millwood) 2011;30:636–45. doi: 10.1377/hlthaff.2010.0526. [DOI] [PubMed] [Google Scholar]
- 21.Trencheva K, Morrissey KP, Wells M, et al. Identifying important predictors for anastomotic leak after colon and rectal resection: prospective study on 616 patients. Annals of surgery. 2013;257:108–13. doi: 10.1097/SLA.0b013e318262a6cd. [DOI] [PubMed] [Google Scholar]
- 22.Bertelsen CA, Andreasen AH, Jorgensen T, et al. Anastomotic leakage after anterior resection for rectal cancer: risk factors. Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland. 2010;12:37–43. doi: 10.1111/j.1463-1318.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- 23.Rullier E, Laurent C, Garrelon JL, et al. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg. 1998;85:355–8. doi: 10.1046/j.1365-2168.1998.00615.x. [DOI] [PubMed] [Google Scholar]
- 24.McDermott FD, Heeney A, Kelly ME, et al. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. 2015;102:462–79. doi: 10.1002/bjs.9697. [DOI] [PubMed] [Google Scholar]
- 25.Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339–51. doi: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Hammond J, Lim S, Wan Y, et al. The burden of gastrointestinal anastomotic leaks: an evaluation of clinical and economic outcomes. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2014;18:1176–85. doi: 10.1007/s11605-014-2506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyman NH, Osler T, Cataldo P, et al. Anastomotic leaks after bowel resection: what does peer review teach us about the relationship to postoperative mortality? J Am Coll Surg. 2009;208:48–52. doi: 10.1016/j.jamcollsurg.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Tevis SE, Carchman EH, Foley EF, et al. Does Anastomotic Leak Contribute to High Failure-to-rescue Rates? Annals of surgery. 2016;263:1148–51. doi: 10.1097/SLA.0000000000001409. [DOI] [PubMed] [Google Scholar]
- 29.Estrov Z, Talpaz M, Mavligit G, et al. Elevated plasma thrombopoietic activity in patients with metastatic cancer-related thrombocytosis. Am J Med. 1995;98:551–8. doi: 10.1016/s0002-9343(99)80013-8. [DOI] [PubMed] [Google Scholar]
- 30.Heits F, Katschinski DM, Wilmsen U, et al. Serum thrombopoietin and interleukin 6 concentrations in tumour patients and response to chemotherapy-induced thrombocytopenia. Eur J Haematol. 1997;59:53–8. doi: 10.1111/j.1600-0609.1997.tb00959.x. [DOI] [PubMed] [Google Scholar]
- 31.Heits F, Stahl M, Ludwig D, et al. Elevated serum thrombopoietin and interleukin-6 concentrations in thrombocytosis associated with inflammatory bowel disease. J Interferon Cytokine Res. 1999;19:757–60. doi: 10.1089/107999099313604. [DOI] [PubMed] [Google Scholar]
- 32.Cerutti A, Custodi P, Duranti M, et al. Thrombopoietin levels in patients with primary and reactive thrombocytosis. Br J Haematol. 1997;99:281–4. doi: 10.1046/j.1365-2141.1997.3823196.x. [DOI] [PubMed] [Google Scholar]
- 33.Krarup PM, Jorgensen LN, Andreasen AH, et al. A nationwide study on anastomotic leakage after colonic cancer surgery. Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland. 2012;14:e661–7. doi: 10.1111/j.1463-1318.2012.03079.x. [DOI] [PubMed] [Google Scholar]
- 34.Jannasch O, Klinge T, Otto R, et al. Risk factors, short and long term outcome of anastomotic leaks in rectal cancer. Oncotarget. 2015;6:36884–93. doi: 10.18632/oncotarget.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorensen LT, Jorgensen T, Kirkeby LT, et al. Smoking and alcohol abuse are major risk factors for anastomotic leakage in colorectal surgery. Br J Surg. 1999;86:927–31. doi: 10.1046/j.1365-2168.1999.01165.x. [DOI] [PubMed] [Google Scholar]
- 36.Peeters KC, Tollenaar RA, Marijnen CA, et al. Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg. 2005;92:211–6. doi: 10.1002/bjs.4806. [DOI] [PubMed] [Google Scholar]
- 37.Birkmeyer JD, Finks JF, O’Reilly A, et al. Surgical skill and complication rates after bariatric surgery. N Engl J Med. 2013;369:1434–42. doi: 10.1056/NEJMsa1300625. [DOI] [PubMed] [Google Scholar]
- 38.Grenda TR, Pradarelli JC, Dimick JB. Using Surgical Video to Improve Technique and Skill. Annals of surgery. 2016 doi: 10.1097/SLA.0000000000001592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon S, Morris A, Billingham R, et al. Routine leak testing in colorectal surgery in the Surgical Care and Outcomes Assessment Program. Arch Surg. 2012;147:345–51. doi: 10.1001/archsurg.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jafari MD, Wexner SD, Martz JE, et al. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg. 2015;220:82–92. e1. doi: 10.1016/j.jamcollsurg.2014.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.