Abstract
Hypoxia is a known feature of aggressive solid tumors as well as a critical hallmark of the niche in aggressive hematologic malignances. Hypoxia is associated with insufficient response to standard therapy, resulting in disease progression and curtailed patients’ survival through maintenance of noncycling cancer stem-like cells. A better understanding of the mechanisms and signaling pathways induced by hypoxia is essential to overcoming these effects. Recent findings demonstrate that bone marrow in the setting of hematologic malignancies is highly hypoxic and that progression of the disease is associated with expansion of hypoxic niches and stabilization of the oncogenic hypoxia-inducible factor-1alpha (HIF-1α). Solid tumors have also been shown to harbor hypoxic areas, maintaining survival of cancer cells via the HIF-1α pathway. Developing new strategies for targeting hypoxia has become a crucial approach in modern cancer therapy. The number of preclinical and clinical trials targeting low-oxygen tumor compartments or the hypoxic bone marrow niche via hypoxia-activated prodrugs is increasing. This review discusses the development of the hypoxia-activated prodrugs and their applicability in treating both hematologic malignancies and solid tumors.
Background
Hypoxia and hypoxia-inducible factors in cancer
Hypoxia is a well-known feature of the microenvironment in solid tumors, usually attributed to rapid tumor growth and insufficient oxygen supply (1). Hypoxic tumors undergo proteomic, genomic, and epigenetic aberrations and are generally resistant to radiotherapy and chemotherapy (2). Hypoxia’s role in poor therapeutic responses and aggressive tumor biology has prompted ongoing efforts to develop diagnostic and therapeutic tools to target hypoxia or its key mediators, such as hypoxia-inducible factors (HIFs) (1,3).
HIFs and its role in hypoxia
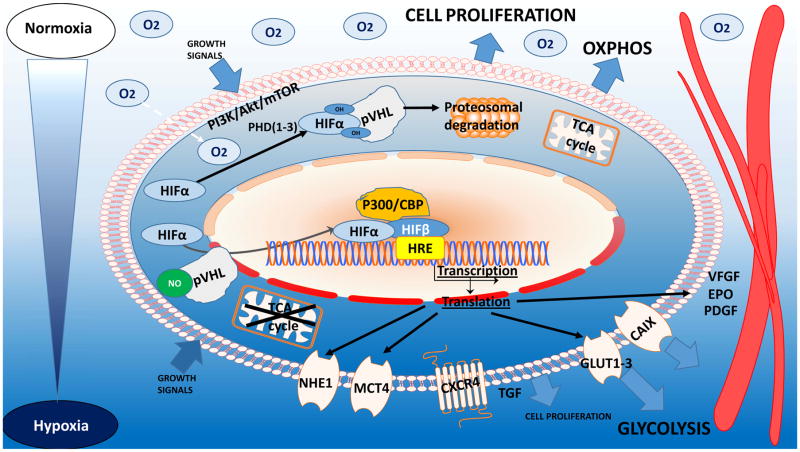
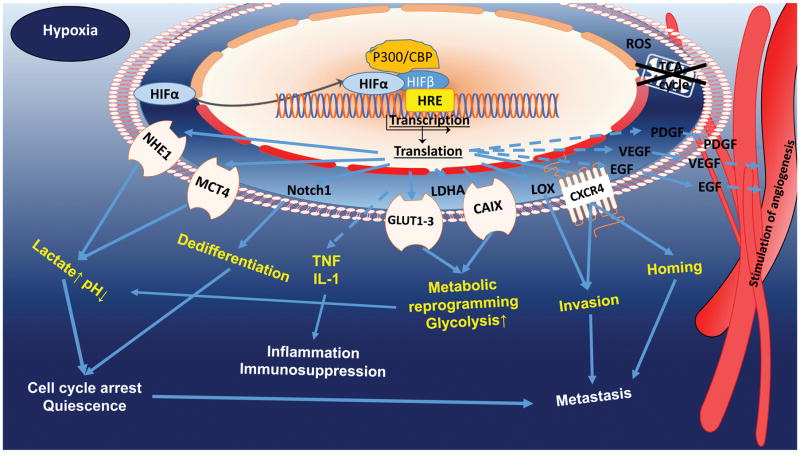
A hypoxic environment maintains changes in tumor cells via the HIF family (4,5). In normoxic conditions, HIF-1α undergoes degradation via catalysis by prolyl hydroxylases and the E3-ubiquitin ligase von Hippel–Lindau tumor suppressor protein complex and by the 26S proteasome (6). In turn, prolyl hydroxylase activity falls in hypoxia, leading to stabilization of HIF-1α, its translocation to the nucleus, and formation of HIF-1α/β heterodimers. The response of HIF subtypes as transcription factors to hypoxia depends on duration of exposure: whereas HIF-1α is a response to acute hypoxia, increasing cell survival and chemoresistance, HIF-2α participates in signal transmission during long-term hypoxia exposure (3), promoting apoptosis (7) and maintaining oncogenic or suppressor activity (8). The role of HIFs in leukemogenesis and carcinogenesis was demonstrated in various models. Generally, suppression of HIF-1α or HIF-2α inhibited the proliferation and growth of hematological malignancies, delaying disease progression (9,10), while HIF overexpression triggered upregulation of hypoxia-driven genes in tumors, inducing activities promoting tumor growth, progression and invasiveness (7,8,11–15) (Fig. 1, 2).
Fig. 1.
HIF-1α maintenance in normoxia, HIF-1α subunits undergo oxygen-dependent degradation via PHD hydroxylation, followed by VHL complex ubiquitination and proteasomal degradation.
Fig. 2.
HIF-1α maintenance in hypoxia, HIF-1α is stabilized and undergoes heterodimerization with subunit β and is then translocated to the nucleus, where it activates hypoxia-dependent gene transcription. HIF plays a key role in hypoxic cells, regulating genetic, epigenetic, and metabolomic reprogramming of cells to survive.
Hypoxia, HIF and the cell cycle
The hypoxic microenvironment is a crucial factor in cancer relapse because of its activities in regulation of the cell cycle, protection from apoptosis, maintenance and quiescence of stem cells, and selection of treatment-resistant noncycling cancer cells (8). Hypoxic tumors upregulate cell cycle inhibitors as a protective mechanism, leading to cell dedifferentiation and arrest or quiescence of the cell cycle (11,16). Chk1 is a central component of genome surveillance pathways required for the initiation of DNA damage checkpoints and is a key regulator of the cell cycle and cell survival. In response to replication stress such as hypoxia, the activation of Chk1 facilitates S and G2 cycle arrest, promoting tumor cell survival, maintained via phosphorylation of tumor suppressor p53. The majority of tumors are deficient in the G1/S DNA damage checkpoint because of mutations in p53, protecting tumor cells from apoptosis. HIFs further attenuate mTOR signaling, that triggers metabolic reprogramming and cancer stem cell quiescence (Fig. 1) (5,7,12).
HIF-1 and maintenance of immunosuppression
Hypoxia in the tumor microenvironment forms a barrier to T cell infiltration and fosters resistance to chemotherapy and radiotherapy. Hypoxia likely also supports immune resistance in tumors by supporting development of suppressive myeloid and T cell populations, by activating immunosuppressive signaling pathways in the tumor and stroma, and by creating a metabolically hostile environment for immune effector cells (17).
Markers of hypoxia
An important issue in targeting hypoxia is identification of appropriate predictive markers. Tools based on immunohistochemical assessment of tumor biopsy specimens detect the distribution of hypoxia by EF5 or pimonidazole staining or by expression of particular hypoxia-associated molecules: HIF-1α, LDH-5, GLUT-1, MCT1, MCT4, or carbonic anhydrase IX (CAIX)(18) (Fig. 1). Specific tumor-imaging techniques such as oxygen-enhanced MRI or PET imaging with [18F]FAZA (NCT01542177), [18F]MISO (NCT02695628), or [18F]HX4 (NCT02233387) have been implemented in clinical trials with the goal of stratifying and identifying patients who would benefit from hypoxia-selective treatment (Table 1)(19). The most common radiotracer is a derivate of nitroimidazole [18F] MISO (20). In hypoxia (< 10mmHg of partial oxygen pressure), these molecules freely diffuse into cells and undergo reduction catalyzed by nitroreductases, serving as electron acceptors. Intracellular reduced species show a significant retention of radiolabeled metabolites cumulating through de-chelation or covalent bond to thiol-rich proteins(19,21).
Table 1.
Overview of hypoxia prodrugs in clinical trials (Update 2/2016, Clinicaltrials.gov). Abbreviation: * -trial included biological or imaging study with FAZA and or evaluation biomarkers; AML-Acute Myeloid Leukemia; ALL-Acute Lymphoblastic Leukemia; CML-Chronic Myeloid Leukemia; CLL-Chronic Lymphoblastic leukemia; 5-FU-5-Fluorouracil; GCSF-Granulocyte Colony Stimulating Factor ; GIST-Gastrointestinal Stromal Tumors; HCC-Hepatocellular Carcinoma; MDS-Myelodysplastic Syndrome; MM-Multiple Myeloma; NHL- Non-Hodgkin Lymphoma; NSCLC-Non-Small Cell Lung Cancer; pNET- Pancreatic Neuroendocrine Tumors; RCC-Renal Cell Carcinoma; RT-Radiotherapy; SCCHN-Squamous Cell Carcinoma of Head and Neck; SCLC-Small Cell Lung Cancer; SLL-Small Lymphocytic Leukemia; TACE- Transcatheter Arterial Chemoembolization; TAE- Transcatheter arterial Embolization; TURB-Transurethral Resection of the Bladder: NR- not recruiting: NYR- not yet recruiting: *** Polychemotherapy: Vincristine+ Irinotecan+ Cyclophosphamide+ Doxorubicin+ Ifosfamide+ Etoposide+ GCSF
| DRUG | INDICATION | PHASE | TREATMENT | TRIAL | STATUS |
|---|---|---|---|---|---|
| TH-302 | Pancreatic Ca | I–III | TH-302 +/− Gemcitabine | NCT01746979, NCT01144455, NCT01833546 | completed |
|
| |||||
| Soft Tissue Sarcoma | I/II–III | TH-302 +/− Doxorubicin | NCT01440088, NCT00742963 | completed | |
|
| |||||
| Biliary Tract Ca | II | TH-302 Monotherapy | NCT02433639 | recruiting | |
|
| |||||
| Glioblastoma | II | TH-302 + Bevacizumab | NCT02342379 | recruiting | |
|
| |||||
| High Grade Glioma | II | TH-302 MonotherapyàBevacizumab | NCT01403610 | completed | |
|
| |||||
| MM | II | TH-302 +/− Dexamethasone +/− Bortezomib/Pomalidomide | NCT01522872 | active, NR | |
|
| |||||
| Melanoma | II* | TH-302 Monotherapy | NCT01864538* | active, NR | |
|
| |||||
| Solid Tumors | I–I/II | TH-302 + Gemcitabine/Docetaxel/Pemetrexed TH-302 + Pazopanib TH-302 Monotherapy |
NCT00743379, NCT00495144, NCT02020226, NCT01485042, NCT01833546, NCT02076230* |
completed active, NR |
|
|
| |||||
| RCC, GIST, pNET | I I* II |
TH-302 + Sunitinib TH-302 [14C] TH-302 + Sunitinib |
NCT01381822 NCT02076230 NCT02402062 |
active completed active, NR |
|
|
| |||||
| HCC | I | TH-302 + Doxorubicin (TACE) | NCT01721941 | NYR | |
|
| |||||
| AML, ALL, CML, MDS | I | TH-302 Monotherapy | NCT01149915 | completed | |
|
| |||||
| TIRAPAZAMINE | SCLC | II* I |
Tirapazamine + Cisplatin, Etoposide, RT Tirapazamine +/− Cisplatin+ Etoposide+ RT |
NCT00066742 NCT00006487 |
completed |
|
| |||||
| NSCLC | III I |
Tirapazamine +/− Carboplatin+Paclitaxel Tirapazamine + Carboplatin, Paclitaxel, RT |
NCT00006484 NCT00033410 |
completed | |
|
| |||||
| Cervical Ca Ovarian Ca Peritoneal Cavity Ca |
III II I |
Tirapazamine +/− Cisplatin, RT Tirapazamine + Cisplatin Tirapazamine + Cisplatin, RT |
NCT00262821 NCT00003369 NCT00098995 NCT00020696 |
completed | |
|
| |||||
| HCC | I | Tirapazamine (TAE) | NCT02174549 | recruiting | |
|
| |||||
| SCCHN | II, III | Tirapazamine +/− Cisplatin, 5-FU, RT | NCT00002774, NCT00174837 | completed | |
|
| |||||
| Childhood Solid Tumor | I | Tirapazamine + Cyclophosphamide+ GCSF | NCT00003288 | completed | |
|
| |||||
| Head and Neck Tumor | III | Tirapazamine +/− Cisplatin, RT | NCT00094081 | completed | |
|
| |||||
| Childhood Rhabdomyosarcoma | II* | Tirapazamine + Polychemotherapy *** | NCT00025363 | completed | |
|
| |||||
| TH-4000 | NSCLC Recurrent/Metastatic SCCHN |
II | TH-4000 Monotherapy | NCT02454842 NCT02449681 |
active, active, NR |
|
| |||||
| EO9 | Bladder Cancer | I–III | EO9 Monotherapy | NCT01373398, NCT00598806, NCT00141531 NCT01469221 |
completed active, NR |
| III | EO9 Monotherapy pre TURB | NCT00461591 NCT02563561 |
completed active, NR |
||
In addition, hypoxia can be assessed indirectly by detecting mRNA or protein expression, incorporating single or multiple hypoxia-driven gene signatures (22,23). Given the intratumor heterogeneity of oxygenation level and vascularization, understanding the distribution pattern of hypoxia and other biomarkers, and its correlation with functional imaging, molecular profiling, and histopathology results might help in the selection of optimal therapy for an individual patient.
Targeting hypoxia and hypoxia-associated signaling pathways
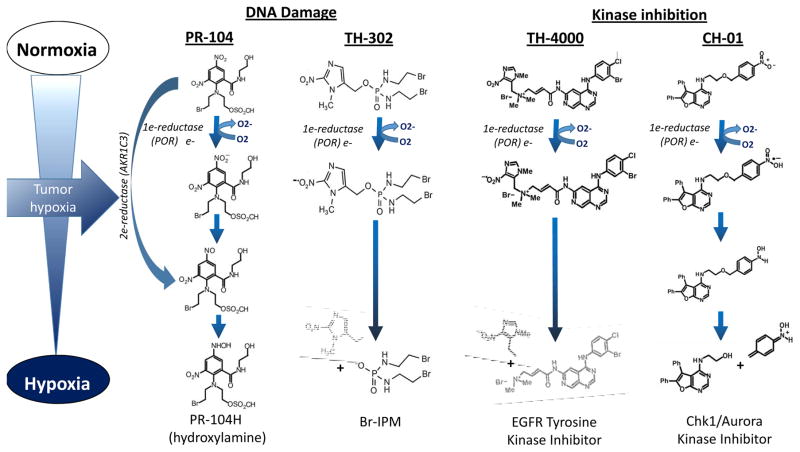
One strategy for targeting hypoxia proposes the inhibition of HIF-1, HIF-2 and their downstream targets or upstream signaling partners. While targeting HIF-1α itself remains challenging, new approaches such as targeting HIF-1α/p300 complex with Chetomin, an inhibitor of HIF-1α/p300 interaction, demonstrated antitumour activity in human myeloma cell lines and primary MM cells from patients (24). Novel selective antagonists of HIF-2α/HIF1β, PT2399 and its analogue PT2385, showed an antitumor activity and selectivity in human ccRCC cell lines and xenografts with higher expression of HIF-2α (HIF-2α dependent tumors) and improved progression-free survival in patients with metastatic extensive pretreated RCC (NCT02293980) (23). Another approach focuses on targeting HIF-1 downstream targets such as CAIX via any of several antibodies or small molecules is a subject of ongoing preclinical and clinical studies (25). Another approach to eradicating hypoxic cells uses bioreductive or hypoxia-activated prodrugs (HAP) (Fig. 3). HAPs are activated by enzymatic reduction under low oxygen tension, sensing hypoxia in a tumor (1).
Fig. 3.
Schematic representation of structure and mechanism of action of selected hypoxia-activated prodrugs (HAP) exemplified by PR-104, TH-302, TH-4000 and CH-01. 1) Nitrobenzamide mustard PR-104: Reduction of the electron-withdrawing nitro group of PR-104A to electron-donating hydroxylamine or amine activates the nitrogen mustard moiety. One electron reductases, such as NADPH:cytochrome P450 oxidoreductase (POR), form a nitro radical that can be re-oxidized by oxygen. Two electron reduction by aldo-keto reductase (AKR1C3) can bypass the nitro radical to affect oxygen-insensitive activation and to generate a DNA-reactive free radical. 2) Th-302: Fragmentation to release the DNA crosslinking agent bromo-isophosphoramide mustard (Br-IPM) occurs primarily from the initial nitro radical and forms a DNA cross-linker. 3) TH-4000: Reduction of TH-4000 prodrug to a nitro radical anion acts as a direct ‘oxygen sensor’ releasing an irreversible EGFR/HER2 tyrosine kinase inhibitor (TKI) under hypoxic conditions 4) CH-01: Under hypoxic conditions, the nitro group of inactive Chk1 prodrug is reduced forming an electron-donating substituent, which generates the active Chk1 and Aurora kinase A inhibitor.
The HAP molecules can be classified by chemical structure as aliphatic and aromatic N-oxides, nitro groups, quinones, and transition metals (1). HAPs also can be separated into two classes according to the hypoxic threshold required for activation by the specific reductase (e.g., POR). Class I requires relatively mild hypoxia for activation and includes, among others, benzotriazine, N-oxides, and tirapazamine. Class II depends on severe hypoxia for activation and encompasses nitro-compounds PR-104A and TH-302 (evofosfamide). Under extreme hypoxia, the prodrug radicals have longer lifetimes and can be reduced more easily to active drugs (1). Agents such as TH-302 have a stable effector molecule that can promote a bystander effect, diffusing from targeted hypoxic cell to neighboring cells. The majority of HAP metabolites result in DNA damage by interfering with DNA replication.
Despite promising preclinical data, several HAPs have failed to show clinical efficacy. A few, however, such as TH-302, tarloxotinib bromide (TH-4000), and tirapazamine, are undergoing ongoing exploration in clinical trials for specific indications (1,26,27) (Table 1).
Clinical–Translational Advances
HAP monotherapy
TH-302 and PR-104
TH-302 is a 2-nitroimidazole radical anion prodrug, activated in hypoxia by one-electron reductases such as POR to the active cytotoxic drug bromo-isophosphoramide mustard (Br-IPM) (Fig. 3) (28). TH-302 exhibits activity against a range of cell lines, especially those with deficiency in homology-directed DNA repair, BRCA1, BRCA2, or FANCA, as well as in H460 multicell spheroids and multicell layer models and a variety of preclinical models of solid tumors (pancreatic and osteolytic breast cancers, non–small cell lung carcinoma [NSCLC], osteosarcoma) (28–39) and hematologic malignancies (multiple myeloma, acute myelogenous leukemia [AML]) (27,40,41).
TH-302 induces γH2AX and generates intra- and inter-strand DNA crosslinks in hypoxic tissue, damaging DNA in both quiescent and proliferating cells and inhibiting cell proliferation and tumor growth through cell cycle arrest and induction of apoptosis. In vivo studies in AML xenograft models demonstrated that TH-302 depleted hypoxic cells, prolonged survival, and reduced the leukemia stem cell pool (41). Administration of TH-302 to mice with residual AML following chemotherapy prolonged survival, suggesting that this approach may be suitable for eliminating chemotherapy-resistant leukemia cells (41), demonstrating the feasibility of targeting hypoxic cells by hypoxia-activated cytotoxins. Because of these encouraging antitumor responses in xenograft models, TH-302 entered Phase I/II clinical trials as monotherapy in solid tumors and acute leukemias (42). Its clinical activity was limited, however; only a few objective responses, mostly transient, were observed (42,43). These results make a compelling case for combination therapies targeting both hypoxic and normoxic neoplastic cells.
PR-104 is a phosphate ester that is rapidly hydrolyzed in vivo to the corresponding alcohol PR104A, which acts as an HAP through its metabolic reduction to activated nitrogen mustards PR-104H and PR-104M (Fig. 3). PR-104 was shown to have significant activity in animal studies, inhibiting disease progression, decreasing tissue infiltration by tumor cells, reducing tumor growth, and prolonging mouse survival, especially in AKR1C3-expressing xenografts (44,45). PR-104 monotherapy elicited significant reductions in growth of hepatocellular carcinoma xenografts, which was reduced even further in mice treated with both PR-104 and sorafenib (46). In Phase I clinical trials, however, PR-104 showed little evidence of therapeutic activity in advanced solid tumors, with several hematologic toxic events (47). In a Phase I/II study in patients with relapsed/refractory AML or acute lymphoblastic leukemia (48), PR-104 was tolerated at doses much higher than the solid tumor maximum tolerated dose; however myelosuppression (neutropenia and thrombocytopenia) was prolonged at the higher doses. Despite reductions of hypoxia markers (HIF-1α, CAIX, and distribution of hypoxia assessed by immunhistochemical staining with pimonidazole) after administration of PR-104, the treatment responses were transient. However, these biomarker studies provided evidence that hypoxia is a prevalent feature of the leukemic microenvironment.
HAPS in combination with chemotherapy or targeted therapy
The activity of TH-302 in combination with conventional chemotherapy or targeted therapy has been reported in multiple preclinical solid tumor models and hematologic malignancies (32,35,37,38,49,50). In various xenograft models, the addition of TH-302 significantly increased DNA damage, apoptosis, and tumor necrosis and reduced stroma density and intratumoral hypoxia, without additive toxicity. In a xenograft model of pancreatic cancer, TH-302 showed promising activity with gemcitabine, decreasing the frequency of tumor-initiating cells from patient-derived xenografts in combination with ionizing radiation (37). TH-302 also improved the efficacy of a gemcitabine and nab-paclitaxel combination in mouse xenograft models of human pancreatic ductal adenocarcinoma (PDAC) (51). The addition of TH-302 to topotecan improved tumor response and prolonged survival in neuroblastoma and rhabdomyosarcoma xenograft models (52). In a murine model of multiple myeloma, the combination of TH-302 with bortezomib significantly prolonged survival (40). A combination of TH-302 with VEGF-A inhibitor pazopanib showed high efficacy in a sarcoma xenograft model, blocking tumor growth, increasing DNA damage and total and endothelial cell–specific apoptosis, and decreasing HIF-1α activity and percentage of sarcoma stem-like cells (53). The same drug combination also increased the benefit of ionizing radiation in metastatic sarcoma (53). In xenograft models of renal cell carcinoma, TH-302 potentiated the antitumor efficacy of mTOR inhibitors, blocking their pro-hypoxia mechanism (54).
The preclinical results have been evaluated in Phase I to III clinical trials in variety of tumor entities. TH-302 has been evaluated in a Phase III study in advanced unresectable or metastatic PDAC at recommended phase 2 dose, however the final report is pending. The efficacy of TH-302 and dexamethasone in combination with bortezomib or pomalidomide was investigated in patients with relapsed or refractory multiple myeloma. The preliminary data from this study showed an International Myeloma Working Group response (MR- Minimal response, PR-Partial response, or CR-complete response) in 29% of extensively pretreated patients at the recommended Phase II dose (55). The combination of TH-302 with pemetrexed in advanced non-squamous NSCLC yielded a mean overall survival duration of 14.9 months (56). In a placebo-controlled, multi-center Phase II study in the same setting, however, this combination as second-line therapy was stopped because it showed no survival benefit compared with pemetrexed alone (NCT02093962).
A novel approach of adding TH-302 to neoadjuvant chemoradiotherapy (paclitaxel, carboplatin, radiotherapy) was proposed for a clinical trial of untreated patients with esophageal cancer (30). This study will utilize several novel tools to monitor and predict the therapy response, including PET/CT scan with hypoxia tracer [18F]HX4 and analysis of CAIX and osteopontin (30). Patients with a complete pathological response after neoadjuvant treatment could then opt for a wait-and-see strategy to omit or postpone surgery. The effect of tumor hypoxia on the response to standard chemoradiation is being investigated by visualizing hypoxia with [18F]HX4 imaging before treatment and 2 weeks after the start of treatment in another ongoing Phase II clinical study (30).
HAPS and immune checkpoint inhibition
Hypoxia drives the establishment of a highly interdependent network of immunosuppressive stromal cells, such as myeloid-derived suppressor cells and myofibroblasts. In the tumor, hypoxic zones might resist infiltration by T cells even in the context of robust T cell infiltration in normoxic areas of the same tumor. To target the reprogrammed hypoxic immunoenvironment of a tumor, Ai et al. proposed a novel combination of immunotherapy and hypoxia-specific chemotherapy, suggesting the potential of such a combination to render some of the most therapeutically resistant cancers, such as prostatic adenocarcinoma, sensitive to checkpoint inhibition (57). Studies in vitro and in a mouse model of prostate cancer suggest that combination of TH-302 and T cell checkpoint blockade promotes an inside-out tumor destruction, with the drug killing at the core, releasing antigen, and diminishing immunosuppression, while the antibodies help expand and protect the activated T cells as a result. Antibody blockade of CTLA-4 and PD-1 in conjunction with TH-302 promoted tumor rejection in a significantly larger percentage of mice than either single agent, promoting uniquely advantageous ratios of effector T cells to myeloid-derived suppressor cells within the tumor microenvironment. This finding provides a strategy for rendering some of the most therapy-resistant cancers sensitive to immunotherapy(57).
HAPs and DNA damage signaling
Chronic hypoxia has been found to induce activation of DNA-dependent protein kinase (DNA-PK) in the absence of applied DNA damage, and this activation was found to promote stabilization of HIF-1α and to increase therapy resistance and treatment failure(58). To overcome resistance based on the DNA repair machinery, two approaches have been explored. Meng et al. showed that TH-302 cytotoxicity was greatly enhanced by Chk1 inhibition in p53-deficient human cancer cell lines. Chk1 inhibitors reduced TH-302–induced cell cycle arrest by increasing histone H3 and Cdc2-Y15 (59). Combination of TH-302 with Chk1 inhibitor AZD-7762 had greater efficacy in a colorectal xenograft model than either agent alone. This sensitization was shown to be due to disruption of the Chk1-mediated DNA damage checkpoint of the cell cycle and induction of apoptosis, providing additional support to the preclinical translational rationale for combining TH-302 with a Chk1 inhibitor (59). Alternatively, the concept of hypoxia-activatable Chk1 inhibitors was realized in the compound CH-01, a HAP that releases Chk1/Aurora A inhibitor following reduction of a 4-nitrobenzyl in hypoxic conditions (Fig. 3) (60).
Another approach was proposed by Lidquist et al. in a study assessing the inhibition of DNA double-strand break repair in hypoxic cells by targeting DNA-PK with BCCA621C, a hypoxia-activated inhibitor of DNA-PK. BCCA621C is enzymatically activated, leading in severely hypoxic conditions to radiosensitization of NCI-H460 cells (61). Recent findings indicate that hypoxia induces resistance to alkylating agents via a distinct molecular pathway involving HIF-1α, p53, and the mTOR target gene NDRG1, resulting in stabilization of O6-methylguanine-DNA methyltransferase (AGT), a key enzyme mediating resistance to alkylating agents in glioblastoma and melanoma (62). Hypoxia-selective 4-nitrobenzyloxycarbonyl derivatives of O6-benzylguanine inhibit AGT and sensitized laromustine-resistant DU145 human prostate carcinoma cells to laromustine under hypoxic conditions (63). This approach could lead to selective depletion of AGT in tumor tissue and sensitization of tumors to O6 guanine–targeting cytotoxic drugs such as temozolomide.
Novel HAPs in ongoing in vitro and in vivo studies
Several novel hypoxia-induced cytotoxins have been generated, translating into hypoxia-activatable DNA-damaging agents. Ikeda et al. developed a new doxorubicin prodrug with improved preclinical efficacy in pancreatic and colon cancers in vitro and in vivo (64). A novel tirapazamine analogue, Q6, showed hypoxia selectivity and topoisomerase II poisoning, and has been proposed for treatment of human hepatocellular carcinoma (65). Schreiber-Brynzak et al. developed compound 2, a HAP of platinum(IV) that inhibited tumor growth in vivo significantly better than other satraplatin drugs (66). Kumar et al. proposed a theranostic prodrug, compound 4, combining fluorophore imaging features and irinotecan metabolite SN38 activity to confirm its tumor-specific localization and inhibition of tumor growth. Its efficacy was proven in cervical and lung cancer cell lines, in tumor cell spheroids, and in a xenograft mouse model (67).
Because of the limited therapeutic window of classical HAPs, a novel approach of hypoxia-selective inhibition of EGFR was recently employed. TH-4000 is an EGFR tyrosine kinase inhibitor HAP designed to release an active inhibitor within hypoxic regions of tumors, offering greater selectivity and lower toxicity than existing EGFR inhibitors (Fig. 3). In preclinical studies, TH-4000 was more active than erlotinib against NSCLC xenografts with either wild-type or mutant EGFR (68). TH-4000 is currently undergoing Phase II clinical evaluation in patients with advanced EGFR-mutant, T790M-negative NSCLC (69) or metastatic squamous cell carcinoma of the head and neck or skin (70). Using a similar concept, a HAP strategy has been developed to release EGFR inhibitors using cobalt (III) as the hypoxia-sensitive trigger group (71).
Another type of HAP, protein prodrug TAT-ODD-procaspase-3 (TOP3), was designed to be activated in HIF-active cancer cells, leading to cell death. Combination of TOP3 with gemcitabine or TS-1 resulted in significantly longer survival in orthotopic pancreatic cancer models, offering a promising new therapeutic option for patients with pancreatic cancer (72). TOP3 in combination with radiotherapy has shown a benefit in xenograft models of cervical and pancreatic cancers, suppressing angiogenesis and inhibiting the growth of irradiated subcutaneous tumors (72).
Summary and Future Perspectives
The fundamental role of hypoxia in tumor biology and in chemoresistance and radioresistance supports continuing emphasis on development of novel strategies to overcome the detrimental consequences of hypoxia and HIFs. Despite enormous progress in HAP development in preclinical settings, the existing strategies have so far failed to show significant clinical benefit either as monotherapy or in combination with standard chemotherapeutic agents, possibly because of their narrow therapeutic windows. Hypoxia-activated molecularly targeted inhibitors might overcome this limitation and provide broader therapeutic efficacy for tumors with driver mutations (e.g., HER2, EGFR, VEGF, AGT, or CHK1). Alternatively, rational combinations with targeted agents, metabolic modulators, or immunotherapies could be highly effective and safe. Novel trial designs, such as the use of HAPs in the neoadjuvant setting or in the setting of minimal residual disease, should be further contemplated to address patients with specific profile of hypoxia biomarkers. Finally, expansion of our knowledge of HIF signaling networks in normal cells, as well as our understanding of HIF-dependent pathways hijacked by cancer cells, is eagerly awaited.
Acknowledgments
Grant Support
Research reported in this publication was supported by the NIH under award number R01 CA155056-05 (to M. Konopleva) and supported in part by the MD Anderson Cancer Center Support Grant P30 CA016672.
M. Konopleva was supported by the Leukemia and Lymphoma Society Scholar in Clinical Research award “Biology and targeting of hypoxic microenvironment in leukemias”, 2189-12
The authors thank Dr. Charles P. Hart, Threshold Pharmaceuticals, Inc., South San Francisco, California and Kathryn Hale, The University of Texas MD Anderson Cancer Center, for editorial help with the manuscript.
Footnotes
Authors’ Contributions
Conception and design: N. Baran, M. Konopleva
Writing, review, and/or revision of the manuscript: N. Baran, M. Konopleva
Disclosure of Potential Conflicts of Interest
Conflicts of interest: Threshold and Proacta research support to MK
References
- 1.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nature reviews Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 2.Semenza GL. Cancer-stromal cell interactions mediated by hypoxia-inducible factors promote angiogenesis, lymphangiogenesis, and metastasis. Oncogene. 2013;32(35):4057–63. doi: 10.1038/onc.2012.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koh MY, Powis G. Passing the baton: the HIF switch. Trends in biochemical sciences. 2012;37(9):364–72. doi: 10.1016/j.tibs.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bredell MG, Ernst J, El-Kochairi I, Dahlem Y, Ikenberg K, Schumann DM. Current relevance of hypoxia in head and neck cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giambra V, Jenkins CE, Lam SH, Hoofd C, Belmonte M, Wang X, et al. Leukemia stem cells in T-ALL require active Hif1alpha and Wnt signaling. Blood. 2015;125(25):3917–27. doi: 10.1182/blood-2014-10-609370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. The Journal of clinical investigation. 2013;123(9):3664–71. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouault-Pierre K, Lopez-Onieva L, Foster K, Anjos-Afonso F, Lamrissi-Garcia I, Serrano-Sanchez M, et al. HIF-2alpha protects human hematopoietic stem/progenitors and acute myeloid leukemic cells from apoptosis induced by endoplasmic reticulum stress. Cell stem cell. 2013;13(5):549–63. doi: 10.1016/j.stem.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nature reviews Cancer. 2012;12(1):9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Liu Y, Malek SN, Zheng P, Liu Y. Targeting HIF1alpha eliminates cancer stem cells in hematological malignancies. Cell stem cell. 2011;8(4):399–411. doi: 10.1016/j.stem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coltella N, Valsecchi R, Ponente M, Ponzoni M, Bernardi R. Synergistic Leukemia Eradication by Combined Treatment with Retinoic Acid and HIF Inhibition by EZN-2208 (PEG-SN38) in Preclinical Models of PML-RARalpha and PLZF-RARalpha-Driven Leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21(16):3685–94. doi: 10.1158/1078-0432.CCR-14-3022. [DOI] [PubMed] [Google Scholar]
- 11.Forristal CE, Winkler IG, Nowlan B, Barbier V, Walkinshaw G, Levesque JP. Pharmacologic stabilization of HIF-1alpha increases hematopoietic stem cell quiescence in vivo and accelerates blood recovery after severe irradiation. Blood. 2013;121(5):759–69. doi: 10.1182/blood-2012-02-408419. [DOI] [PubMed] [Google Scholar]
- 12.Masson N, Ratcliffe PJ. Hypoxia signaling pathways in cancer metabolism: the importance of co-selecting interconnected physiological pathways. Cancer & metabolism. 2014;2(1):3. doi: 10.1186/2049-3002-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Beucken T, Koch E, Chu K, Rupaimoole R, Prickaerts P, Adriaens M, et al. Hypoxia promotes stem cell phenotypes and poor prognosis through epigenetic regulation of DICER. Nature communications. 2014;5:5203. doi: 10.1038/ncomms6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forristal CE, Brown AL, Helwani FM, Winkler IG, Nowlan B, Barbier V, et al. Hypoxia inducible factor (HIF)-2alpha accelerates disease progression in mouse models of leukemia and lymphoma but is not a poor prognosis factor in human AML. Leukemia. 2015;29(10):2075–85. doi: 10.1038/leu.2015.102. [DOI] [PubMed] [Google Scholar]
- 15.Xiong Z, Guo M, Yu Y, Zhang FF, Ge MK, Chen GQ, et al. Downregulation of AIF by HIF-1 contributes to hypoxia-induced epithelial-mesenchymal transition of colon cancer. Carcinogenesis. 2016 doi: 10.1093/carcin/bgw089. [DOI] [PubMed] [Google Scholar]
- 16.Hogel H, Miikkulainen P, Bino L, Jaakkola PM. Hypoxia inducible prolyl hydroxylase PHD3 maintains carcinoma cell growth by decreasing the stability of p27. Molecular cancer. 2015;14:143. doi: 10.1186/s12943-015-0410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chouaib S, Noman MZ, Kosmatopoulos K, Curran MA. Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene. 2016 doi: 10.1038/onc.2016.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rademakers SE, Lok J, van der Kogel AJ, Bussink J, Kaanders JH. Metabolic markers in relation to hypoxia; staining patterns and colocalization of pimonidazole, HIF-1alpha, CAIX, LDH-5, GLUT-1, MCT1 and MCT4. BMC cancer. 2011;11:167. doi: 10.1186/1471-2407-11-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connor JP, Boult JK, Jamin Y, Babur M, Finegan KG, Williams KJ, et al. Oxygen-Enhanced MRI Accurately Identifies, Quantifies, and Maps Tumor Hypoxia in Preclinical Cancer Models. Cancer research. 2016;76(4):787–95. doi: 10.1158/0008-5472.CAN-15-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell C, Dowson N, Fay M, Thomas P, Puttick S, Gal Y, et al. Hypoxia imaging in gliomas with 18F-fluoromisonidazole PET: toward clinical translation. Seminars in nuclear medicine. 2015;45(2):136–50. doi: 10.1053/j.semnuclmed.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Vaupel P, Mayer A. The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxidants & redox signaling. 2015;22(10):878–80. doi: 10.1089/ars.2014.6155. [DOI] [PubMed] [Google Scholar]
- 22.Toustrup K, Sorensen BS, Nordsmark M, Busk M, Wiuf C, Alsner J, et al. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer research. 2011;71(17):5923–31. doi: 10.1158/0008-5472.CAN-11-1182. [DOI] [PubMed] [Google Scholar]
- 23.Chen W, Hill H, Christie A, Kim MS, Holloman E, Pavia-Jimenez A, et al. Targeting Renal Cell Carcinoma with a HIF-2 antagonist. Nature. 2016 doi: 10.1038/nature19796. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viziteu E, Grandmougin C, Goldschmidt H, Seckinger A, Hose D, Klein B, et al. Chetomin, targeting HIF-1alpha/p300 complex, exhibits antitumour activity in multiple myeloma. British journal of cancer. 2016;114(5):519–23. doi: 10.1038/bjc.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald PC, Winum JY, Supuran CT, Dedhar S. Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget. 2012;3(1):84–97. doi: 10.18632/oncotarget.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu YL, Jahangiri A, De Lay M, Aghi MK. Hypoxia-induced tumor cell autophagy mediates resistance to anti-angiogenic therapy. Autophagy. 2012;8(6):979–81. doi: 10.4161/auto.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Portwood S, Lal D, Hsu YC, Vargas R, Johnson MK, Wetzler M, et al. Activity of the hypoxia-activated prodrug, TH-302, in preclinical human acute myeloid leukemia models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(23):6506–19. doi: 10.1158/1078-0432.CCR-13-0674. [DOI] [PubMed] [Google Scholar]
- 28.Meng F, Evans JW, Bhupathi D, Banica M, Lan L, Lorente G, et al. Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302. Mol Cancer Ther. 2012;11(3):740–51. doi: 10.1158/1535-7163.MCT-11-0634. [DOI] [PubMed] [Google Scholar]
- 29.Sun JD, Liu Q, Ahluwalia D, Ferraro DJ, Wang Y, Jung D, et al. Comparison of hypoxia-activated prodrug evofosfamide (TH-302) and ifosfamide in preclinical non-small cell lung cancer models. Cancer biology & therapy. 2016:1–10. doi: 10.1080/15384047.2016.1139268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larue RT, Van De Voorde L, Berbee M, van Elmpt WJ, Dubois LJ, Panth KM, et al. A phase 1 ‘window-of-opportunity’ trial testing evofosfamide (TH-302), a tumour-selective hypoxia-activated cytotoxic prodrug, with preoperative chemoradiotherapy in oesophageal adenocarcinoma patients. BMC cancer. 2016;16:644. doi: 10.1186/s12885-016-2709-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Wojtkowiak JW, Martinez GV, Cornnell HH, Hart CP, Baker AF, et al. MR Imaging Biomarkers to Monitor Early Response to Hypoxia-Activated Prodrug TH-302 in Pancreatic Cancer Xenografts. PloS one. 2016;11(5):e0155289. doi: 10.1371/journal.pone.0155289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liapis V, Labrinidis A, Zinonos I, Hay S, Ponomarev V, Panagopoulos V, et al. Hypoxia-activated pro-drug TH-302 exhibits potent tumor suppressive activity and cooperates with chemotherapy against osteosarcoma. Cancer letters. 2015;357(1):160–9. doi: 10.1016/j.canlet.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liapis V, Zinonos I, Labrinidis A, Hay S, Ponomarev V, Panagopoulos V, et al. Anticancer efficacy of the hypoxia-activated prodrug evofosfamide (TH-302) in osteolytic breast cancer murine models. Cancer medicine. 2016;5(3):534–45. doi: 10.1002/cam4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardenas-Rodriguez J, Li Y, Galons JP, Cornnell H, Gillies RJ, Pagel MD, et al. Imaging biomarkers to monitor response to the hypoxia-activated prodrug TH-302 in the MiaPaCa2 flank xenograft model. Magnetic resonance imaging. 2012;30(7):1002–9. doi: 10.1016/j.mri.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Q, Sun JD, Wang J, Ahluwalia D, Baker AF, Cranmer LD, et al. TH-302, a hypoxia-activated prodrug with broad in vivo preclinical combination therapy efficacy: optimization of dosing regimens and schedules. Cancer chemotherapy and pharmacology. 2012;69(6):1487–98. doi: 10.1007/s00280-012-1852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun JD, Liu Q, Wang J, Ahluwalia D, Ferraro D, Wang Y, et al. Selective tumor hypoxia targeting by hypoxia-activated prodrug TH-302 inhibits tumor growth in preclinical models of cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(3):758–70. doi: 10.1158/1078-0432.CCR-11-1980. [DOI] [PubMed] [Google Scholar]
- 37.Lohse I, Rasowski J, Cao P, Pintilie M, Do T, Tsao MS, et al. Targeting hypoxic microenvironment of pancreatic xenografts with the hypoxia-activated prodrug TH-302. Oncotarget. 2016 doi: 10.18632/oncotarget.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saggar JK, Tannock IF. Chemotherapy Rescues Hypoxic Tumor Cells and Induces Their Reoxygenation and Repopulation-An Effect That Is Inhibited by the Hypoxia-Activated Prodrug TH-302. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21(9):2107–14. doi: 10.1158/1078-0432.CCR-14-2298. [DOI] [PubMed] [Google Scholar]
- 39.Wojtkowiak JW, Cornnell HC, Matsumoto S, Saito K, Takakusagi Y, Dutta P, et al. Pyruvate sensitizes pancreatic tumors to hypoxia-activated prodrug TH-302. Cancer & metabolism. 2015;3(1):2. doi: 10.1186/s40170-014-0026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu J, Handisides DR, Van Valckenborgh E, De Raeve H, Menu E, Vande Broek I, et al. Targeting the multiple myeloma hypoxic niche with TH-302, a hypoxia-activated prodrug. Blood. 2010;116(9):1524–7. doi: 10.1182/blood-2010-02-269126. [DOI] [PubMed] [Google Scholar]
- 41.Benito J, Ramirez MS, Millward NZ, Velez J, Harutyunyan KG, Lu H, et al. Hypoxia-Activated Prodrug TH-302 Targets Hypoxic Bone Marrow Niches in Preclinical Leukemia Models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(7):1687–98. doi: 10.1158/1078-0432.CCR-14-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Badar T, Handisides DR, Benito JM, Richie MA, Borthakur G, Jabbour E, et al. Phase I study of evofosfamide, an investigational hypoxia-activated prodrug, in patients with advanced leukemia. American journal of hematology. 2016;91(8):800–5. doi: 10.1002/ajh.24415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss GJ, Infante JR, Chiorean EG, Borad MJ, Bendell JC, Molina JR, et al. Phase 1 study of the safety, tolerability, and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(9):2997–3004. doi: 10.1158/1078-0432.CCR-10-3425. [DOI] [PubMed] [Google Scholar]
- 44.Benito J, Shi Y, Szymanska B, Carol H, Boehm I, Lu H, et al. Pronounced hypoxia in models of murine and human leukemia: high efficacy of hypoxia-activated prodrug PR-104. PloS one. 2011;6(8):e23108. doi: 10.1371/journal.pone.0023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moradi Manesh D, El-Hoss J, Evans K, Richmond J, Toscan CE, Bracken LS, et al. AKR1C3 is a biomarker of sensitivity to PR-104 in preclinical models of T-cell acute lymphoblastic leukemia. Blood. 2015;126(10):1193–202. doi: 10.1182/blood-2014-12-618900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abbattista MR, Jamieson SM, Gu Y, Nickel JE, Pullen SM, Patterson AV, et al. Pre-clinical activity of PR-104 as monotherapy and in combination with sorafenib in hepatocellular carcinoma. Cancer biology & therapy. 2015;16(4):610–22. doi: 10.1080/15384047.2015.1017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKeage MJ, Gu Y, Wilson WR, Hill A, Amies K, Melink TJ, et al. A phase I trial of PR-104, a pre-prodrug of the bioreductive prodrug PR-104A, given weekly to solid tumour patients. BMC cancer. 2011;11:432. doi: 10.1186/1471-2407-11-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konopleva M, Thall PF, Yi CA, Borthakur G, Coveler A, Bueso-Ramos C, et al. Phase I/II study of the hypoxia-activated prodrug PR104 in refractory/relapsed acute myeloid leukemia and acute lymphoblastic leukemia. Haematologica. 2015;100(7):927–34. doi: 10.3324/haematol.2014.118455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peeters SG, Zegers CM, Biemans R, Lieuwes NG, van Stiphout RG, Yaromina A, et al. TH-302 in Combination with Radiotherapy Enhances the Therapeutic Outcome and Is Associated with Pretreatment [18F]HX4 Hypoxia PET Imaging. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21(13):2984–92. doi: 10.1158/1078-0432.CCR-15-0018. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Marrano P, Wu B, Kumar S, Thorner P, Baruchel S. Combined Antitumor Therapy with Metronomic Topotecan and Hypoxia-Activated Prodrug, Evofosfamide, in Neuroblastoma and Rhabdomyosarcoma Preclinical Models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015 doi: 10.1158/1078-0432.CCR-15-1853. [DOI] [PubMed] [Google Scholar]
- 51.Sun JD, Liu Q, Ahluwalia D, Li W, Meng F, Wang Y, et al. Efficacy and safety of the hypoxia-activated prodrug TH-302 in combination with gemcitabine and nab-paclitaxel in human tumor xenograft models of pancreatic cancer. Cancer biology & therapy. 2015;16(3):438–49. doi: 10.1080/15384047.2014.1003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Marrano P, Wu B, Kumar S, Thorner P, Baruchel S. Combined Antitumor Therapy with Metronomic Topotecan and Hypoxia-Activated Prodrug, Evofosfamide, in Neuroblastoma and Rhabdomyosarcoma Preclinical Models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(11):2697–708. doi: 10.1158/1078-0432.CCR-15-1853. [DOI] [PubMed] [Google Scholar]
- 53.Yoon C, Chang KK, Lee JH, Tap WD, Hart CP, Simon MC, et al. Multimodal targeting of tumor vasculature and cancer stem-like cells in sarcomas with VEGF-A inhibition, HIF-1alpha inhibition, and hypoxia-activated chemotherapy. Oncotarget. 2016 doi: 10.18632/oncotarget.10212. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Sun JD, Ahluwalia D, Liu Q, Li W, Wang Y, Meng F, et al. Combination treatment with hypoxia-activated prodrug evofosfamide (TH-302) and mTOR inhibitors results in enhanced antitumor efficacy in preclinical renal cell carcinoma models. American journal of cancer research. 2015;5(7):2139–55. [PMC free article] [PubMed] [Google Scholar]
- 55.Laubach J, Raje NS, Yee AJ, Armand P, Schlossman RL, Rosenblatt J, et al. Preliminary safety and efficacy of evofosfamide (TH-302), an investigational hypoxia-activated prodrug, combined with bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma (RR MM) Journal of Clinical Oncology. 2015;33(15_suppl) [Abstr 8579] [Google Scholar]
- 56.Goldman J, Belani C, Novello S, von Pawel J, Csoszi T, Orlov S, et al. 142TiPRandomized, double-blind, placebo-controlled trial of evofosfamide (TH-302) in combination with pemetrexed in advanced non-squamous non-small cell lung cancer. Annals of Oncology. 2015;26(suppl 1):i44. [Google Scholar]
- 57.Ai M, Budhani P, Sheng J, Balasubramanyam S, Bartkowiak T, Jaiswal AR, et al. Tumor hypoxia drives immune suppression and immunotherapy resistance. Journal for ImmunoTherapy of Cancer. 2015;3(2):1. [Google Scholar]
- 58.Barker HE, Paget JTE, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nature reviews Cancer. 2015;15(7):409–25. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meng F, Bhupathi D, Sun JD, Liu Q, Ahluwalia D, Wang Y, et al. Enhancement of hypoxia-activated prodrug TH-302 anti-tumor activity by Chk1 inhibition. BMC cancer. 2015;15:422. doi: 10.1186/s12885-015-1387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cazares-Korner C, Pires IM, Swallow ID, Grayer SC, O’Connor LJ, Olcina MM, et al. CH-01 is a hypoxia-activated prodrug that sensitizes cells to hypoxia/reoxygenation through inhibition of Chk1 and Aurora A. ACS chemical biology. 2013;8(7):1451–9. doi: 10.1021/cb4001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindquist K, Cran J, Kordic K, Winters G, Chua P, Tan J, et al. Synthesis and Development of Prodrug BCCA621C: a Hypoxia Triggered DNA-PK Inhibitor. European journal of cancer. 2012;48:86. [Google Scholar]
- 62.Weiler M, Blaes J, Pusch S, Sahm F, Czabanka M, Luger S, et al. mTOR target NDRG1 confers MGMT-dependent resistance to alkylating chemotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(1):409–14. doi: 10.1073/pnas.1314469111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu R, Liu MC, Luo MZ, Penketh PG, Baumann RP, Shyam K, et al. 4-nitrobenzyloxycarbonyl derivatives of O(6)-benzylguanine as hypoxia-activated prodrug inhibitors of O(6)-alkylguanine-DNA alkyltransferase (AGT), which produces resistance to agents targeting the O-6 position of DNA guanine. Journal of medicinal chemistry. 2011;54(21):7720–8. doi: 10.1021/jm201115f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ikeda Y, Hisano H, Nishikawa Y, Nagasaki Y. Targeting and Treatment of Tumor Hypoxia by Newly Designed Prodrug Possessing High Permeability in Solid Tumors. Molecular pharmaceutics. 2016;13(7):2283–9. doi: 10.1021/acs.molpharmaceut.6b00011. [DOI] [PubMed] [Google Scholar]
- 65.Liu XW, Cai TY, Zhu H, Cao J, Su Y, Hu YZ, et al. Q6, a novel hypoxia-targeted drug, regulates hypoxia-inducible factor signaling via an autophagy-dependent mechanism in hepatocellular carcinoma. Autophagy. 2014;10(1):111–22. doi: 10.4161/auto.26838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schreiber-Brynzak E, Pichler V, Heffeter P, Hanson B, Theiner S, Lichtscheidl-Schultz I, et al. Behavior of platinum(iv) complexes in models of tumor hypoxia: cytotoxicity, compound distribution and accumulation. Metallomics : integrated biometal science. 2016;8(4):422–33. doi: 10.1039/c5mt00312a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar R, Kim EJ, Han J, Lee H, Shin WS, Kim HM, et al. Hypoxia-directed and activated theranostic agent: Imaging and treatment of solid tumor. Biomaterials. 2016;104:119–28. doi: 10.1016/j.biomaterials.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 68.Patterson AV, Silva S, Guise C, Bull M, Abbattista M, Hsu A, et al. TH-4000, a hypoxia-activated EGFR/Her2 inhibitor to treat EGFR-TKI resistant T790M-negative NSCLC. Journal of Clinical Oncology. 2015;33(15_suppl) [Abstr e13548] [Google Scholar]
- 69.Liu SV, Aggarwal C, Brzezniak C, Gerber DE, Gitlitz B, Horn L, et al. A Phase 2 Study (NCT02454842) of Tarloxotinib Bromide (TH-4000) in Patients with EGFR Mutant, T790M-Negative, Advanced NSCLC Progressing on an EGFR TKI. Journal of Clinical Oncology. 2016;34(16_suppl) [Abstr TPS9100] [Google Scholar]
- 70.Rischin DBM, Brzezniak CE, Colevas AD, Doebele RC, Gilbert J, et al. A phase 2 study of tarloxotinib bromide (TRLX) in patients (Pts) with recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN) or skin (SCCS) J Clin Oncol. 2016;34 Chicago. 2016 (suppl; abstr TPS6105) [Google Scholar]
- 71.Karnthaler-Benbakka C, Groza D, Kryeziu K, Pichler V, Roller A, Berger W, et al. Tumor-targeting of EGFR inhibitors by hypoxia-mediated activation. Angewandte Chemie. 2014;53(47):12930–5. doi: 10.1002/anie.201403936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoang NT, Kadonosono T, Kuchimaru T, Kizaka-Kondoh S. Hypoxia-inducible factor-targeting prodrug TOP3 combined with gemcitabine or TS-1 improves pancreatic cancer survival in an orthotopic model. Cancer science. 2016;107(8):1151–8. doi: 10.1111/cas.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]