Abstract
Objective
We sought to explore potential mechanisms underlying hospital sepsis case volume-mortality associations by investigating implementation of evidence-based processes of care.
Design
Retrospective cohort study. We determined associations of sepsis case-volume with three evidence-based processes of care (lactate measurement during first hospital day, norepinephrine as first vasopressor, and avoidance of starch-based colloids) and assessed their role in mediation of case-volume-mortality associations.
Setting
Enhanced administrative data (Premier, Charlotte, NC) from 534 U.S. hospitals.
Subjects
287,914 adult patients with sepsis present on admission between July 2010 and December 2012 of whom 58,045 received a vasopressor for septic shock during the first two days of hospitalization.
Interventions
None.
Measurements and Main Results
Among patients with sepsis, 1.9% received starch, and among patients with septic shock, 68.3% had lactate measured and 64% received norepinephrine as initial vasopressor. Patients at hospitals with the highest case-volume were more likely to have lactate measured (Adjusted odds ratio Quartile 4 vs. Quartile 1 [AORQ4:Q1] 2.8, 95%CI 2.1–3.7) and receive norepinephrine as initial vasopressor (AORQ4:Q1 2.1, 95%CI 1.6–2.7). Case-volume was not associated with avoidance of starch products (AORQ4:Q1 0.73, 95%CI 0.45–1.2). Adherence to evidence-based care was associated with lower hospital mortality (AOR 0.81, 95% CI: 0.70–0.94), but did not strongly mediate case-volume-mortality associations (point estimate change ≤2%).
Conclusions
In a large cohort of US patients with sepsis, select evidence-based processes of care were more likely implemented at high volume hospitals but did not strongly mediate case-volume-mortality associations. Considering processes and case-volume when regionalizing sepsis care may maximize patient outcomes.
Keywords: sepsis, septic shock, Multiple Organ Failure, Outcome and Process Assessment, Delivery of Health Care, High-Volume Hospitals
Introduction
Sepsis is characterized by a dysregulated immune response to infection and life-threatening organ dysfunction (1), with an annual incidence of 535 per 100,000 residents in the United States (2), Sepsis case-fatality rates have markedly declined over the past two decades (3, 4), though remain at approximately 20–30% (5–7), In the absence of novel, specific therapies targeted to treat sepsis, declining sepsis mortality rates have been largely attributed to improvements in processes of care (8).
A growing body of literature documenting associations between higher hospital sepsis case volume and reduced sepsis mortality supports the assertion that improvements in healthcare delivery processes may improve sepsis outcomes (9–11). The theory that “practice makes perfect” in critical care has led to proposals for regionalization of intensive care in order to take advantage of economies of scale and potentially maximize positive patient outcomes (12). However, regionalization may introduce new problems, including delayed treatment during transport, compromised quality at referring hospitals, geographic separation of patients from their families, and overcrowding at referral centers (13, 14). Investigating potential mechanisms underlying case volume-outcome associations for sepsis may enhance efforts to transfer effective processes of care across hospitals and inform programs that seek to regionalize critical care (15, 16). Differences in processes of care between hospitals according to sepsis case volumes are currently unclear. We hypothesized that evidence-based processes of care would be more common at hospitals with higher sepsis case volume, and that increased use of evidence-based processes may mediate associations between sepsis case volume and mortality.
Materials and Methods
Patient Sample
We identified adult patients with sepsis (defined by presence of infection and organ dysfunction, formerly “severe sepsis” (17)) present on admission between July 2010 and December 2012 using a modified version of a high positive predictive value (>95%) algorithm described by Martin et al, based on explicit sepsis ICD-9 codes (0.38x, 995.91, 995.92, 785.52) and at least one acute organ failure (circulatory, respiratory, renal, neurologic, hematologic, metabolic, hepatic) (18, 19), from the Premier (Premier, Charlotte, NC) enhanced administrative database (https://goo.gl/6nl6QJ) (20). Premier data includes standard hospital discharge files as well as date-stamped pharmacy and laboratory billing information from over 500 hospitals across all geographic regions of the US. We also identified a subgroup of patients with septic shock requiring a vasopressor (dopamine, epinephrine, norepinephrine, phenylephrine) within the first two days of sepsis hospitalization (21). The two day period was chosen in order to capture at least 24 hours of hospitalization in all patients since the first recorded hospital day may represent a partial day. Patients who received more than one vasopressor during the initial hospital day of vasopressor administration were excluded because we were unable to distinguish initial vasopressor if more than one was administered on the same day.
Case Volume
The number of cases per month of sepsis or septic shock treated at each hospital during the study period was calculated by dividing the total number of cases by the hospital’s total reporting period (6 months for 2010, 12 months each for 2011 and 2012). Patients transferred in from another hospital were included when calculating the accepting hospital’s case volume but excluded from the analysis of practice patterns, since there was no documentation of initial testing or interventions. Hospitals were divided into quartiles (Q1–Q4) of sepsis case volume.
Processes of Care
Using the pharmacy and laboratory billing information available in the Premier database we identified three evidence-based/guideline-recommended processes of care (22–24) during the time period of the study amenable to identification using enhanced administrative data: 1) measurement of lactate during the first hospital day (to assist in recognition of high risk patients in need of immediate resuscitation) (5, 23, 25), 2) use of norepinephrine as first vasopressor in septic shock (associated with reduced arrhythmias and lower mortality as compared with dopamine) (21–23), and 3) avoidance of hydroxyethyl starch (HES) products for volume expansion (associated with increased risk of acute renal failure and increased mortality) (26–28). A query of the pharmacy data for starch-based volume expanders available in the US revealed that only tetrastarch and hetastarch were administered. Although other processes of care (e.g., time from onset of hypotension (29) or organ failure (30) to initiation of antibiotics) may mediate sepsis survival and potentially be associated with case volume, identification of additional evidence-based processes was not feasible using available administrative data that is granular to day of medication administration (but not to hour), and does not record time of admission, organ failure or hypotension.
Statistical Analysis
We reported baseline characteristics as percentages for categorical variables and means with standard deviations or medians with interquartile ranges for continuous variables depending on distribution. Given the potential for non-clinically significant, but statistically significant, differences with the large sample sizes in our dataset, we evaluated differences in baseline characteristics across sepsis case volume quartiles using standardized differences with a threshold of 0.1, which corresponds to a 0.25% variance in the outcome of interest (31, 32). Initial vasopressor use and lactate measurement were analyzed only among patients with septic shock, while the avoidance of HES was analyzed in the full sepsis cohort. We evaluated within-hospital correlations of rates of each paired combination of the three processes of care using Spearman rank coefficients and visualized the relationships using scatter plots with a fitted quadratic regression line to account for non-linear associations.
We used generalized estimating equations accounting for hospital-level clustering to determine associations between sepsis case volume and patient receipt of each of the three processes of care of interest. Potential confounding variables incorporated as independent variables in multivariable models included year of hospitalization, patient demographics, hospital characteristics, attending physician specialty, location of admission, comorbid conditions, and acute organ failures present on admission (see Supplemental Digital Content - Table 1 for complete list of covariates).
We calculated the hospital-level rate of adherence to each practice pattern (% of patients receiving evidence-based practice) and of a composite variable representing adherence to all three practice patterns of interest. We created individual multivariable models to investigate the association of hospital mortality with the rate of each process of care and with quartiles of the composite “% hospital adherence” process variable. To evaluate the potential role of adherence to evidence based processes of care as a mediator of the case volume-mortality association we created a multivariable model for hospital mortality using number of organ failures as a potential effect modifier (9) and included sepsis case volume and adherence to evidence based processes of care in the model separately and simultaneously. We assessed the change in effect estimate for the multivariable-adjusted case-volume-mortality association after including in the model the hospital-level rate of the composite variable representing adherence to all three processes of care (33, 34). We used SAS version 9.4 (Cary, NC) for all analyses. This study qualified for a waiver from the Institutional Review Board at Boston University who determined that it does not constitute research involving human subjects.
Results
We identified 287,914 patients with sepsis of whom 58,045 received a vasopressor for shock during the first two days of hospitalization. Patients in the sepsis cohort had an average age of 68.6 ± 16 years, 50.6% were female and 67.5% were white, with average hospital mortality of 16.7%. Characteristics were similar among patients with septic shock, except average hospital mortality was 25%. The median monthly case volume of sepsis or septic shock was 17.3 cases (25–75th percentile: 7.9–29.4, range 0.1–99.1 cases/month) among the 534 hospitals; baseline variables according to hospital case volume quartile are shown in Table 1.
Table 1.
Baseline characteristics of patients with sepsis by hospital sepsis case volume quartile
| Variable [n (%) unless otherwise noted] | Hospital Sepsis Case Volume Quartile | ||||
|---|---|---|---|---|---|
| 1st Quartile N = 11,476 (0.1–7.8 cases/month) |
2nd Quartile N = 41,066 (7.9–17.3 cases/month) |
3rd Quartile N = 76,606 (17.4–29.3 cases/month) |
4th Quartile N = 158,766 (29.4–99.1 cases/month) |
Standardized Differencea | |
| Age [median (IQR)] | 73 (23) | 71 (23) | 71 (23) | 70 (24) | 0.05 |
| Sex (female) | 6042 (52.7) | 20956 (51) | 38581 (50.4) | 80332 (50.6) | 0.03 |
| Race | 0.23b | ||||
| White | 9319 (81.2) | 29727 (72.4) | 50444 (65.9) | 104783 (66) | |
| Black | 739 (6.4) | 4492 (10.9) | 10023 (13.1) | 24508 (15.4) | |
| Hispanic | 130 (1.1) | 296 (0.7) | 1513 (2) | 2630 (1.7) | |
| Other/unknown | 1288 (11.2) | 6551 (16) | 14626 (19.1) | 26845 (16.9) | |
| Geographic location | 0.42b | ||||
| Northeast | 1111 (9.7) | 9105 (22.2) | 12405 (16.2) | 24380 (15.4) | |
| Midwest | 2543 (22.2) | 8290 (20.2) | 19201 (25.1) | 26831 (16.9) | |
| South | 6324 (55.1) | 16096 (39.2) | 31671 (41.3) | 70779 (44.6) | |
| West | 1498 (13.1) | 7575 (18.5) | 13329 (17.4) | 36776 (23.2) | |
| Teaching hospital status | 1143 (10) | 9737 (23.7) | 20801 (27.2) | 76506 (48.2) | 0.37b |
| Specialty of attending physician | 0.17b | ||||
| Pulmonary/Critical Care Medicine | 194 (1.7) | 1861 (4.5) | 4187 (5.5) | 11671 (7.4) | |
| Cardiology | 97 (0.9) | 286 (0.7) | 868 (1.1) | 1743 (1.1) | |
| Other Medical Specialty | 10878 (94.8) | 37965 (92.5) | 69261 (90.4) | 139625 (87.9) | |
| Surgery | 307 (2.7) | 954 (2.3) | 2290 (3) | 5727 (3.6) | |
| Nonhealthcare facility point of origin | 9163 (79.8) | 34354 (83.7) | 61693 (80.5) | 128385 (80.9) | 0.1b |
| Intensive Care Unit Stay | 5800 (50.5) | 20469 (49.8) | 36907 (48.2) | 68943 (43.4) | 0.01 |
| Prevalent Comorbidity [mean (SD)] | 2.9 (1.8) | 2.9 (1.8) | 3 (1.9) | 3 (1.8) | 0.01 |
| Diabetes Mellitus | 4298 (37.5) | 15445 (37.6) | 29579 (38.6) | 60722 (38.3) | 0.003 |
| Hypertension | 7453 (64.9) | 26533 (64.6) | 50833 (66.4) | 105283 (66.3) | 0.007 |
| Heart failure | 3067 (26.7) | 11369 (27.7) | 21316 (27.8) | 45033 (28.4) | 0.02 |
| Ischemic stroke or transient ischemic attack | 192 (1.7) | 741 (1.8) | 1344 (1.8) | 2965 (1.9) | 0.01 |
| Atrial Fibrillation | 2494 (21.7) | 9057 (22.1) | 16020 (20.9) | 3341 (21.1) | 0.008 |
| Ischemic heart disease | 3149 (27.4) | 11210 (27.3) | 21286 (27.8) | 43221 (27.2) | 0.003 |
| Renal insufficiency | 4337 (37.8) | 15047 (36.6) | 29900 (39) | 59990 (37.8) | 0.02 |
| Chronic pulmonary disease | 3883 (33.8) | 13607 (33.1) | 24163 (31.5) | 49006 (30.9) | 0.01 |
| Valvular heart disease | 892 (7.8) | 3165 (7.7) | 6367 (8.3) | 13154 (8.3) | 0.003 |
| Peripheral vascular disease | 1125 (9.8) | 4441 (10.8) | 8933 (11.7) | 17721 (11.2) | 0.03 |
| Venous thromboembolic disease | 228 (2) | 860 (2.1) | 1947 (2.5) | 4466 (2.8) | 0.008 |
| Cancer | 1124 (9.8) | 4664 (11.4) | 9000 (11.8) | 20464 (12.9) | 0.05 |
| Dementia | 811 (7.1) | 2712 (6.6) | 4903 (6.4) | 9656 (6.1) | 0.02 |
| Cirrhosis | 545 (4.8) | 2101 (5.1) | 4117 (5.4) | 9105 (5.7) | 0.02 |
| Additional acute organ failures [mean (SD)] | 1.4 (0.9) | 1.6 (0.9) | 1.6 (1) | 1.6 (1) | 0.15b |
| Shock requiring vasopressor within the first 48 hours of hospitalization | 2837 (24.7) | 12379 (30.1) | 22128 (28.9) | 45068 (28.4) | 0.12b |
| Only 1 vasopressor during first hospital day (septic shock subgroup) [n (%) of total requiring vasopressor] | 2177 (76.7) | 8884 (71.8) | 15736 (71.1) | 31248 (69.3) | 0.11b |
| Respiratory | 2982 (26) | 12304 (30) | 23356 (30.5) | 49750 (31.3) | 0.09 |
| Renal | 7688 (67) | 27821 (67.8) | 53293 (69.6) | 108629 (68.4) | 0.02 |
| Neurologic | 1513 (13.2) | 6380 (15.5) | 13227 (17.3) | 29306 (18.5) | 0.07 |
| Hematologic | 1798 (15.7) | 6868 (16.7) | 13001 (17) | 27966 (17.6) | 0.03 |
| Metabolic/acidosis | 1907 (16.6) | 8763 (21.3) | 17232 (22.5) | 35918 (22.6) | 0.12b |
| Hepatic | 403 (3.5) | 1771 (4.3) | 3377 (4.4) | 6939 (4.4) | 0.04 |
| Mechanical Ventilation (Day 1) | 958 (8.4) | 5274 (12.8) | 10087 (13.2) | 20726 (13.1) | 0.15b |
| Length of stay [mean days (SD)] | 6.9 (7.5) | 8.5 (9.1) | 9.1 (10.1) | 9.3 (10.8) | 0.19b |
| Year of sepsis hospitalization | 0.03 | ||||
| 2010 | 2132 (18.6) | 7191 (17.5) | 14445 (18.9) | 30870 (19.4) | |
| 2011 | 4376 (38.1) | 16114 (39.2) | 30644 (40) | 62784 (39.5) | |
| 2012 | 4968 (43.3) | 17761 (43.3) | 31517 (41.1) | 65112 (41) | |
| Died during hospitalization | 1628 (14.2) | 6958 (16.9) | 13012 (17) | 26348 (16.6) | 0.08 |
Absolute standardized difference ≥ 0.1 denotes a significant difference between groups.
Significant difference between groups.
IQR = interquartile range, SD = standard deviation.
Compared to highest case-volume quartile hospitals (Q4), the lowest quartile of sepsis case volume hospitals (Q1) were more likely non-teaching hospitals, in the Southern US, with fewer patients staffed by pulmonary/critical care attending physicians or insured by Medicaid. Patients at lowest sepsis case volume hospitals were more likely to be white, older, female, hospitalized in the ICU, and had fewer acute organ failures at admission.
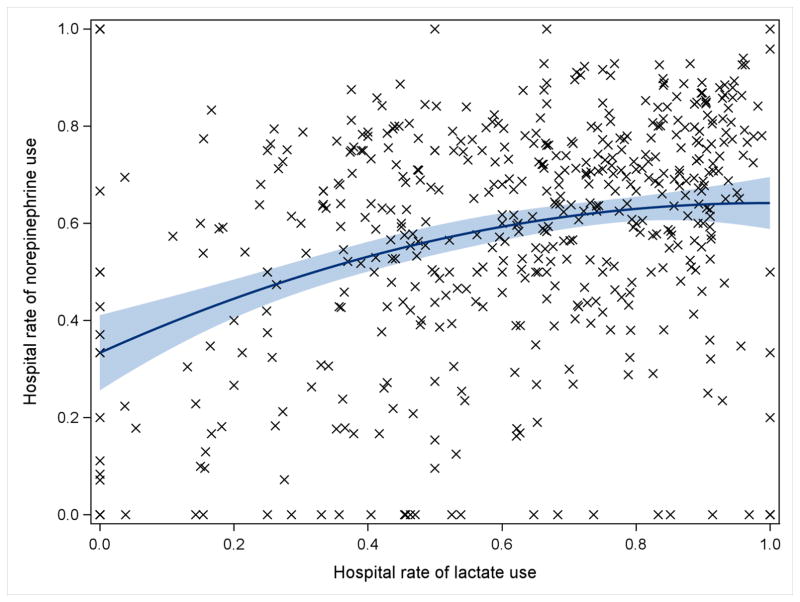
Among 58,045 patients with septic shock, lactate was measured for 39,633 (68.3%) and norepinephrine was the first vasopressor administered to 37,153 (64%). In multivariable-adjusted analysis, patients admitted to hospitals with higher sepsis case volume had nearly threefold increased odds of lactate measurement during the first day of hospitalization (Q4 vs. Q1 of case volume: Adjusted odds ratio [AOR] 2.8, 95% CI 2.1–3.7) and twofold increased norepinephrine use as first vasopressor for septic shock (AOR 2.1, 95% CI 1.6–2.7) (Figure 1). HES was administered to 5,371 (1.9%) of 287,914 patients with sepsis. Rates of HES use during sepsis trended lower among higher case volume hospitals but did not reach statistical significance (AOR 0.73, 95% CI 0.45–1.2). Among patients with septic shock, hospital rates of lactate correlated moderately with norepinephrine utilization (Spearman’s ρ = 0.29, p < 0.001; Figure 2). There was no correlation between hospital rates of HES use and rates of either lactate or norepinephrine (see Supplemental Digital Content - Figure 1). Average hospital-level rates of adherence to all three processes of care increased from the lowest (20.5 ± 21.5%) to highest (48 ± 19.1%) quartile of sepsis case volume, with lower variability in process adherence within each sepsis case volume quartile as case volume increased (Q1 coefficient of variation: 1.05 vs. Q4: 0.4, p <0.001, Figure 3).
Figure 1.
Association of hospital sepsis case volume quartile and evidence-based processes of care [Adjusted Odds Ratio (95% Confidence Interval)]. NE = Norepinephrine.
Figure 2.
Correlation of within-hospital rates of lactate and norepinephrine use with quadratic best fit line and 95% confidence interval. Spearman’s ρ = 0.29, p < 0.001.
Figure 3.
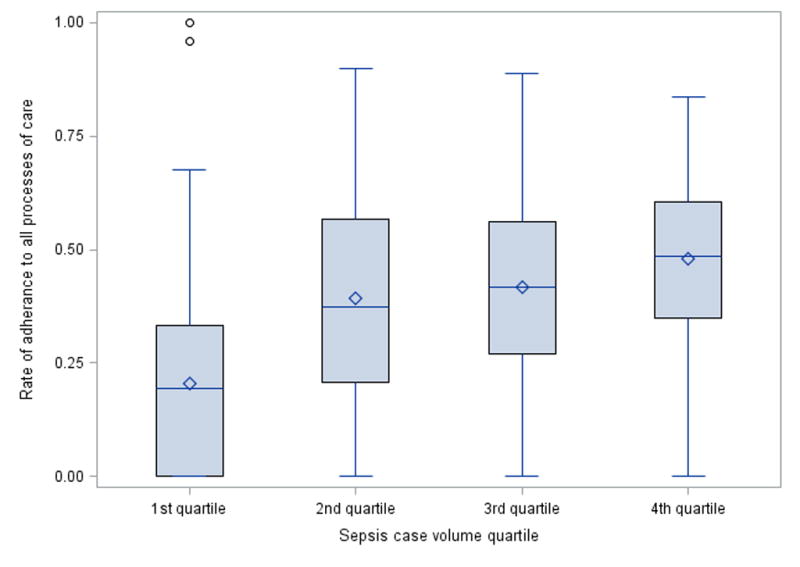
Box plot of hospital-level rate of adherence to all processes of care of interest (measuring lactate, norepinephrine as first vasopressor, and avoiding starch products) showing median, interquartile range, range (with outliers), and mean (diamonds).
Among all patients with septic shock, hospital case volume was not significantly associated with hospital mortality (Q1 vs. Q4 of case volume: AOR 0.95, 95% CI: 0.82–1.12). Associations between case-volume and mortality differed depending upon the number of acute organ failures present at admission (p for interaction = 0.04), thus analyses were stratified by number to acute organ failures. The highest quartile of sepsis case volume was associated with lower hospital mortality compared to the lowest quartile among patients with a single organ failure (AOR 0.77, 95% CI: 0.60–0.99), but not among patients with more than one organ failure (AOR 0.98, 95% CI: 0.83–1.2). Mediation analysis (33, 34) adding the variable for hospital-level adherence to processes of care changed the effect estimate for case volume by 2% or less among the full cohort, and among subgroups with one organ failure and two or more organ failures when comparing the highest to lowest case volume quartile.
Among the full cohort with septic shock, the highest quartile of adherence to evidence-based processes of care was associated with lower hospital mortality compared with the lowest quartile (AOR 0.81, 95% CI: 0.70–0.94). The association between process adherence and mortality was independent of hospital case-volume and persisted after controlling for case-volume as a confounding variable (Table 2). Furthermore, the associations between mortality and adherence to measured processes of care did not differ by number of acute organ failures (p for interaction = 0.75) (Table 2).
Table 2.
Association of case volume and adherence to all three processes of care studied (measurement of lactate within the first day, use of norepinephrine as initial vasopressor, and avoidance of hydroxyethyl starch products) with mortality in the septic shock cohort stratified by number of acute organ failures
| Whole Septic Shock Cohort
| |||
|---|---|---|---|
| Case Volume | Processes of Care | Processes of Care (controlling for case volume) | |
| 1st Quartile | Reference | Reference | Reference |
| 2nd Quartile | 1.0 (0.85–1.2) | 0.99 (0.88–1.1) | 0.99 (0.88–1.1) |
| 3rd Quartile | 0.96 (0.82–1.1) | 0.86 (0.76–0.98)a | 0.87 (0.76–0.99)a |
| 4th Quartile | 0.95 (0.82–1.1) | 0.81 (0.70–0.94)a | 0.81 (0.71–0.94)a |
|
| |||
| Single Organ Failure | |||
|
| |||
| Case Volume | Processes of Care | Processes of Care (controlling for case volume) | |
| 1st Quartile | Reference | Reference | Reference |
| 2nd Quartile | 0.89 (0.68–1.2) | 0.93 (0.79–1.1) | 0.96 (0.81–1.1) |
| 3rd Quartile | 0.94 (0.73–1.2) | 0.90 (0.75–1.1) | 0.93 (0.78–1.1) |
| 4th Quartile | 0.77 (0.60–0.99)a | 0.82 (0.68–0.99)a | 0.85 (0.70–1.0) |
|
| |||
| Two or More Organ Failures | |||
|
| |||
| Case Volume | Processes of Care | Processes of Care (controlling for case volume) | |
| 1st Quartile | Reference | Reference | Reference |
| 2nd Quartile | 1.04 (0.88–1.2) | 0.99 (0.89–1.1) | 0.99 (0.89–1.1) |
| 3rd Quartile | 0.99 (0.83–1.2) | 0.88 (0.77–1.0) | 0.88 (0.77–1.0) |
| 4th Quartile | 0.98 (0.83–1.2) | 0.82 (0.71–0.95)a | 0.82 (0.71–0.95)a |
All models adjusted for age, sex, race, year of hospitalization, geographic location, teaching hospital status, specialty of attending physician, point of origin, prevalent comorbidities, intensive care unit stay, mechanical ventilation during the first hospital day, acute organ failures, and source of infection.
Statistically significant adjusted odds ratio.
Discussion
We investigated associations between evidence-based processes of care and hospital case volume using a large national sample of patients with sepsis in the United States. Two processes of care (lactate measurement and norepinephrine as initial vasopressor during shock) were implemented for approximately two-thirds of patients, leaving 1 in 3 patients with sepsis without receipt of evidence-based sepsis care based upon three process measures. Hospitals with the highest sepsis case volume were 2–3 fold times more likely to implement evidence-based processes than hospitals with the lowest case volume. In line with prior studies (9), associations between case-volume and hospital mortality depended upon patient severity of illness, but case-volume mortality associations among patients with lower sepsis severity were not mediated by measured evidence-based care processes. Patients admitted to hospitals with greater adherence to evidence-based practice had lower hospital mortality, regardless of illness severity or case-volume. Although hospitals with high case volume were more likely to use evidence-based processes of care, hospital case volume and use of evidence-based care processes may act through different mechanisms to achieve lower sepsis mortality. Our results inform debates regarding regionalization of sepsis care: sepsis process measures more reliably predicted better sepsis outcomes than case volume alone.
Our findings in sepsis expand upon prior studies of processes of care and case volume in other conditions. For example, investigations of the treatment of head and neck cancer (35), mitral valve surgery (36), heart failure (37), and breast cancer (38) have shown that hospitals and physicians with higher case volume used significantly higher rates of guideline-recommended processes. However, the association between hospital case volume and adherence to processes of care has not been borne out for all conditions. Adherence to guideline-recommended processes was unassociated with case volume among patients hospitalized with exacerbation of chronic obstructive pulmonary disease (39), and was inversely related to case volume for pneumonia (40). Defining characteristics of conditions in which processes of care are likely to improve with increased case volume warrants further study, with ramifications for the design of future models of healthcare delivery.
Our findings indicate that hospitals caring for a greater number of patients with sepsis were more likely to enact evidence-based processes of care. While we were unable to directly link specific process measures to mediation of the case-volume-mortality association, we demonstrated an association between hospital process adherence and lower mortality among patients with sepsis that was independent of case volume. Given that: 1) hospital-level use of evidence-based processes of care was more strongly associated with lower mortality than the patient-level associations reported previously (21), and 2) use of evidence-based care processes correlated within hospitals, it is likely that the processes we were able to measure may represent other, unmeasured factors that are more strongly associated with mortality during sepsis. The observation that evidence-based processes of care correlated within hospitals also suggests that the proliferation of quality measures requiring potentially burdensome reporting of multiple care processes may be unnecessary (41). Further studies exploring associations among other processes of care during sepsis, including time to antibiotics, are warranted.
Our findings expand the literature suggesting that high sepsis case volume may be a component of achieving improved sepsis outcomes. Consistent with prior studies (9), we identified that higher case volume was associated with improved outcomes only among patients with lower illness severity. Although case volume and evidence-based processes were associated, our findings suggest that high case volume and evidence-based processes may independently contribute to improved sepsis outcomes. Thus, regionalization of sepsis care to high volume centers also would require continued efforts to disseminate and implement evidence based practices in order to achieve maximum benefits which is in line with multi-dimensional approaches that have been suggested (14). Our finding that patients admitted to hospitals with greater adherence to evidence-based processes of care experienced better outcomes is supported by prior studies evaluating implementation of Surviving Sepsis campaign guidelines, demonstrating that evidence uptake was associated with improved outcomes (42). Finally, prior studies investigating evidence uptake in sepsis have shown that participation in quality improvement efforts was associated with improved outcomes, regardless of whether the specific processes of care specified in sepsis bundles were adopted (42, 43). Future studies should investigate how case volume may predict engagement in, or alter responsiveness to, quality improvement interventions.
Our study has limitations. Few evidence-based processes of care for sepsis can be currently identified from enhanced claims data, thus our study could not comprehensively investigate evidence-based practices. In addition, our dataset did not contain information regarding structures of care such as nurse-to-patient ratios, intensivist staffing, or hospital technological indices that may mediate improved processes and outcomes at high volume hospitals. Future studies using multicenter electronic health record data linked to American Hospital Association files may identify additional practices and structures of care associated with case volume. Differences in sepsis recognition and ICD-9 coding practices between hospitals may introduce a misclassification bias and is a limitation to using administrative data. However, patients at high sepsis case volume hospitals appeared to have markers of greater illness severity (more comorbidities and acute organ failures) that would tend to bias associations between case-volume and outcome towards the null, away from potential benefits of higher case volume. Use of administrative criteria for identifying sepsis cases is limited by variable sensitivity between hospitals and may miss up to one-third of cases diagnosed through clinical criteria, but has high specificity (44). Case identification in this study was based on an algorithm with high positive predictive value at two tertiary hospitals, but may have different performance characteristics at hospitals with lower sepsis prevalence. The granularity of the timing of processes of care to “hospital day” required us to exclude patients who received more than one vasopressor on the same initial day of vasopressor administration, which limits the generalizability of our findings. Further, claims data presents limitations in the ability to adjust illness severity and analysis may be affected by unmeasured confounding. We were likewise unable to account for situations where deviation from evidence-based or guideline recommended practice may have been warranted. Despite these limitations, few other currently available sources of data would allow the study of case volume, practice patterns and patient outcomes across multiple hospitals.
In conclusion, we investigated associations between hospital sepsis case volume and evidence-based care practices. Despite measuring only 3 care processes, we identified a large proportion of patients who did not receive care in line with evidence-based or guideline-recommended practices. Evidence-based practice patterns for sepsis clustered together within hospitals and were more likely to occur at hospitals with higher sepsis case volume. Although the processes identified in our study did not appear to mediate the association between case volume and outcomes in sepsis, greater adherence to evidence-based processes of care was associated with lower sepsis mortality. Seemingly independent associations between mortality, case volume and evidence-based processes of care, demonstrate that both factors are likely important to maximize favorable sepsis outcomes. Unless further studies identify transferrable processes that mediate case-volume-outcome associations, greater investigation of regionalization of sepsis care should focus on identification of “centers of excellence” that can provide the complex combination of processes and case volume that may lead to improved outcomes for patients of varying disease acuity.
Supplementary Material
Correlation of within-hospital rates of norepinephrine (top) and lactate (bottom) use with hydroxyethyl starch use including quadratic best fit line and 95% confidence interval.
Acknowledgments
Sources of support: NIH NHLBI K01HL116768
Footnotes
Copyright form disclosure: Dr. Fawzy received support for article research from the National Institutes of Health (NIH). Dr. Walkey received support for article research from the NIH, his institution received funding from the NIH, and he received funding from UptoDate.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walkey AJ, Lagu T, Lindenauer PK. Trends in sepsis and infection sources in the United States. A population-based study. Ann Am Thorac Soc. 2015;12:216–220. doi: 10.1513/AnnalsATS.201411-498BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson EK, Rubenstein AR, Radin GT, et al. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med. 2014;42:625–631. doi: 10.1097/CCM.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 6.Investigators A. Group ACT, Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 7.Pro CI, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rimmer E, Kumar A, Doucette S, et al. Activated protein C and septic shock: a propensity-matched cohort study. Crit Care Med. 2012;40:2974–2981. doi: 10.1097/CCM.0b013e31825fd6d9. [DOI] [PubMed] [Google Scholar]
- 9.Gaieski DF, Edwards JM, Kallan MJ, et al. The relationship between hospital volume and mortality in severe sepsis. Am J Respir Crit Care Med. 2014;190:665–674. doi: 10.1164/rccm.201402-0289OC. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin AJ, Simpson KN, Ford DW. Volume-Mortality Relationships during Hospitalization with Severe Sepsis Exist Only at Low Case Volumes. Ann Am Thorac Soc. 2015;12:1177–1184. doi: 10.1513/AnnalsATS.201406-287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahul S, Hacker MR, Novack V, et al. The effect of hospital volume on mortality in patients admitted with severe sepsis. PLoS One. 2014;9:e108754. doi: 10.1371/journal.pone.0108754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahn JM, Linde-Zwirble WT, Wunsch H, et al. Potential value of regionalized intensive care for mechanically ventilated medical patients. Am J Respir Crit Care Med. 2008;177:285–291. doi: 10.1164/rccm.200708-1214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scales DC, Rubenfeld GD, editors. The organization of critical care: an evidence-based approach to improving quality. New York: Humana Press; 2014. [Google Scholar]
- 14.Nguyen YL, Kahn JM, Angus DC. Reorganizing adult critical care delivery: the role of regionalization, telemedicine, and community outreach. Am J Respir Crit Care Med. 2010;181:1164–1169. doi: 10.1164/rccm.200909-1441CP. [DOI] [PubMed] [Google Scholar]
- 15.Kahn JM, Goss CH, Heagerty PJ, et al. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355:41–50. doi: 10.1056/NEJMsa053993. [DOI] [PubMed] [Google Scholar]
- 16.Walkey AJ, Wiener RS. Hospital case volume and outcomes among patients hospitalized with severe sepsis. Am J Respir Crit Care Med. 2014;189:548–555. doi: 10.1164/rccm.201311-1967OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 18.Iwashyna TJ, Odden A, Rohde J, et al. Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care. 2014;52:e39–43. doi: 10.1097/MLR.0b013e318268ac86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 20.Lindenauer PK, Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 21.Fawzy A, Evans SR, Walkey AJ. Practice Patterns and Outcomes Associated With Choice of Initial Vasopressor Therapy for Septic Shock. Crit Care Med. 2015;43:2141–2146. doi: 10.1097/CCM.0000000000001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 23.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel A, Waheed U, Brett SJ. Randomised trials of 6% tetrastarch (hydroxyethyl starch 130/0.4 or 0. 42) for severe sepsis reporting mortality: systematic review and meta-analysis. Intensive Care Med. 2013;39:811–822. doi: 10.1007/s00134-013-2863-6. [DOI] [PubMed] [Google Scholar]
- 25.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 26.Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 27.Schortgen F, Lacherade JC, Bruneel F, et al. Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet. 2001;357:911–916. doi: 10.1016/S0140-6736(00)04211-2. [DOI] [PubMed] [Google Scholar]
- 28.Wiedermann CJ. Systematic review of randomized clinical trials on the use of hydroxyethyl starch for fluid management in sepsis. BMC Emerg Med. 2008;8:1. doi: 10.1186/1471-227X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 30.Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749–1755. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 31.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, N.J: L. Erlbaum Associates; 1988. [Google Scholar]
- 33.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 34.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 35.Eskander A, Monteiro E, Irish J, et al. Adherence to guideline-recommended process measures for squamous cell carcinoma of the head and neck in Ontario: Impact of surgeon and hospital volume. Head Neck. 2016;38(Suppl 1):E1987–1992. doi: 10.1002/hed.24364. [DOI] [PubMed] [Google Scholar]
- 36.Gammie JS, O’Brien SM, Griffith BP, et al. Influence of hospital procedural volume on care process and mortality for patients undergoing elective surgery for mitral regurgitation. Circulation. 2007;115:881–887. doi: 10.1161/CIRCULATIONAHA.106.634436. [DOI] [PubMed] [Google Scholar]
- 37.Joynt KE, Orav EJ, Jha AK. The association between hospital volume and processes, outcomes, and costs of care for congestive heart failure. Ann Intern Med. 2011;154:94–102. doi: 10.1059/0003-4819-154-2-201101180-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vrijens F, Stordeur S, Beirens K, et al. Effect of hospital volume on processes of care and 5-year survival after breast cancer: a population-based study on 25000 women. Breast. 2012;21:261–266. doi: 10.1016/j.breast.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Lindenauer PK, Pekow P, Gao S, et al. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144:894–903. doi: 10.7326/0003-4819-144-12-200606200-00006. [DOI] [PubMed] [Google Scholar]
- 40.Lindenauer PK, Behal R, Murray CK, et al. Volume, quality of care, and outcome in pneumonia. Ann Intern Med. 2006;144:262–269. doi: 10.7326/0003-4819-144-4-200602210-00008. [DOI] [PubMed] [Google Scholar]
- 41.National Quality Forum. NQF #0500 Severe Sepsis and Septic Shock: Management Bundle. 2012 Oct 5;2012 [Google Scholar]
- 42.Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7. 5-year study. Intensive Care Med. 2014;40:1623–1633. doi: 10.1007/s00134-014-3496-0. [DOI] [PubMed] [Google Scholar]
- 43.Miller RR, 3rd, Dong L, Nelson NC, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med. 2013;188:77–82. doi: 10.1164/rccm.201212-2199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss SL, Parker B, Bullock ME, et al. Defining pediatric sepsis by different criteria: discrepancies in populations and implications for clinical practice. Pediatr Crit Care Med. 2012;13:e219–226. doi: 10.1097/PCC.0b013e31823c98da. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation of within-hospital rates of norepinephrine (top) and lactate (bottom) use with hydroxyethyl starch use including quadratic best fit line and 95% confidence interval.