Abstract
Purpose
The prevalence of kidney stones has increased globally in recent decades. However, studies investigating the association between temporal changes in risk of stone formation and stone types are scarce. We investigated temporal changes in stone composition, demographic, serum and urinary parameters of kidney stone formers from 1980–2015.
Materials and Methods
Retrospective analysis of 1516 patients diagnosed with either calcium or uric acid stones at initial visits in a university kidney stone clinic from 1980–2015.
Results
From 1980–2015, the proportion of uric acid stones within all stone formers increased from 7% to 14%. While age and BMI of both uric acid and calcium stone formers increased over time, uric acid stone formers were consistently older, had higher BMI, and lower urinary pH than calcium stone formers. While the proportion of females with stones has increased over time, the increase in female gender was more prominent among calcium stone formers. Urinary pH, phosphorus, oxalate, and sodium increased over time within calcium stone formers, but remained unchanged in uric acid stone formers. After accounting for various parameters of stone risk, the strongest clinical discriminant of uric acid vs. calcium stone was urinary pH. Limitations of this study include the retrospective single center design and available number of patients with stone analysis.
Conclusions
From 1980 to 2015, the proportion of uric acid stones increased significantly. With time, there were proportionately more female calcium but not uric acid stone formers. Urinary pH is the most prominent factor distinguishing uric acid from calcium stones.
Key Terms: nephrolithiasis, uric acid stones, calcium stones, urinary pH
Introduction
Over the past decades, the prevalence of kidney stones (KS) has increased significantly in both genders in the United States1. The greater prevalence of the metabolic syndrome (MS) contributed to this increase in KS 2, and is predicted to impact its economic burden 3. Several pathophysiological models are proposed to explain the causal relationship between obesity, MS, and KS formation 4–8. However, studies investigating the association between temporal changes in obesity with KS formation and stone type are limited 9, 10. Two prior studies showed lower urinary pH, and increasing urinary calcium, phosphorus, and uric acid with time as risk factors for KS 9, 10.
In this study, we comprehensively examined stone risk profile and clinical variables in KS formers over the past 35 years.
Material and Methods
Study participants
We reviewed charts of 2,166 kidney stone formers (KSF) with available stone analysis who underwent initial metabolic evaluation at their initial visit to University of Texas Southwestern Medical Center (UTSWMC) from 1980 to 2015. Patients were mostly recurrent stone formers who were referred to our Center for further evaluation. Excluded were patients ≤ 21 years old, with primary hyperparathyroidism, cystine stones, infection-related stones, chronic kidney disease stage 4 or higher, renal tubular acidosis, or chronic diarrheal states. This study was approved by the Institutional Review Board at UTSWMC.
Data collection and measurements
Stone analysis was assessed at the Urolithiasis Laboratory in Houston TX, by optical crystallography (Zeiss/Olympus petrographic microscope). Patients with stones ≥ 70% in calcium content were classified as calcium stone formers (CaSF), whereas those with > 30% in uric acid content were classified as uric acid stone formers (UASF) 11. Patients were instructed to hold all medications known to influence urinary KS profiles such as alkali treatment, allopurinol, and thiazide diuretics, for two weeks prior to stone analysis. Demographic characteristics recorded at the first visit included age, gender, body mass index (BMI), family history of KS, and personal history of urinary tract infections.
Initial stone visits included two 24-hour urine samples obtained while the subjects continued their ad-lib diet. Results of the two 24-hour urine results were averaged. At the completion of urine collections, a fasting venous serum was obtained for calcium (sCa, normal lab values (NL) 2.18–2.58 mmol/L), uric acid (sUA, NL 180–420 μmol/L), glucose (sGlu, NL 3.3–5.8 mmol/L), and triglyceride (sTG, NL <1.7 mmol/L), all measured by autoanalyzer (Synchron CX9ALX; Beckman, Brea, CA). The 24-hour urine samples were analyzed for total volume (uTV), creatinine (uCr) by picric acid method (Olympus AU400), upH (NL 5–7) by digital pH electrode, sodium (uNa, NL 100–260 mmol/day) and potassium (uK, NL 25–100 mmol/day) by flame photometry (BWB Technologies, UK), calcium (uCa, NL 2.5–7.5 mmol/day) by atomic absorption spectrophotometry (Varian-Agilent Technologies, Palo Alto, CA), uric acid (uUA, NL 1.48–4.43 mmol/day) by uricase, oxalate (uOx, NL 0.11–0.46 mmol/day) and sulfate (uSO4, NL 14–84 mEQ/day) by ion chromatography (Dionex, Sunnyvale, CA), citrate (uCit) by citrate lyase (Cobas Fara, Roche, NJ), phosphorus (uP), chloride (uCl, NL 80– 250 mmol/day), and ammonium (uNH4+) by the glutamate dehydrogenase. Urinary parameters were also normalized to body weight (BW). The following parameters were calculated: 24-hour creatinine clearance (CrCl), net gastrointestinal absorption of alkali (NGIA) 12, urinary bicarbonate (HCO3−) estimated from upH, and titratable acidity (TA) 13 for calculation of net acid excretion (NAE): (uNH4+ + TA) -(uCit2/3- + HCO3−), expressed in milliequivalents (mEq).
Statistical analyses
Continuous variables were reported as mean±standard deviation or median [25th, 75th percentile]. Categorical variables were reported as counts or percentages. Baseline characteristics were compared between UASF and CaSF groups by Cochran-Armitage tests and unpaired t-test/non-parametric test for categorical and continuous variables respectively. Two-way analysis of variance (ANOVA) with polynomial trend analysis was implemented for comparing changes in UASF and CaSF over three time periods and assessing the interaction between stone types and among time periods. Results analyzing study year as a continuous variable were similar (not shown). For ANOVA results, the main effect p-values from the F statistics for stone group and time trend within each group are reported separately. As a secondary analysis, stone risk factors were evaluated for single variable and multivariable logistic regression and receiver operating characteristic (ROC) analysis to evaluate their association with stone type (dependent variable UASF with CaSF as the reference group). Internal validation of the logistic model and area under the ROC curve was performed using 5,000 bootstrap replications. P<0.05 was considered statistically significant. Statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
Demographic and biochemical characteristics of KSF
Table 1 presents the demographic, serum and urine biochemical characteristics of the cohort grouped according to stone type. 1516 subjects were included, of whom 1365 (90%) were CaSF and 151 (10%) were UASF. UASF were significantly older, had higher BMI, and included fewer females (UASF vs. CaSF: 25% vs. 34%, p=0.03) compared with CaSF. UASF had significantly higher sUA, sGlu, and sTG compared with CaSF (Table 1).
Table 1.
Demographic, serum, and urinary parameters of all subjects, and comparison between calcium stone formers and uric acid stone formers. Continuous variables are summarized as mean ± standard deviation or median [25th, 75th percentile].
| Total Sample | Calcium Stone Formers (CaSF) |
Uric Acid Stone Formers (UASF) |
P-value (UASF vs. CaSF) |
|
|---|---|---|---|---|
| Demographics | ||||
| Sample Size (n) | 1516 | 1365 | 151 | |
| Proportion in Total Population (%) | 90% | 10% | ||
| Age (years) | 43.7±12.6 | 43.1±12.6 | 49.6±11.7 | <0.0001 |
| Race/Ethnicity | 0.69 | |||
| White | 1414 | 1271 | 143 | |
| Black | 45 | 41 | 4 | |
| Hispanic | 16 | 15 | 1 | |
| Asian | 29 | 28 | 1 | |
| American Indians | 5 | 5 | 0 | |
| Weight (kg) | 81.5±20.0 | 80.3±19.5 | 92.8±20.8 | <0.0001 |
| BMI (kg/m2) | 27.4±6.0 | 27.0±5.8 | 30.7±6.7 | <0.0001 |
| % Female | 33% | 34% | 25% | 0.03 |
| Serum | ||||
| Calcium (mmol/L) | 2.37±0.11 | 2.37±0.11 | 2.37±0.11 | 0.46 |
| Uric Acid(µmol/L) | 352±96 | 348±93 | 394±110 | <0.0001 |
| Glucose (mmol/L) | 5.2±1.2 | 5.1±1.1 | 5.9±1.8 | <0.0001 |
| Triglyceride (mmol/L) | 1.7±1.4 | 1.7±1.4 | 2.1±1.7 | 0.01 |
| Urine | ||||
| Volume (mL/day) | 1808±927 | 1809±936 | 1801±849 | 0.94 |
| pH | 5.99±0.45 | 6.03±0.44 | 5.65±0.44 | <0.0001 |
| Cr-Clearance (mL/min/1.73m2) | 97.5±27.9 | 97.6±27.4 | 95.8±31.6 | 0.57 |
| Creatinine (mmol/day) | 14.0±4.5 | 13.8±4.4 | 15.6±4.4 | <0.0001 |
| Sodium (mEq/day) | 175±82 | 173±82 | 189±84 | 0.02 |
| Potassium (mEq/day) | 52±22 | 51±22 | 56±23 | 0.01 |
| Calcium (mmol/day) | 6.1±3.1 | 6.2±3.1 | 5.7±3.4 | 0.13 |
| Oxalate (mmol/day) | 0.4±0.2 | 0.4±0.2 | 0.4±0.2 | 0.29 |
| Uric Acid (mmol/day) | 3.5±1.3 | 3.5±1.3 | 3.3±1.4 | 0.08 |
| Citrate (mmol/day) | 2.68±1.62 | 2.66±1.57 | 2.82±2.03 | 0.40 |
| Phosphorus (mmol/day) | 31±12 | 30±11 | 34±12 | 0.001 |
| Sulfate (mEq/day) | 39.8±19.3 | 38.7±17.7 | 46.9±22.4 | <0.0001 |
| Ammonium (mEq/day) | 36.8±17.2 | 36.2±17.2 | 39.9±17.0 | 0.05 |
| NAE (mEq/day) | 48.2±29.3 | 46.2±29.4 | 59.3±25.7 | <0.0001 |
| NAE/Sulfate (mEq/mEq) | 1.19 [0.88, 1.61] | 1.18 [0.87, 1.61] | 1.26 [0.96, 1.59] | 0.30 |
| Ammonium/NAE (mEq/mEq) | 0.75 [0.64, 0.91] | 0.77 [0.66, 0.92] | 0.65 [0.59, 0.77] | <0.0001 |
| Ammonium/Sulfate (mEq/mEq) | 0.90 [0.68, 1.21] | 0.91 [0.69, 1.21] | 0.79 [0.61, 1.11] | 0.01 |
| NGIA (mEq/day) | 28.5±42.6 | 30.0±42.0 | 18.0±45.1 | 0.01 |
| NGIA/Potassium (mEq/mEq) | 0.54 [0.28, 0.81] | 0.56 [0.29, 0.83] | 0.40 [0.11, 0.64] | 0.0004 |
| Urine per Kg Bodyweight | ||||
| Creatinine (µmol/day/kg) | 173.2±45.5 | 173.4±45.5 | 171.3±45.3 | 0.59 |
| Sodium (mEq/day/kg) | 2.17±0.94 | 2.18±0.95 | 2.07±0.88 | 0.14 |
| Potassium (mEq/day/kg) | 0.65±0.27 | 0.65±0.28 | 0.62±0.25 | 0.14 |
| Calcium (mmol/day/kg) | 0.08±0.04 | 0.08±0.04 | 0.06±0.04 | <0.0001 |
| Oxalate (µmol/day/kg) | 4.8±2.4 | 4.9±2.4 | 4.4±1.7 | 0.004 |
| Uric Acid (µmol/day/kg) | 43.6±15.6 | 44.4±15.3 | 36.5±16.1 | <0.0001 |
| Citrate (µmol/day/kg) | 33.2±20.2 | 33.6±20.1 | 29.9±21.2 | 0.01 |
| Phosphorus (mmol/day/kg) | 0.38±0.13 | 0.38±0.13 | 0.37±0.12 | 0.24 |
| Sulfate (mEq/day/kg) | 0.48±0.21 | 0.48±0.20 | 0.50±0.22 | 0.34 |
| Ammonium (mEq/day/kg) | 0.44±0.22 | 0.44±0.22 | 0.43±0.21 | 0.70 |
| NAE (mEq/day/kg) | 0.56±0.36 | 0.55±0.37 | 0.63±0.30 | 0.02 |
| NGIA (mEq/day/kg) | 0.35±0.48 | 0.38±0.48 | 0.19±0.49 | <0.0001 |
Cr: Creatinine; NAE: Net Acid Excretion; NGIA: Net Gastrointestinal Absorption of Alkali; BW: Body Weight.
24-hour urine biochemical variables of UASF showed higher uNa, uK, and uP compared with CaSF (Table 1). 24-hour urine acid-base profiles of UASF showed lower upH but higher uSO4 and NAE compared with CaSF, while uCit was not statistically different between the two groups. NGIA was lower in UASF compared with CaSF. UASF had higher uNH4+, but lower uNH4+/NAE (UASF vs. CaSF, expressed as median [25th, 75th percentile]: 0.65 [0.59, 0.77] vs. 0.77 [0.66, 0.92] mEq/mEq, p<0.0001) and uNH4+/uSO4 (UASF vs. CaSF: 0.79 [0.61, 1.11] vs. 0.91 [0.69, 1.21] mEq/mEq, p=0.01) compared with CaSF (Table 1).
Since the subjects differed in body weight, we normalized the 24-hour urinary parameters to BW. When corrected to BW, uNa, uK, and uP were similar between the two groups. UASF had lower uCa, uOx, and uUA compared with CaSF (Table 1). 24-hour urine acid-base profiles corrected to BW demonstrated similar uSO4 and uNH4+ between UASF and CaSF. UASF had higher NAE, but lower NGIA and uCit excretion compared with CaSF (Table 1).
Time trends in demographics and biochemical characteristics in UASF versus CaSF
Patients were stratified based on year of initial visit: 661 (44%) were evaluated from 1980–1990, 445 (29%) from 1990–2000, and 410 (27%) from 2000–2015.
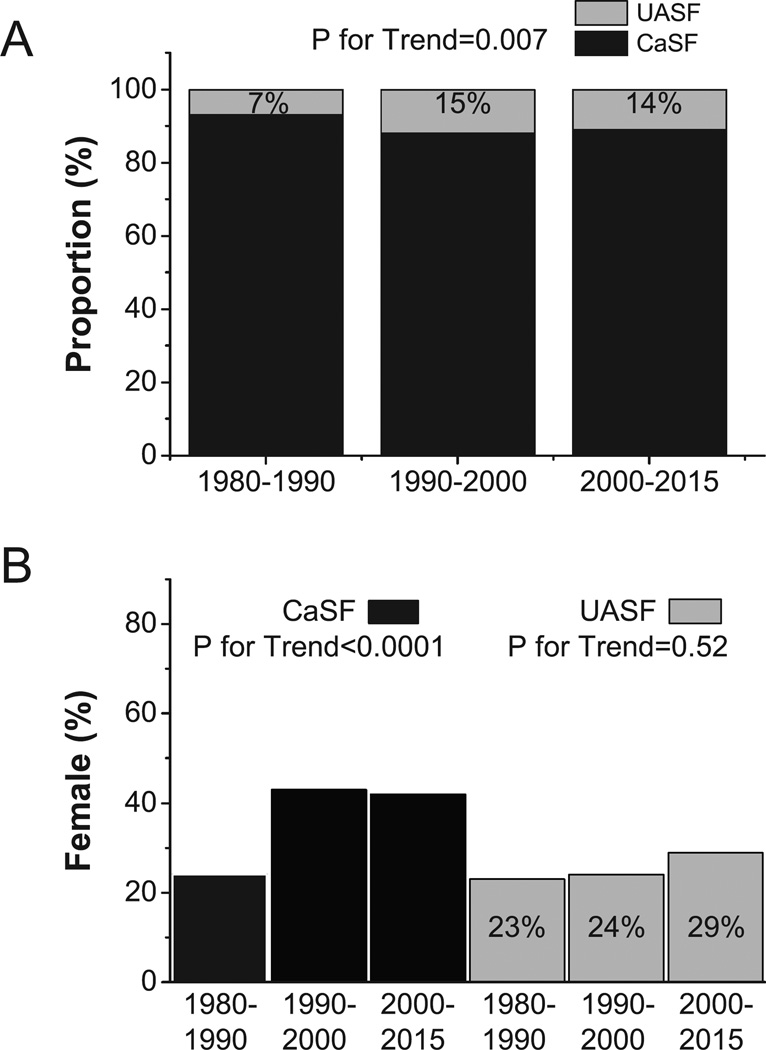
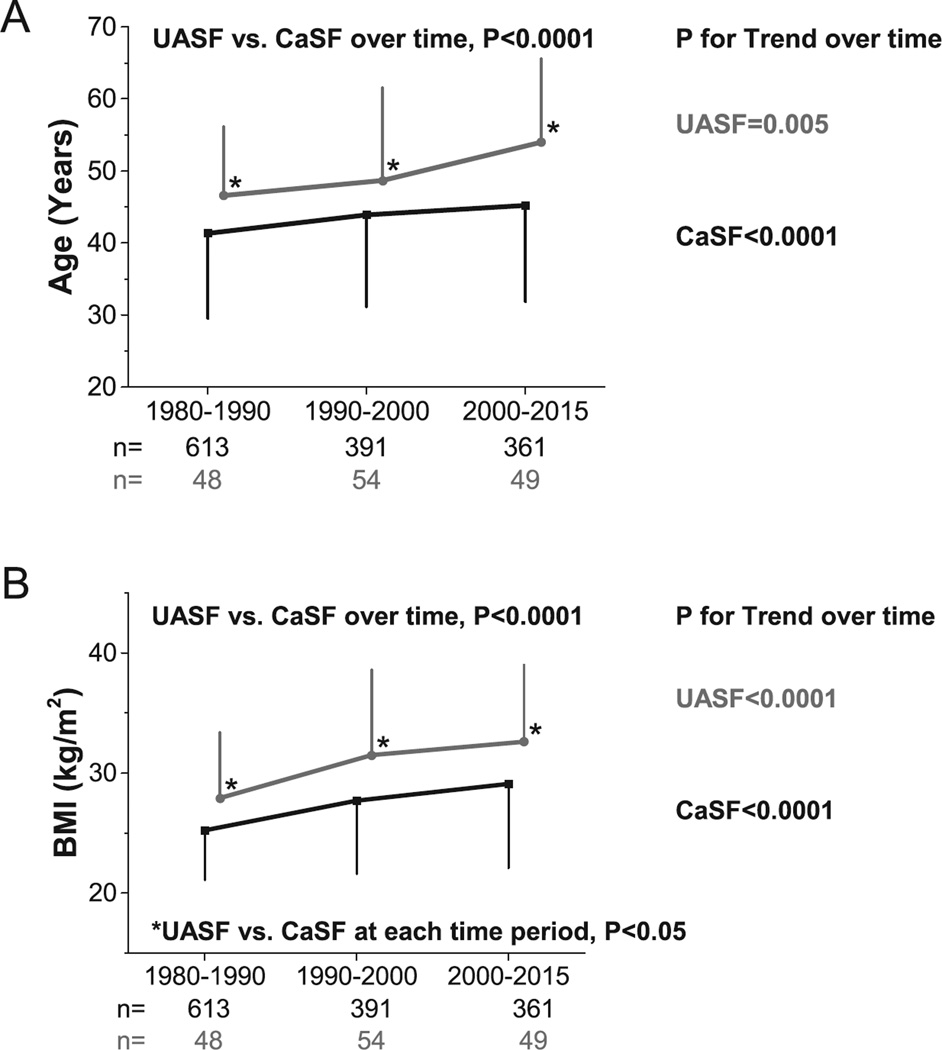
Over the time periods, the proportion of UASF increased significantly (from 7% to 14%, p=0.007) (Figure 1A). The proportion of female patients did not change among UASF (p=0.52), but increased among CaSF over the three time periods from 24% to 42% (p<0.001) (Figure 1B). With time, and in both groups, there was a significant increase in age (p=0.005 in UASF, and p<0.0001 in CaSF, Figure 2A) and BMI (p<0.0001 in both groups, Figure 2B). Within each time period, both age and BMI were significantly higher in UASF than CaSF (p<0.0001 for both, Figures 2A and 2B). UASF had consistently higher sUA (p<0.0001), sGlu (p<0.0001), and sTG (p=0.0007) compared with CaSF in all time periods (Supplemental Figures 1A, 1B and 1C).
Figure 1.
A) Proportion of UASF/CaSF at different time periods; B) Female percentage among UASF/CaSF at different time periods.
Figure 2.
A) Age trends of UASF/CaSF at different time periods; B) BMI trends of UASF/CaSF at different time periods. P-value at the top of each figure compares UASF vs. CaSF group effect, while p-values to the right of each line corresponds to test for linear trend across time periods within each group. Asterisk (*) indicates p<0.05 between for CaSF vs. UASF at corresponding time period.
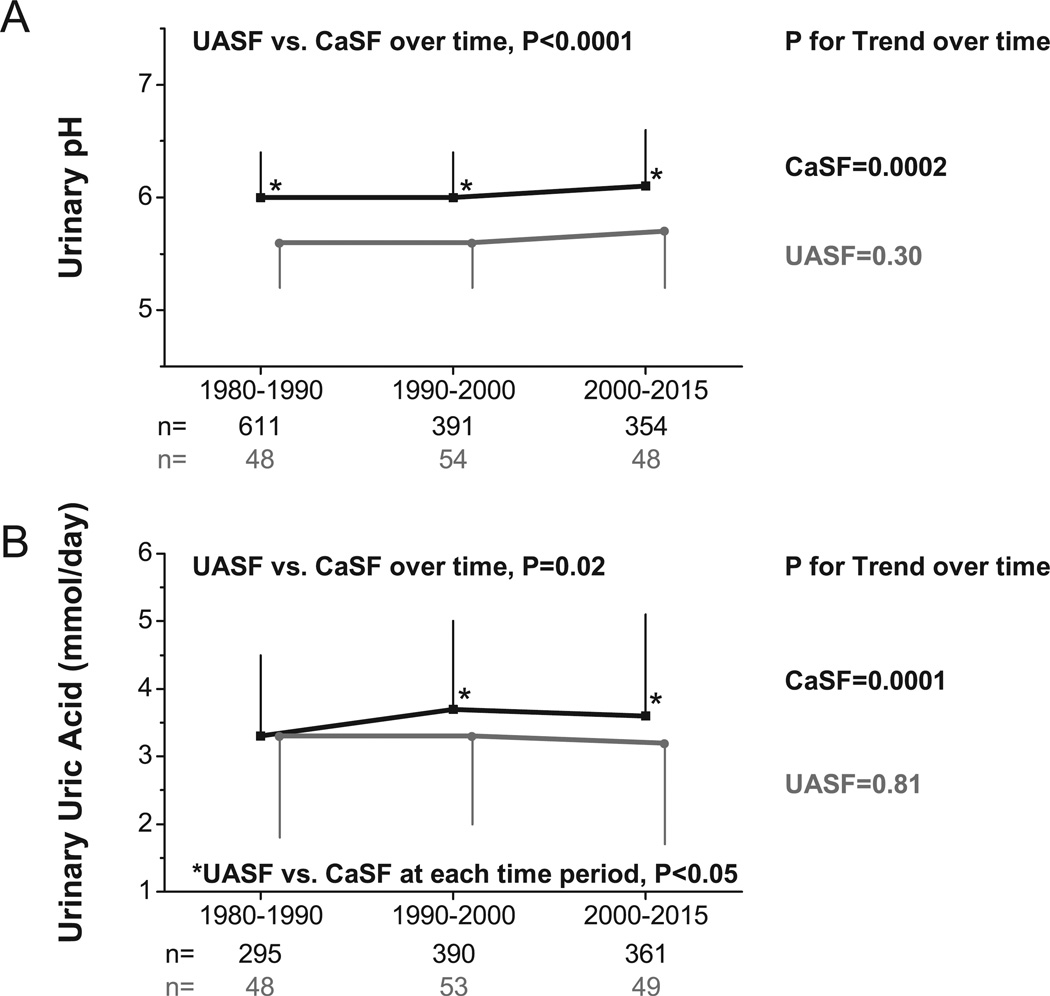
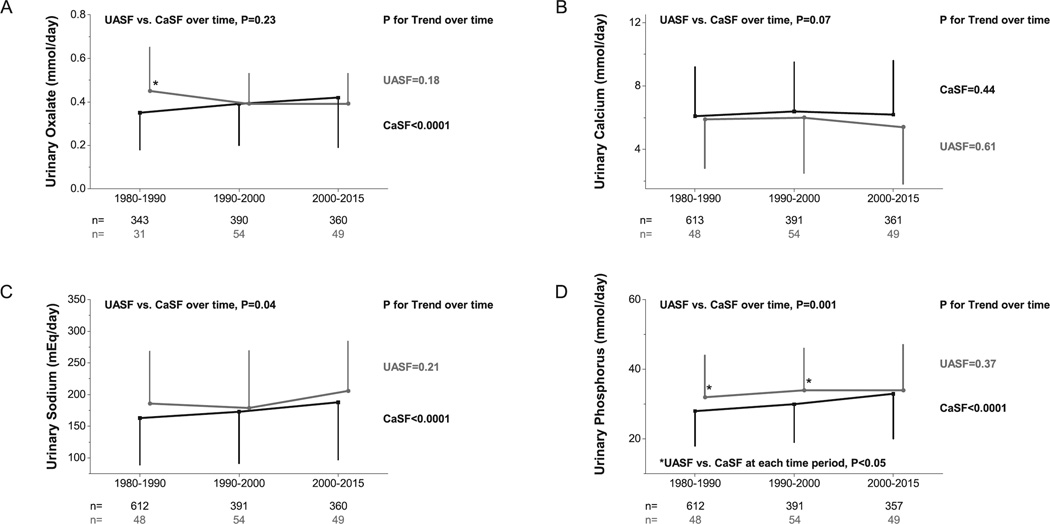
Temporal changes in urinary profiles with time as a continuous variable are shown in Figures 3 and 4. UASF had lower upH (p<0.0001) and uUA (p=0.02) than CaSF (Figures 3A and 3B). UpH and UA remained constant within UASF (P for Trend=0.30 for upH and 0.81 for uUA), but increased within CaSF (P for Trend=0.0002 for upH and 0.0001 for uUA). Both groups had similar uOx (p=0.23) and Ca (p=0.07) (Figures 4A and 4B). UOx increased within CaSF (P for Trend<0.0001) but remained constant within UASF (P for Trend=0.18). UASF and CaSF had distinct uNa (UASF vs. CaSF, p=0.04) and uP (UASF vs. CaSF, p=0.001) (Figures 4C and 4D), with uNa and uP increasing significantly within CaSF (P for Trend<0.0001 for both) while remaining constant within UASF (P for Trend=0.21 and 0.37, respectively).
Figure 3.
A) Urinary pH trends of UASF/CaSF at different time periods; B) Urinary uric acid trends of UASF/CaSF at different time periods; P-value at the top of each figure compares UASF vs. CaSF group effect, while p-values to the right of each line corresponds to test for linear trend across time periods within each group. Asterisk (*) indicates p<0.05 between for UASF vs. CaSF at corresponding time period.
Figure 4.
A) Urinary oxalate trends of UASF/CaSF at different time periods; B) Urinary calcium trends of UASF/CaSF at different time periods. C) Urinary sodium trends of UASF/CaSF at different time periods. D) Urinary phosphorus trends of UASF/CaSF at different time periods. P-value at the top of each figure compares UASF vs. CaSF group effect, while p-values to the right of each line corresponds to test for linear trend across time periods within each group. Asterisk (*) indicates p<0.05 between for UASF vs. CaSF at corresponding time period.
Urinary parameters shown in Supplemental Figure 2 demonstrated similar changes over time in uCit, NAE, and NGIA, but a significant difference in uSO4 (p<0.0001) and uK (p=0.01) between the two groups.
For each stone group, we examined the interaction between gender and time. While there were sex differences for some urinary variables (e.g., pH, urinary calcium, etc.) the patterns over time were similar for these urinary variables as well as age and BMI (i.e., non-significant time-gender interactions).
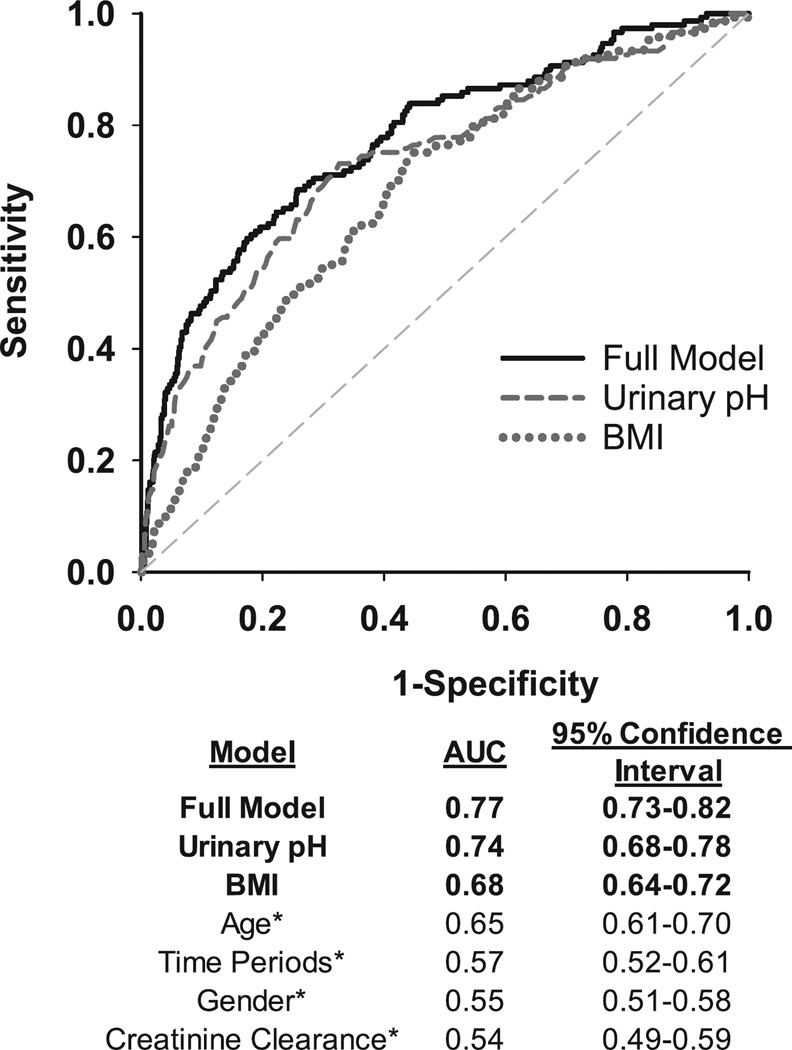
ROC analysis, clinical predictors of uric acid KS formation
A receiver operating characteristic (ROC) model was used to evaluate the effects of upH, and other variables in estimating the risk of uric acid stone formation (Figure 5). UpH had the strongest association with uric acid stone formation [Area under curve (AUC) for pH=0.74, BMI=0.68, Age=0.65, Time periods=0.57, Gender=0.55, and CrCl=0.54, Figure 5). The AUC for the full multivariable model was marginally higher than upH alone at 0.77 (95% CI: 0.73–0.82). The bootstrap-corrected AUC from internal validation was 0.76 (optimism correction=0.0096).
Figure 5.
Receiver Operating Characteristic (ROC) analysis and areas under the curves (AUC) for clinical predictors of uric acid stone formation. Multivariable full model includes: urinary pH, BMI, time period, gender, and creatinine clearance. Urinary pH and BMI from univariable analysis are shown. Asterisks (*) indicate variables that are not shown on the figure.
Discussion
To our knowledge, this is the longest longitudinal retrospective study of a large number of KSF evaluated over 35 years. The three principal findings of this study are: 1) The rising trend in proportion of uric acid stones associated with increased BMI and low upH with time; 2) The substantial growth in the proportion of female CaSF during the study period; and 3) UpH alone is a powerful clinical discriminant of whether a stone former has uric acid or calcium stones. Several minor findings are: 1) The increasing trends of uOx, uP, and uNa only in CaSF; 2) Similar uCa in both groups; and 3) 24-hour CrCl is not a significant predictor of stone types.
MS and obesity are major health burdens in the U.S., affecting 30% of general population 14, 15. Excessively acidic urine, the principal abnormality and promoter of uric acid crystallization and uric acid stone 16, was reported in aging patients 17, 18, obesity, and type 2 diabetes mellitus 19. Consistent with these findings, we observed an association between uric acid stones and increased BMI, older age, and low upH (Figures 2A, 2B and 3A). A proposed mechanism is increased acid load to the kidney coupled with a sustained increased delivery of fat to the kidneys that occurs with high-fat Western diet, which could lead to renal proximal tubular injury (lipotoxicity) and consequently defective renal ammoniagenesis 20, 21 and lower urine pH due impaired buffering by ammonium. This is supported by studies in non-stone forming human subjects who exhibited an inverse relationship between visceral fat, upH and uNH4+ 6, 22, and higher renal fat content with greater BMI 22, 23, as well as in animal and cell culture models 20, 21. The lower uNH4+/NAE and uNH4+/uSO4 ratios (uSO4: a surrogate marker of dietary acid) in UASF compared with CaSF (p=0.01, Table 1) are compatible with the blunted ammoniagenic response to acid load in UASF. The defective NH4+ excretion is not the sole factor in causing abnormally acidic urine; acid load is also increased since UASF have higher NAE and lower NGIA per body weight compared to CaSF (p<0.0001, Table 1).
The National Health and Nutrition Examination Survey (NHANES) demonstrate a narrowing in the gender gap reported in KS prevalence in the United States 1. A recent study by Moses et al also confirmed the narrowing gender gap by showing significant increase of female stone formers with >50% UA content 24. Our results confirm the NHANES findings, with an increase in female CaSF proportion from 24% to 42% over time (Figure 1B), but differ from Moses et al’s findings, possibly due to the use of different stone analysis cutoff (70% vs. 50% 24) . Another study by Yang et al from China indicated that the occurrence of uric acid stone decreased from 2002 to 2014 25, while we observed the opposite. The difference may be related due to ethnic group and/or BMI differences. However, our results resemble what is reported in the U.S population 18. While gender influences excretion of key urinary factors related to stone formation 26, the underlying cause(s) for the rising female KSF proportion over time has not yet been fully elucidated. Nevertheless, the magnitude of the impact of obesity on increasing the risk of KS formation has been reported to be greater in women than in men 8. Other hypothesized mechanism for the narrowing gender gap in the incidence of nephrolithiasis including an aging female population with estrogen lack with potential skeletal calcium and alkali loss, calcium/vitamin D supplement use, and post-menopausal hormone replacement. In terms of stone type, our logistic model did not find gender to be a significant predictive risk factor of stone type (Figure 5).
A third major finding is the ability of upH to serve as strong discriminant (AUC for pH=0.74) of UA stone in our results, followed by BMI (AUC=0.68) and Age (AUC=0.65), and the lowest three factors were time, gender, and CrCl (Figure 5). This finding may be clinically tool to predict patient’s stone type and appropriate treatment plan during initial visit, when imaging and/or stone analysis is not available readily. Chronic kidney disease (CKD) may alter urine pH, by reducing ammonium excretion and impacting other urinary parameters. CKD has also been hypothesized as a risk factor for stone formation in previous studies, thus we chose to evaluate creatinine clearance. Still, 24-hour CrCl was not a strong predictor in our model (Figure 5), contrasting previous studies that demonstrated renal function (eGFR) may affect stone type 26–29.
Some minor findings are also noteworthy. The lack of temporal change in uCa in CaSF despite increasing sodium intake (Figure 4C) 30 may be due to increased intestinal binding of calcium by higher dietary phosphate blunting intestinal calcium absorption and reducing uCa excretion (Figure 4B). The increasing trend in uP in CaSF (Figure 4D) confirmed the possibility of increased dietary phosphate intake over time. Besides changes in dietary intake of oxalate precursors, the increasing trend in uOx excretion in CaSF (Figure 4A) may be explained by increased dietary phosphate intake mainly due to increasing addition of inorganic phosphate in processed foods over time (Figure 4D), complexing intestinal luminal Ca, resulting in enhanced intestinal Ox absorption 7, 9, 10.
One advantage of our analysis over prior studies is that we relied on urinary biochemical profiles as part of a structured protocol. This study explored a large number of KSF through cross sectional sampling over time, taking into account of gender, age, and BMI. The statistical analysis also attempted to identify clinical predictors of stone type that may be available to clinicians who may not readily have access to stone composition analysis/imaging or, in some instances, even to 24-hour urinary analysis.
Limitations include: 1. the observational single center design with possible selection bias; 2. lack of follow-up data; and 3. absence of information on dietary intake at the time of urine collection. We utilized a 70% cut-off for classifying stone composition 11. Use of different definitions/cut-offs by various investigators impacts comparison across centers and study populations.
Conclusion
Three principal findings of this study include trending rise in proportion of UASF associated with increased BMI and low upH, growth in proportion of female CaSF during the study period, and low upH being a strong clinical discriminant of uric acid stone occurrence.
Supplementary Material
Supplemental Figure 1. A) Serum uric acid trends of UASF/CaSF at different time periods; B) Serum glucose trends of UASF/CaSF at different time periods; C) Serum triglyceride trends of UASF/CaSF at different time periods. P-value at the top of each figure compares UASF vs. CaSF group effect, while p-values to the right of each line corresponds to test for linear trend across time periods within each group. Asterisk (*) indicates p<0.05 between for CaSF vs. UASF at corresponding time period.
Supplemental Figure 2. A) Creatinine clearance trends of UASF/CaSF at different time periods; B) Urinary sulfate trends of UASF/CaSF at different time periods; C) Urinary potassium trends of UASF/CaSF at different time periods; D) Urinary citrate trends of UASF/CaSF at different time periods; E) Net acid excretion trends of UASF/CaSF at different time periods; F) Net gastrointestinal absorption of alkali trends of UASF/CaSF at different time periods. P-value at the top of each figure compares UASF vs. CaSF group effect. Asterisk (*) indicates p<0.05 between for UASF vs. CaSF at corresponding time period. Cr: Creatinine; NAE: Net Acid Excretion; NGIA: Net Gastrointestinal Absorption of Alkali.
Acknowledgments
The authors acknowledge Ms. Crystal Aguirre and Ms. Tamara Crowe for assistance in the preparation of this manuscript. The studies were supported by the National Institute of Health (R01-DK081423, R01-DK081423-06, R01- DK092461, P01-DK20543 and P30 DK-079328). LHRX was supported by the Truelson Fellowship Grant from the Charles and Jane Pak Center for Mineral Metabolism and Clinical Research.
Abbreviation (in order of appearance)
- KS
Kidney Stones
- MS
Metabolic Syndrome
- upH
Urinary pH
- KSF
Kidney Stone Formers
- UASF
Uric Acid Stone Formers
- CaSF
Calcium stone Formers
- BMI
Body Mass Index
- NL
Normal Lab values
- sCa
Serum Calcium
- sUA
Serum Uric Acid
- sGlu
Serum Glucose
- sTG
Serum Triglyceride
- uTV
Urinary Total Volume
- uCr
Urinary Creatinine
- uNa
Urinary Sodium
- uK
Urinary Potassium
- uCa
Urinary Calcium
- uUA
Urinary Uric Acid
- uOx
Urinary Oxalate
- uSO4
Urinary Sulfate
- uCit
Urinary Citrate
- uP
Urinary Phosphorus
- uCl
Urinary Chloride
- uNH4+
Urinary Ammonium
- BW
Body Weight
- CrCl
Creatinine Clearance
- NGIA
net gastrointestinal absorption of alkali
- HCO3−
Bicarbonate
- NAE
Net Acid Excretion
- mEq
Milliequivalents
- ROC
Receiver Operating Characteristic
- AUC
Area Under the Curve
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Summary
The authors have nothing to disclose.
Reference
- 1.Scales CD, Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.West B, Luke A, Durazo-Arvizu RA, et al. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988–1994. Am J Kidney Dis. 2008;51:741. doi: 10.1053/j.ajkd.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 3.Antonelli JA, Maalouf NM, Pearle MS, et al. Use of the National Health and Nutrition Examination Survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. Eur Urol. 2014;66:724. doi: 10.1016/j.eururo.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maalouf NM, Cameron MA, Moe OW, et al. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883. doi: 10.2215/CJN.00670207. [DOI] [PubMed] [Google Scholar]
- 5.Sakhaee K, Capolongo G, Maalouf NM, et al. Metabolic syndrome and the risk of calcium stones. Nephrol Dial Transplant. 2012;27:3201. doi: 10.1093/ndt/gfr703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pigna F, Sakhaee K, Adams-Huet B, et al. Body fat content and distribution and urinary risk factors for nephrolithiasis. Clin J Am Soc Nephrol. 2014;9:159. doi: 10.2215/CJN.06180613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemann J, Jr, Pleuss JA, Worcester EM, et al. Urinary oxalate excretion increases with body size and decreases with increasing dietary calcium intake among healthy adults. Kidney Int. 1996;49:200. doi: 10.1038/ki.1996.27. [DOI] [PubMed] [Google Scholar]
- 8.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 9.Shavit L, Ferraro PM, Johri N, et al. Effect of being overweight on urinary metabolic risk factors for kidney stone formation. Nephrol Dial Transplant. 2015;30:607. doi: 10.1093/ndt/gfu350. [DOI] [PubMed] [Google Scholar]
- 10.Rendina D, De Filippo G, De Pascale F, et al. The changing profile of patients with calcium nephrolithiasis and the ascendancy of overweight and obesity: a comparison of two patient series observed 25 years apart. Nephrol Dial Transplant. 2013;28(Suppl 4):iv146. doi: 10.1093/ndt/gft076. [DOI] [PubMed] [Google Scholar]
- 11.Pak CY, Poindexter JR, Adams-Huet B, et al. Predictive value of kidney stone composition in the detection of metabolic abnormalities. Am J Med. 2003;115:26. doi: 10.1016/s0002-9343(03)00201-8. [DOI] [PubMed] [Google Scholar]
- 12.Oh MS. A new method for estimating G-I absorption of alkali. Kidney Int. 1989;36:915. doi: 10.1038/ki.1989.280. [DOI] [PubMed] [Google Scholar]
- 13.Kok DJ, Poindexter J, Pak CY. Calculation of titratable acidity from urinary stone risk factors. Kidney Int. 1993;44:120. doi: 10.1038/ki.1993.221. [DOI] [PubMed] [Google Scholar]
- 14.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care. 2004;27:2444. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 15.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 16.Sakhaee K, Adams-Huet B, Moe OW, et al. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 17.Knoll T, Schubert AB, Fahlenkamp D, et al. Urolithiasis through the ages: data on more than 200,000 urinary stone analyses. J Urol. 2011;185:1304. doi: 10.1016/j.juro.2010.11.073. [DOI] [PubMed] [Google Scholar]
- 18.Daudon M, Dore JC, Jungers P, et al. Changes in stone composition according to age and gender of patients: a multivariate epidemiological approach. Urol Res. 2004;32:241. doi: 10.1007/s00240-004-0421-y. [DOI] [PubMed] [Google Scholar]
- 19.Daudon M, Traxer O, Conort P, et al. Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol. 2006;17:2026. doi: 10.1681/ASN.2006030262. [DOI] [PubMed] [Google Scholar]
- 20.Bobulescu IA, Dubree M, Zhang J, et al. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol. 2008;294:F1315. doi: 10.1152/ajprenal.00550.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bobulescu IA, Dubree M, Zhang J, et al. Reduction of renal triglyceride accumulation: effects on proximal tubule Na+/H+ exchange and urinary acidification. Am J Physiol Renal Physiol. 2009;297:F1419. doi: 10.1152/ajprenal.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bobulescu IA, Lotan Y, Zhang J, et al. Triglycerides in the human kidney cortex: relationship with body size. PLoS One. 2014;9:e101285. doi: 10.1371/journal.pone.0101285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoo T, Clark HR, Pedrosa I, et al. Quantification of renal steatosis in type II diabetes mellitus using dixon-based MRI. J Magn Reson Imaging. 2016 doi: 10.1002/jmri.25252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moses R, Pais VM, Jr, Ursiny M, et al. Changes in stone composition over two decades: evaluation of over 10,000 stone analyses. Urolithiasis. 2015;43:135. doi: 10.1007/s00240-015-0756-6. [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Zhang C, Qi S, et al. Multivariate Analyses of Urinary Calculi Composition: A 13-Year Single-Center Study. J Clin Lab Anal. 2016;30:873. doi: 10.1002/jcla.21950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perinpam M, Ware EB, Smith JA, et al. Effect of Demographics on Excretion of Key Urinary Factors Related to Kidney Stone Risk. Urology. 2015;86:690. doi: 10.1016/j.urology.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoag J, Halpern J, Goldfarb DS, et al. Risk of chronic and end stage kidney disease in patients with nephrolithiasis. J Urol. 2014;192:1440. doi: 10.1016/j.juro.2014.05.117. [DOI] [PubMed] [Google Scholar]
- 28.Rule AD, Krambeck AE, Lieske JC. Chronic kidney disease in kidney stone formers. Clin J Am Soc Nephrol. 2011;6:2069. doi: 10.2215/CJN.10651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedlander JI, Moreira DM, Hartman C, et al. Comparison of the metabolic profile of mixed calcium oxalate/uric acid stone formers to that of pure calcium oxalate and pure uric acid stone formers. Urology. 2014;84:289. doi: 10.1016/j.urology.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Ticinesi A, Nouvenne A, Maalouf NM, et al. Salt and nephrolithiasis. Nephrol Dial Transplant. 2016;31:39. doi: 10.1093/ndt/gfu243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. A) Serum uric acid trends of UASF/CaSF at different time periods; B) Serum glucose trends of UASF/CaSF at different time periods; C) Serum triglyceride trends of UASF/CaSF at different time periods. P-value at the top of each figure compares UASF vs. CaSF group effect, while p-values to the right of each line corresponds to test for linear trend across time periods within each group. Asterisk (*) indicates p<0.05 between for CaSF vs. UASF at corresponding time period.
Supplemental Figure 2. A) Creatinine clearance trends of UASF/CaSF at different time periods; B) Urinary sulfate trends of UASF/CaSF at different time periods; C) Urinary potassium trends of UASF/CaSF at different time periods; D) Urinary citrate trends of UASF/CaSF at different time periods; E) Net acid excretion trends of UASF/CaSF at different time periods; F) Net gastrointestinal absorption of alkali trends of UASF/CaSF at different time periods. P-value at the top of each figure compares UASF vs. CaSF group effect. Asterisk (*) indicates p<0.05 between for UASF vs. CaSF at corresponding time period. Cr: Creatinine; NAE: Net Acid Excretion; NGIA: Net Gastrointestinal Absorption of Alkali.