Abstract
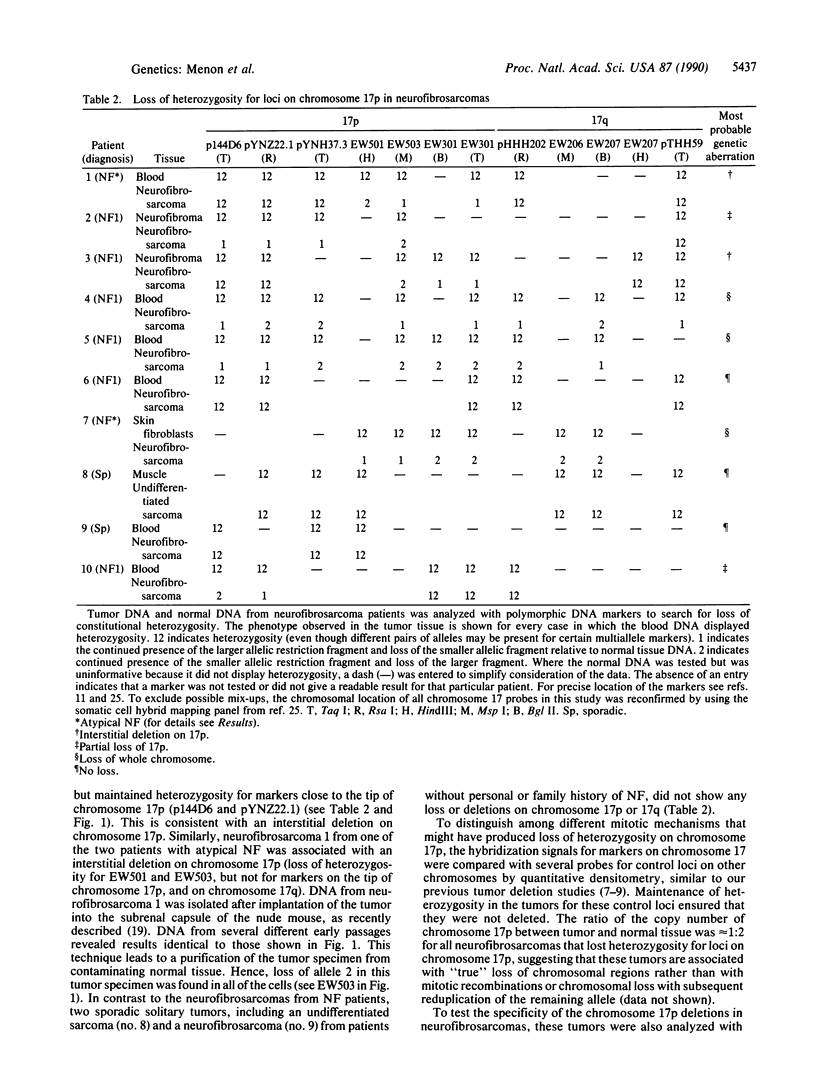
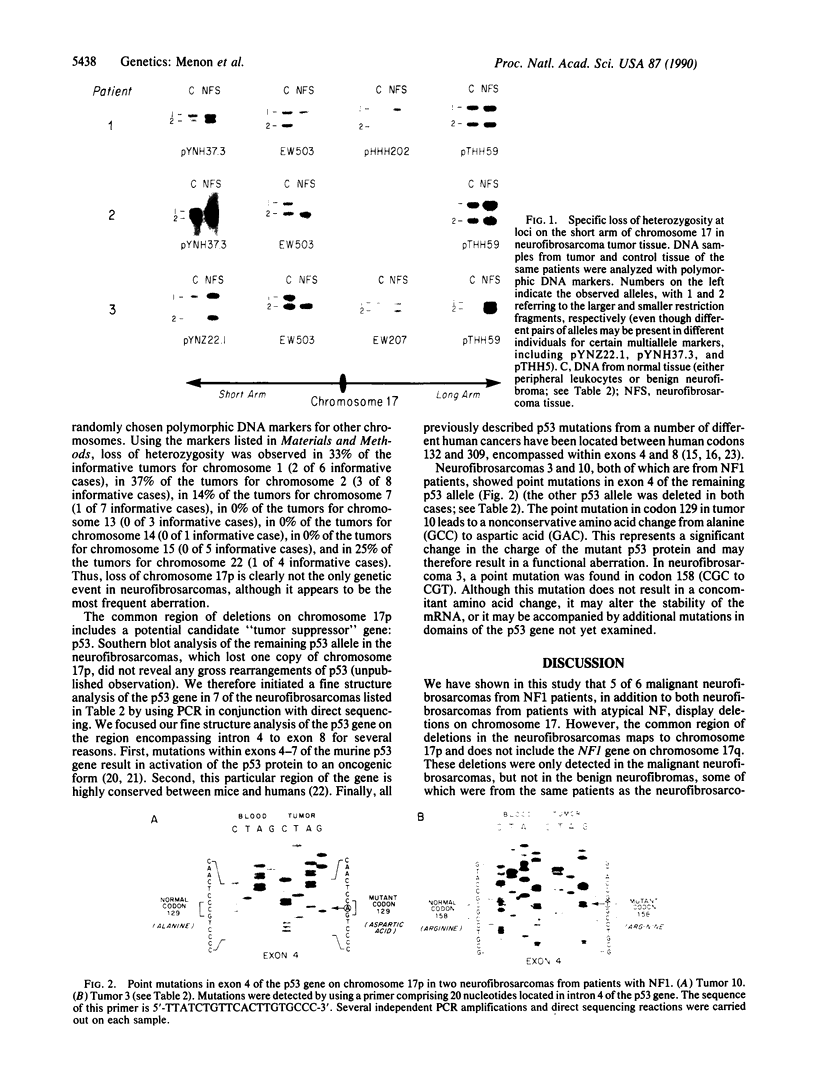
von Recklinghausen neurofibromatosis (NF1) is a common hereditary disorder characterized by neural crest-derived tumors, particularly benign neurofibromas whose malignant transformation to neurofibrosarcomas can be fatal. The NF1 gene has been mapped to a small region of chromosome 17q, but neither the nature of the primary defect nor the mechanisms involved in tumor progression are understood. We have tested whether NF1 might be caused by the inactivation of a tumor suppressor gene on 17q, analogous to that on chromosome 22 in NF2, by searching for deletions of chromosome 17 in NF1-derived tumor specimens. Both neurofibrosarcomas from patients with "atypical" NF and 5 of 6 neurofibrosarcomas from NF1 patients displayed loss of alleles for polymorphic DNA markers on chromosome 17. However, the common region of deletion was on 17p and did not include the NF1 region of 17q. Since no loss of markers on chromosome 17 was observed in any of 30 benign tumors from NF1 patients, the 17p deletions seen in neurofibrosarcomas are probably associated with tumor progression and/or malignancy. This region contains a candidate gene for tumor progression, p53, which has recently been implicated in the progression of a broad array of human cancers. In a preliminary search for p53 aberrations by direct sequencing of polymerase chain reaction-amplified DNA from 7 neurofibrosarcomas, 2 tumors that contained point mutations in exon 4 of the p53 gene were found, suggesting a role for this gene in at least some neurofibrosarcomas. Thus the formation of malignant neurofibrosarcomas may result from several independent genetic events including mutation of the NF1 gene, whose mechanism of tumorigenesis remains uncertain, and subsequent loss of a "tumor suppressor" gene on 17p, most likely p53.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auer H. E., Glick D. M. The mechanism of activation of porcine pepsinogen. Nature. 1986 Aug 14;322(6080):664–664. doi: 10.1038/322664a0. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Barker D., Wright E., Nguyen K., Cannon L., Fain P., Goldgar D., Bishop D. T., Carey J., Baty B., Kivlin J. Gene for von Recklinghausen neurofibromatosis is in the pericentromeric region of chromosome 17. Science. 1987 May 29;236(4805):1100–1102. doi: 10.1126/science.3107130. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Fountain J. W., Wallace M. R., Bruce M. A., Seizinger B. R., Menon A. G., Gusella J. F., Michels V. V., Schmidt M. A., Dewald G. W., Collins F. S. Physical mapping of a translocation breakpoint in neurofibromatosis. Science. 1989 Jun 2;244(4908):1085–1087. doi: 10.1126/science.2543076. [DOI] [PubMed] [Google Scholar]
- Hinds P., Finlay C., Levine A. J. Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol. 1989 Feb;63(2):739–746. doi: 10.1128/jvi.63.2.739-746.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb P., Crawford L. Characterization of the human p53 gene. Mol Cell Biol. 1986 May;6(5):1379–1385. doi: 10.1128/mcb.6.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter D. H., Ledbetter S. A., vanTuinen P., Summers K. M., Robinson T. J., Nakamura Y., Wolff R., White R., Barker D. F., Wallace M. R. Molecular dissection of a contiguous gene syndrome: frequent submicroscopic deletions, evolutionarily conserved sequences, and a hypomethylated "island" in the Miller-Dieker chromosome region. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5136–5140. doi: 10.1073/pnas.86.13.5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhkour A., Van Roey M., Sobel R. A., Fingert H. J., Lee J., Martuza R. L. Implantation of human meningiomas into the subrenal capsule of the nude mouse. A model for studies of tumor growth. J Neurosurg. 1989 Oct;71(4):545–550. doi: 10.3171/jns.1989.71.4.0545. [DOI] [PubMed] [Google Scholar]
- Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988 May;45(5):575–578. [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- O'Connell P., Leach R., Cawthon R. M., Culver M., Stevens J., Viskochil D., Fournier R. E., Rich D. C., Ledbetter D. H., White R. Two NF1 translocations map within a 600-kilobase segment of 17q11.2. Science. 1989 Jun 2;244(4908):1087–1088. doi: 10.1126/science.2543077. [DOI] [PubMed] [Google Scholar]
- Riccardi V. M. Von Recklinghausen neurofibromatosis. N Engl J Med. 1981 Dec 31;305(27):1617–1627. doi: 10.1056/NEJM198112313052704. [DOI] [PubMed] [Google Scholar]
- Rouleau G. A., Wertelecki W., Haines J. L., Hobbs W. J., Trofatter J. A., Seizinger B. R., Martuza R. L., Superneau D. W., Conneally P. M., Gusella J. F. Genetic linkage of bilateral acoustic neurofibromatosis to a DNA marker on chromosome 22. Nature. 1987 Sep 17;329(6136):246–248. doi: 10.1038/329246a0. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Rouleau G., Ozelius L. J., Lane A. H., St George-Hyslop P., Huson S., Gusella J. F., Martuza R. L. Common pathogenetic mechanism for three tumor types in bilateral acoustic neurofibromatosis. Science. 1987 Apr 17;236(4799):317–319. doi: 10.1126/science.3105060. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., de la Monte S., Atkins L., Gusella J. F., Martuza R. L. Molecular genetic approach to human meningioma: loss of genes on chromosome 22. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5419–5423. doi: 10.1073/pnas.84.15.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen S. A., Mulvihill J. J., Nielsen A. Long-term follow-up of von Recklinghausen neurofibromatosis. Survival and malignant neoplasms. N Engl J Med. 1986 Apr 17;314(16):1010–1015. doi: 10.1056/NEJM198604173141603. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- Weiss S. W., Langloss J. M., Enzinger F. M. Value of S-100 protein in the diagnosis of soft tissue tumors with particular reference to benign and malignant Schwann cell tumors. Lab Invest. 1983 Sep;49(3):299–308. [PubMed] [Google Scholar]
- Yandell D. W., Campbell T. A., Dayton S. H., Petersen R., Walton D., Little J. B., McConkie-Rosell A., Buckley E. G., Dryja T. P. Oncogenic point mutations in the human retinoblastoma gene: their application to genetic counseling. N Engl J Med. 1989 Dec 21;321(25):1689–1695. doi: 10.1056/NEJM198912213212501. [DOI] [PubMed] [Google Scholar]
- Zakut-Houri R., Bienz-Tadmor B., Givol D., Oren M. Human p53 cellular tumor antigen: cDNA sequence and expression in COS cells. EMBO J. 1985 May;4(5):1251–1255. doi: 10.1002/j.1460-2075.1985.tb03768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vanTuinen P., Dobyns W. B., Rich D. C., Summers K. M., Robinson T. J., Nakamura Y., Ledbetter D. H. Molecular detection of microscopic and submicroscopic deletions associated with Miller-Dieker syndrome. Am J Hum Genet. 1988 Nov;43(5):587–596. [PMC free article] [PubMed] [Google Scholar]






