Abstract
Objectives
Characterization of urinary bacterial microbiome and antimicrobial peptides (AMPs) after burn injury to identify potential mechanisms leading to urinary tract infections (UTIs) and associated morbidities in burn patients.
Design
Retrospective cohort study using human urine from control and burn subjects.
Setting
University Research Laboratory.
Patients
Burn patients.
Interventions
None.
Measurements and Main Results
Urine samples from catheterized burn patients were collected hourly for up to 40 hours. Control urine was collected from “healthy” volunteers. The urinary bacterial microbiome and AMP levels and activity were compared with patient outcomes. We observed a significant increase in urinary microbial diversity in burn patients vs. controls, which positively correlated with a larger percent burn and with the development of UTI and sepsis post-admission, regardless of age or gender. Urinary psoriasin and β-defensin AMP levels were significantly reduced in burn patients at 1 and 40 hours post-admission. We observed a shift in AMP hydrophobicity and activity between control and burn patients when urinary fractions were tested against Escherichia coli and Enterococcus faecalis UTI isolates. Furthermore, the AMP activity in burn patients was more effective against E. coli than E. faecalis. UTI-positive burn patients with altered urinary AMP activity developed either an E. faecalis or Pseudomonas aeruginosa UTI, suggesting a role for urinary AMPs in susceptibility to select uropathogens.
Conclusions
Our data reveal potential links for UTI development and several morbidities in burn patients through alterations in the urinary microbiome and AMPs. Overall, this study supports the concept that early assessment of urinary AMP responses and the bacterial microbiome may be used to predict susceptibility to UTIs and sepsis in burn patients.
Keywords: burn injury, urinary tract infection, antimicrobial peptides, sepsis, urinary microbiome
Introduction
Pathologic immunomodulation is a major complication of burns[1, 2]. This increases the susceptibility to UTIs, particularly in burn patients with indwelling urinary catheters, which can lead to pyelonephritis and sepsis, costing more than $425 million annually[3]. Recent evidence shows that dysregulation of the microbiome[4, 5] and AMPs[6-9] are associated with UTI susceptibility. Contrary to the previous dogma[10], the urinary tract is not sterile and comprises a diverse microbiome[5, 11]. The urinary microbiome likely stimulates, or is influenced by, innate immune molecules (e.g., AMPs).
AMPs are conserved components of the innate immune system which exhibit microbicidal activity, stimulate inflammation, and facilitate epithelial barrier homeostasis to protect against invading microbes[10, 12-14]. Production of urinary AMPs, including human β-defensin-1 (hBD1), hBD2, psoriasin, lactoferrin, and hepcidin, is influenced by bacteria and inflammation[15-20]. Certain “protective” microbes induce select AMPs to control the pathogen colonization and/or growth[21]. Thus, urinary AMP dysregulation following burn injury could augment UTI susceptibility.
We reported that UTI risk in women undergoing pelvic floor surgery correlated with specific urinary bacteria and AMP levels[8], and that cutaneous burn injury in mice impairs AMP production in distal sites from the burn, including the urinary tract[13]. We further determined that AMP responses and the cutaneous microbiome in autologous donor skin significantly differs from unburned controls and correlates with infectious outcomes (unpublished results and [22]). Therefore, we hypothesized that burn injury would promote alterations in urinary AMP levels and activity, resulting in a more pathogenic urinary microbiome profile.
To our knowledge, the impact of burn injury on urinary AMPs and the microbiome in the context of UTI and sepsis risk has not been evaluated. This study determined that select urinary AMP production is impaired in human burn patients, which parallels a shift in AMP activity against common uropathogens. Furthermore, burn patients exhibit a rapid shift in the urinary microbiome to a more diverse bacterial profile. Finally, we determined that the changes in urinary AMPs and the bacterial microbiome statistically correlate with several post-burn complications. These findings, along with our previous observations, suggest that the colonizing bacteria in the skin and urinary tract, may be used as a tool to predict morbidity in burn patients.
Materials and Methods
Human Patients and Urine Processing
All protocols were approved by the Loyola University Chicago Institutional Review Board. All included patients had a urinary catheter inserted upon arrival to the burn intensive care unit (BICU). See Supplementary Methods for exclusion criteria, clinical characteristics and post-burn complications. Discarded catheter urine was collected hourly for up to 40 hours in burn subjects; voided volunteer urine served as control. Specimens were centrifuged at 5000xg for 20 minutes, and supernatants sterile-filtered and stored at -80°C.
Microbiome Analyses
DNA was isolated from urine cell pellets, and 16S rDNA amplified and sequenced (see Supplementary Methods).
ELISAs and AMP Analyses
AMP protein levels were analyzed by ELISA (see Supplementary Methods). Antimicrobial activity was analyzed using established radial diffusion assays[8, 23], against UTI isolates of Escherichia coli and Enterococcus faecalis, two common uropathogens in our patient population. Zones of bacterial growth inhibition were quantified using ImageJ Software.
Statistical Analyses
All quantitative data are described as mean±standard error of the mean (SEM). Comparisons were performed by Mann-Whitney test. P values <0.05 were considered statistically significant. Microbial diversity indices were computed as described in Supplementary Methods.
RESULTS
Clinical Assessments and Patient Demographics
Urine samples from 30 BICU patients aged 20-80 years (median age: 45 years) were evaluated. Control urine samples were obtained from 8 non-burned volunteers aged 27-63 years (median age: 41 years) (Supplementary Digital Content-Table 1). 10 patients (33%) developed a culture-positive UTI. Of these 10 patients, 60% developed positive blood cultures during their hospitalization. Only 4 patients without a UTI (20%) developed positive blood cultures (Supplementary Digital Content-Table 1). The mortality rate was 20% for all patients in the study group; and those who succumbed to their injury were >66 years old and exhibited a burn total body surface area (TBSA) of >43%.
Burn Injury Augments Microbial Diversity and Correlates with Clinical Outcomes
To identify whether burn injury shifts the urinary bacterial microbiome to a more “pathogenic” profile, we assessed whether urinary bacterial diversity (e.g., the number of unique bacterial species and/or how diverse the population is relative to each cohort) was significantly different between controls and burn patients. Using a non-metric multidimensional scaling (NMDS) based on Bray-Curtis dissimilarity plot (Figure 1A), we observed that control patients clustered together, whereas the burn patients clustered away from the controls, indicating that the urinary microbiome from burn patients was significantly different from controls (PERMANOVA test p<0.001, either with or without age, gender, and ethnic group as confounding factors). To assess the relationship between specific bacteria and patient cohorts, we identified the 8 most abundant bacterial genera (Figure 1B). In control patients, Lactobacillus and Streptococcus genera were highly abundant, with a relative abundance of 72.28% and 8.59% of total 16S rRNA reads, respectively. Enterococcus (42.36%), Aeribacillus (18.03%), Nesterenkonia (10.06%), Propionibacterium (3.99%) and Halomonas (3.64%) were the most abundant genera in urine from burn patients at either 1 or 40 hours post-admission. Table 1A shows a summary of the genera determined to be statistically more or less abundant in controls vs. burn patients.
Figure 1. Burn injury significantly alters the diversity of the urinary microbiome.
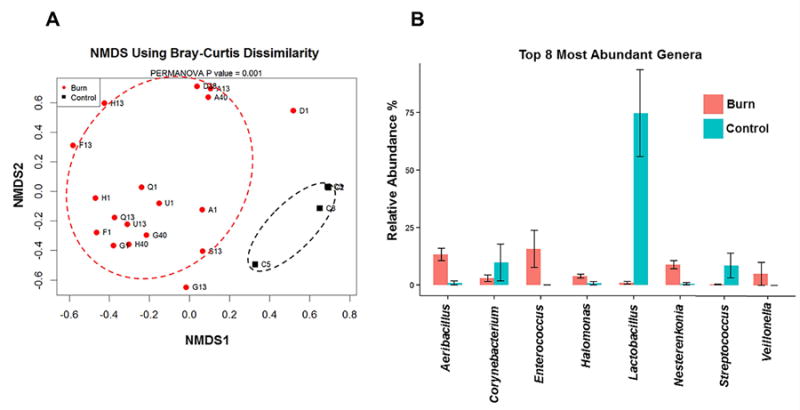
A) NMDS using Bray-Curtis Dissimilarity analysis comparing the bacterial diversity at the Genus level within the urinary microbiome of controls (black squares) and burn patients (red circles). The letter represents the subject identification, while the number represents the time (hours) post-admission for each subject. The patients within the black dotted circle represent control patients, which cluster away from burn patients within the red dotted circle at all time-points. B) The most abundant bacterial genera in controls and burn patients are indicated in horizontal bar graphs. Controls are indicated by blue bars. Burn subjects are indicated by red bars. All genera shown are significantly different between the 2 cohorts (p<0.05).
Table 1.
A. Significant differences between genera within the urinary bacterial community structure of control urine or urine from burn subjects (1hr or 40hr post-burn).
| Control vs. 1hr Post-Burn | Genera | Corrected P value | % in Control | % in 1hr Post-Burn |
|---|---|---|---|---|
| Lactobacillus | 6.94E-10 | 74.72% | 1.62% | |
| Propionibacterium | 3.95E-05 | 0.15% | 3.99% | |
| Caldalkalibacillus | 0.000172604 | 0.13% | 2.61% | |
| Nesterenkonia | 0.000177684 | 0.55% | 10.06% | |
| Aeribacillus | 0.000424733 | 0.90% | 18.03% | |
| Staphylococcus | 0.004739377 | 0.35% | 4.34% | |
| Halomonas | 0.012388841 | 0.74% | 5.09% | |
| Flavobacterium | 0.012388841 | 0 | 1.23% | |
| Enterococcus | 0.014662622 | 0 | 1.73% | |
| Control vs. 40hr Post-burn | Genera | Corrected P value | % in Control | % in 40hr Post-burn |
| Lactobacillus | 7.35E-163 | 74.96% | 1.52% | |
| Streptococcus | 7.94E-20 | 8.59% | 0.81% | |
| Enterococcus | 5.66E-18 | 0 | 42.36% | |
| Halomonas | 4.73E-15 | 0.74% | 3.64% | |
| 1hr vs. 40hr Post-burn | Genera | Corrected P value | % in 1hr Post-burn | % in 40hr Post-burn |
| Anaerococcus | 0.010707066 | 0.29% | 1.12% | |
| B. Significant correlations between genera within the urinary bacterial community structure of UTI positive (UTI+) and UTI negative (UTI-) burn subjects at 1 hour post-burn, after accounting for age and gender. | ||||
| UTI+ vs. UTI- in 1hr Post-burn | Genera | Corrected P value | % in UTI+ | % in UTI- |
| Corynebacterium | 1.65E-77 | 4.83% | 0.87% | |
| Veillonella | 1.72E-15 | 0 | 12.82% | |
| Aeribacillus | 4.85E-12 | 18.72% | 14.26% | |
| Nesterenkonia | 7.00E-11 | 11.89% | 8.76% | |
| Bifidobacterium | 2.12E-05 | 1.20% | 0 | |
| Diaphorobacter | 0.002093995 | 0 | 2.20% | |
| Gardnerella | 0.004108313 | 0.02% | 0.32% | |
| Atopobium | 0.031680484 | 0 | 0.21% | |
| C. Significant correlations between genera within the urinary bacterial community structure of Sepsis positive (Sepsis+) and Sepsis negative (Sepsis-) burn subjects at 1 hour post-burn, after accounting for age and gender. | ||||
| Sepsis+ vs. Sepsis- in 1hr Post-burn | Genera | Corrected P value | % in Sepsis+ | % in Sepsis- |
| Aeribacillus | 1.62E-29 | 22.17% | 13.53% | |
| Nesterenkonia | 2.23E-16 | 14.42% | 8.20% | |
| Lactobacillus | 5.54E-14 | 0.99% | 1.80% | |
| Corynebacterium | 1.37E-12 | 0.92% | 2.83% | |
| Halomonas | 8.61E-12 | 6.26% | 3.92% | |
| Gardnerella | 1.36E-10 | 0 | 0.29% | |
We then determined that a larger TBSA positively correlated with greater urinary microbial diversity 1 hour post-admission (Supplementary Digital Content-Table 2), and subsequently compared this data with several clinical parameters. We further identified a significant positive correlation between greater urinary microbial diversity and the development of a UTI 1 hour post-admission, as well as sepsis development at 1 hour post-admission, with greater significance occurring over time (Supplementary Digital Content-Table 2). Thus, the larger the urinary microbial diversity, the more likely the burn patient was to develop a UTI and/or sepsis post-admission, regardless of age or gender. Tables 1B and 1C show a summary of the genera determined to be statistically more or less abundant in UTI-positive/UTI-negative and sepsis-positive/sepsis-negative burn subjects 1 hour post-burn, respectively.
Burn Injury Decreases Urinary AMPs
To identify whether burn injury promotes alterations in urinary AMP production, we quantified several urinary AMPs by ELISA, which were normalized to total urinary protein (Figure 2). Psoriasin levels were significantly decreased at 1 and 40 hours post-admission in burn patients compared to controls (p<0.001 and p<0.0001, respectively), and exhibited the most robust changes as compared to the other AMPs (Figure 2A). Lactoferrin levels were not significantly different (Figure 2B). In burn patients, hBD1 levels were reduced by over 50% both 1 and 40 hours post-admission (p<0.05 and p<0.001, respectively; Figure 2C), while hBD2 levels were reduced by ~70% at 40 hours post-admission (p<0.05; Figure 2D). HMGB1 levels were evaluated as a urinary biomarker for UTI susceptibility in burn patients, as blood measurements serve as a marker for tissue necrosis and late sepsis[24, 25] and was recently found to correlate with cystitis-associated bladder pain[26], but no significant differences were observed (Figure 2E). No statistically significant differences were observed for hepcidin concentrations in controls vs. burn patients (Figure 2F). We further determined that the urinary microbial diversity at the genus level (inverse Simpson test:Shannon, p=0.032), significantly correlated inversely with urinary hepcidin levels at 40 hours post-admission, indicating that higher hepcidin values correlate with a more diverse urinary microbiome. Other AMPs (e.g., psoriasin, hBD1, hBD2, lactoferrin) did not correlate with urinary microbial diversity.
Figure 2. Urinary protein levels of AMPs after burn injury.
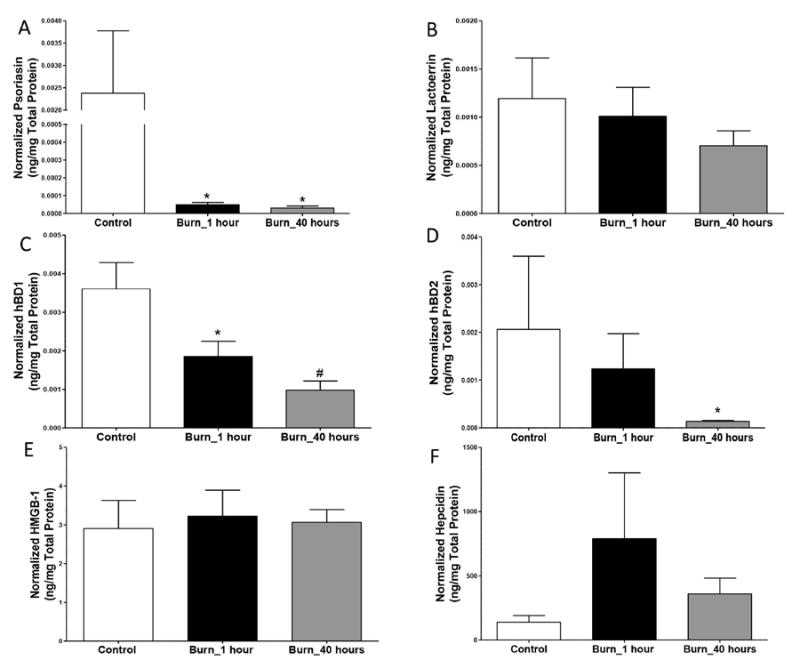
A) Psoriasin levels are significantly decreased after burn injury at 1 and 40 hours post-admission. B) Lactoferrin levels are not significantly different after burn injury. C) hBD1 levels are significantly decreased after burn injury at hour 1 and 40 hours post-admission. D) hBD2 levels are significantly decreased at 40 hours post-admission. E) HMGB-1 levels are not statistically different at either time-point. F) Hepcidin levels are not statistically different at either time-point. * = p<0.05 and # = p<0.001 with Mann Whitney test, n = 8-24.
Burn Injury Impairs Urinary Bactericidal Activity
We next assessed the capacity of urinary AMPs to limit the growth of typical uropathogens by radial diffusion assay (RDA)[8, 23]. HPLC was used to purify peptides from urine specimens based upon hydrophobicity, as peptide hydrophobicity can dictate bactericidal capacity of AMPs[27]. Fractions from each randomly selected urine specimen (6 control specimens and 12 specimens from 6 burn patients: 1 hour and 40 hours post-admission) were normalized to the same peptide concentration and then tested in RDAs to assess their capacity to inhibit growth of two of the most prevalent bacteria cultured from urine of our institution’s burn patients, E. coli and E. faecalis[28]. Select urine fractions demonstrated distinct AMP activity against E. coli when comparing controls vs. burn patients at 1 or 40 hours post-admission (Figure 3A and 3B). For example, AMP activity in fractions 10-11 was elevated in urine from select burn patients 1 hour post-admission (median:~18mm2) vs. controls (median:0-0.16 mm2), which may represent secreted AMPs in response to urothelial barrier defects induced by the systemic response to burn injury[13]. In contrast, minimal change in AMP activity in fractions 17-20 was observed at 1 hour when comparing burn patients (median: 0.08-0.35 mm2) vs. controls (median 0.16-0.89 mm2). When comparing AMP activity between controls and burn patient samples collected at 1 hour or 40 hours hour post-admission, we observed a robust increase in AMP activity in fractions 2, 13, and 17-20 (Figure 3B). The majority of fractions from burn patients at either 1 or 40 hours post-admission exhibited greater AMP activity compared to controls. Fractions from burn patients also exhibited a more diverse range of AMP activity overall. Differences in AMP activity were also seen between fractions taken at hour 1 compared to hour 40, as a robust decrease in AMP activity was observed for fraction 10 (median: 18.33 vs. 0.89 mm2), suggesting progressive changes in urinary AMP activity over time.
Figure 3. Bacterial growth inhibition of E. coli with urine fractions from control patients compared to the same fractions of burned patients at 1 hour and 40 hours post-admission.
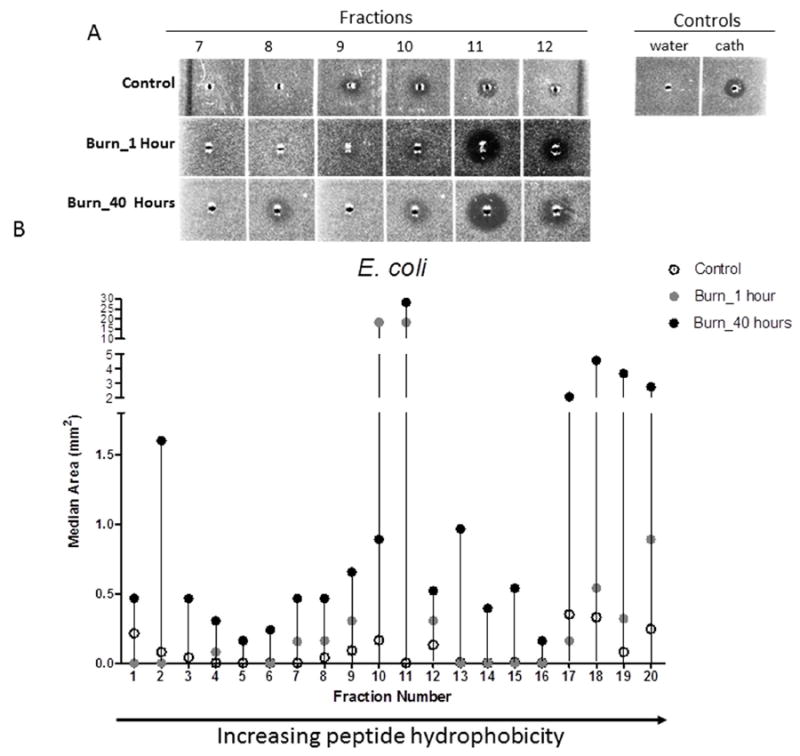
A) Areas of clearing in E. coli bacterial lawns in fractions 7-12 from urine samples of a representative control and burn patient at 1 and 40 hours post-admission. B) Antimicrobial activity against E. coli demonstrates notable changes in multiple fractions, primarily in fractions 2, 10-11, and 17-20. Arrow to the right indicates increasing peptide hydrophobicity. The median is shown for all groups: open circles=controls; grey circles=burn patients at 1 hour post-admission; black circles=burn patients at 40 hours post-admission. Fractions were assessed in duplicate with wells containing 1μl of sterile water and 1 μl LL-37 (100 μM; GeneScript) as negative and positive controls, respectively.
When incubated with E. faecalis, fractions from burn patients exhibited a different profile than E. coli, with some burn fractions exhibiting lower AMP activity compared to those from controls. For example, the AMP activity of fraction 9 (Figure 4A) was much more robust in controls (median: 17.07 mm2) than burn patients at 1 or 40 hours (median: 0.33-0.62 mm2). In addition, fractions 15-17 and 20 from controls (median: 2.6-5.26 mm2) exhibited greater AMP activity than burn patients (median: 0.62-5.63 mm2). In fractions 1-7, there was a greater increase in the bactericidal capacity of urine from burn patients, as compared to later fractions where the controls exhibited greater AMP activity (fractions 9-10, 15-17, 20) (Figure 4B). Collectively, the shift in AMP activity seen in early fractions compared to that observed in later fractions suggests that AMP hydrophobicity may change over time after burn injury, and that susceptibility of urinary pathogens may be mediated by altered AMP activity.
Figure 4. Bacterial growth inhibition of E. faecalis with urine fractions from control patients compared to the same fractions of burned patients at 1 hour and 40 hours post-admission.
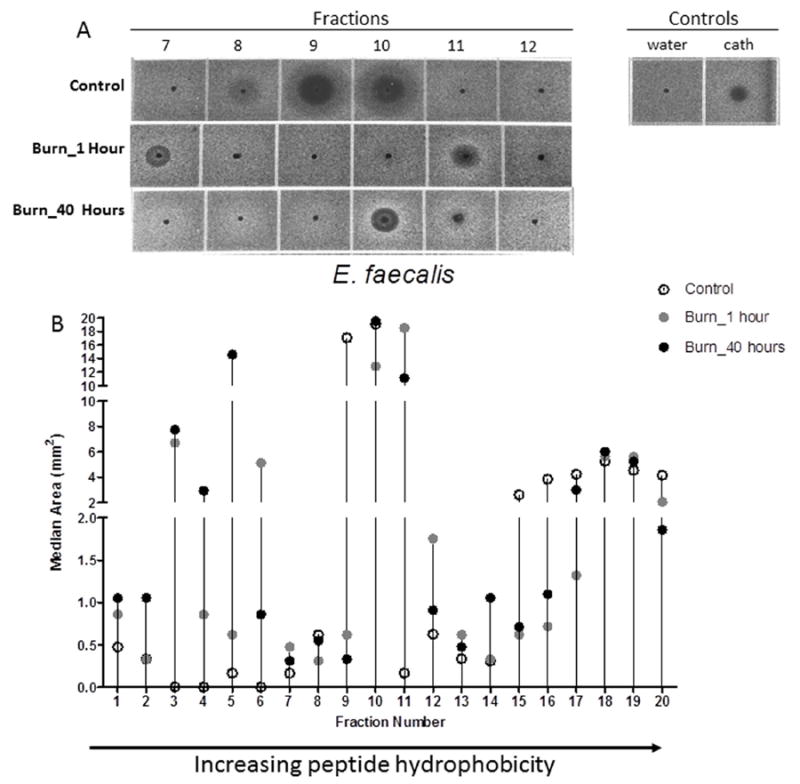
A) Zones of bacterial growth inhibition of E. faecalis present in fractions 7-12 withcontrol urine and fractions from burn patients 1 and 40 hours post-admission. B) Antimicrobial activity against E. faecalis is altered following burn injury in the majority of urine fractions and antimicrobial activity against E. faecalis demonstrates notable changes in multiple fractions. Arrow to the right indicates increasing peptide hydrophobicity. The median is shown for all groups: open circles: controls; grey circles: burn at 1 hour post-admission; black circles: burn at 40 hours post-admission. Fractions were assessed in duplicate with wells containing 1μl of sterile water and 1 μl LL-37 (100 μM; GeneScript) as negative and positive controls, respectively.
Discussion
The importance of AMPs and the cutaneous microbiome has been consistently demonstrated in acute trauma and chronic wounds[13, 29, 30]. However, no studies to date have addressed the impact of cutaneous burn injury on AMP responses in the urothelium, although UTI and subsequent urosepsis is frequently observed in burn patients[10, 13, 29, 31]. The present study is the first to identify potential mechanisms by which burn patients exhibit a greater UTI susceptibility after injury, in part, via shifts in the urinary microbiome and AMPs. The parallel reduction in urinary AMPs and shift in the microbiome positively correlated with the propensity to develop a UTI and sepsis in burn patients.
We conclude that burn injury potentiates a systemic effect on epithelial AMP production, as supported by our previous studies in mice[13], which may facilitate a reduction in “protective” urinary microorganisms and overgrowth of uropathogens after burn injury. We recently found that the cutaneous microbiome of autologous donor skin of burn patients significantly differs from non-burned controls (Plichta et al., submitted). These data indicate that the tissue-specific mechanisms for microbiome and AMP regulation may be altered after burn injury, but also that a burn injury elicits a systemic effect on epithelial innate immune and microbial responses.
Our assessment of the urinary bacterial microbiome in burn patients is the first to demonstrate that burn injury promotes a rapid change in the urinary bacterial microbiome, which likely influences and is regulated by changes in endogenous urinary AMPs. We identified several statistical correlations between the diversity of the microbiome and the development of both UTIs and sepsis, which may be used to predict morbidity among burn patients. Extensive studies are necessary to further determine whether the identified uropathogens/microbial shifts are related to the type of injury, patient response/comorbidities, and/or the magnitude of the injury, as well as the specific mechanisms that regulate their responses.
In our study population, we observed that the microbiome in the controls was composed primarily of Lactobacillus, as compared to burn urine, which was comprised of multiple other genera. These results parallel our[8] and other urinary studies, which demonstrate a predominance of Lactobacillus in the “healthy” or control (i.e. non-diseased) cohort[32, 33]. We speculate that the abundance of these other genera (e.g. Enterococcus, Staphylococcus), as well as the rapid change in the urinary microbial diversity after burn injury, is the result of increased gut epithelial barrier permeability, which requires further scrutiny. Recently, a meta-analysis showed that following UTI, patients given a vaginal suppository of Lactobacillus probiotic were significantly less likely to develop a recurrent infection[34], illustrating the possible role of a “protective” microbiome in preventing UTIs[35, 36]. Our colleagues previously determined that urinary bacteria detected by 16S rRNA sequencing is not due to specimen contamination or due to the method of collection (catheterized vs. clean-catch)[5]. However, it must be determined whether the observed bacterial microbiome is a part of intracellular bacterial communities or biofilms [37, 38] within the urothelium, or whether the bacteria are planktonic in nature.
Intriguingly, we observed that several unique bacterial taxa were enriched in burn subjects overall, and more so in UTI-positive and sepsis-positive burn subjects (Table 1): Aeribacillus, Nesterenkonia, Halomonas, and Caldalkalibacillus. These taxa are related as they are characterized as halophilic or thermophilic (e.g. extremophiles), and tend to be isolated from water and soil sources[39-41]. We surmise that enrichment of these taxa may be derived, in part, from cutaneous absorption following exposure to hospital steam sources during wound debridement procedures, as we previously determined that skin barrier permeability increases after burn injury[13, 22]. Alternatively, changes in urinary osmolarity/salt concentrations, caused by a disturbance of the local ionic environment (e.g. renal insufficiency; urea production; hormone secretion) following burn injury[42, 43], may enable their proliferation by providing key metabolites that are normally limited in the urine. A more detailed assessment of these potential mechanisms are necessary to better elucidate the positive correlation between these taxa and the development of UTI and sepsis in our burn population.
AMPs participate in the regulation of epithelial microbiota and maintenance of epithelial barrier integrity by directly killing microbes and stimulating innate and adaptive immune responses[8, 13, 23, 29, 30]. Although the protein levels of urinary AMPs have been assessed in a limited number of urinary pathologies[8, 44, 45], no studies to date have assessed changes in urinary AMP levels or activity as a mechanism for the development of post-burn UTIs. In our studies, we observed a significant reduction in specific AMPs, rather than global suppression. Psoriasin exhibits its antimicrobial activity against E. coli by zinc sequestration[46]. The significant psoriasin decrease following burn injury could indicate an attenuated activity against E. coli, possibly due to changes in urinary ion composition, as fluid balance and ionic homeostasis are critical after burn injury[45]. Reportedly, hBD1 may encourage bacterial tolerance in the urinary tract and limit growth of pathogenic microbes[47, 48]. We previously demonstrated that hBD1 was critical in protecting women with pelvic organ prolapse from UTI[8], suggesting an essential role for hBD1 in protection from uropathogens. In contrast, hBD2 is known to be upregulated in pyelonephritis[48], while our observed reduction suggests impairment of this AMP induction. Alternatively, stress mediator (e.g. acetylcholine or glucocorticoid) production following traumatic burn injury may suppress urothelial AMP production, as this mechanism has been demonstrated in models of epithelial injury and infection[23, 49], and requires further exploration.
Our studies also determined that urinary AMP activity from burn patients exhibited robust changes in AMP hydrophobicity, as indicated by greater AMP activity in later fractions against E. coli, and greater AMP activity in earlier fractions against E. faecalis. These changes in peptide hydrophobicity and AMP activity dictate how well urinary AMPs exert their activity[12, 14] against pathogenic microbes and influence local inflammation in the urinary tract[8, 50]. Furthermore, we observed that AMP activity from burn patients was more effective against E. coli vs. E. faecalis. Both of our UTI-positive burn patients (2 of 6) developed either an E. faecalis or Pseudomonas aeruginosa UTI. These clinical data suggest that reduced urinary AMP activity may directly increase one’s susceptibility to specific uropathogens, and that testing of urinary AMP activity early after admission may predict UTI. Of note, none of our burn patients received systemic antibiotics during the urine collection period; thus, none of the urinary AMP activity could be attributed to excreted systemic antibiotics.
In summary, our data demonstrate significant alterations in host urinary AMPs and the urinary bacterial microbiome soon after burn injury. These changes likely have direct or indirect implications for UTI and sepsis development in burn patients. Further evaluation of the mechanisms by which burn injury modulates the urinary AMP and bacterial microenvironment will be critical to our understanding of the host-pathogen interactions after traumatic injury.
Supplementary Material
Acknowledgments
Financial Support: Research reported in this publication was supported by the National Institutes of Health grant NIH T32 GM008750 (RLG) and the Dr. Ralph and Marian C. Falk Medical Research Trust (KAR and RLG).
Footnotes
Conflict of Interest: The authors have declared that no conflict of interest exists.
References
- 1.Sharma BR. Infection in Patients with Severe Burns: Causes and Prevention Thereof. 2007;21:745–759. doi: 10.1016/j.idc.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Sihler KC, Raghavendran K, Westerman M, et al. Hepcidin in trauma: linking injury, inflammation, and anemia. The Journal of trauma. 2010;69:831–837. doi: 10.1097/TA.0b013e3181f066d5. [DOI] [PubMed] [Google Scholar]
- 3.Christ-libertin C, Black S, Latacki T, et al. Evidence-Based Prevent Catheter-Associated Urinary Tract Infections Guidelines and Burn-Injured Patients : A Pilot Study. 2014:1–6. doi: 10.1097/BCR.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 4.Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. Journal of clinical microbiology. 2014;52(3):871–876. doi: 10.1128/JCM.02876-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfe AJ, Toh E, Shibata N, et al. Evidence of uncultivated bacteria in the adult female bladder. Journal of clinical microbiology. 2012;50(4):1376–1383. doi: 10.1128/JCM.05852-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chromek M, Slamová Z, Bergman P, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nature medicine. 2006;12(6):636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen KL, Dynesen P, Larsen P, et al. Role of urinary cathelicidin LL-37 and human β-defensin 1 in uncomplicated escherichia coli urinary tract infections. Infection and Immunity. 2014;82(4):1572–1578. doi: 10.1128/IAI.01393-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nienhouse V, Gao X, Dong Q, et al. Interplay between Bladder Microbiota and Urinary Antimicrobial Peptides : Mechanisms for Human Urinary Tract Infection Risk and Symptom Severity. 2014:1–26. doi: 10.1371/journal.pone.0114185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer JD, Schwaderer AL, Dirosario JD, et al. Ribonuclease 7 is a potent antimicrobial peptide within the human urinary tract. Kidney international. 2011;80:174–180. doi: 10.1038/ki.2011.109. [DOI] [PubMed] [Google Scholar]
- 10.Zasloff M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. Journal of the American Society of Nephrology : JASN. 2007;18:2810–2816. doi: 10.1681/ASN.2007050611. [DOI] [PubMed] [Google Scholar]
- 11.Nelson DE, Van Der Pol B, Dong Q, et al. Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PloS one. 2010;5(11):e14116. doi: 10.1371/journal.pone.0014116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Guarnieri MT, Vasil AI, et al. Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrobial agents and chemotherapy. 2007;51:1398–1406. doi: 10.1128/AAC.00925-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plichta JK, Droho S, Curtis BJ, et al. Local Burn Injury Impairs Epithelial Permeability and Antimicrobial Peptide Barrier Function in Distal Unburned Skin. Critical Care Medicine. 2014;42(6):e420–431. doi: 10.1097/CCM.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tossi A, Sandri L, Giangaspero A. Amphipathic, α-helical antimicrobial peptides. Peptide Science. 2000;55:4–30. doi: 10.1002/1097-0282(2000)55:1<4::AID-BIP30>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 15.Spencer JD, Schwaderer A, McHugh K, et al. The demographics and costs of inpatient vesicoureteral reflux management in the USA. Pediatric nephrology. 2011;26(11):1995–2001. doi: 10.1007/s00467-011-1900-3. [DOI] [PubMed] [Google Scholar]
- 16.Becknell B, Hains DS, Schwaderer AL, et al. Impact of urinary tract infection on inpatient healthcare for congenital obstructive uropathy. Journal of pediatric urology. 2012;8(5):470–476. doi: 10.1016/j.jpurol.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Fouts DE, Pieper R, Szpakowski S, et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. Journal of translational medicine. 2012;10:174. doi: 10.1186/1479-5876-10-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valore EV, Park CH, Quayle AJ, et al. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. The Journal of clinical investigation. 1998;101(8):1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13(8):975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 20.Park CH, Valore EV, Waring AJ, et al. Hepcidin, a Urinary Antimicrobial Peptide Synthesized in the Liver. Journal of Biological Chemistry. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 21.Giorgetti G, Brandimarte G, Fabiocchi F, et al. Interactions between Innate Immunity, Microbiota, and Probiotics. J Immunol Res. 2015;2015:501361. doi: 10.1155/2015/501361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plichta JK, Holmes CJ, Gamelli RL, et al. Local Burn Injury Promotes Defects in the Epidermal Lipid and Antimicrobial Peptide Barriers in Human Autograft Skin and Burn Margin: Implications for Burn Wound Healing and Graft Survival. J Burn Care Res. 2016 doi: 10.1097/BCR.0000000000000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radek KA, Elias PM, Taupenot L, et al. Neuroendocrine nicotinic receptor activation increases susceptibility to bacterial infections by suppressing antimicrobial peptide production. Cell Host and Microbe. 2010;7:277–289. doi: 10.1016/j.chom.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang LT, Yao YM, Lu JQ, et al. Recombinant bactericidal/permeability-increasing protein inhibits endotoxin-induced high-mobility group box 1 protein gene expression in sepsis. Shock. 2008;29(2):278–284. doi: 10.1097/shk.0b013e31811ff581. [DOI] [PubMed] [Google Scholar]
- 25.Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, et al. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33(3):564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka J, Yamaguchi K, Ishikura H, et al. Bladder pain relief by HMGB1 neutralization and soluble thrombomodulin in mice with cyclophosphamide-induced cystitis. Neuropharmacology. 2014;79:112–118. doi: 10.1016/j.neuropharm.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Guarnieri MT, Vasil AI, et al. Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrob Agents Chemother. 2007;51(4):1398–1406. doi: 10.1128/AAC.00925-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehrer RI, Rosenman M, Harwig SS, et al. Ultrasensitive assays for endogenous antimicrobial polypeptides. Journal of immunological methods. 1991;137(2):167–173. doi: 10.1016/0022-1759(91)90021-7. [DOI] [PubMed] [Google Scholar]
- 29.Holmes CJ, Plichta JK, Gamelli RL, et al. Dynamic Role of Host Stress Responses in Modulating the Cutaneous Microbiome : Implications for Wound Healing and Infection. 2015;4(1):24–37. doi: 10.1089/wound.2014.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radek Ka. Antimicrobial anxiety: the impact of stress on antimicrobial immunity. Journal of leukocyte biology. 2010;88(2):263–277. doi: 10.1189/jlb.1109740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siddiqui H, Lagesen K, Nederbragt AJ, et al. Alterations of microbiota in urine from women with interstitial cystitis. 2012;12:205–205. doi: 10.1186/1471-2180-12-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio. 2014;5(4):e01283–01214. doi: 10.1128/mBio.01283-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khasriya R, Sathiananthamoorthy S, Ismail S, et al. Spectrum of bacterial colonization associated with urothelial cells from patients with chronic lower urinary tract symptoms. Journal of clinical microbiology. 2013;51(7):2054–2062. doi: 10.1128/JCM.03314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stapleton AE. Urinary tract infection pathogenesis. Host factors. Infectious Disease Clinics of North America. 2014;28(1):150–159. doi: 10.1016/j.idc.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Pearce MM, Zilliox MJ, Rosenfeld AB, et al. The female urinary microbiome in urgency urinary incontinence. Am J Obstet Gynecol. 2015;213(3):347 e341–311. doi: 10.1016/j.ajog.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brubaker L, Nager CW, Richter HE, et al. Urinary bacteria in adult women with urgency urinary incontinence. Int Urogynecol J. 2014;25(9):1179–1184. doi: 10.1007/s00192-013-2325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Justice SS, Hung C, Theriot JA, et al. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A. 2004;101(5):1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson GG, Palermo JJ, Schilling JD, et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301(5629):105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 39.Minana-Galbis D, Pinzon DL, Loren JG, et al. Reclassification of Geobacillus pallidus (Scholz et al 1988) Banat et al. 2004 as Aeribacillus pallidus gen. nov., comb. Nov. Int J Syst Evol Microbiol. 2010;60(Pt 7):1600–1604. doi: 10.1099/ijs.0.003699-0. [DOI] [PubMed] [Google Scholar]
- 40.Zhao W, Zhang CL, Romanek CS, et al. Description of Caldalkalibacillus uzonensis sp. nov. and emended description of the genus Caldalkalibacillus. Int J Syst Evol Microbiol. 2008;58(Pt 5):1106–1108. doi: 10.1099/ijs.0.65363-0. [DOI] [PubMed] [Google Scholar]
- 41.Li WJ, Zhang YQ, Schumann P, et al. Nesterenkonia halophila sp. nov., a moderately halophilic, alkalitolerant actinobacterium isolated from a saline soil. Int J Syst Evol Microbiol. 2008;58(Pt 6):1359–1363. doi: 10.1099/ijs.0.64226-0. [DOI] [PubMed] [Google Scholar]
- 42.Cioffi WG, Jr, Vaughan GM, Heironimus JD, et al. Dissociation of blood volume and flow in regulation of salt and water balance in burn patients. Ann Surg. 1991;214(3):213–218. doi: 10.1097/00000658-199109000-00004. discussion 218-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balogh D, Benzer A, Hackl JM, et al. Sodium balance and osmolarity in burn patients. Intensive Care Med. 1986;12(2):100–103. doi: 10.1007/BF00254520. [DOI] [PubMed] [Google Scholar]
- 44.Oottamasathien S, Jia W, McCoard L, et al. A murine model of inflammatory bladder disease: cathelicidin peptide induced bladder inflammation and treatment with sulfated polysaccharides. The Journal of urology. 2011;186(4 Suppl):1684–1692. doi: 10.1016/j.juro.2011.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Celis JE, Rasmussen HH, Vorum H, et al. Bladder squamous cell carcinomas express psoriasin and externalize it to the urine. The Journal of urology. 1996;155(6):2105–2112. [PubMed] [Google Scholar]
- 46.Glaser R, Harder J, Lange H, et al. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nature immunology. 2005;6(1):57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- 47.Hiratsuka T, Nakazato M, Ihi T, et al. Structural analysis of human beta-defensin-1 and its significance in urinary tract infection. Nephron. 2000;85(1):34–40. doi: 10.1159/000045627. [DOI] [PubMed] [Google Scholar]
- 48.Lehmann J, Retz M, Harder J, et al. Expression of human beta-defensins 1 and 2 in kidneys with chronic bacterial infection. BMC infectious diseases. 2002;2:20. doi: 10.1186/1471-2334-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aberg KM, Radek KA, Choi EH, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. The Journal of clinical investigation. 2007;117(11):3339–3349. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radek K, Gallo R. Antimicrobial peptides: natural effectors of the innate immune system. Seminars in immunopathology. 2007;29(1):27–43. doi: 10.1007/s00281-007-0064-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


