Abstract
Terminal sterilization of hydrogel-based biomaterials is crucial for their clinically relevant applications. The authors synthesized nonfouling zwitterionic hydrogels consisting of carboxybetaine (CB) acrylamide monomer and a carboxybetaine dimethacrylate crosslinker. The mechanical and biological stability of nonfouling hydrogels were investigated using three main terminal sterilization techniques, i.e., steam autoclave, ethylene oxide gas, and gamma irradiation. It was found that CB hydrogels are very stable at high temperature and pressure and in oxidative gas environments without changing their stress, modulus, and nonfouling properties. Gamma irradiation of CB hydrogels in dry state showed high mechanical and nonfouling stability by avoiding the adverse effect of the free radicals resulted from water inside the hydrogel network. The CB hydrogels can be dehydrated and hydrated back and forward reversibly in several cycles without any loss in mechanical properties, which is desirable for hydrogel storage, handling, and sterilization. The CB hydrogel tubes are easily prepared using a simple procedure, and they are uniformly transparent and tough after swelling. Furthermore, the good mechanical properties of the CB hydrogel tubes and their resistance to red blood cells indicate great potential of this nonfouling material for medical applications.
I. INTRODUCTION
Medical device sterilization is required in clinical applications to control hospital-acquired infections. These devices are sterilized before entering the market and/or before clinical usage to effectively eliminate microorganisms by a harsh sterilization process. Different terminal sterilization methods are chosen, depending on the purpose of sterilization and the material to be sterilized.1 While there are a number of sterilization techniques available,2–4 gamma irradiation, ethylene oxide gas (EtO), and steam autoclave5 are three main sterilization techniques.
In the past few decades, there has been a dramatic increase in the development of polymeric biomaterials.6 Among these biomaterials, polymeric hydrogels have been used extensively7,8 for soft tissue engineering applications due to their hydrophilicity, biocompatibility, and tunable mechanical properties similar to those of the tissue components of the body. These biomaterials offering the potential for regenerating tissue and organs of the human body must undergo sterilization before implantation.9 Different sterilization methods10–15 have been carried out on different polymeric biomaterials.16–19 However, many sterilization procedures have shown significant effects on the performance of polymeric materials.20,21 Sterilization of hydrogels is a much more challenging task because of the properties of their soft nature, 3D network architecture, and water content. Commonly, hydrogel samples have been lyophilized to remove all water before sterilization and reswollen upon applications. However, lyophilization has been shown to damage hydrogel structure, impacting the final mechanical properties and the native properties of the hydrogel.22,23
Zwitterionic hydrogels are demonstrated to have ultra-low-fouling properties, resulting from the formation of a highly hydrated layer around the opposing charges24,25 for medical applications.26,27 Previously, we have demonstrated the stability of poly(carboxybetaine methacrylate) hydrogels to resist degradation in harsh oxidative environments as well as in acidic and basic conditions over a long period of time.28 However, studies on the stability of hydrogels under different sterilization conditions are limited,29–32 especially on zwitterionic hydrogels in a wet state. Due to their zwitterionic nature, the stability of hydrogels will be examined against changes in mechanical and nonfouling properties. In this work, a zwitterionic carboxybetaine (CB) acrylamide monomer and a carboxybetaine crosslinker (CBX) were used to prepare zwitterionic hydrogels. Three main terminal sterilization techniques, i.e., steam autoclave, EtO, and gamma irradiation, were selected to test the stability in the mechanical and nonfouling properties of hydrogels.
II. EXPERIMENTAL SECTION
A. Materials
N,N,N′,N′-tetramethylethylenediamine (TEMED) and ammonium persulfate (APS) were purchased from Sigma Aldrich (St. Louis, MO). o-Phenylenediamine dihydrochloride (OPD) was purchased from Pierce (Rockford, IL). Hydrogen peroxide was purchased from J. T. Baker (Phillipsburg, NJ). Human plasma fibrinogen was purchased from Sigma–Aldrich (Milwaukee, WI). Horeseradish peroxidase (HRP)-conjugated antifibrinogen was purchased from Alpha Diagnostics. Phosphate buffered saline (PBS) solution (0.01 M, pH 7.4) was prepared from PBS powder purchased from Sigma-Aldrich. All water used had been purified to 18.2 mΩ cm on a Millipore Simplicity water purification system.
The zwitterionic monomer, 1-carboxy-N,N-dimethyl-N-(3-acrylamidopropyl) ethanaminium inner salt (CB) was synthesized as previously reported.33 The zwitterionic crosslinker, 1-carboxy-N-methyl-N,N-di(2-methacryloyloxy-ethyl) methanaminium inner salt (CBX), was synthesized as previously reported.34
B. Preparation of hydrogels
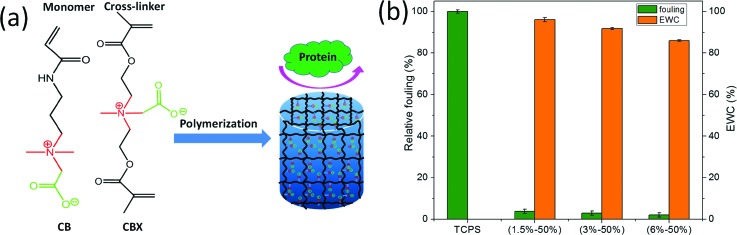
Hydrogels were fabricated by a redox polymerization of precursor solutions. The structures of the monomer and the crosslinker used are shown in Fig. 1. Two molds of different geometries were used: (1) a plastic syringe mold with typical dimensions of a 4 mm outer diameter, 3 mm inner diameter, and 10 mm depth and (2) a flat glass mold with a typical 0.8 mm-thick spacer frame. An appropriate amount of CB monomer, crosslinker CBX, and TEMED accelerator (0.3 wt. %, relative to total monomer mass) were dissolved in water and were vigorously mixed at room temperature to form a homogenous precursor solution. The initiator APS was dissolved in water to make a 10 wt. % solution. The hydrogel solution was prepared by adding a certain amount of initiator solution into the precursor solution and was quickly mixed by a vortex. The concentration of APS initiator was fixed at 1.6 wt. % relative to the total monomer mass. After being homogenized, the hydrogel solution was injected into the mold immediately. Different formulations were made with a constant monomer weight while increasing crosslinker amount and decreasing water content. Polymerization was carried out for 24 h at room temperature unless otherwise stated. All the hydrogels were equilibrated in water or in PBS for at least three days prior to tests. Hydrogel samples were named as x%-y%, where x% is the crosslinker concentration relative to total mass of monomer and y% is the monomer concentration relative to the total mass of water.
Fig. 1.
(a) Illustration of the formation of a zwitterionic hydrogel from the CB monomer and CBX crosslinker and (b) the equilibrium water content of CB hydrogels and their protein adsorption normalized to that of TCPS. Data are presented as mean ± standard error (n = 3).
C. Characterization of hydrogel
1. Equilibrium water content
Freshly prepared hydrogel disks (5-mm in diameter) were soaked in water at room temperature for at least 3 days after gelation. The swelling medium was refreshed every 12 h. The swollen hydrogel disks were withdrawn on a filter paper after swelling in water at equilibrium. After removal of the excess superficial water, the weight of the samples in the swollen state (mwet) was measured. Then, the hydrogel disks were dehydrated in a vacuum oven at room temperature until the weight was kept constant. Dried hydrogel disks were weighed (mdry). The equilibrium water content (EWC) was determined based on the change in weight relative to the initial sample weight according to EWC = 100(%) × (mwet − mdry)/mwet, where mwet is the mass of the wet hydrogel and mdry is the mass of the dry hydrogel. All samples were measured at least in triplicate.
2. Measurements of mechanical properties
The compressive modulus and fracture stress of the hydrogels were characterized by compressive stress–strain measurements which were performed on swollen gels using an Instron 5543 Single Column Testing System. The hydrogel sheets were cut into small circular pieces with 5 mm in diameter and 2 mm in thicknesses. For compression test, the hydrogel samples were put on the lower plate and compressed by the upper plate, which was connected to a load cell, at a strain rate of 1.0 mm/min. The compressive modulus was calculated from 5% to 10% strain while the fracture stress was recorded as the compressive stress at which a sample fails due to fracture. Five parallel samples per measurement were performed and the values obtained were averaged.
3. Hydration–dehydration measurements
Hydrogel disks (5-mm diameter) and hydrogel tubes were used to test the effect of hydration–dehydration processes on the mechanical and morphological properties of the samples. Freshly prepared hydrogel samples were soaked in water at room temperature for at least three days after gelation. The swollen hydrogel disks were withdrawn on a Teflon sheet and put in cabinet at room temperature for two days to obtain the partially dehydrated samples. Then, the partially dehydrated samples were rehydrated in water at room temperature for one day. At least three days were needed for one cycle. Teflon sheets were used as the substrate for the hydrogel to decrease it deformation in the dehydration process.
4. Assessment of protein adsorption
The protein adsorption behaviors of different hydrogels were evaluated using an enzyme-linked immune sorbent assay (ELISA). To measure fibrinogen adsorption, the swollen hydrogel sheets of three different formulations (1.5%–50%, 3%–50%, and 6%–50%) were cut into hydrogel disks of 5-mm in diameter. Nine samples (three samples for each formulation) and three samples of tissue culture grade polystyrene (TCPS) substrates were incubated with 1 mg/ml fibrinogen in a well plate for 30 min at room temperature, followed by five washes with PBS buffer. The hydrogels were transferred to new wells and incubated with 1 μg/ml of HRP-conjugated anti-fibrinogen in PBS for 30 min, followed by another five washes with the PBS buffer. Then, all samples were moved to new wells. Each well had 500 μl of OPD solution of 1 mg/ml OPD in 0.1 M citrate-phosphate buffer, pH 5.5, containing 0.03% hydrogen peroxide added to each sample. The samples incubated in the OPD solution away from light. Enzyme activity was stopped after 30 min with addition of an equal volume of 3N H2SO4. The supernatant was removed from each hydrogel disk and transferred to a new well. The tangerine color of the supernatants, where intensity is proportional to the amount of protein adsorption, was measured at 492 nm with a microplate reader (BioTek). Standard TCPS 96-well plates were used for quantitative comparison and the average protein adsorption of TCPS substrates was normalized to 100%.
5. Sterilization methods
Three main terminal sterilization techniques, i.e., steam autoclave, EtO, and gamma irradiation, were used to test the stability of the CB hydrogel. For the steam sterilization, hydrogel sheets were sterilized in an autoclave under a standard condition at 121 °C for 30 min. For the EtO sterilization, hydrogel sheets were packed and subjected to the standard EtO sterilization procedure in the University of Washington hospital. The hydrogel sheets both in hydrated and in dehydrated state were subjected to 60Co gamma-ray irradiation at a dose of 25 kGy (2.5 Mrad) at the Nuclear Radiation Center of Washington State University. Ten disks per sample were sterilized for each method, and the values obtained were averaged.
III. RESULTS AND DISCUSSION
Free radical polymerization initiated by a redox initiator system is a fast and controllable method to prepare a hydrogel under facile ambient or physiological conditions.35,36 Here, three CB hydrogel samples with different crosslinking densities (1.5%, 3%, and 6% CBX/CB) and the same monomer concentration (50% CB/water) were prepared via redox polymerization. All the resulting hydrogels were uniformly transparent in appearance with high water content. Figure 1(b) shows the EWC of CB hydrogels. As expected, an increase in crosslinker content resulted in a decrease in hydration and swelling. For 1.5%–50% sample, the highest hydration achieved was approximately 96% of EWC. At 6%–50%, hydration decreased to 86% of EWC, which is still high.
Previously, we have demonstrated low protein adsorption on CB hydrogels37 since the hydrogels were prepared from a CB monomer and a CB-based crosslinker (CBX), both contained ultralow fouling zwitterionic moieties. The nonfouling properties of hydrogels were evaluated using an ELISA with fibrinogen. Figure 1(b) shows the relative protein adsorption of hydrogels of different formulations with respect to the TCPS control. Results showed that protein adsorption on all CB hydrogels was significantly lower than on that on the TCPS control due to the zwitterionic nature of CB hydrogels.38
A. Mechanical and nonfouling stability of CB hydrogels under different sterilization methods
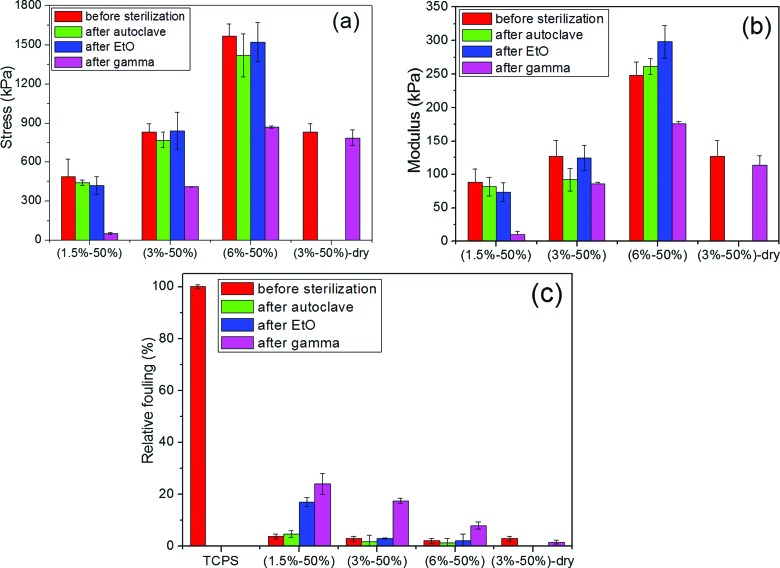
At a fixed monomer concentration for three formulations of 1.5%–50%, 3%–50%, and 6%–50%, an evident compressive fracture stress and compressive modulus dependency of hydrogels on the crosslinker contents was seen. The CB hydrogels tested in this study achieved compressive fracture stress from 500 to 1600 kPa and compressive modulus from 85 to 300 kPa by varying the crosslinking density. Results showed that compressive modulus was more sensitive to the crosslinking density than compressive fracture stress. The mechanical properties of these hydrogels were similar to those of self-healing zwitterionic hydrogels reported previously.39,40
High-temperature steam sterilization was first selected, because it is considered to be one of the safest and most practical means of sterilizing fluids and hydrogel containing fluids.30 Different from temperature sensitive poly(sulfobetaine methacrylate),41 poly(carboxybetaine acrylamide) is a temperature-insensitive zwitterionic polymer due to no self-aggregation. As expected, these CB hydrogels showed good mechanical stability under heating conditions, even at crosslinking density as low as 1.5%. Both nonsterilized and autoclaved hydrogels demonstrated low fouling properties as shown in Fig. 2(c). All the autoclaved hydrogel samples displayed fouling lower than 10% of the control surface. Unlike hyaluronic acid gels, high temperature and pressure did not compromise the mechanical and nonfouling properties of CB hydrogels.41
Fig. 2.
Mechanical properties (a, b) and relative fouling properties (c) of three hydrated CB hydrogel samples (1.5%–50%, 3%–50%, and 6%–50%) and one dry CB sample (3%–50%) before and after steam autoclave, EtO sterilization, and gamma irradiation sterilization. Data are presented as mean ± standard error (n = 3).
Different from the steam sterilization, the EtO sterilization was expected to dehydrate the hydrogel to a dried sample in the degas procedure, which was necessary to remove the trapped ethylene oxide within the material.42 Obvious changes were observed from the visual appearance of the samples, where the hydrogel was dehydrated and shrank to half of the original size after EtO sterilization. The dehydrated hydrogel sheets were reswollen in water for one day, and their mechanical and fouling properties were evaluated and compared with that of the nonsterilized hydrogels. The sterilized hydrogels that were recovered in water demonstrated no change in size or shape compared to nonsterilized hydrogels. Amazingly, there was no change in the mechanical and nonfouling properties of the hydrogels after sterilization despite the undergoing a dehydration and reswelling process and a sterilization procedure, indicating that the dehydration and oxidation of the hydrogel samples did not destroy the mechanical properties after reswelling in water.
Gamma irradiation sterilization, as a physically cold process, has been widely used for the sterilization of health care products.43,44 Although the sterilization dose must be set for each type of product, depending on its bioburden as recommended by the International Organization for Standardization (ISO), we used a minimum dose of 25 kGy.45 Although the dose of 25 kGy of gamma irradiation was a harsh condition to hydrogels, it did not cause any changes to the visual appearance of the samples, neither hazy nor brittle. Different from the steam sterilization and EtO oxidation, the mechanical and fouling performance of the gamma-sterilized hydrogel was more sensitive to the crosslinking density. Lower crosslinked hydrogels had more significant loss in compression strength and fouling properties. A higher crosslinked network and lower EWC showed stronger resistance to destruction from the gamma irradiation. In fact, these results are not surprising. Gamma irradiation is an ionizing irradiation, which can affect hydrogel network either directly by energy deposition or indirectly by the interaction of irradiation with water.18 In particular, irradiation interacts with water, leading to the formation of free radicals46 that can damage polymeric network. If the hydrogel is in a dry dehydrated state, then the hydrogel can be sterilized by gamma irradiation with little adverse effects on the mechanical and nonfouling properties. As shown in Fig. 2(c), one gamma-sterilized hydrogel sample in dry state showed very low fouling after it was rehydrated. This was consistent with the previous finding that gamma irradiation was a suitable sterilization method for the dried hydrogel sponges of hydroxyethyl methacrylate.5
B. Hydration–dehydration process of CB hydrogels
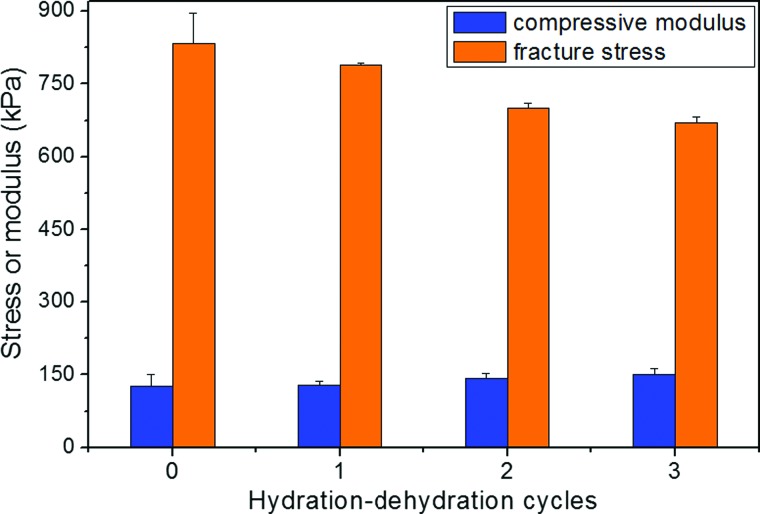
As aforementioned, hydrogels are commonly dehydrated or lyophilized to remove all water before being packaged or sterilized before usage.31 The dehydrated hydrogel matrix is reswollen upon applications. This process requires good mechanical stability of hydrogels after dehydration and reswelling. Figure 3 shows the mechanical properties of CB hydrogels over three hydration–dehydration cycles. Although the hydrogels were dehydrated thoroughly for 48 h in fume hood at room temperature, they were reswollen successfully after rinsing in water for 4 h without any obvious change in shape and size. The mechanical properties of the rehydrated hydrogels were tested 24 h after rehydration to ensure sufficient reswelling. Results shown in Fig. 3 demonstrate that the hydrogel retained their compressive fracture stress and compressive modulus after three hydration–dehydration cycles with only slight change. The mechanical properties of CB hydrogels were kept relatively stable as compared to the reported copolymer gel prepared from blends of polyvinyl alcohol and polyvinyl pyrrolidone, where its modulus of the copolymer gel (after completely dehydrated and then rehydrated) increased nearly three times than that of the as-prepared samples.47 When the CB hydrogels were subject to a hydration–dehydration process, ionic interactions and hydrogen bonding between CB chains underwent a breaking and reforming process. These hydrogen bonds as well as electrostatic interactions between the negatively charged groups and the positively charged groups serve as secondary, physical crosslinks and allow the recovery of the hydrogel when the dehydrated hydrogel is rehydrated.
Fig. 3.
Compressive modulus and fracture stress of the CB hydrogels (3%–50%) after different hydration–dehydration cycles. Data are presented as mean + standard error (n = 3).
C. Fabrication and characterization of CB hydrogel tubes
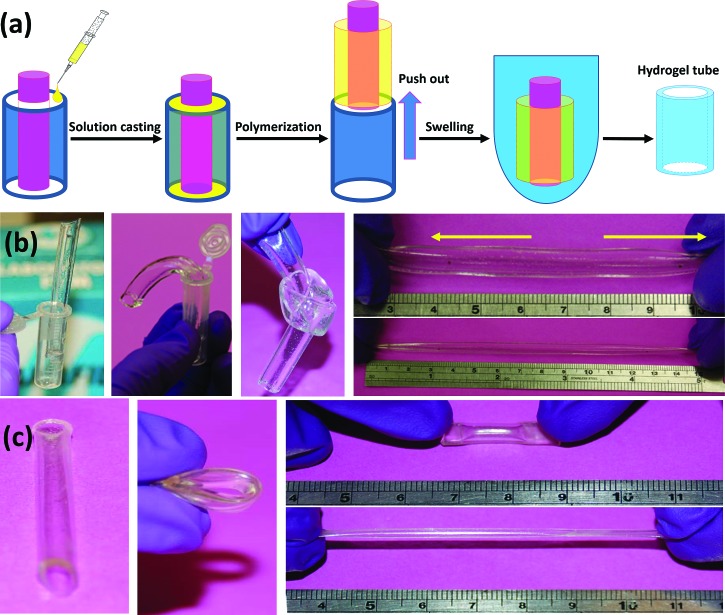
CB hydrogel tubes were fabricated using a simple mold, and the formulation of 3%–50% was selected. Due to ease in handling and mild gelling conditions, CB hydrogel tubes were formulated from a simple preparation procedure unlike the previously reported method.48 Figure 4 illustrates the device used to prepare the hydrogel tubes. It can be seen that the hydrogel tubes were transparent, soft, and tough. Although the polymerized hydrogel tubes underwent a strong shear force when they were pushed out from the mold, they stayed intact with a smooth wall surface. Additionally, the hydrogel tubes could be knotted freely and stretched up to twofold length and then fully recovered to their original dimensions rapidly without destroying their mechanical integrity. Results showed that the hydrogels were strong enough to be free-shaped in different molds. In this work, zwitterionic monomers were used as the building blocks where the crosslinked network served as the structural framework. The ionic interactions between the cationic and anionic groups on the side chains allowed reversible breaking and reforming of the structure. Cross-linked network, ionic interaction and hydrogen bonding all contributed to the self-recovery of the hydrogels.49
Fig. 4.
Illustration of the device used to prepare the hydrogel tubes (a). The transparent hydrated CB hydrogel (3%–50%) tubes with good mechanical properties: stand-alone, bending, knotting, and stretching (b). The dehydrated CB hydrogel (3%–50%) tube still retained its transparency and its overall shape and could be bent and stretched without breaking (c). After being rehydrated in water for 24 h, the hydrogel retained its original shape, size and appearance. The outer diameter was 8 ± 0.3 mm, while the inner diameter was 6 ± 0.2 mm with a wall thickness of 1 ± 0.1 mm.
Interestingly, the dehydrated CB hydrogel tubes also demonstrated good flexibility. The dehydrated CB hydrogel tubes were readily bent and compressed without any damage or breaks. After being stretched at least 6 times without fracture, the dehydrated hydrogel tube could be recovered back to the original shape and size after being stored in room temperature for 24 h. The flexibility of the dehydrated hydrogels, the reversibility of the stretched and bent dehydrated hydrogels, and the stability of the hydration–dehydration cycle provide ease in manipulation, which is very useful for practical applications, such as storage, handling, and sterilization. Therefore, the hydrogel tubes were morphologically and mechanically robust in maintaining their physical integrity and preserving their compressive modulus.
Here, we evaluated permeability and coagulation visually by using undiluted whole blood (Fig. 5). The hydrogel tube after swelled in PBS buffer for three days were soaked in blood, and then followed by rinsing with PBS after the blood clotted. It was observed that there was no blood leakage from the CB hydrogel tube. In addition, after being soaked in the blood, the hydrogel tube was rinsed and washed in PBS solution and then examined under a microscope. No blood cells were observed for CB hydrogel. The soaked part of the hydrogel tube remained optically transparent and void of any red color, indicating the hydrogels resisted the attachment of red blood cells. Since both monomer and crosslinker contained ultralow fouling zwitterionic moieties, the low fouling of the CB hydrogels was expected, similar to the previous results.37 Here, resistance of the CB hydrogels to red blood cells was attributed to low protein adsorption.50
Fig. 5.
(a) Hydrogel tubes incubated in whole blood at 37 °C for 1 h. The CB hydrogel (3%–50%) tube was folded in the vial with two openings upward and then whole blood was loaded into the hydrogel tube; (b) hydrogel tubes after rinsed by PBS for at least five times. No obvious difference was observed between the soaked and unsoaked parts of the hydrogel tube.
IV. CONCLUSIONS
Zwitterionic carboxybetaine hydrogels with excellent nonfouling and tunable mechanical properties were prepared. These CB hydrogels were uniformly transparent with high equilibrium water content and could stand several hydration–dehydration cycles without compromising their mechanical properties. The mechanical stability in both swollen and dehydrated states ensured that they could sustain the three most commonly used sterilization methods, i.e., steam sterilization, ethylene oxide (EtO) gas sterilization, and gamma irradiation sterilization. The hydrogels with higher crosslinking densities preserved their mechanical integrity and nonfouling properties, especially for steam sterilization and EtO oxidation in wet state and for gamma irradiation in dry state. Hydrogel tubes were fabricated using a simple method. Both hydrated and dehydrated hydrogel tubes were soft and tough and could be bent, knotted, and stretched freely. CB hydrogel tubes were found to effectively resist nonspecific protein adsorption and cell adhesion when in contact with whole blood.
ACKNOWLEDGMENTS
S.J. acknowledges financial support from the Office of Naval Research (Nos. N00014-14-1-0099, N00014-15-1-2277, and N00014-16-1-3084). X.H. acknowledges financial support from the National Natural Science Foundation of China (Grant No. 21376073).
References
- 1. Lambert B. J., Mendelson T. A., and Craven M. D., AAPS PharmSciTech 12, 1116 (2011). 10.1208/s12249-011-9644-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karajanagi S. S., Yoganathan R., Mammucari R., Park H., Cox J., Zeitels S. M., Langer R., and Foster N. R., Biotechnol. Bioeng. 108, 1716 (2011). 10.1002/bit.23105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. White A., Burns D., and Christensen T. W.. J. Biotechnol. 123, 504 (2006). 10.1016/j.jbiotec.2005.12.033 [DOI] [PubMed] [Google Scholar]
- 4. Bernhardt A., Wehrl M., Paul B., Hochmuth T., Schumacher M., Schutz K., and Gelinsky M., PloS One 10, e0129205 (2015). 10.1371/journal.pone.0129205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eljarrat-Binstock E., Bentolila A., Kumar N., Harel H., and Domb A. J., Polym. Adv. Technol. 18, 720 (2007). 10.1002/pat.948 [DOI] [Google Scholar]
- 6. Seal B. L., Otero T. C., and Panitch A., Mater. Sci. Eng., R 34, 147 (2001). 10.1016/S0927-796X(01)00035-3 [DOI] [Google Scholar]
- 7. Saha N., Saarai A., Roy N., Kitano T., and Saha P., J. Biomater. Nanobiotechnol. 2, 85 (2011). 10.4236/jbnb.2011.21011 [DOI] [Google Scholar]
- 8. Patel A. and Mequanint K., Hydrogel Biomaterials, Biomedical Engineering-Frontiers and Challenges, edited by Fazel R. ( InTech, Croatia, 2011), pp. 275–296. [Google Scholar]
- 9. Dhandayuthapani B., Yoshida Y., Maekawa T., and Kumar D. S., Int. J. Polym. Sci. 2011, 290602. 10.1155/2011/290602 [DOI] [Google Scholar]
- 10. Hirata N., Matsumoto K. I., Inishita T., Takenaka Y., Suma Y., and Shintani H., Radiat. Phys. Chem. 46, 377 (1995). 10.1016/0969-806X(94)00134-6 [DOI] [Google Scholar]
- 11. Hooper K. A., Cox J. D., and Kohn J., J. Appl. Polym. Sci. 63, 1499 (1997). [DOI] [Google Scholar]
- 12. França R., Mbeh D. A., Samani T. D., Le Tien C., Mateescu M. A., Yahia L., and Sacher E., J. Biomed. Mater. Res. 101B, 1444 (2013). 10.1002/jbm.b.32964 [DOI] [PubMed] [Google Scholar]
- 13. Odelius K., Plikk P., and Albertsson A. C., Biomaterials 29, 129 (2008). 10.1016/j.biomaterials.2007.08.046 [DOI] [PubMed] [Google Scholar]
- 14. Müller F. A., Müller L., Hofmann I., Greil P., Wenzel M. M., and Staudenmaier R., Biomaterials 27, 3955 (2006). 10.1016/j.biomaterials.2006.02.031 [DOI] [PubMed] [Google Scholar]
- 15. Jiménez A., Zhang J., and Matthews M. A., Biotechnol. Bioeng. 101, 1344 (2008). 10.1002/bit.21983 [DOI] [PubMed] [Google Scholar]
- 16. Morejón-Alonso L., Carrodeguas R. G., García-Menocal J. Á. D., Pérez J. A. A., and Manent S. M., Mater. Res. 10, 15 (2007). 10.1590/S1516-14392007000100005 [DOI] [Google Scholar]
- 17. Türker N. S., Özer A. Y., Kutlu B., Nohutcu R., Sungur A., Bilgili H., Ekizoglu M., and Özalp M., Tissue Eng. Regener. Med. 11, 341 (2014). 10.1007/s13770-014-0016-9 [DOI] [Google Scholar]
- 18. Ferraris S., Pan G., Cassinelli C., Mazzucco L., Vernè E., and Spriano S., Biomed. Mater. 7, 054102 (2012). 10.1088/1748-6041/7/5/054102 [DOI] [PubMed] [Google Scholar]
- 19. Holy C. E., Cheng C., Davies J. E., and Shoichet M. S., Biomaterials 22, 25 (2000). 10.1016/S0142-9612(00)00136-8 [DOI] [PubMed] [Google Scholar]
- 20. Moraes M. A., Weska R. F., and Beppu M. M., J. Biomed. Mater. Res. 102B, 869 (2014). 10.1002/jbm.b.33069 [DOI] [PubMed] [Google Scholar]
- 21. Marreco P. R., Moreira P. D. L., Genari S. C., and Moraes Â. M., J. Biomed. Mater. Res. 71B, 268 (2004). 10.1002/jbm.b.30081 [DOI] [PubMed] [Google Scholar]
- 22. Huebsch N., Gilbert M., and Healy K. E., J. Biomed. Mater. Res. 74B, 440 (2005). 10.1002/jbm.b.30155 [DOI] [PubMed] [Google Scholar]
- 23. Annabi N., Nichol J. W., Zhong X., Ji C., Koshy S., Khademhosseini A., and Dehghani F., Tissue Eng., Part B 16, 371 (2010). 10.1089/ten.teb.2009.0639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shao Q. and Jiang S., Adv. Mater. 27, 15 (2015). 10.1002/adma.201404059 [DOI] [PubMed] [Google Scholar]
- 25. Chen S., Li L., Zhao C., and Zheng J., Polymer 51, 5283 (2010). 10.1016/j.polymer.2010.08.022 [DOI] [Google Scholar]
- 26. Ahmed E. M., J. Adv. Res. 6, 105 (2015). 10.1016/j.jare.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cao B., Tang Q., Li L., Lee C.-J., Wang H., Zhang Y., Castaneda H., and Cheng G., Chem. Sci. 6, 782 (2015). 10.1039/C4SC02200A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carr L. R., Zhou Y., Krause J. E., Xue H., and Jiang S., Biomaterials 32, 6893 (2011). 10.1016/j.biomaterials.2011.06.006 [DOI] [PubMed] [Google Scholar]
- 29. Kanjickal D., Lopina S., Evancho-Chapman M. M., Schmidt S., and Donovan D., J. Biomed. Mater. Res. 87A, 608 (2008). 10.1002/jbm.a.31811 [DOI] [PubMed] [Google Scholar]
- 30. Jarry C., Chaput C., Chenite A., Renaud M.-A., Buschmann M., and Leroux J.-C., J. Biomed. Mater. Res. 58B, 127 (2001). 10.1002/1097-4636(2001)58:1%3C127::AID-JBM190%3D3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- 31. Stoppel W. L., White J. C., Horava S. D., Henry A. C., Roberts S. C., and Bhatia S. R., J. Biomed. Mater. Res. 102B, 877 (2014). 10.1002/jbm.b.33070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matuska A. M. and McFetridge P. S., J. Biomed. Mater. Res. 103B, 397 (2015). 10.1002/jbm.b.33213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vaisocherova H., Yang W., Zhang Z., Cao Z., Cheng G., Piliarik M., Homola J., and Jiang S., Anal. Chem. 80, 7894 (2008). 10.1021/ac8015888 [DOI] [PubMed] [Google Scholar]
- 34. Carr L. R., Xue H., and Jiang S., Biomaterials 32, 961 (2011). 10.1016/j.biomaterials.2010.09.067 [DOI] [PubMed] [Google Scholar]
- 35. Hennink W. E. and Van Nostrum C. F., Adv. Drug Delivery Rev. 54, 13 (2002). 10.1016/S0169-409X(01)00240-X [DOI] [PubMed] [Google Scholar]
- 36. Nguyen K. T. and West J. L., Biomaterials 23, 4307 (2002). 10.1016/S0142-9612(02)00175-8 [DOI] [PubMed] [Google Scholar]
- 37. Carr L. R., Krause J. E., Ella-Menye J.-R., and Jiang S., Biomaterials 32, 8456 (2011). 10.1016/j.biomaterials.2011.07.062 [DOI] [PubMed] [Google Scholar]
- 38. Zhang L., Cao Z., Bai T., Carr L., Ella-Menye J.-R., Irvin C., Ratner B. D., and Jiang S., Nat. Biotechnol. 31, 553 (2013). 10.1038/nbt.2580 [DOI] [PubMed] [Google Scholar]
- 39. Bai T., Liu S., Sun F., Sinclair A., Zhang L., Shao Q., and Jiang S., Biomaterials 35, 3926 (2014). 10.1016/j.biomaterials.2014.01.077 [DOI] [PubMed] [Google Scholar]
- 40. Shao Q., Mi L., Han X., Bai T., Liu S., Li Y., and Jiang S., J. Phys. Chem. B 118, 6956 (2014). 10.1021/jp503473u [DOI] [PubMed] [Google Scholar]
- 41. Szabó A., Szabó B., Balogh E., Zelkó R., and Antal I., Polym. Test. 32, 1322 (2013). 10.1016/j.polymertesting.2013.08.006 [DOI] [Google Scholar]
- 42. Mendes G. C. C., Brandão T. R. S., and Silva C. L. M., Am. J. Infect. Control 35, 574 (2007). 10.1016/j.ajic.2006.10.014 [DOI] [PubMed] [Google Scholar]
- 43. Hammad A., “ Microbiological aspects of radiation sterilization,” in Trends in Radiation Sterilization of Health Care Products ( IAEA, Vienna, 2008), p. 119. [Google Scholar]
- 44. Moyne P., Botella A., Peyrouset A., and Rey L., Radiat. Phys. Chem. 63, 703 (2002). 10.1016/S0969-806X(01)00563-1 [DOI] [Google Scholar]
- 45.ISO 11137-2, Part 2: Establishing the Sterilisation dose. Sterilisation of Health Care Products-Radiation ( International Standard Organisation, Geneva, 2006), p. 60. [Google Scholar]
- 46. Kanjickal D., Lopina S., Evancho-Chapman M. M., Schmidt S., Inbaraj J. J., Cardon T. B., and Lorigan G. A., J. Biomed. Mater. Res. 88A, 409 (2009). 10.1002/jbm.a.31717 [DOI] [PubMed] [Google Scholar]
- 47. Thomas J., Gomes K., Lowman A., and Marcolongo M., J. Biomed. Mater. Res. 69B, 135 (2004). 10.1002/jbm.b.20023 [DOI] [PubMed] [Google Scholar]
- 48. Sadr N., Zhu M., Osaki T., Kakegawa T., Yang Y., Moretti M., Fukuda J., and Khademhosseini A., Biomaterials 32, 7479 (2011). 10.1016/j.biomaterials.2011.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sun T. L., Kurokawa T., Kuroda S., Ihsan A. B., Akasaki T., Sato K., Haque Md. A., Nakajima T., and Gong J. P., Nat. Mater. 12, 932 (2013). 10.1038/nmat3713 [DOI] [PubMed] [Google Scholar]
- 50. Ji F., Lin W., Wang Z., Wang L., Zhang J., Ma G., and Chen S., ACS Appl. Mater. Interfaces 5, 10489 (2013). 10.1021/am403657t [DOI] [PubMed] [Google Scholar]