Abstract
Objective
Prior studies have suggested that traumatic brain injury (TBI) may affect cardiac function. Our study aims were to determine the incidence, longitudinal course, and admission risk factors for systolic dysfunction in patients with moderate-severe TBI.
Design
Prospective cohort study
Setting
Level 1 trauma center
Measurements
Transthoracic echocardiogram (TTE) within 1 day and over the first week after moderate-severe TBI; TTE within 1 day after mild TBI (comparison group).
Measurements and Main Results
Systolic function was assessed by TTE, and systolic dysfunction was defined as fractional shortening (FS) < 25%. Multivariable Poisson regression models examined admission risk factors for systolic dysfunction. Systolic function in 32 patients with isolated moderate-severe TBI and 32 patients with isolated mild TBI (comparison group) was assessed with TTE. Seven (22%) moderate-severe TBI and 0 (0%) mild TBI patients had systolic dysfunction within the first day after injury (p<0.01). All patients with early systolic dysfunction recovered in one week. Younger age (RR 0.87, 95% CI 0.79 – 0.94, for one year increase in age) and lower admission GCS score (RR 0.34, 95% CI 0.20 – 0.58, for one unit increase in GCS) were independently associated with the development of systolic dysfunction among moderate-severe TBI patients.
Conclusions
Early systolic dysfunction can occur in previously healthy patients with moderate-severe TBI, and it is reversible over the first week of hospitalization. Younger age and lower admission GCS score are independently associated with the development of systolic dysfunction after moderate-severe TBI.
Keywords: traumatic brain injury, stress cardiomyopathy, echocardiography, trauma
Introduction
Traumatic brain injury (TBI) is sustained by more than 1.7 million individuals annually, and contributes to 30% of all injury-related deaths in the United States(1). Patients with moderate-severe TBI experience hypotension [defined commonly as systolic blood pressure (SBP) < 90 mmHg] early after hospitalization(2), which can lead to poor blood flow to an injured brain(3, 4) and worse mortality and functional outcomes following TBI(5, 6). Experimental studies and clinical observations in other non-TBI neurologic disease paradigms, such as subarachnoid hemorrhage (SAH), suggest that acute systolic cardiac dysfunction may be responsible for the early hypotension that is often associated with catastrophic neurologic processes(7, 8).
The approach to fluid management and selection of vasoactive agents should be directed by the status of cardiac function in TBI. For example, current data suggests that intravenous phenylephrine is the most commonly used vasopressor following severe TBI (9). However, without knowledge of the status of the heart, it is difficult to examine which vasoactive agent will best improve cerebral perfusion in an individual TBI patient. Currently, outside of a retrospective study (10) and case reports (11), there is little prospective data on what happens to cardiac function after TBI. To increase our understanding of brain-heart interactions and to provide information that would guide the management of systemic and cerebral hemodynamics following TBI, we aimed to determine the incidence, longitudinal course, and admission risk factors for systolic dysfunction in patients hospitalized with isolated moderate-severe TBI. We hypothesized that systolic dysfunction would be relatively common after moderate-severe TBI and greater TBI severity would result in more systolic dysfunction.
Materials and Methods
We conducted a prospective cohort study among moderate-severe TBI patients, using mild TBI patients as a comparison group, at Harborview Medical Center, a 413-bed tertiary care center and the only Level 1 adult and pediatric trauma center for a 4-state region in the United States (Washington, Alaska, Montana, and Idaho). The study was approved by the University of Washington Institutional Review Board.
Study Population
Patients were screened for a diagnosis of mild TBI and moderate-severe TBI within 24 hours of injury. TBI was defined according to the Centers for Disease Control and Prevention(12), and TBI severity was based on the admission Glasgow Coma Scale score (GCS) after resuscitation(13, 14). Mild TBI was defined by a GCS score ≥ 13, and moderate-severe TBI was defined by a GCS score ≤ 12. We excluded patients older than 65 years and any patient with a documented history of ischemic heart disease, congenital heart disease, moderate or severe valvular heart disease, and systolic or diastolic heart failure. We excluded patients with severe systemic comorbidities (liver cirrhosis, greater than stage 2 chronic kidney disease, human immunodeficiency virus infection, a history of chemotherapy, greater than stage 2 chronic obstructive pulmonary disease, pulmonary hypertension, or a history of cerebrovascular disease). Furthermore, we excluded patients with a body region Abbreviated Injury Scale (AIS) score of greater than 2 in the chest or abdomen, patients with spinal cord injuries, patients who sustained a cardiac arrest prior to enrollment, and any patients requiring greater than 2 units of packed red blood cells as part of their initial resuscitation in the emergency department and (if required) operating room.
Study Procedures
Following recruitment, a transthoracic echocardiogram (TTE) was performed within the day following injury. For patients with mild TBI, a single TTE exam was performed; and for patients with moderate-severe TBI, the initial TTE was performed, along with a repeat TTE exam within 2–4 days and 7–9 days following injury. Among patients in whom clinical instability or medical procedures (i.e. surgery) precluded a research TTE within the specified time frame, the TTE was performed as early as possible after clinical stability was achieved. Data were collected from clinical records for demographic, clinical, radiographic, and hemodynamic data.
Transthoracic Echocardiography
All TTE examinations were performed by an anesthesiologist-intensivist (VK) with certification in echocardiography, using a Philips iE-33 ultrasound system (Bothell, WA) and utilizing two-dimensional and Doppler imaging technology according to the American Society of Echocardiography guidelines(15). The focused exam consisted of evaluation of left ventricular systolic and diastolic function, primarily assessed in the parasternal, apical, and subcostal windows. As many moderate-severe TBI patients had labile intracranial pressures, especially with changes in position, all research TTE exams were performed in the supine position; therefore, systolic function was primarily assessed in the parasternal long-axis window (basal fractional shortening) rather than in the apical windows (ejection fraction, which requires adequate imaging of the cardiac apex without foreshortening(16), and is best obtained in the left lateral decubitus position). The study cardiologist (EG), blinded to the patient status or clinical details of patient management, reviewed all echocardiogram examinations offline for data quality, and any exams with inadequate imaging windows for assessment of systolic function were excluded from analysis. The study certified cardiac sonographer (CP), also blinded to patient exposure status or clinical details, performed all cardiac measurements offline, including left ventricular diameters, areas, and Doppler measurements of mitral inflow and septal tissue velocity. A randomly selected group of images, representing approximately 15% of the patient population, was selected for repeated ventricular diameter measurement by CP (6 weeks after the initial measurements) and EG for determination of intraobserver and interobserver variability, respectively.
Echocardiographic Outcomes
Systolic function was assessed using endocardial fractional shortening [(left ventricular internal diameter in diastole – left ventricular internal diameter in systole) / (left ventricular internal diameter in diastole)], a highly reproducible and validated method for linear assessment of left ventricular function that has been used in multiple clinical studies(15). In comparing the mild versus moderate-severe TBI groups, our primary outcome was systolic dysfunction, defined as a fractional shortening less than 25%(15), recorded on the first echocardiogram after injury. Secondary outcomes included diastolic dysfunction, defined as a mitral annular septal tissue velocity [e′(s)] < 8 cm/s(17), and change in systolic and diastolic function over the first week of hospitalization in patients with moderate-severe TBI.
Statistical Analysis
Sample size calculations were based on the expected incidence of systolic dysfunction in moderate-severe TBI versus mild TBI derived from retrospective studies from our research group(10, 18) and previous literature from other neurologic injury paradigms (approximately 20% versus <1%, respectively). We calculated that 62 patients (31 patients in each group) would be required to reject the null hypothesis of no difference in systolic function between groups with mild and moderate-severe TBI, with an alpha level of 0.05 and power of 0.8. Assuming that approximately 10% of patients would not have adequate echocardiographic windows to assess fractional shortening, we aimed to recruit 35 patients per group. Descriptive statistics examined the demographic, clinical, radiographic, and echocardiographic characteristics of the cohort. The incidence of systolic dysfunction within the day after injury was calculated. For comparison of echocardiographic parameters between groups with mild and moderate-severe TBI, a Student’s t-test or a Fisher’s exact test was used. A Fisher’s exact test was used to examine the differences in the proportions of patients with systolic and diastolic dysfunction in the moderate-severe (exposed) and mild (comparison) TBI groups. Univariate and multivariable Poisson regression models (with adjustment for age, gender, intracranial lesion, Glasgow Coma Scale score, systolic blood pressure, fluid balance, sedative use, vasopressor use, osmotherapy use, and need for intracranial surgery) with robust standard errors were used to calculate the relative risk of factors present on admission for the development of systolic dysfunction among patients with moderate-severe TBI. Interobserver and intraobserver variability was evaluated using Bland-Altman analysis(19), and expressed as a mean difference (bias) and limits of agreement. All analyses were performed using Stata version 13.0 statistical software (StataCorp, Texas, USA).
Results
During June to August 2015, 99 TBI patients were met criteria for screening and 29 patients were excluded, primarily due to refusal to participate in the study, the presence of polytrauma, and the presence of underlying cardiac disease. A total of 70 patients were recruited, with 64 patients (32 with moderate-severe TBI and 32 with mild TBI) having adequate echocardiographic windows for final analysis. In eight patients, a research TTE within the first day following injury was unable to be performed due to clinical circumstances (prolonged transport time, resuscitation, clinical procedures, or surgery) and the initial TTE was performed within 2 days after injury.
Baseline demographic and clinical characteristics in the patients with mild and moderate-severe TBI are shown in Table 1. Both groups were relatively young (mean age 36.2 and 36.5 years, respectively), primarily male (69% and 84%, respectively), and mainly free from medical comorbidities. A greater proportion of the moderate-severe TBI group had intracranial hemorrhage on initial head CT than in the mild TBI group, with the majority of moderate-severe TBI patients (66%) having multiple types of hemorrhage on initial head CT. Mean admission systolic blood pressure (SBP) was greater in the moderate-severe TBI than the mild TBI group (132.3 mmHg vs. 125.5 mmHg), although a greater proportion of moderate-severe TBI than mild TBI patients (34% vs. 13%, respectively) experienced hypotension (SBP ≤ 90 mmHg) within 24 hours of admission, with 19% requiring vasopressors.
Table 1.
Demographic and Clinical Characteristics in Mild and Moderate-Severe Traumatic Brain Injury Patientsa
| Variable | Mild TBI (n=32) | Moderate-Severe TBI (n=32) |
|---|---|---|
| Age (years) | 36.2 (11.0) | 36.5 (13.3) |
| Race | ||
| White | 18 (56%) | 21 (66%) |
| Black | 6 (19%) | 3 (9%) |
| Hispanic | 5 (16%) | 3 (9%) |
| Asian / Pacific Islander | 3 (9%) | 2 (6%) |
| Native American | 0 (0%) | 3 (9%) |
| Male Gender | 22 (69%) | 27 (84%) |
| Medical Co-morbidities | ||
| Pulmonary | 0 (0%) | 0 (0%) |
| Hypertension | 1 (3%) | 1 (3%) |
| Diabetes | 0 (0%) | 2 (6%) |
| Renal Disease | 0 (0%) | 0 (0%) |
| Injury Mechanism | ||
| Fall | 6 (19%) | 10 (31%) |
| Motor vehicle crash | 11 (34%) | 10 (31%) |
| Vehicle vs. pedestrian | 3 (9%) | 5 (16%) |
| Bicycle crash | 3 (9%) | 1 (3%) |
| Gunshot to head | 0 (0%) | 1 (3%) |
| Assault | 6 (19%) | 3 (9%) |
| Other | 3 (9%) | 2 (6%) |
| Initial Head CT Findingsb | ||
| Epidural hemorrhage | 4 (13%) | 5 (16%) |
| Subdural hemorrhage | 7 (22%) | 24 (75%) |
| Subarachnoid hemorrhage | 2 (6%) | 23 (72%) |
| Intraparenchymal hemorrhage | 4 (13%) | 15 (47%) |
| Glasgow Coma Scale | ||
| Admission GCS | 14.8 (0.4) | 5.2 (2.5) |
| Highest GCS (within 24 hours) | 15.0 (0.2) | 9.0 (3.0) |
| Lowest GCS (within 24 hours) | 14.5 (0.8) | 4.8 (2.4) |
| Admission Hematocrit (%) | 40.6 (4.5) | 38.0 (5.3) |
Values are mean(SD) for continuous variables and n(%) for categorical variables.
Some patients had multiple head CT findings.
CT=Computed Tomography; GCS=Glasgow Coma Scale; bpm=beats per minute; MAP=mean arterial pressure
Initial echocardiogram findings are shown in Table 2. The incidence of early systolic dysfunction in patients with mild TBI was 0%, compared to 22% in patients with moderate-severe TBI (p<0.01). Patients with moderate-severe TBI had a greater left ventricle area in diastole and systole, as well as greater left ventricle internal diameter at end-systole. Mean fractional shortening was significantly lower in moderate-severe TBI patients, compared to mild TBI patients (p=0.01). The mild and moderate-severe TBI groups both had similar values of most diastolic parameters, although moderate-severe TBI patients had a significantly lower mean deceleration time compared to mild TBI patients (122.5 versus 162.6 msec).
Table 2.
Early Echocardiographic Findings in Mild and Moderate-Severe Traumatic Brain Injurya
| Cardiac Functional Parameters | Mild TBI (n=32) | Moderate-Severe TBI (n=32) | p |
|---|---|---|---|
| Systolic Functionb,c | |||
| Left Ventricle Area End-Diastole (cm2) | 16.15 (3.68) | 18.60 (4.88) | 0.04 |
| Left Ventricle Area End-Systole (cm2) | 6.99 (1.93) | 8.90 (3.39) | 0.01 |
| Fractional Area Change (cm2) | 0.57 (0.06) | 0.53 (0.11) | 0.10 |
| Left Ventricle Internal Diameter End-Diastole (cm) | 4.55 (0.45) | 4.64 (0.61) | 0.47 |
| Left Ventricle Internal Diameter End-Systole (cm) | 3.02 (0.43) | 3.28 (0.60) | 0.05 |
| Fractional Shortening | 0.34 (0.06) | 0.30 (0.07) | 0.01 |
| Mitral Annular Septal Tissue Velocity [S′(s) (cm/s)] | 8.21 (1.92) | 9.49 (2.36) | 0.05 |
| Mitral Annular Septal Tissue Velocity [S′(s)] < 6 cm/s | 3 (9%) | 2 (6%) | 0.42 |
| Systolic Dysfunction (Fractional Shortening < 0.25) | 0 (0%) | 7 (22%) | <0.01 |
| Diastolic Functiond,e | |||
| Mitral Inflow Peak Early Filling [E wave (cm/s)] | 69.65 (12.53) | 67.44 (19.64) | 0.62 |
| Mitral Inflow Peak Late Filling [A wave (cm/s)] | 52.01 (14.08) | 46.52 (12.83) | 0.17 |
| E-wave to A-wave Ratio | 1.43 (0.42) | 1.55 (0.57) | 0.41 |
| E-wave to A-wave Ratio < 1 | 4 (13%) | 5 (16%) | 0.47 |
| E-wave to A-wave Ratio > 2 | 3 (9%) | 5 (16%) | 0.33 |
| Mitral Inflow E-wave Deceleration Time (msec) | 162.59 (39.18) | 122.5 (43.51) | <0.01 |
| Mitral Annular Septal Tissue Velocity [e′(s) (cm/s)] | 10.28 (2.46) | 9.46 (2.74) | 0.28 |
| E-wave to e′(s) Ratio | 7.05 (1.85) | 7.33 (2.01) | 0.62 |
| E-wave to e′(s) Ratio > 8 | 6 (19%) | 11 (34%) | 0.22 |
| Mitral Annular Septal Tissue Velocity [e′(s)] < 8 cm/s | 5 (16%) | 9 (28%) | 0.31 |
Values are mean(SD) for continuous variables and n(%) for categorical variables
Systolic area, diastolic area, and fractional area change from data available in 28 subjects mild TBI and 26 with moderate-severe TBI
Mitral annular tissue Doppler velocities from data available in 21 subjects with mild TBI and 26 with moderate-severe TBI
Among available echocardiograms within the day after injury all patients with moderate-severe TBI, the median (IQR) fractional shortening was 29% (25% – 34%), with improvement to 33% (30% – 36%) by 7–9 days after injury. Figure 1 describes the change in fractional shortening over the first week of hospitalization in moderate-severe TBI patients with early systolic dysfunction. Among available echocardiograms with the day after injury, the median (IQR) fractional shortening was 20% (16% – 21%), with improvement to 32% (29% – 34%) by 7–9 days after injury.
Figure 1. Change in Fractional Shortening Over the First Week of Hospitalization in Patients with Moderate-Severe Traumatic Brain Injury and Initial Systolic Dysfunction a,b.
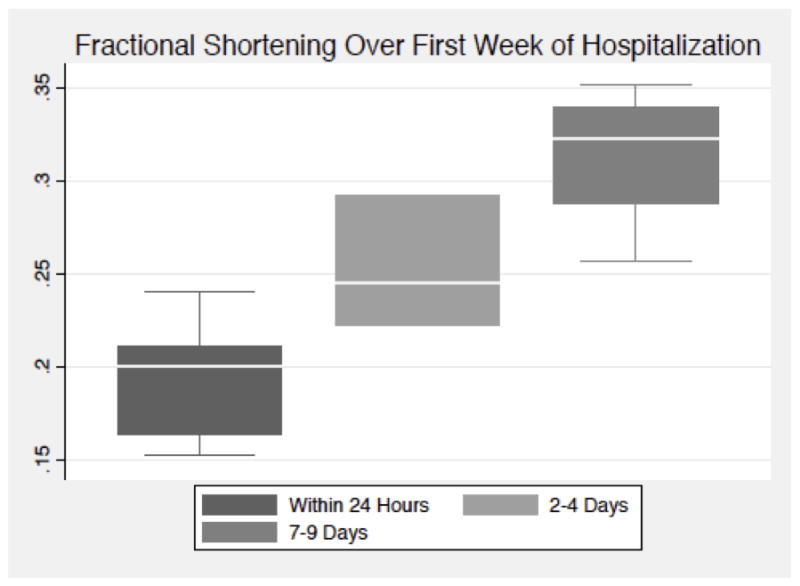
aIn above boxplots, solid line represents median value, box represents interquartile range, and whiskers represent adjacent values
bEchocardiograms at approximately 3 days and 1 week after injury were performed in the majority to patients, although some exams are missing secondary to clinical procedures, patient death, or patient discharge from the hospital
Clinical care during the first 24 hours after injury in patients with moderate-severe TBI is shown in Table 3. Vasopressors were used in 29% of patients with systolic dysfunction, compared to 16% of patients without systolic dysfunction. The use of mannitol (57% versus 40%, respectively) and hypertonic saline (43% versus 16%, respectively) was more common in patients with systolic dysfunction than patients without systolic dysfunction. In-hospital mortality occurred in 3 (43%) patients with systolic dysfunction and 1 (4%) patient without systolic dysfunction. Table 4 shows admission risk factors that are associated with the development of systolic dysfunction in the moderate-severe TBI cohort. On multivariable analysis, age (RR 0.87, 95% CI 0.79 – 0.94, p=0.001) and GCS score on admission (RR 0.34, 95% CI 0.20 – 0.58, p < 0.0001) were independently associated with the development of systolic dysfunction following moderate-severe TBI.
Table 3.
Clinical Care in Moderate-Severe TBI Patientsa
| Variable | No systolic dysfunction (n=25) | Systolic dysfunction (n=7) | p |
|---|---|---|---|
| Age (years) | 39.1 (13.7) | 27.1 (5.6) | 0.03 |
| Male Gender | 21 (84%) | 6 (86%) | 0.91 |
| Glasgow Coma Scale | |||
| Admission GCS | 5.7 (2.5) | 3.4 (1.1) | 0.03 |
| Highest GCS (within 24 hours) | 9.3 (2.1) | 7.7 (5.1) | 0.22 |
| Lowest GCS (within 24 hours) | 4.8 (2.0) | 4.9 (3.8) | 0.99 |
| Admission Hematocrit (%) | 38.0 (5.2) | 37.9 (6.2) | 0.96 |
| Systolic Blood Pressure (mmHg) | |||
| Admission SBP | 131.9 (25.2) | 133.9 (19.4) | 0.85 |
| Lowest SBP in first 24 hours | 98.4 (12.6) | 96.1 (19.5) | 0.71 |
| Hypotension (SBP≤90) in first 24 hours | 8 (32%) | 3 (43%) | 0.59 |
| Hypertension (SBP≥140) in first 24 hours | 19 (76%) | 6 (86%) | 0.58 |
| Admission Oxygen Saturation (%) | 96.4 (3.2) | 92.9 (8.1) | 0.09 |
| Vasopressorb | 4 (16%) | 2 (29%) | 0.45 |
| Phenylephrine | 3 (12%) | 1 (14%) | 0.87 |
| Maximum Dose (mcg/kg/min) | 1.5 (0) | 15 (0) | n/a |
| Norepinephrine | 1 (4%) | 1 (14%) | 0.32 |
| Maximum Dose (mcg/kg/min) | 0.2 (0) | 0.2 (0) | n/a |
| Anti-Hypertensive Useb | 0 (0%) | 0 (0%) | n/a |
| Intracranial Proceduresb | |||
| ICP monitor placement | 12 (48%) | 4 (57%) | 0.67 |
| Need for intracranial surgery | 9 (36%) | 3 (43%) | 0.74 |
| Mechanical Ventilationb | 25 (100%) | 7 (100%) | n/a |
| Propofol Sedationb | |||
| Any Use | 25 (100%) | 7 (100%) | n/a |
| Maximum Dose (mcg/kg/min) | 43.6 (24.9) | 53.6 (21.0) | 0.34 |
| Osmotherapyb | |||
| Mannitol | 10 (40%) | 4 (57%) | 0.42 |
| Hypertonic Saline | 4 (16%) | 3 (43%) | 0.13 |
| Fluid Balance (mL)b | |||
| Intake | 5395 (2558.0) | 6776 (4692.4) | 0.31 |
| Output | 3098 (1440.8) | 3688 (1269.1) | 0.33 |
| Fluid Balance | 2297 (2404.6) | 3088 (4202.6) | 0.52 |
Values are mean(SD) for continuous variables and n(%) for categorical variables.
In first 24 hours after admission
GCS=Glasgow Coma Scale; SBP = systolic blood pressure; mmHg = millimeters of mercury; mL = milliliters
Table 4.
Admission Risk Factors for Systolic Dysfunction Following Moderate-Severe TBI
| Variable | Univariatea | Multivariableb,c | ||||
|---|---|---|---|---|---|---|
| Relative Risk | 95% CI | p | Relative Risk | 95 % CI | p | |
| Age | 0.93 | 0.88 – 0.97 | 0.001 | 0.87 | 0.79 – 0.94 | 0.001 |
| Male Gender | 1.11 | 0.16 – 7.58 | 0.91 | 1.19 | 0.17 – 8.58 | 0.86 |
| Initial Head CT Findings | ||||||
| Epidural Hemorrhage | 0.9 | 0.13 – 6.14 | 0.91 | 2.42 | 0.37 – 15.68 | 0.13 |
| Subarachnoid Hemorrhage | 0.52 | 0.14 – 1.92 | 0.33 | 2.29 | 0.58 – 9.06 | 0.24 |
| Intraparenchymal Hemorrhage | 0.85 | 0.22 – 3.27 | 0.81 | 0.04 | 0.001 – 1.77 | 0.09 |
| Subdural Hemorrhage | 0.83 | 0.19 – 3.57 | 0.81 | 1.02 | 0.25 – 4.18 | 0.97 |
| Admission Glasgow Coma Scale | 0.6 | 0.37 – 0.99 | 0.05 | 0.34 | 0.20 – 0.58 | <0.0001 |
| Admission Systolic Blood Pressure | 1.00 | 0.98 – 1.03 | 0.82 | 0.95 | 0.91 – 1.00 | 0.05 |
Poisson regression models with robust standard errors
Adjusted for all admission risk factors in the univariate analysis, in addition to 24-hour fluid balance, propofol sedation, vasopressor use, osmotherapy use, mechanical ventilation, and need for intracranial surgery
Interobserver reliability determined the mean difference (bias) in left ventricular internal diameter in diastole measurements between observers to be 0.15 cm, with all observations falling within the 95% limits of agreement (−0.80 – 0.50). Intraobserver reliability determined the mean difference (bias) in left ventricular internal diameter in diastole measurements within the same observer (6 weeks apart) to be 0.12 cm, with all observations falling within the 95% limits of agreement (−0.43 – 0.18).
Discussion
In this study, we aimed to provide information on the incidence, trajectory and risk factors associated with cardiac dysfunction after TBI. The primary findings of our study are that: 1) Early systolic dysfunction can occur in previously healthy patients following moderate-severe TBI, 2) Systolic function recovers within the week following injury, and 3) Younger age and greater TBI severity (as measured by admission GCS score) are independently associated with the development of systolic dysfunction early after TBI. To our knowledge, this is the first study to prospectively document and examine the trajectory of systolic function after moderate-severe TBI.
Very little and methodologically limited data exists on cardiac function following TBI. A retrospective study documented the occurrence of systolic dysfunction among TBI patients who underwent echocardiography(10), but TTE was performed at the discretion of the clinical team and the study findings may have been limited by selection bias. In contrast, our current study evaluated cardiac function in all moderate-severe TBI patients meeting stringent criteria (which excluded patients with a high probability of pre-existing cardiac disease or non-TBI induced cardiac dysfunction) – this approach limited selection bias and improved the ability to the isolate the effect of TBI on the heart. Another prospective study(20) demonstrated troponin elevation in 31% of patients following moderate-severe TBI; while echocardiographic dysfunction was not demonstrated in that study, the majority of echocardiograms were performed several days after injury, a period by which most patients in our study had recovered normal systolic function. Acute systolic dysfunction has also been observed after several severe acute non-TBI neurologic diseases including acute emotional distress(21) (classic Takotsubo’s cardiomyopathy), SAH(22), ischemic stroke(23, 24), epilepsy(25, 26), and brain death(27).
In this study, we observed that patients with systolic dysfunction showed marked improvement in cardiac function over their first week of hospitalization. This pattern of improvement is faster than observed following SAH(22), and potentially mirrors the improvement in cerebral edema over the first 2–3 days of injury, rather than the more protracted course of SAH, which includes both the initial hemorrhage and delayed cerebral ischemia from vasospasm(28). It is also possible that the most intense early treatments for cerebral perfusion (i.e. sedation to control ICP, vasopressors, and aggressive resuscitation) are generally de-escalated by 3–7 days after injury, accounting for improved cardiac loading conditions and improved systolic function. Future studies should use more load-independent measures of systolic function, such as the utilization of cardiac MRI or myocardial deformation imaging(29), to better measure intrinsic myocardial dysfunction versus changes in loading conditions.
We found younger age and lower GCS score as the only independent admission risk factors for the development of systolic dysfunction among moderate-severe TBI patients. Our findings also suggested that lower admission systolic blood pressure may be associated with systolic dysfunction, and future studies should examine the hemodynamic implications of systolic dysfunction in more detail. Furthermore, mechanistic studies should better elucidate whether hypotension is a cause or effect of systolic dysfunction following TBI. While systolic dysfunction following neurologic injury is postulated to occur secondary to both dysregulated systemic inflammation and a catecholamine-excess state(30), myocardial catecholamine responsiveness decreases with age(31, 32) and may represent one possible explanation for our finding of a greater risk of systolic dysfunction with younger age. The relationship between initial severity of neurologic injury and systolic dysfunction has been described in other neurologic diseases. For example, in subarachnoid hemorrhage, greater myocardial injury is associated with worse admission clinical symptoms and greater blood load on the initial CT scan(33). Our study findings further contribute to the understanding of the relationship between severity of neurologic injury and the development of systolic dysfunction in neurocritically ill patients.
Apart from a lower deceleration time (a suggestion of restrictive filling independent of elevated E/e′ ratio), we did not observe worse diastolic function in moderate-severe TBI patients, compared to mild TBI patients. One possible reason for this is that the traditional definitions of diastolic dysfunction have been derived in patients with chronic cardiac disease, and these definitions may be inadequate to identify patients with acute diastolic dysfunction secondary to brain-heart interactions or acute changes in loading conditions due to fluid resuscitation. While most studies in the neurocardiac literature have focused mainly on systolic dysfunction, diastolic dysfunction may be a clinically important finding, and future studies should evaluate diastolic function following TBI in more detail.
The findings of our study have clinical relevance. Currently, the approach to early hemodynamic management after TBI does not involve evaluation of the heart, and abnormalities are assumed to be a result of the physiologic stress of brain injury(34), fluid shifts, and/or effects of sedatives(35, 36). Knowledge of early systolic function may allow a more rational use of fluids and vasopressors to optimize cerebral blood flow following TBI. For example, international guidelines(37) suggest maintenance of a cerebral perfusion pressure (CPP) of 50–70 mmHg, given a high risk of the acute respiratory distress syndrome (ARDS) at CPP > 70 mmHg(38). Unfortunately, data informing this recommendation are devoid of the consideration of cardiac function, as presumed ARDS may have been the result of cardiogenic edema in patients with undiagnosed systolic dysfunction who required high doses of vasopressors and fluid infusions in an attempt to reach CPP targets. Thus, knowledge of cardiac function may add to the multimodal data that could help inform rational vasopressor choices and individualize CPP targets in this patient population. Furthermore, as adequate cardiac output is critical for maintenance of cerebral blood flow(39), prevention of the development systolic dysfunction (for example, through the use of low-dose beta-blockade) may also represent a therapeutic target; interestingly, through mechanisms which have not been delineated, early exposure to beta-blockers have been shown to be associated with a survival benefit following TBI(40).
There are some limitations to our study. First, patient care interventions such as sedation, vasopressors, or fluid resuscitation make it impossible to tease out the natural effect of brain injury from subsequent clinical management in causing systolic dysfunction; while we did control for these variables to the best extent possible, there remains the possibility of residual confounding. Thus, the mechanistic underpinnings of our findings require further studies. Second, we chose fractional shortening as our main measure of systolic function rather than the more traditional measure of ejection fraction – the reason for this choice centers around the difficulty in placing many patients in a left lateral decubitus position (due to labile intracranial pressures with patient movement), thus limiting imaging of the true cardiac apex. Fractional shortening may overestimate systolic function in patients with regional wall motion abnormalities beyond the cardiac base, and may lead to an underestimation of the burden of systolic dysfunction in the TBI population. However, calculation of fractional shortening has a strong record of reproducibility and has been used successfully in many clinical studies(41). Third, it is impossible to fully establish that none of our patients had systolic dysfunction prior to their TBI, but this would be unlikely as we sampled a population that was young and had no history of cardiovascular disease prior to their injury. Furthermore, patients with systolic dysfunction recovered to normal function over the first week, suggesting a stress (rather than pre-existing) cardiomyopathy. Lastly, due to the small sample size of our study, our findings should be confirmed in larger and heterogeneous TBI populations.
Conclusions
We found that systolic dysfunction occurred in 22% of previously healthy patients following moderate-severe TBI; furthermore, younger age and lower admission GCS score were independently associated with the development of systolic dysfunction after injury. Our findings provide new information, and suggest that TBI severity adversely impacts cardiac function. Future research should correlate cardiac function with cardiac biomarker changes, examine underlying mechanisms, examine factors associated with improvement in cardiac function, and test therapies to optimize cardiac function following TBI.
Acknowledgments
Funding: NIH – National Research Service Award (T32 GM086270)
Washington State Society of Anesthesiologists Seafair Research Grant
Footnotes
Conflicts of Interest: None
Copyright form disclosure: Dr. Krishnamoorthy’s institution received funding from the National Institutes of Health (NIH) and Washington State Society of Anesthesiologists; and he received support for article research from the NIH. Dr. Temkin’s institution received funding from multiple grant and applications to different US federal sources, and she received funding from consulting for Data and Safety Monitoring Boards and Statistical Consulting (unrelated to paper topic). Dr. Luk received support for article research from the NIH. Dr. Graves received support for article research from the NIH and Foundation Dr. Vavilala received support for article research from the NIH. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Rutland-Brown W, Langlois JA, Thomas KE, et al. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21(6):544–8. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Mascia L, Sakr Y, Pasero D, et al. Extracranial complications in patients with acute brain injury: a post-hoc analysis of the SOAP study. Intensive care medicine. 2008;34(4):720–7. doi: 10.1007/s00134-007-0974-7. [DOI] [PubMed] [Google Scholar]
- 3.Jeremitsky E, Omert L, Dunham CM, et al. Harbingers of poor outcome the day after severe brain injury: hypothermia, hypoxia, and hypoperfusion. J Trauma. 2003;54(2):312–9. doi: 10.1097/01.TA.0000037876.37236.D6. [DOI] [PubMed] [Google Scholar]
- 4.Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34(2):216–22. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Zafar SN, Millham FH, Chang Y, et al. Presenting blood pressure in traumatic brain injury: a bimodal distribution of death. J Trauma. 2011;71(5):1179–84. doi: 10.1097/TA.0b013e3182140d38. [DOI] [PubMed] [Google Scholar]
- 6.Pietropaoli JA, Rogers FB, Shackford SR, et al. The deleterious effects of intraoperative hypotension on outcome in patients with severe head injuries. J Trauma. 1992;33(3):403–7. doi: 10.1097/00005373-199209000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Samuels MA. The brain-heart connection. Circulation. 2007;116(1):77–84. doi: 10.1161/CIRCULATIONAHA.106.678995. [DOI] [PubMed] [Google Scholar]
- 8.Piek J, Chesnut RM, Marshall LF, et al. Extracranial complications of severe head injury. Journal of neurosurgery. 1992;77(6):901–7. doi: 10.3171/jns.1992.77.6.0901. [DOI] [PubMed] [Google Scholar]
- 9.Sookplung P, Siriussawakul A, Malakouti A, et al. Vasopressor use and effect on blood pressure after severe adult traumatic brain injury. Neurocritical care. 2011;15(1):46–54. doi: 10.1007/s12028-010-9448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prathep S, Sharma D, Hallman M, et al. Preliminary report on cardiac dysfunction after isolated traumatic brain injury. Crit Care Med. 2014;42(1):142–7. doi: 10.1097/CCM.0b013e318298a890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnamoorthy V, Sharma D, Prathep S, et al. Myocardial dysfunction in acute traumatic brain injury relieved by surgical decompression. Case Rep Anesthesiol. 2013;2013:482596. doi: 10.1155/2013/482596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marr AL, Coronado VG. Central nervous system injury surveillance data submission standards—2002. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2004. [Google Scholar]
- 13.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 14.Rimel RW, Jane JA, Edlich RF. An injury severity scale for comprehensive management of central nervous system trauma. JACEP. 1979;8(2):64–7. doi: 10.1016/s0361-1124(79)80039-8. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Gottdiener JS, Bednarz J, Devereux R, et al. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17(10):1086–119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Borbely XI, Krishnamoorthy V, Modi S, et al. Temporal Changes in Left Ventricular Systolic Function and Use of Echocardiography in Adult Heart Donors. Neurocritical care. 2015 doi: 10.1007/s12028-014-0101-x. [DOI] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 20.Serri K, El Rayes M, Giraldeau G, et al. Traumatic brain injury is not associated with significant myocardial dysfunction: an observational pilot study. Scand J Trauma Resusc Emerg Med. 2016;24:31. doi: 10.1186/s13049-016-0217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539–48. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 22.Banki N, Kopelnik A, Tung P, et al. Prospective analysis of prevalence, distribution, and rate of recovery of left ventricular systolic dysfunction in patients with subarachnoid hemorrhage. Journal of neurosurgery. 2006;105(1):15–20. doi: 10.3171/jns.2006.105.1.15. [DOI] [PubMed] [Google Scholar]
- 23.Wang TD, Wu CC, Lee YT. Myocardial stunning after cerebral infarction. Int J Cardiol. 1997;58(3):308–11. doi: 10.1016/s0167-5273(96)02879-3. [DOI] [PubMed] [Google Scholar]
- 24.Ay H, Koroshetz WJ, Benner T, et al. Neuroanatomic correlates of stroke-related myocardial injury. Neurology. 2006;66(9):1325–9. doi: 10.1212/01.wnl.0000206077.13705.6d. [DOI] [PubMed] [Google Scholar]
- 25.Strzelczyk A, Adjei P, Scott CA, et al. Postictal increase in T-wave alternans after generalized tonic-clonic seizures. Epilepsia. 2011;52(11):2112–7. doi: 10.1111/j.1528-1167.2011.03266.x. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch LJ, Hauser WA. Can sudden unexplained death in epilepsy be prevented? Lancet. 2004;364(9452):2157–8. doi: 10.1016/S0140-6736(04)17605-8. [DOI] [PubMed] [Google Scholar]
- 27.Krishnamoorthy V, Borbely X, Rowhani-Rahbar A, et al. Cardiac dysfunction following brain death in children: prevalence, normalization, and transplantation. Pediatr Crit Care Med. 2015;16(4):e107–12. doi: 10.1097/PCC.0000000000000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naredi S, Lambert G, Eden E, et al. Increased sympathetic nervous activity in patients with nontraumatic subarachnoid hemorrhage. Stroke. 2000;31(4):901–6. doi: 10.1161/01.str.31.4.901. [DOI] [PubMed] [Google Scholar]
- 29.Shah AM, Solomon SD. Myocardial deformation imaging: current status and future directions. Circulation. 2012;125(2):e244–8. doi: 10.1161/CIRCULATIONAHA.111.086348. [DOI] [PubMed] [Google Scholar]
- 30.Krishnamoorthy V, Mackensen GB, Gibbons EF, et al. Cardiac Dysfunction After Neurologic Injury: What Do We Know and Where Are We Going? Chest. 2016;149(5):1325–31. doi: 10.1016/j.chest.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kendall MJ, Woods KL, Wilkins MR, et al. Responsiveness to beta-adrenergic receptor stimulation: the effects of age are cardioselective. Br J Clin Pharmacol. 1982;14(6):821–6. doi: 10.1111/j.1365-2125.1982.tb02043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebstein RP, Stessman J, Eliakim R, et al. The effect of age on beta-adrenergic function in man: a review. Isr J Med Sci. 1985;21(3):302–11. [PubMed] [Google Scholar]
- 33.Hravnak M, Frangiskakis JM, Crago EA, et al. Elevated cardiac troponin I and relationship to persistence of electrocardiographic and echocardiographic abnormalities after aneurysmal subarachnoid hemorrhage. Stroke. 2009;40(11):3478–84. doi: 10.1161/STROKEAHA.109.556753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grunsfeld A, Fletcher JJ, Nathan BR. Cardiopulmonary complications of brain injury. Current neurology and neuroscience reports. 2005;5(6):488–93. doi: 10.1007/s11910-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 35.Filipovic M, Wang J, Michaux I, et al. Effects of halothane, sevoflurane and propofol on left ventricular diastolic function in humans during spontaneous and mechanical ventilation. British journal of anaesthesia. 2005;94(2):186–92. doi: 10.1093/bja/aei028. [DOI] [PubMed] [Google Scholar]
- 36.Bolliger D, Seeberger MD, Kasper J, et al. Different effects of sevoflurane, desflurane, and isoflurane on early and late left ventricular diastolic function in young healthy adults. British journal of anaesthesia. 2010;104(5):547–54. doi: 10.1093/bja/aeq066. [DOI] [PubMed] [Google Scholar]
- 37.Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. I. Blood pressure and oxygenation. Journal of neurotrauma. 2007;24(Suppl 1):S7–13. doi: 10.1089/neu.2007.9995. [DOI] [PubMed] [Google Scholar]
- 38.Prabhakar H, Sandhu K, Bhagat H, et al. Current concepts of optimal cerebral perfusion pressure in traumatic brain injury. J Anaesthesiol Clin Pharmacol. 2014;30(3):318–27. doi: 10.4103/0970-9185.137260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng L, Hou W, Chui J, et al. Cardiac Output and Cerebral Blood Flow: The Integrated Regulation of Brain Perfusion in Adult Humans. Anesthesiology. 2015;123(5):1198–208. doi: 10.1097/ALN.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 40.Alali AS, McCredie VA, Golan E, et al. Beta blockers for acute traumatic brain injury: a systematic review and meta-analysis. Neurocritical care. 2014;20(3):514–23. doi: 10.1007/s12028-013-9903-5. [DOI] [PubMed] [Google Scholar]
- 41.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–70. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]


