Abstract
No studies to date have prospectively examined early autism spectrum disorder (ASD) markers in infants with fragile X syndrome (FXS), who are at elevated risk for ASD. This paper describes the developmental profiles of eight infants with FXS from 9 to 24 months of age. Four meet diagnostic criteria for ASD at 24 months of age, and four do not. Trends in these case studies suggest that early social-communicative deficits differentiate infants with and without later ASD diagnoses in ways that are similar to later-born siblings of children with ASD. Repetitive behaviors and cognitive and adaptive impairments are present in all FXS infants throughout development, suggesting that these deficits reflect the general FXS phenotype and not ASD in FXS specifically.
Keywords: Fragile X Syndrome, Autism Spectrum Disorder, Infants, Case Studies
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by social-communicative deficits and repetitive and stereotyped behaviors (DSM-5; American Psychiatric Association, 2013). The prevalence of ASD is quite high, with 1 in 68 children diagnosed (CDC, 2014). Given evidence of the impact of early intervention to optimize outcomes, efforts have increasingly focused on identification of ASD markers in the first two years of life, in order to confer eligibility for services and also to target treatment on specific developmental impairments (Jones, Gliga, Bedford, Charman, & Johnson, 2014). One approach to identifying early markers of ASD is through the study of infants at elevated genetic risk for ASD, including infants who have an older sibling with ASD and infants with a genetic syndrome associated with ASD such as fragile X syndrome (FXS). Prospectively studying these infants enables researchers to elucidate the timing and nature of the earliest symptoms of ASD as well as other markers of genetic liability to ASD. The majority of this research has focused on infants with an older sibling diagnosed with ASD, with only a handful of studies on other high-risk groups.
ASD Symptoms in High-Risk Infants with Non-Syndromic ASD
Later-born siblings of children with ASD are at 20 times higher risk to be diagnosed with ASD themselves (Ozonoff et al., 2011). To date, few reliable behavioral indicators of ASD have been consistently documented in infant siblings younger than 12 months, with most work reporting typical development for the first 6 months and subtle behavioral differences manifesting by the first birthday (for review, see Jones et al., 2014; Zwaigenbaum, Bryson, & Garon, 2013). Potential markers identified in the first 12 months include a decline in eye fixation, reduced social gaze, disrupted attention to social stimuli, enhanced visual search abilities, and increased atypical vocalizations (Chawarska, Macari, & Shic, 2013; Gliga, Bedford, Charman, Johnson, & Team, 2015; Jones et al., 2016; Jones & Klin, 2013). Specific temperament profiles, including reduced Positive Affect, increased Negative Affect, and difficulty regulating attention, have also been reported (Clifford et al., 2013; Filliter et al., 2015; Zwaigenbaum et al., 2005).
The predictive validity of distinguishable markers of ASD increases between 12 and 24 months of age, with atypical eye gaze, reduced response to joint attention, lack of response to name, and aberrant visual attention emerging as primary social-communicative indicators (Bedford et al., 2012; Bryson, Zwaigenbaum, McDermott, Rombough, & Brian, 2008; Gammer et al., 2015; Sacrey, Bryson, & Zwaigenbaum, 2013). Elevated rates of repetitive play behaviors and motor movements also emerge as distinguishing features during the second year of life (Christensen et al., 2010; Damiano, Nahmias, Hogan-Brown, & Stone, 2013). Additionally, an early temperament profile marked by high Negative Affect, low Positive Affect, and poor Effortful Control has been noted to distinguish those who meet criteria for ASD (Brian et al., 2008; Bryson et al., 2007; Clifford et al., 2013; Filliter et al., 2015; Garon et al., 2016).
Not surprisingly, there is significant heterogeneity in the developmental trajectories of behavioral markers of ASD in the first two years of life. Evidence suggests that increased severity of ASD at 24 months of age is associated with earlier emergence of atypical development (Estes et al., 2015). Specifically, poor motor abilities and reduced visual reception skills at 6 months of age and impairments in cognitive and adaptive function at 12 months distinguished a subgroup of high-risk infant siblings diagnosed with ASD with severe impairments at 24 months. On the other hand, those diagnosed with ASD with mild to moderate severity at 24 months did not demonstrate delays at 6 or 12 months of age.
In sum, prospective studies of high-risk infant siblings have provided invaluable insight into how ASD symptoms emerge and develop in the first years of life. However, this work has been almost completely restricted to studies of infant siblings of children with ASD, with an absence of etiologically distinct groups such as those at elevated risk of ASD due to a known genetic disorder. Given that identifiable genetic disorders account for 10% of children with ASD (Cohen, Pichard, & Tordjman, 2005), inclusion of genetic disorder cohorts will be useful to parse out the heterogeneity of ASD as well as to identify the generalizability of ASD markers.
ASD Symptoms in Infants with Fragile X Syndrome
Fragile X syndrome (FXS) is the leading known genetic cause of ASD, accounting for 2–6% of ASD cases (Cohen et al., 2005). Furthermore, up to 75% of children with FXS meet diagnostic criteria for ASD (Budimirovic et al., 2006; Harris et al., 2008; Kaufmann et al., 2004; Rogers, Wehner, & Hagerman, 2001). Fragile X syndrome is a monogenic disorder caused by a cytosine-guanine-guanine (CGG) repeat expansion on the Fragile X Mental Retardation-1 (FMR1) gene on the X chromosome. The disorder occurs in approximately 1 in 3,600 males and 1 in 2,700 females and is the most common inherited cause of intellectual disability (Coffee et al., 2009; Fernandez-Carvajal et al., 2009; Kidd et al., 2014). The co-occurrence of ASD in FXS is associated with debilitating effects, so efforts to understand the nature of ASD in FXS are of critical importance (Bailey, Hatton, Skinner, & Mesibov, 2001; Kaufmann et al., 2004; Rogers et al., 2001).
The diagnosis of ASD in FXS is complex and controversial, with some evidence that ASD is qualitatively and mechanistically similar across FXS and idiopathic ASD (iASD; ASD without an identified cause) (Yu & Berry-Kravis, 2014). On the other hand, data also indicate unique behavioral and neurobiological mechanisms, suggesting that ASD features in FXS are distinct from those in iASD and represent part of the FXS phenotype and not a separate disorder (Budimirovic et al., 2006; Hall, Lightbody, Hirt, Rezvani, & Reiss, 2010; Thurman, McDuffie, Kover, Hagerman, & Abbeduto, 2015). Thus, consensus has not been reached on the nature of ASD in FXS as it is not yet clear whether overlapping features in iASD and FXS represent true shared phenotypes or are simply features that look similar but stem from divergent underlying etiology. Cross-syndrome studies of early ASD markers can provide much-needed insight into the heterogeneity of ASD and can contribute to refinement of the FXS phenotype.
To date, only a handful of studies have focused on infants with FXS, and most have targeted features not specific to ASD. Results indicate that developmental scores in male infants with FXS aged 6 to 28 months were dissociated from those of typically developing and high-risk infant siblings of children with ASD as early as 6 months of age, with a trajectory of decreasing standard scores that was not observed in either comparison group (Roberts, McCary, Shinkareva, & Bailey, 2016). Greater impairments in the Visual Reception and Fine Motor domains on the Mullen Scales of Early Learning (MSEL; Mullen, 1995) were particularly salient in discriminating infants with FXS from high-risk siblings. Approximately 68% of the infants with FXS were accurately discriminated from the high-risk siblings based on Mullen profiles indicating the presence of both shared and distinct features across these groups of infants.
Temperament and regulation profiles have also been studied in infants with FXS. Results suggest that Negative Affect predicts anxiety, but not ASD, symptoms in infants and preschool males with FXS (Tonnsen et al., 2013). These findings are especially interesting in contrast to findings in studies of children with iASD, in which Negative and Positive Affect, as well as poor Effortful Control, are robust predictors of ASD symptomatology later in development (Clifford et al., 2013; Filliter et al., 2015; Garon et al., 2016). Attentional control is another potential ASD marker, and infants with FXS have been reported to display impaired attention shifting, which was associated with elevated severity of ASD features (Roberts et al., 2012). These attentional differences were associated with shallower heart rate decelerations during attention, suggesting immature physiological regulation and less efficient physiological coupling. A separate study also documented that physiological regulation, indexed by vagal tone, was associated with ASD symptomatology in infants and toddlers with FXS, with a non-linear relationship observed between heart rate and ASD symptoms (Roberts, Tonnsen, Robinson, & Shinkareva, 2012).
In the only study to date aimed at identifying ASD-specific features in infants with FXS, Roberts, Tonnsen, et al. (2016) reported that 53% of 12-month-old infants with FXS fell in the “at risk” range on the Autism Observation Scale for Infants (AOSI; Bryson et al., 2008), a screening measure for infants at risk for ASD, suggesting that many infants with FXS exhibit early behavioral markers of ASD. The prevalence of infants with FXS exhibiting ASD features was higher than age-matched high-risk infant siblings of children with ASD and low-risk typical controls, of whom 17% and 6% were identified as “at risk” respectively. The AOSI scores at 12 months were moderately related to scores on the Autism Diagnostic Observation Schedule (ADOS-2; Lord, Rutter, et al., 2012) at 24 months (r = .43) in those with FXS. Increased sensory behaviors and insistence on sameness, as well as decreased babbling, eye contact, and social referencing differentiated infants with FXS from high-risk infant siblings. However, atypicalities in motor behavior and motor control were the most striking behaviors exhibited by the FXS group, with more than 80% of infants with FXS exhibiting elevated rates of these atypical behaviors contrasted to less than 20% of high-risk infant siblings. Thus, early motor atypicalities may be salient behavioral markers of ASD in infants with FXS or may be distinctive features of the phenotype of FXS more generally.
In sum, though small in number, group design studies have begun to identify the early markers associated with ASD in FXS across a variety of domains. However, additional work is required before the nature and trajectory of ASD in FXS is better understood. Increased precision in the identification of ASD markers in FXS has important implications for addressing the phenotypic heterogeneity of ASD and its presentation in distinct ASD subtypes. Furthermore, because ASD is associated with poorer outcomes in FXS (Bailey et al., 2001; Kaufmann et al., 2004; Rogers et al., 2001), an understanding of early ASD markers in FXS could facilitate early identification of children. This early identification would, in turn, enable early intervention for ASD symptoms in infants with FXS, providing valuable opportunities to optimize outcomes and improve quality of life in children with FXS and their families.
The objective of this paper is to identify behaviors that might predict later ASD diagnoses in infants with FXS. Here, we present detailed case reviews of eight children with FXS in their first two years of life, focusing primarily on the early emergence of ASD symptoms and other related domains, such as language, cognitive and adaptive abilities, and temperament. Thus, this paper complements existing group design studies by offering detailed and nuanced descriptions of emergent behavior across multiple domains on an individual person-centered level as modeled in a parallel case study report for iASD (Bryson et al., 2007).
Methods
Participants
Cases included eight infants with a confirmed diagnosis of FXS (six males, two females) who were participants in an ongoing longitudinal study of ASD symptoms in infants with FXS. We included the first eight participants who were assessed from 9 months of age (or younger) through 24 months with a clinical best estimate diagnosis at 24 months. The first three males and first female diagnosed with ASD (FXS-ASD) and the first three males and first female not diagnosed with ASD (FXS-O) were included. Participants were recruited through collaboration with ongoing studies on FXS as well as notices posted on FXS listservs and the [BLINDED] Research Registry (https://[BLINDED]). The presence of FXS was confirmed through the review of previous genetics reports provided by the parents of the participants. All study procedures were approved by the [BLINDED] Institutional Review Board and informed consent was obtained from all parents prior to the assessment of their infants.
Measures
Developmental level
The Mullen Scales of Early Learning (MSEL; Mullen, 1995) was administered at the 9-, 12-, and 24-month assessments for an index of general cognitive abilities. The MSEL is for children from birth to 68 months of age, and it includes five subscales: Gross Motor, Visual Reception, Fine Motor, Receptive Language, and Expressive Language. An Early Learning Composite (ELC) score is derived from all subscales except Gross Motor. Internal reliability is .75 to .83 for subscales, and .91 for the ELC (Mullen, 1995).
Adaptive behavior
The Vineland Adaptive Behavior Scales - Second Edition (VABS-II; Sparrow, Cicchetti, & Balla, 2005) measured adaptive functioning at 9, 12, and 24 months. The VABS-II is a semi-structured interview for individuals from birth through 90 years of age across four domains: Communication, Daily Living Skills, Socialization, and Motor Skills with an Adaptive Behavior Composite (ABC). Internal and test-retest reliability are .81 to .90 for subscales, and .94 to .97 for the ABC (Sparrow, Balla, & Cicchetti, 1984).
Temperament
Temperament was assessed at 9 and 12 months of age using the Infant Behavior Questionnaire – Revised (IBQ-R; Gartstein & Rothbart, 2003) and at 24 months of age using the Early Childhood Behavior Questionnaire (ECBQ; Putnam, Gartstein, & Rothbart, 2006). Both measures are well-validated parent-report questionnaires, with the IBQ-R targeting infants aged 3 to 12 months and the ECBQ designed for infants aged 18 to 36 months. Internal reliability for the IBQ-R subscales is good (αs > .70) (Parade & Leerkes, 2008) with internal consistency for ECBQ ranging from .60 to .89 (Putnam et al., 2006).
Three factor scores were calculated for the IBQ-R and the ECBQ to reflect the primary temperament domains: Surgency, Negative Affect, and Effortful Control (Putnam, Rothbart, & Gartstein, 2008). The Surgency factor includes subscales such as Activity Level, Positive Anticipation, High-Intensity Pleasure, and Sociability. The Negative Affect factor includes Fear, Frustration/Distress, and Sadness. The Effortful Control factor takes into account subscales such as Attention, Cuddliness, Soothability, and Inhibitory Control. To compare temperament in FXS to typically-developing (TD) infants, 25 typically-developing, low-risk controls matched in age and sex were drawn from the larger longitudinal study.
ASD symptoms in infants
At the 9- and 12-month assessments, the Autism Observation Scale for Infants (AOSI; Bryson et al., 2008) was used to measure early ASD symptoms and behaviors. The AOSI is a semi-structured play observation designed to identify signs of ASD in infants between the ages of 6 and 18 months. There is a Total Score ranging from 0–50 across 16 items and a Total Marker Score ranging from 0–16. A Total Score of 9 or higher and a Total Marker score of 7 or higher are indicators of elevated ASD risk (Bryson et al., 2008; Zwaigenbaum et al., 2005). The AOSI has test-retest reliability of .61 for Total Score and .68 for Total Marker Score (Bryson et al., 2008), and strong sensitivity (84%) and specificity (98%) for ASD at 12 months (Zwaigenbaum et al., 2005). For the larger longitudinal study, item-level inter-rater agreement (81%) was checked for 20% of administrations.
ASD symptoms at 24 months
The Autism Diagnostic Observation Schedule-2 Toddler Module (ADOS-2; Lord, Rutter, et al., 2012) was administered at 24 months to quantify ASD symptomatology and to assist in clinical best estimate ASD diagnoses. The ADOS-2 is a semi-structured, standardized assessment of communication, social interaction, play, and restricted/repetitive behaviors. The sensitivity of the ADOS-2 ranges from .83 to .91, and the specificity ranges from .86 to .94 (Lord, Rutter, et al., 2012). For the larger longitudinal study, inter-rater item-level agreement was evaluated for 20% of administrations for all items (84%) and diagnostic algorithm items (82%).
Procedure
Parents provided written informed consent. All available time points are presented, with missing data due to incomplete parent forms or missed assessments resulting from scheduling conflicts. Participants were assessed in their homes at 9 and 24 months of age and were assessed in the laboratory at [BLINDED] at 12 months of age. The AOSI and ADOS-2 were administered and scored by research reliable project staff consisting primarily of doctoral students, postdoctoral fellows, and Ph.D.-level investigators. A clinical best estimate (CBE) diagnosis of ASD, adapted from standard procedures (Lord, Petkova, Hus, & et al., 2012; Lord et al., 2006), was determined based on a review of data collected at the 24-month visit. These diagnoses were determined by a multidisciplinary team that included a licensed psychologist and licensed speech-language pathologist who were both research reliable on the ADOS-2. Diagnostic certainty estimates were determined for each case, and are included in the case summaries.
Case Summaries
Below, summaries are provided for each child at each assessment visit. Cases 1–4 represent children diagnosed with ASD (FXS-ASD) while cases 5–8 represent children not diagnosed with ASD (FXS-O) at the 24-month assessment. Three males and one female are included for each group. Throughout the case descriptions, acronyms are used to identify the source of the information provided (e.g., Mullen, AOSI). The key for this shorthand is in Table 1. Scores for each measure at each assessment are listed in Tables 2 and 3.
Table 1.
Shorthand Used in Case Summaries to Identify the Source of Information
| Shorthand | Name of Measure |
|---|---|
| M | Mullen Scales of Early Learning (MSEL) |
| V | Vineland Adaptive Behavior Scales (VABS) |
| T | Infant Behavior Questionnaire – Revised (IBQ-R) or Early Childhood Behavior Questionnaire (ECBQ) |
| AO | Autism Observation Scale for Infants (AOSI) |
| AD | Autism Diagnostic Observation Schedule (ADOS) |
Table 2.
Participant Descriptive Data
| Case | Sex | Chronological Age (months; days) | AOSI Total Score |
AOSI Total Markers |
ADOS Severity Scorea |
Mullen ELC Composite |
Vineland ABC Composite |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9m | 12m | 24m | 9m | 12m | 9m | 12m | Total | SA | RRB | 9m | 12m | 24m | 9m | 12m | 24m | ||
| FXS-ASD | |||||||||||||||||
| 1 | M | 8;29 | 12;27 | 25;13 | 13 | 13 | 8 | 9 | 6 | 5 | 10 | 97 | 91 | 61 | 97 | 79 | 81 |
| 2 | M | 8;25 | 12;11 | 24;19 | 17 | 13 | 11 | 9 | 8 | 9 | 6 | 85 | 64 | 65 | 81 | 78 | 89 |
| 3 | M | 9;20 | 12;14 | 26;14 | 24 | 14 | 11 | 8 | 9 | 7 | 10 | 50 | 51 | 49 | 56 | 60 | 58 |
| 4 | F | 7;22 | -- | 23;15 | 11 | -- | 7 | -- | 6 | 6 | 8 | 85 | -- | 58 | 76 | -- | 66 |
| FXS-O | |||||||||||||||||
| 5 | M | 8;24 | 13;9 | 23;25 | 21 | 7 | 12 | 3 | 3 | 1 | 7 | 70 | 64 | 55 | 74 | 77 | 74 |
| 6 | M | 9;12 | 25;12 | 26;17 | 10 | 14 | 6 | 8 | 1 | 1 | 6 | 76 | 77 | 61 | 99 | 83 | 83 |
| 7 | M | 9;28 | 12;7 | 24;8 | 13 | 7 | 7 | 4 | 3 | 2 | 7 | 84 | 65 | 61 | 78 | 72 | 85 |
| 8 | F | 9;7 | 12;27 | 23;00 | 3 | 1 | 2 | 1 | 2 | 2 | 5 | 98 | 112 | 106 | 86 | 96 | 99 |
ADOS Severity Scores at 24 months; SA = Social Affect; RRB = Repetitive/Restricted Behavior.
Table 3.
Parent-Rated Temperament Factor Scores from the IBQ-R and ECBQ
| Case | Sex | Surgency | Negative Affect | Effortful Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 9m | 12m | 24m | 9m | 12m | 24m | 9m | 12m | 24m | ||
| FXS-ASD | ||||||||||
| 1 | M | 5.34 | 4.00 | 3.62 | 4.17 | 3.41 | 2.62 | 4.44 | 4.22 | 4.78 |
| 2 | M | 6.86 | 5.04 | 4.54 | 5.65 | 4.35 | 2.79 | -- | 4.45 | 4.90 |
| 3 | M | -- | 3.83 | 1.74 | -- | 4.73 | 3.19 | -- | -- | 3.35 |
| 4 | F | 4.63 | 4.62 | 4.66 | 3.71 | 4.39 | 3.16 | 2.95 | 2.55 | 2.94 |
| FXS-O | ||||||||||
| 5 | M | 4.41 | 4.88 | 4.45 | 3.19 | 3.63 | 3.34 | 5.46 | 4.35 | 4.26 |
| 6 | M | 5.22 | 5.65 | 5.58 | 3.45 | 3.52 | 2.99 | 5.78 | 5.03 | 4.57 |
| 7 | M | 4.84 | 4.81 | 4.99 | 4.22 | 3.58 | 2.94 | 5.21 | 4.67 | 4.53 |
| 8 | F | 4.05 | 5.26 | 5.25 | 3.21 | 4.03 | 3.08 | 4.52 | 4.51 | 4.92 |
| TD (n = 25) | 5.17 | 5.23 | 5.13 | 3.73 | 4.17 | 3.01 | 4.65 | 4.82 | 4.49 | |
Case 1: FXS-ASD Male
Nine months
Overall developmental functioning and motor skills are average (M,V); visual tracking of objects and partial object permanence are present (M); expressive language marked by sounds of pleasure (e.g., laughing) and vowel sounds (M); responsive to the examiner’s voice (M). Surgency and Effortful Control are consistent with TD comparisons but Negative Affect is slightly elevated (T). Social interaction is atypical (e.g. limited eye contact, absence of pleasure in interaction with examiner, delayed response to social smile; AO); motor imitation and social babbling are absent (AO); inconsistent response to name and trouble modulating attention to stimuli (AO); atypical motor or sensory behaviors not observed (AO).
Twelve months
Overall developmental functioning and motor are average with expressive language somewhat low (M, V); no words or word approximations spoken (M,V); sounds and gestures used to get attention (V); parallel play has begun to emerge, but consistent interest in other children not clear (V). Surgency, Negative Affect, and Effortful Control are now lower than typical controls (T). Eye contact, response to social smile, and interest in interactions with the examiner are limited (AO); motor imitation is present as is reciprocal social babbling (AO); abnormal reactivity to environmental stimuli, fleeting attention, and excessive interest in particular objects and activities (AO); atypical sensory and motor behaviors are evident (AO).
Twenty-four months
Development is now significantly delayed across all domains (M); combination of jargon/words and gestures is present; however, only a few single words have developed (M); parents report use of 75 communicative signs (V); comprehension of one-step instructions is present (M,V). Surgency is now quite low, while Negative Affect and Effortful Control scores are closer to typical controls (T). Demonstrates interest in other children but cooperative play is inconsistent (V); eye contact is atypical (AD); communicative strategies not well integrated (AD); some evidence of giving and showing (AD); sensory interests, stereotyped behaviors, hand posturing, and complex body mannerisms are observed (AD). Clinical best estimate diagnosis of ASD with Global Developmental Delay (Certainty Estimate: 60–80%).
Case 2: FXS-ASD Male
Nine months
Overall developmental functioning and motor in the low average range (M,V); object permanence not mastered (M); however, visual tracking of objects present (M); vocalizations limited to babbling, but no two-syllable strings used (M); not consistently responding to familiar names or words (M); some interest in same-aged peers (V); will respond to social interaction but does not initiate (V). Surgency exceeds typical level with elevated Negative Affect as well (T). Social interaction limited with poor eye contact (AO); atypical motor or sensory behaviors not present (AO); motor imitation absent and vocalizations not reciprocal in nature (AO); eye contact reduced and response to name variable (AO, M).
Twelve months
Development significantly delayed across all domains (M); object permanence absent (M); two-syllable sounds observed, but frequency reduced (M,V); interested in other children with inconsistent parallel play (V). Surgency close to age expectations, as is Negative Affect with Effortful Control slightly low (T). Eye contact remains limited and reciprocal vocalizations not observed (AO); response to name and motor imitation present (AO); atypical sensory and motor behaviors remain absent (AO).
Twenty-four months
Significant delays in all domains (M); follows one-step instructions consistently, but comprehension of two-step instructions not mastered (M,V); uses fewer than 10 words consistently (M,V). Fewer Surgency-related behaviors than expected, but Negative Affect and Effortful Control are nearer to age expectations (T). Cooperative play is emerging, but limited to siblings (V); eye contact reduced and poorly modulated (AD); directed facial expressions limited to infrequent smiles; rarely integrates communicative strategies (AD); does not show for social purposes, with giving behaviors used primarily to request (AD); play is repetitive and immature (AD); atypical sensory behaviors include visual peering (AD); atypical hand and finger mannerisms present (AD). Clinical best estimate diagnosis of ASD with Global Developmental Delay (Certainty Estimate: 60–80%).
Case 3: FXS-ASD Male
Nine months
Pervasive and significant developmental delays evident (M); single-syllable vocalizations present (M); responsive to parent’s voice, but will not orient to peripheral sounds (M); does not use adaptive strategies to get others’ attention (V); tracks objects, however, no object permanence (M); eye contact is limited (AO,V); social responsiveness is below age expectations, no response to name and absence of social anticipation (AO); atypical sensory and motor behaviors are not present (AO).
Twelve months
Pervasive and significant developmental delays evident (M); plays with sounds, but reduplicative babbling is not observed (M,AO); orients toward environmental sounds (M) but response to name is inconsistent (M,AO); attempts to gain others’ attention but not consistently (V); does not share affection with family members (V); does not reach to others or respond to their reaches to be held (V); parallel play not developed (V). Surgency and Negative Affect scores slightly elevated (T). Motor imitation absent (AO); eye contact atypical (AO); inconsistent interest in the examiner observed (AO); atypical motor behaviors and excessive interests in objects are present (AO).
Twenty-four months
Pervasive and significant developmental delays (M); vocalizations absent (M,V,AD); does not demonstrate interest in same aged-peers but engages in some parallel play (V). Surgency scores are quite low; yet, Negative Affect is close to age expectations (T); modulation of eye contact is inconsistent (AD); does not integrate communicative strategies (AD); giving and showing behaviors extremely limited (AD); social overtures employed solely to seek comfort (AD); no functional play or motor imitation (AD); definite sensory interests, stereotyped behaviors, hand posturing, and complex body mannerisms (AD). Clinical best estimate diagnosis of ASD with Global Developmental Delay (Certainty Estimate: 80–100%).
Case 4: FXS-ASD Female
Nine months
Development is in the low average range (M); partial object permanence is observed (M), visual tracking is present (M,AO), expressive language consists of babbling with vowel and consonant sounds, however sounds of pleasure not reported (V); responsiveness to familiar words and own name limited (M,V). Surgency and Effortful Control scores are slightly lower than age expectations, but Negative Affect appears normal (T). Eye contact is sustained (AO); inconsistent social responsiveness (e.g. interest and pleasure only in response to games with a physical component, social smile absent) (AO); shows some affection (V); atypical sensory and motor behaviors are not observed (AO).
Twelve months
No data available.
Twenty-four months
Global and significant developmental delays are present (M); less than seven words, and no evidence of the combination of words and gestures (M); names for caregivers and familiar members are not used (V); does not point to request or express interest (V); comprehension of simple commands absent (M); cooperative play with peers not yet developed (V). Lower Effortful Control and lower Surgency than typical controls, but Negative Affect not different (T). Social behaviors limited and eye contact not well modulated (AD); integration of communication is absent (AD); giving and showing behaviors extremely limited (AD); social overtures are primarily to seek help or get comfort (AD); functional play is limited and underdeveloped (AD); definite sensory interests (e.g. licking, visual peering) and repetitive interests (e.g. repetitive play with objects) (AD). Clinical best estimate diagnosis of ASD with Global Developmental Delay (Certainty Estimate: 80–100%).
Case 5: FXS-O Male
Nine months
Global developmental delays present (M); visually tracks objects, however does not disengage attention or shift gaze between objects (M); expressive language is average with vocalizations and reduplicative babbling present, however, babbling is infrequent and not yet meaningful or socially reciprocal (M,V,AO); response to social games has emerged, but not consistent (V). Surgency slightly lower than typical controls, as is Negative Affect, but Effortful Control is higher (T). Some evidence of initiation of social interaction (V); eye contact is fleeting (AO); social smile is inconsistent (AO); social anticipatory response and motor imitation have not yet developed (AO); no atypical sensory or motor behaviors are observed (AO).
Twelve months
Global and significant developmental delays remain (M); object permanence has developed (M); reduplicative babbling is present (M); sometimes uses vocalizations to get others’ attention (V); attends to words and names (M); interested in peers, with early interactive play emerging (V). Surgency and Negative Affect continue to be slightly lower than age expectations, and Effortful Control now lower as well (T). Eye contact consistent and sustained (AO); age appropriate social reciprocity and motor imitation present (AO); social babbling absent with minimal vocalizations (AO); no atypical sensory or motor behaviors present (AO).
Twenty-Four Months
Global and significant developmental delays (M); receptive and expressive language remain delayed; single words have developed, and gesture/word combinations (e.g. “bye-bye” + wave) are present (M, AD); understands simple verbal input (M); one-step instructions are followed (V); one-word requests used to get needs met (V). Surgency remains slightly below age expectations, but Negative Affect and Effortful Control scores are similar to typical controls (T). Social skills a relative strength, and appropriate eye contact, direction of facial expressions, and response to name (AD); some sensory and repetitive behaviors observed (e.g. licking of objects, repetitively throwing) (AD); no atypical hand, finger, or body movements (AD); overactivity, mild aggression, and anxiety observed (AD). Clinical best estimate diagnosis of Global Developmental Delay (Certainty Estimate: 60–80%).
Case 6: FXS-O Male
Nine months
Overall developmental functioning significantly delayed (M); expressive language average with vocalizations and reduplicative babbling observed (M); receptive language less developed, with no evidence of attention to words (M); shows interest in same-aged peers (V); Surgency at age expectations, Negative Affect slightly below that of TD controls, and Effortful Control is elevated relative to controls (T). Social interaction is inconsistent with social smile absent to examiner but present with parents, as well as fleeting social enjoyment and interest (AO,V); atypical motor behaviors (i.e. flapping) observed (AO); however atypical sensory behaviors not noted (AO).
Twelve months
Global and significant developmental delays are present (M); attention to words and names (including own name) is present (M); fine motor skills include a partial pincer grasp and coordination of two hands together (M); gross motor skills within normal limits (M). Surgency and Effortful Control near age expectations, and Negative Affect lower than typical controls (T). Eye contact consistent and sustained (AO); social interaction is inconsistent with delayed social smile, and inconsistent social interest and shared affect (AO); motor imitation present (AO); social babbling absent with minimal vocalizations (AO); no atypical sensory behaviors (AO); exhibits atypical motor behaviors, but not repetitive interests (AO).
Twenty Four Months
Global and significant developmental delays across all domains (M); beginning to follow one-step directions (M,V); engages in cooperative play with peers (V); able to share concern for others through actions (V). Effortful Control, Negative Affect and Surgency are similar to same-aged TD controls (T). Social skills remain a relative strength with appropriate eye contact and direction of facial expressions (AD); response to name limited to parent (AD); social behaviors of requesting, showing, and giving are observed and used with integrated communication (AD); engages in repetitive behaviors (e.g. throwing toys) (AD); no atypical sensory interests noted (AD); brief finger flicking observed (AD); mild overactivity noted (AD). Clinical best estimate diagnosis of Global Developmental Delay (Certainty Estimate: 80–100%).
Case 7: FXS-O Male
Nine months
Overall developmental functioning is in the low average range (M); visually tracks objects (M); expressive language average with vocalizations and babbling observed (M); uses sounds and gestures to get caregivers’ attention (V); receptive language is average, evidenced by attention to words and names (M); engages in parallel play with peers (V). Surgency is slightly lower than typical controls, and Negative Affect and Effortful Control are slightly elevated (T). Social interaction inconsistent, with typical eye contact and social smiling but absent social babbling and erratic social interest (AO); atypical motor behaviors, such as whole body rocking, observed (AO); no atypical sensory or repetitive behaviors (AO).
Twelve months
Global and significant developmental delays across all domains (M); vocalizations limited to single-sound babbles (M); attention to words and familiar names is present (M); limited interest in same-aged children (V). Surgency and Negative Affect somewhat lower than typical controls, but Effortful Control is at expected level (T). Eye contact consistent and sustained (AO); social interest and shared affect inconsistent (AO); age-appropriate motor imitation (AO); social babbling absent with minimal vocalizations (AO); no atypical sensory behaviors (AO); engages in repetitive motor behaviors (AO).
Twenty-four months
Global and significant developmental delays across all domains except gross motor (M); uses caregivers’ names, and initiates one word requests (V); follows one-step instructions, though inconsistently (V); social skills include appropriate eye contact and direction of facial expressions (AD); shows interest in peers and engages in early friendship-seeking behaviors (V); displays clear desire to please others (V). Effortful Control, Surgency, and Negative Affect are similar to typical controls (T). Age-appropriate social behaviors of requesting and showing (AD); repetitive behaviors (e.g., hand and finger posturing) present (AD); no atypical sensory interests (AD). Clinical best estimate diagnosis of Global Developmental Delay (Certainty Estimate: 80–100%).
Case 8: FXS-O Female
Nine months
Overall developmental functioning is average (M); vocalizations not yet consistently directed (AO); receptive language is average with attention to words and names present (M); shows understanding of the word “no” (M,V); uses sounds and gestures to get others’ attention (V); shows interest in same-aged peers and engages in parallel play (V). Surgency is below age expectations, as is Negative Affect, but Effortful Control scores are similar to typical controls (T). Age-appropriate social skills noted, including eye contact, social interest, and social anticipation (AO); atypical sensory or motor behaviors not observed (AO).
Twelve months
Overall developmental functioning is average (M); expressive language comprised of approximately five single words as well as jargon and gesture combinations (M); names of caregivers consistently used (V); distal pointing is used (V); receptive language marked by object identification and comprehension of simple commands (M). Effortful Control, Surgency, and Negative Affect are similar to TD controls (T). Social skills include typical social interest and shared affect, reciprocal social smiling, social anticipation, and sustained eye contact (AO); no atypical sensory or motor behaviors or repetitive interests observed (AO).
Twenty-four months
Global development is in average range, but expressive language skills above average with 3–4 word sentences and at least 100 recognizable words (M,V); able to match objects and sort by size and shape (M); follows one-step and “if-then” instructions consistently (V); appropriate eye contact and direction of facial expressions (AD); plays cooperatively with peers (V). Surgency and Negative Affect consistent with typical controls, with Effortful Control slightly elevated (T); requesting and giving observed, but showing inconsistent (AD); no sensory interests, though some subtle hand flapping exhibited (AD). Clinical best estimate diagnosis of No Clinical Features. (Certainty Estimate: 80–100%).
Results
Though the primary objective of this case series paper was to qualitatively examine patterns of development in infants with FXS who do and do not later receive a comorbid diagnosis of ASD, statistical analyses were utilized to provide quantitative evidence of the qualitative profiles observed. Because of the extremely small sample size (n = 4) in each outcome group, results should be interpreted with caution and effect size estimates should be the primary focus.
Independent samples t-tests were employed to explore the visual trends observed in the AOSI scores. Results from these analyses can be found in Table 4. To determine whether proportions of children that exceeded the “risk” cutoff differed between the FXS-ASD and FXS-O children, Fisher’s exact tests were run. At nine months, 100% (n = 4) of the FXS-ASD cases and 75% (n = 3) of the FXS-O cases exceeded the Total Score Cutoff, a difference that is not significant, p = .50. Similarly, groups did not differ in the proportion of children exceeding the Total Marker Cutoff, p = .21 (FXS-ASD: 100%; n = 4; FXS-O: 50%; n = 2). At 12 months of age, 100% (n = 3) of FXS-ASD cases and 25% (n = 1) of FXS-O cases exceeded the Total Score Cutoff, a marginal difference, p = .11. Similarly, 100% (n = 3) of FXS-ASD cases and 25% (n = 1) of FXS-O cases exceeded the Total Marker Cutoff at 12 months, p = .11. Results from independent samples t-tests comparing ADOS severity scores between the groups at 24 months can be found in Table 4.
Table 4.
Results of independent samples t-tests
| Measure | FXS-ASD M (SD) | FXS-O M (SD) | Test statistic | p-value | Effect size (Cohen’s d) |
|---|---|---|---|---|---|
| Early ASD Risk Markers (AOSI) | |||||
| Total Score – 9m | 16.25 (5.74) | 7.25 (5.32) | t(6) = 0.96 | .38 | 1.63 |
| Total Score – 12m | 13.33 (0.58) | 7.50 (4.93) | t(5) = 1.99 | .10 | 1.66 |
| Marker Score – 9m | 9.25 (2.06) | 6.75 (4.11) | t(6) = 1.09 | .32 | 0.77 |
| Marker Score – 12m | 8.67 (0.58) | 4.00 (2.94) | t(5) = 2.65 | <.05 | 2.20 |
| ASD Severity (ADOS) | |||||
| Total Severity Score – 24m | 7.75 (1.26) | 2.25 (0.96) | t(6) = 6.97 | <.001 | 4.91 |
| SA Severity Score – 24m | 6.75 (1.71) | 1.50 (0.58) | t(6) = 5.82 | <.01 | 4.11 |
| RRB Severity Score – 24m | 8.50 (1.91) | 6.25 (0.96) | t(6) = 2.10 | .08 | 1.49 |
| Temperament Factors (IBQ-R & ECBQ) | |||||
| Surgency – 9m | 5.61 (1.14) | 4.63 (0.51) | t(5) = 1.56 | .18 | 1.11 |
| Surgency – 12m | 4.38 (0.56) | 5.17 (0.37) | t(6) = −2.37 | .06 | 0.44 |
| Surgency – 24m | 3.61 (1.35) | 4.93 (0.57) | t(6) = −1.76 | .13 | 1.27 |
| Negative Affect – 9m | 4.51 (1.01) | 3.26 (0.13) | t(5) = 2.52 | .05 | 1.74 |
| Negative Affect – 12m | 4.22 (0.57) | 3.70 (0.22) | t(6) = 1.70 | .14 | 1.20 |
| Negative Affect – 24m | 2.94 (0.28) | 3.19 (0.18) | t(6) = −1.49 | .19 | 1.06 |
| Effortful Control – 9m | 3.70 (1.05) | 5.31 (0.54) | t(4) = −2.63 | .06 | 1.93 |
| Effortful Control – 12m | 3.74 (1.04) | 4.56 (0.32) | t(5) = −1.53 | .19 | 1.07 |
| Effortful Control – 24m | 3.99 (0.99) | 4.50 (0.31) | t(6) = −0.98 | .37 | 0.70 |
| Change in Surgency | −1.34 (1.22) | 0.30 (0.67) | t(5) = −2.30 | .07 | 1.67 |
| Change in Negative Affect | −1.65 (1.16) | −0.07 (0.29) | t(5) = −2.70 | <.05 | 1.87 |
| Change in Effortful Control | 0.17 (0.25) | −0.81 (0.80) | t(4) = 1.58 | .19 | 1.65 |
| Developmental Level (MSEL) | |||||
| Raw Score Total – 9m | 28.50 (5.00) | 35.75 (8.09) | t(6) = −1.52 | .18 | 1.08 |
| Raw Score Total – 12m | 40.67 (12.22) | 50.75 (8.77) | t(5) = −1.28 | .26 | 0.95 |
| Raw Score Total – 24m | 58.50 (17.71) | 75.75 (17.67) | t(6) = −1.38 | .22 | 0.98 |
| Change in Raw Score Total | 30.00 (12.99) | 40.00 (11.92) | t(6) = −1.14 | .30 | 0.80 |
| Adaptive Abilities (VABS-II) | |||||
| Raw Score Total – 9m | 56.75 (20.27) | 79.25 (19.43) | t(6) = −1.61 | .16 | 1.13 |
| Raw Score Total – 12m | 76.67 (28.45) | 104.50 (19.43) | t(5) = −1.55 | .18 | 1.14 |
| Raw Score Total – 24m | 138.25 (59.47) | 191.50 (30.91) | t(6) = −1.59 | .16 | 1.12 |
| Change in Raw Score Total | 81.50 (42.19) | 112.25 (20.56) | t(6) = −1.31 | .24 | 0.93 |
To compare trajectories of change in temperament factors over time, change scores were calculated for each factor, defined as the score at 24 months minus the score at 9 months. Independent samples t-tests were employed to investigate group differences in change in each factor. Temperament factor scores were also compared at each timepoint using independent samples t-tests. (See Table 4).
Trajectories of change in developmental level over time were examined by quantifying change in scores on the MSEL over time. Raw score totals were calculated by summing the Visual Reception, Fine Motor, Receptive Language, and Expressive Language raw scores at each timepoint. Change was defined as raw score total at 24 months minus raw score total at 9 months. Independent samples t-tests were used to examine group differences in change. Table 4 depicts these results.
To compare trajectories of change in adaptive skills over time, VABS-II raw score totals were calculated by summing the Communication, Daily Living, Socialization, and Motor raw scores at each timepoint. Change was defined as raw score total at 24 months minus raw score total at 9 months. Independent samples t-tests were used to examine group differences in change in adaptive skills. Results are outlined in Table 4.
Discussion
Fragile X syndrome is the leading known genetic cause of ASD, with up to 75% of children meeting diagnostic criteria for ASD (Budimirovic et al., 2006; Harris et al., 2008; Rogers et al., 2001). As such, efforts to identify the early features of ASD in FXS have great utility for refining the infant phenotype in FXS, directing treatment efforts in FXS, and characterizing the latent heterogeneity in ASD. The aim of this paper is to describe the emergence of ASD in FXS using a case study approach, contrasting infants with FXS who meet diagnostic criteria for ASD at 24 months of age to those who do not. Overall, we report that specific features of ASD, along with accompanying developmental and temperament profiles, are apparent within the first year of life in infants with FXS who receive a clinical best estimate diagnosis of ASD at 24 months of age. However, many early markers of ASD risk are subtle or absent at 9 and 12 months of age, with complex and dynamic interactions among variables for outcomes associated with and without ASD.
ASD Symptoms and Diagnoses
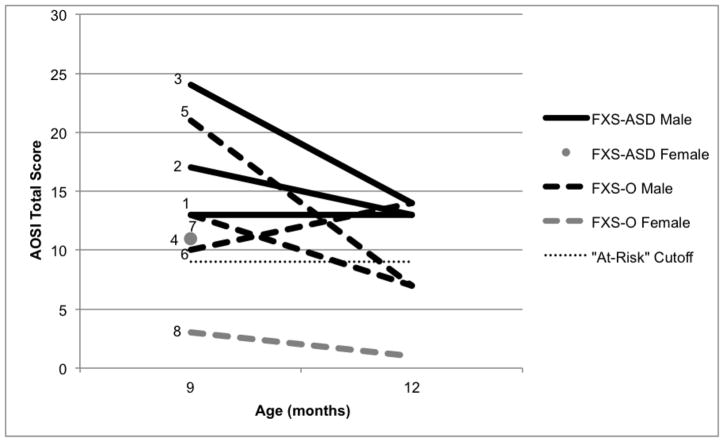
Using the AOSI, a screening measure of ASD, we observed elevated rates exceeding the “at risk” cutoff in all four infants with FXS-ASD at both 9 and 12 months of age. However, at 9 months of age, three of the four infants in the FXS-O group were also above the total score cutoff, though only one of these infants had an elevated score at 12 months (see Figure 1). Thus, while the majority of infants with FXS had elevated scores at 9 months of age (seven out of eight), those who later received a diagnosis of ASD maintained elevated scores on the AOSI through 12 months of age. Results from quantitative analyses support our qualitative findings, in that groups differed marginally on the Total Score and significantly on the Marker Score at 12 months, but not 9 months. This indicates that in FXS infants, the AOSI may be more sensitive at 12 months of age than at 9 months of age, which aligns with findings in high-risk siblings of children with ASD indicating increased specificity with age (Gammer et al., 2015). Also, these case profiles suggest that the AOSI may have better specificity with true positive cases than with false positive cases, which is consistent with our group-level findings in infants with FXS [BLINDED].
Figure 1.
AOSI Total Score at 9 and 12 months. Infants who received a diagnosis of ASD at 24 months (FXS-ASD) are indicated by solid lines, and infants who did not receive a diagnosis of ASD (FXS-O) are indicated by dashed lines. Males are represented in black and females in gray.
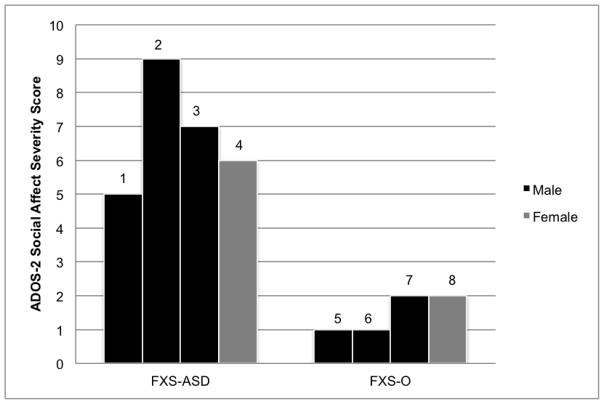
In terms of the core features of ASD, deficits in social communication appear to be the most striking and earliest emerging ASD marker in this sample. By nine months of age, infants with FXS-ASD have clear social-affective deficits including reduced eye contact, limited social interest, lack of social smiling, and no social babbling. While the infants in the FXS-O group are not exhibiting age-appropriate social behaviors, they demonstrate clear strengths (e.g., sustained eye contact). Furthermore, their behavior is characterized more by inconsistencies as opposed to a discrete absence of social behaviors. Thus, children with FXS-O may be better described as having delayed social development, whereas children with FXS-ASD may be described as having aberrant social development. These distinctions are confirmed by contrasting the ADOS-2 Social Affect severity scores, with clear patterns distinguishing the groups (Figure 2). Statistical analyses also suggest that groups differed significantly on the Social Affect severity score (Table 4).
Figure 2.
ADOS-2 Social Affect Severity Scores at 24 Months. Males are represented in black and females in gray.
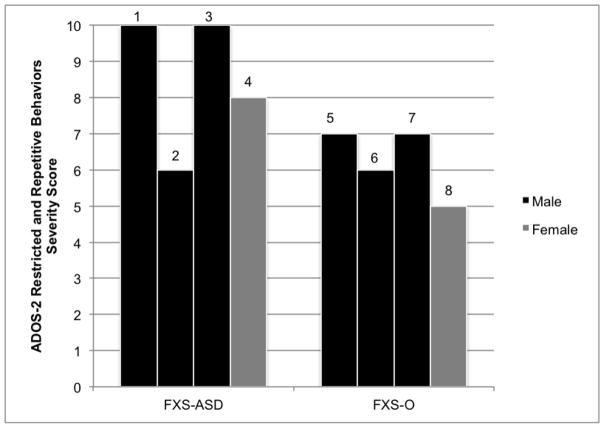
Early repetitive motor mannerisms, such as hand flapping and body rocking, may not be specific to early features of ASD in FXS as these were actually present in a greater number of the FXS-O infants than the FXS-ASD infants. Instead, it may be later-emerging sensory interests and repetitive play behaviors that differentiate those with ASD and those without. It is possible that atypical motor behaviors are simply a feature of FXS itself, and not uniquely related to ASD in this population (Thurman et al., 2015). These distinctions are confirmed by the ADOS-2 profiles at 24 months, which illustrate that all eight children exhibited some form of repetitive or restricted behavior (Figure 3). Alternatively, motor abnormalities in FXS could represent complex relationships, with lower order and repetitive motor movements (e.g., body rocking, hand flapping) present in FXS independent of ASD and higher order motor and repetitive features representing a unique marker for ASD in FXS. These relationships may differ qualitatively from those with idiopathic (non-FXS) ASD or may reflect developmental factors (Oakes, Thurman, McDuffie, Bullard, Hagerman, & Abbeduto, 2016; Wolff, Bodfish, Hazlett, Lightbody, Reiss, & Piven, 2012). This is supported by evidence from our group that atypical motor behavior and poor motor control (e.g., hand flapping, body rocking, finger posturing) were present in 80% of 12-month-old infants with FXS, a rate that was elevated in contrast to just 20% of high-risk siblings of children with ASD [BLINDED].
Figure 3.
ADOS-2 Restricted and Repetitive Behaviors Severity Scores at 24 Months. Males are represented in black and females in gray.
Developmental and Adaptive Skills
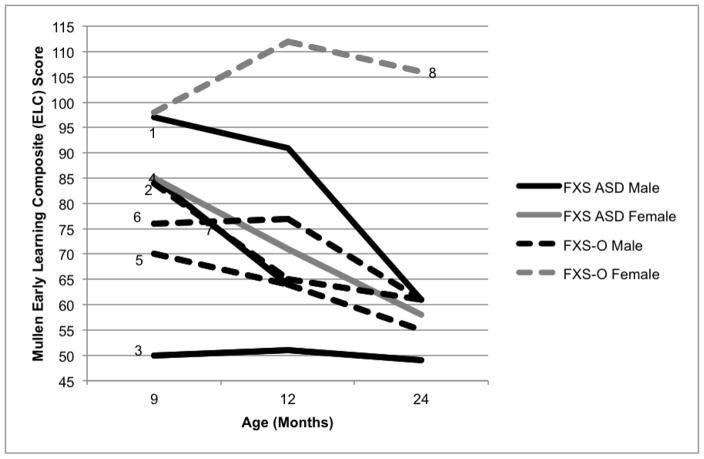
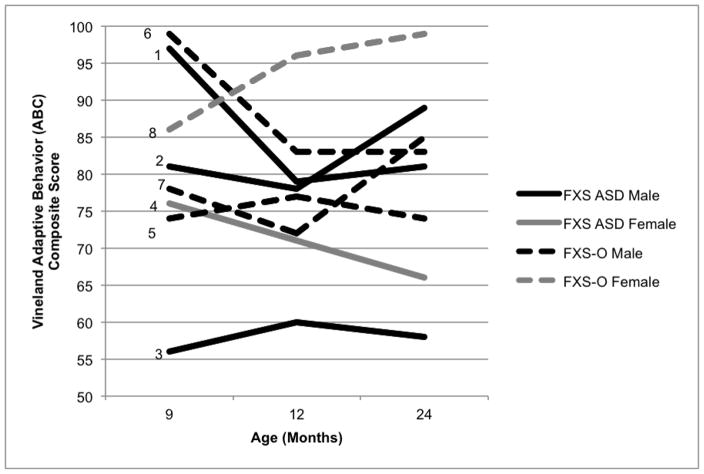
Males in both groups showed developmental and adaptive trajectories consistent with previous FXS group reports (Roberts et al., 2016), demonstrating delays by nine months of age that became more pronounced over time (see Figure 4). Surprisingly, adaptive skills were quite inconsistent in the FXS-O group with varying trajectories over time (see Figure 5). The female in the FXS-O group displayed developmental and adaptive skills that were in the average range consistently across age. This aligns with reports that a subset of females with the full mutation have average cognitive skills, while the majority have subtle to pronounced delays (Bailey, Sideris, Roberts, & Hatton, 2008; Hunter et al., 2014). In contrast, and surprisingly, the FXS-ASD group demonstrated greater variability in developmental skills, with three of the infants (two males, one female) displaying composite scores in the average range at nine months of age, which is somewhat atypical (Roberts et al., 2016). However, all three of these infants displayed fairly marked declines in their developmental trajectories that emerged by 12 or 24 months of age. As was observed in the FXS-O group, adaptive skills also showed a great deal of variability in the FXS-ASD children, both male and female. Results from quantitative analyses suggest that groups did not differ on developmental or adaptive scales at any timepoint, and that no group differences in change in scores over time emerged (Table 4).
Figure 4.
Mullen ELC Composite Scores by Age in Months. Infants who received a diagnosis of ASD at 24 months (FXS-ASD) are indicated by solid lines, and infants who did not receive a diagnosis of ASD (FXS-O) are indicated by dashed lines. Males are represented in black and females in gray.
Figure 5.
Vineland ABC Composite Scores by Age in Months. Infants who received a diagnosis of ASD at 24 months (FXS-ASD) are indicated by solid lines, and infants who did not receive a diagnosis of ASD (FXS-O) are indicated by dashed lines. Males are represented in black and females in gray.
Our findings are quite consistent with profiles reported for high-risk infant siblings of children with ASD, which are characterized by decreasing developmental scores from 6 to 24 months of age as a marker for ASD, with those having the steepest decline of scores at risk for the most severe ASD outcomes (Estes et al., 2015; Gammer et al., 2015). Our results extend the work in idiopathic (non-FXS) ASD by suggesting that these trends may also exist in a low-functioning etiologically-specific condition. However, the severity and consistency of the developmental delays appear more appreciable in our FXS sample, as would be expected given that intellectual disability is one of the most common outcomes in FXS, especially in males.
Temperament
Temperament profiles suggest that subtle differences may exist between those children who receive an ASD diagnosis, and those who do not, particularly for males. The most salient temperament profile differences were related to Surgency, the factor that indexes social approach behaviors, reactions to exciting activities, and physical activity. Males with FXS-ASD tended to score higher on this factor at nine months of age with steady declines across age and lower scores than controls and the FXS-O group by 24 months. In contrast, Surgency scores for males without ASD and both females stay relatively stable across development (see Table 3). Statistical analyses confirmed that groups demonstrated a marginally different trajectory in Surgency scores over time (Table 4). A similar, but subtler, pattern was noted for the Negative Affect factor scores. Males with FXS-ASD exhibited decreasing scores over time, starting off slightly higher than the FXS-O males and both females. Most FXS-O boys and both females exhibited relatively stable Negative Affect scores across development. Interestingly, by 24 months of age, all children had similar levels of parent-reported Negative Affect. Results from independent samples t-tests provide support of this qualitative pattern, with the FXS-ASD demonstrating a significantly larger decrease in Negative Affect scores from 9 to 24 months (see Table 4). Subtle differences appear present on the Effortful Control factor with a slight shift from elevated to more normalized scores observed for three of the males from the FXS-O group, in contrast to the males with FXS-ASD, who showed stable scores that aligned with the typical controls, and a consistently lower score for the female with FXS-ASD.
These initial findings from our study represent somewhat stark contrasts to temperament profiles reported for high-risk infants of children with ASD who are later diagnosed with ASD. Reduced Surgency at 7 and 14 months, reduced Effortful Control at 14 and 24 months, and increased Negative Affect across the second year of life have been identified as traits that distinguished high-risk infant siblings from typical controls (Clifford et al., 2013). Low Behavioral Approach (Garon et al., 2009) and low Positive Affect (Zwaigenbaum et al., 2005) have also been reported as associated with elevated risk for ASD. Thus, non-FXS high-risk infants appear to display a temperament profile characterized by a general blunting of reactivity and regulation with increased Negative Affect. In contrast, infants with FXS later diagnosed with ASD display initial elevated reactivity and approach at nine months and decreasing Negative Affect over time, and previous work suggests that this profile may be more closely associated with anxiety as opposed to ASD (Tonnsen et al., 2013).
Limitations and Future Directions
This is the first qualitative case series paper to examine developmental trajectories in infants with FXS with and without later ASD diagnoses. Similar work has been published in other high-risk cohorts, including later-born siblings of children with ASD (Bryson et al., 2006). The present study examines early development through a unique lens and serves as an important complement to group-design studies by providing detailed case summaries and drawing conclusions based on qualitative patterns observed across cases. Thus, this series of case studies provides insight that is not possible to glean from group-design studies and also sheds light on preliminary patterns that may be examined in larger samples in future group-design studies. However, there are some limitations inherent in the case study approach, particularly the very small sample size, which limits the conclusions that can be drawn from the data presented and also prohibits generalization to other infants with FXS. Additionally, due to the scope of the longitudinal infant study which focused on the emergence of autism features at 9, 12 and 24 months of age, it was not possible to examine long-term ASD outcomes or diagnostic stability in this case series. In fact, little work has focused on this important topic, and much remains to be understood regarding the early presentation and stability of ASD in FXS. Work out of our lab has recently begun to examine the longitudinal trajectories of ASD features and diagnoses to determine what factors contribute to the stability, or lack thereof, from infancy into the preschool years. We hypothesize that, similar to high-risk infant sibling findings, subgroups will emerge, with some infants showing early-emerging and enduring ASD features, others displaying few ASD features across early development, and yet another cohort that presents with unstable ASD feature presentation. This work is important to refine the infant phenotype in FXS and to contribute to the debate regarding the shared and unique ASD features and trajectories in FXS contrasted to idiopathic non-syndromic ASD.
Summary and Implications
Utilizing a prospective case study approach to highlight individual differences across multiple measures this study demonstrated that early indicators of ASD apppear early as a rather ubiquitous feature in infants with FXS at 9 months of age. By 12 months of age, however, elevated ASD features appear to remain primarily for those who are later diagnosed with ASD. Social-communication deficits and elevated Surgency may represent the earliest presentation of ASD features in FXS, with steep declines in developmental scores across age as another potential marker. The early presentation of social-communication deficits and declining developmental trajectories is consistent with other high-risk infants who are later diagnosed with ASD (e.g. infant siblings of children with ASD), while differences with elevated Surgency in early infancy and reductions in Surgency and Negative Affect across age may be unique to infants with ASD associated with FXS. Follow-up studies with larger samples to confirm these initial findings are underway.
Findings from this study provide partial support for shared behavioral trajectories in infants with FXS to those with idiopathic (non-syndromic) ASD. This is important as it suggests that early markers of ASD that have been found useful for early detection in idiopathic ASD may also apply to infants with FXS. Likewise, if the behavioral profiles of ASD are shared across ASD associated with FXS and non-syndromic causes, then treatments reported as beneficial in non-syndromic ASD could be recommended for ASD associated with FXS. This is particularly important given the challenges of large scale randomized clinical trials in infants with FXS associated with the relatively low prevelance. In fact, no behavioral treatment studies have been published with infants and toddlers with FXS to date. One cautionary note is that similar behavioral profiles do not necessarily reflect shared mechanistic underpinings, so future work should include both behavioral and mechanistic features to derive a firmer conclusion regarding etiology and treatment.
Acknowledgments
The authors are grateful to the families who participated in this research. Special thanks to John Colombo for his thoughtful feedback on an earlier draft of this manuscript. This research was funded by the National Institute of Mental Health (R01MH107573; R01MH0901194; PI: Roberts).
Footnotes
Statement of Human Rights: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- American Psychiatric Association. 5. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- Bailey DB, Hatton DD, Skinner M, Mesibov G. Autistic behavior, FMR1 protein, and developmental trajectories in young males with Fragile X syndrome. Journal of Autism and Developmental Disorders. 2001;31(2):165–174. doi: 10.1023/a:1010747131386. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Sideris J, Roberts J, Hatton D. Child and genetic variables associated with maternal adaptation to fragile X syndrome: A multidimensional analysis. American Journal of Medical Genetics Part A. 2008;146(6):720–729. doi: 10.1002/ajmg.a.32240. [DOI] [PubMed] [Google Scholar]
- Bedford R, Elsabbagh M, Gliga T, Pickles A, Senju A, Charman T, et al. Precursors to social and communication difficulties in infants at-risk for autism: Gaze following and attentional engagement. Journal of Autism and Developmental Disorders. 2012;42(10):2208–2218. doi: 10.1007/s10803-012-1450-y. [DOI] [PubMed] [Google Scholar]
- Brian J, Bryson SE, Garon N, Roberts W, Smith IM, Szatmari P, Zwaigenbaum L. Clinical assessment of autism in high-risk 18-month-olds. Autism. 2008;12(5):433–456. doi: 10.1177/1362361308094500. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, McDermott C. A prospective case series of high-risk infants who developed autism. Journal of Autism and Developmental Disorders. 2007;37(1):12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, Brian J. The autism observation scale for infants: Scale development and reliability data. Journal of Autism and Developmental Disorders. 2008;38(4):731–738. doi: 10.1007/s10803-007-0440-y. [DOI] [PubMed] [Google Scholar]
- Budimirovic DB, Bukelis I, Cox C, Gray RM, Tierney E, Kaufmann WE. Autism spectrum disorder in fragile X syndrome: Differential contribution of adaptive socialization and social withdrawal. American Journal of Medical Genetics Part A. 2006;9999:1–13. doi: 10.1002/ajmg.a.31405. [DOI] [PubMed] [Google Scholar]
- CDC. Prevalence of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. Morbidity and Mortality Weekly Report Surveillance Summaries. 2014;64(SS02):1–21. [PubMed] [Google Scholar]
- Chawarska K, Macari S, Shic F. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biological Psychiatry. 2013;74(3):195–203. doi: 10.1016/j.biopsych.2012.11.022. http://dx.doi.org/10.1016/j.biopsych.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen L, Hutman T, Rozga A, Young GS, Ozonoff S, Rogers SJ, et al. Play and developmental outcomes in infant siblings of children with autism. Journal of Autism and Developmental Disorders. 2010;40(8) doi: 10.1007/s10803-010-0941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford SM, Hudry K, Elsabbagh M, Charman T, Johnson MH, et al. Temperament in the first 2 years of life in infants at high-risk for autism spectrum disorders. Journal of Autism and Developmental Disorders. 2013;43(3):673–686. doi: 10.1007/s10803-012-1612-y. [DOI] [PubMed] [Google Scholar]
- Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL, Warren ST. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. American Journal of Human Genetics. 2009;85(4):503–514. doi: 10.1016/j.ajhg.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Pichard N, Tordjman S. Specific genetic disorders and autism: clinical contribution towards their identification. Journal of Autism and Developmental Disorders. 2005;35(1):103. doi: 10.1007/s10803-004-1038-2. [DOI] [PubMed] [Google Scholar]
- Damiano CR, Nahmias A, Hogan-Brown AL, Stone WL. What do repetitive and stereotyped movements mean for infant siblings of children with autism spectrum disorders? Journal of Autism and Developmental Disorders. 2013;43(6):1326–1335. doi: 10.1007/s10803-012-1681-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes A, Zwaigenbaum L, Gu HB, St John T, Paterson S, Elison JT, et al. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. Journal of Neurodevelopmental Disorders. 2015;7:10. doi: 10.1186/s11689-015-9117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Carvajal I, Walichiewicz P, Xiaosen X, Pan R, Hagerman PJ, Tassone F. Screening for expanded alleles of the FMR1 gene in blood spots from newborn males in a Spanish population. Journal of Molecular Diagnostics. 2009;11(4):324–329. doi: 10.2353/jmoldx.2009.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliter JH, Longard J, Lawrence MA, Zwaigenbaum L, Brian J, Garon N, et al. Positive affect in infant siblings of children diagnosed with autism spectrum disorder. Journal of Abnormal Child Psychology. 2015;43(3):567–575. doi: 10.1007/s10802-014-9921-6. [DOI] [PubMed] [Google Scholar]
- Gammer I, Bedford R, Elsabbagh M, Garwood H, Pasco G, Tucker L, et al. Behavioural markers for autism in infancy: Scores on the Autism Observational Scale for Infants in a prospective study of at-risk siblings. Infant Behavior & Development. 2015;38:107–115. doi: 10.1016/j.infbeh.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon N, Bryson SE, Zwaigenbaum L, Smith IM, Brian J, Roberts W, Szatmari P. Temperament and its relationship to autistic symptoms in a high-risk infant sib cohort. Journal of Abnormal Child Psychology. 2009;37(1):59–78. doi: 10.1007/s10802-008-9258-0. [DOI] [PubMed] [Google Scholar]
- Garon N, Zwaigenbaum L, Bryson S, Smith IM, Brian J, Roncadin C, … Roberts W. Temperament and its association with autism symptoms in a high-risk population. Journal of Abnormal Child Psychology. 2016;44(4):757–769. doi: 10.1007/s10802-015-0064-1. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior and Development. 2003;26(1):64–86. doi: 10.1016/s0163-6383(02)00169-8. [DOI] [Google Scholar]
- Gliga T, Bedford R, Charman T, Johnson MH, Team B. Enhanced visual search in infancy predicts emerging autism symptoms. Current Biology. 2015;25(13):1727–1730. doi: 10.1016/j.cub.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Hirt M, Rezvani A, Reiss AL. Autism in fragile X syndrome: A category mistake? Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(9):921–933. doi: 10.1016/j.jaac.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones BL, Ferranti J, Bacalman S, Barbato I, et al. Autism profiles of males with fragile X syndrome. American Journal on Mental Retardation. 2008;113(6):427–438. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JE, Rivero-Arias O, Angelov A, Kim E, Fotheringham I, Leal J. Epidemiology of fragile X syndrome: A systematic review and meta-analysis. American Journal of Medical Genetics Part A. 2014;16A:1648–1658. doi: 10.1002/ajmg.a.36511. [DOI] [PubMed] [Google Scholar]
- Jones EJH, Gliga T, Bedford R, Charman T, Johnson MH. Developmental pathways to autism: A review of prospective studies of infants at risk. Neuroscience and Biobehavioral Reviews. 2014;39:1–33. doi: 10.1016/j.neubiorev.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJH, Venema K, Earl R, Lowy R, Barnes K, Estes A, et al. Reduced engagement with social stimuli in 6-month-old infants with later autism spectrum disorder: A longitudinal prospective study of infants at high familial risk. Journal of Neurodevelopmental Disorders. 2016;8(7) doi: 10.1186/s11689-016-9139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature. 2013;504(7480):427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, et al. Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. American Journal of Medical Genetics Part A. 2004;129A(3):225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Kidd SA, Lachiewicz A, Barbouth D, Blitz RK, Delahunty C, McBrien D, et al. Fragile X syndrome: A review of associated medical problems. Pediatrics. 2014;134(5):995–1005. doi: 10.1542/peds.2013-4301. [DOI] [PubMed] [Google Scholar]
- Lord C, Petkova E, Hus V, Gan W, Lu F, Martin DM, et al. A multisite study of the clinical diagnosis of different autism spectrum disorders. Archives of General Psychiatry. 2012;69(3):306–313. doi: 10.1001/archgenpsychiatry.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PC, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Archives of General Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Los Angeles, CA: Western Psychological Services; 2012. [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning: AGS edition. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Oakes A, Thurman AJ, McDuffie A, Bullard LM, Hagerman RJ, Abbeduto L. Characterising repetitive behaviours in young boys with fragile X syndrome. Journal of Intellectual Disability Research. 2016;60(1):54–67. doi: 10.1111/jir.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics. 2011;128(3):e1–e8. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parade SH, Leerkes EM. The reliability and validity of the Infant Behavior Questionnaire-Revised. Infant Behavior & Development. 2008;31(4):637–646. doi: 10.1016/j.infbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam SP, Gartstein MA, Rothbart MK. Measurement of fine-grained aspects of toddler temperament: The early childhood behavior questionnaire. Infant Behavior & Development. 2006;29(3):386–401. doi: 10.1016/j.infbeh.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam SP, Rothbart MK, Gartstein MA. Homotypic and heterotypic continuity of fine-grained temperament during infancy, toddlerhood, and early childhood. Infant and Child Development. 2008;17(4):387–405. doi: 10.1002/icd.582. [DOI] [Google Scholar]
- Roberts JE, Hatton DD, Long AC, Anello V, Colombo J. Visual attention and autistic behavior in infants with fragile X syndrome. Journal of Autism and Developmental Disorders. 2012;42(6):937–946. doi: 10.1007/s10803-011-1316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, McCary LM, Shinkareva SV, Bailey DB. Infant development in fragile X syndrome: Cross-syndrome comparisons. Journal of Autism and Developmental Disorders. 2016;46(6):2088–2099. doi: 10.1007/s10803-016-2737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen BL, McCary LM, Caravella KE, Shinkareva SV. Brief report: Autism symptoms in infants with fragile X syndrome. Journal of Autism and Developmental Disorders. 2016;46(12):3830–3837. doi: 10.1007/s10803-016-2903-5. http://doi.org/10.1007/s10803-016-2903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen BL, Robinson A, Shinkareva SV. Heart activity and autistic behavior in infants and toddlers with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities. 2012;117(2):90–102. doi: 10.1352/1944-7558-117.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: Symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Journal of Developmental Behavioral Pediatrics. 2001;22(6):409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Sacrey LAR, Bryson SE, Zwaigenbaum L. Prospective examination of visual attention during play in infants at high-risk for autism spectrum disorder: A longitudinal study from 6 to 36 months of age. Behavioural Brain Research. 2013;256:441–450. doi: 10.1016/j.bbr.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales - Second Edition. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- Thurman AJ, McDuffie A, Kover ST, Hagerman RJ, Abbeduto L. Autism symptomatology in boys with fragile X syndrome: A Cross sectional developmental trajectories comparison with nonsyndromic autism spectrum disorder. Journal of Autism and Developmental Disorders. 2015;45(9):2816–2832. doi: 10.1007/s10803-015-2443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnsen BL, Malone PS, Hatton DD, Roberts JE. Early negative affect predicts anxiety, not autism, in preschool boys with fragile X syndrome. Journal of Abnormal Child Psychology. 2013;41(2):267–280. doi: 10.1007/s10802-012-9671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Bodfish JW, Hazlett HC, Lightbody AA, Reiss AL, Piven J. Evidence of a distinct behavioral phenotype in young boys with fragile X syndrome and autism. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51(12):1324–1332. doi: 10.1016/j.jaac.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TW, Berry-Kravis E. Autism and fragile X syndrome. Seminars in Neurology. 2014;34(3):258–265. doi: 10.1055/s-0034-1386764. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Garon N. Early identification of autism spectrum disorders. Behavioural Brain Research. 2013;251:133–146. doi: 10.1016/j.bbr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23(2–3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]