Abstract
Ghrelin is a stomach-produced hormone that stimulates ingestive behavior and increases motivated behavior to obtain palatable foods. Ghrelin receptors (growth hormone secretagogue receptors; Ghsr) are expressed in the lateral hypothalamic area (LHA), and LHA-targeted ghrelin application increases ingestive behavior in male rodents. However, the effects of LHA ghrelin signaling in females are unexplored. Here we investigated whether LHA ghrelin signaling is necessary and sufficient for control of ingestive and motivated behavior for food in male and female rats. Ghrelin delivered to the LHA increased food intake and motivated behavior for sucrose in both male and female rats, whereas increased food-seeking behavior and body weight were only observed in females. Females had slightly higher Ghsr levels in the LHA compared to males, and importantly, acute blockade of the Ghsr in the LHA significantly reduced food intake, body weight, and motivated behavior for sucrose in female but not male rats. Chronic LHA Ghsr reduction in female rats achieved by RNA inference-mediated Ghsr knockdown resulting in a 25% reduction in LHA Ghsr mRNA abolished the reward-driven behavioral effects of LHA-targeted ghrelin, but was not sufficient to affect baseline food intake or food reward responding. Collectively we show that ghrelin acts in the LHA to alter ingestive and motivated behaviors in a sex-specific manner.
Keywords: ghrelin, lateral hypothalamus, body weight, operant conditioning, food motivation
1. Introduction
Ghrelin is an orexigenic peptide produced mainly in the stomach [1-5]. Circulating levels of ghrelin are highest during fasting and prior to anticipated meals, and a sharp reduction in ghrelin is detected immediately following food ingestion [1, 6, 7]. Both peripheral and central administration of ghrelin induces hyperphagia, whereas pharmacological blockade of ghrelin receptors reduces food intake [8]. The orexigenic effect of ghrelin is mediated by the activation of the growth hormone secretagogue receptor 1A (Ghsr), with CNS targets for Ghrs-mediated orexigenic effects originally thought to be the arcuate and paraventricular nuclei of the hypothalamus and the nucleus of the solitary tract in the hindbrain [9-12]. However, recent work has identified extra-hypothalamic and extra-hindbrain neural targets through which ghrelin stimulates ingestive behavior [13-15]. For example, ghrelin increases motivation to obtain food through Ghsr action in the ventral tegmental area (VTA), the nucleus accumbens, and the ventral hippocampus [15-18]. The endogenous role of CNS ghrelin signaling in reward regulation is supported by data showing that pharmacological or genetic blockade of Ghsr signaling decreases motivated behavior for food [18-21].
Ghsr expression is distributed throughout multiple areas of the brain, including the lateral hypothalamic area (LHA) [22], a key area involved with ingestive behavior and reward-based behavior [23-26]. Activation of LHA neurons stimulates food seeking and eating (even in satiated rats) [27] and rodents will lever press to stimulate LHA neurons without satiation [28-30]. More recent studies have confirmed a functional connection between ghrelin signaling and the LHA. Ghrelin injections into the lateral ventricle, or directly into the LHA, activate LHA neurons, as illustrated by increased c-Fos levels in this nucleus following ghrelin injections [31, 32]. Furthermore, several groups have demonstrated that ghrelin indirectly, or directly, activates LHA neurons that produce the orexigenic neuropeptide, orexin (aka hypocretin) [16, 20, 31, 33]. Orexin neurons connect the LHA with the mesolimbic dopamine neurotransmission [34] and thus, findings linking ghrelin and orexin signaling suggest that the LHA is a critical gateway for ghrelin to affect phasic dopamine transmission [35]. While direct intra-LHA ghrelin administration has been shown to stimulate ingestive behavior for less palatable chow [35-37]; the role of LHA ghrelin signaling in motivated responding for palatable food remains unexplored.
The overwhelming majority of research investigating the hyperphagic effects of CNS ghrelin signaling has been conducted exclusively in male rodents. Very little is known about sex differences in this system. However, recent literature suggests a possible modulatory role of sex on ghrelinergic feeding effects. For example, ghrelin mRNA levels increase after ovariectomy, an effect reversed by estrogen replacement [38]. Additionally, both males and ovariectomized females are more sensitive to systemic or ventricular injection of ghrelin than intact females, suggesting that the orexigenic potency of ghrelin is modulated by estrogen [39]. On the other hand, only female Ghsr-null mice fed with standard chow display reduced body weight and adiposity compared to wild type animals [40]. Thus, existing data give little clues on how the LH ghrelin responses may differ in males and females, but they certainly suggest that sex differences after LH-ghrelin manipulation are likely.
In this study we investigated the role of LHA ghrelin signaling on food intake and motivated responding for palatable food in male and female rats. We first determined whether ghrelin stimulates ingestive behavior and operant responding for sucrose in males and females when administered directly into the LHA. We also assessed the effects of ghrelin intra-LHA microinjection on hypothalamic expression of orexin in females, which has been reported to increase in male rats after ghrelin administration. To assess whether ghrelin signaling in the LHA is necessary for food reward behavior, we measured food ingestion, food seeking, and motivated responding for palatable food after pharmacologically blocking LHA Ghsr signaling. Finally, we determined the effect of chronic blockade of Ghsr by specifically knocking down Ghsr in the LHA of female rats using an adenovirus carrying short hairpin RNAs specifically targeting the Ghsr transcript.
2. Material and Methods
2.1 Animals
Male and female Sprague-Dawley rats purchased from Charles River, Germany (5 weeks at arrival) were housed in individual cages in a 12h light/dark cycle with ad libitum access to chow and water, unless otherwise stated. Female rats were free cycling throughout the study. The estrous cycle was followed in order to ensure that testing days were not biased towards a particular phase. All phases were represented on each testing day for most testing days. Behavioral testing was conducted during the mid-light cycle, unless otherwise stated. All studies were carried out with ethical permissions from the Animal Welfare Committee of the University of Gothenburg, in accordance with legal requirements of the European Community (Decree 86/609/EEC).
2.2 Drugs
Ghrelin and the Ghsr antagonist (YIL 781) [41] were purchased from Tocris and dissolved in artificial cerebrospinal fluid (aCSF; Tocris), also used as vehicle. Drugs were stored as aliquots at -20°C.
2.3 Brain cannulation
Guide cannulas were implanted into the LHA using the following coordinates adapted from [42]: ±1.5 mm from midline, 2.8 mm posterior to bregma, and 6.8 mm ventral from the surface of the skull, with injector aimed 8.8 mm ventral to skull. These coordinates were chosen to position the tip of the injector in the LHA, yet as far away as possible from other hypothalamic Ghsr-expressing sites like the paraventricular nucleus or the dorsomedial hypothalamus. As a result of this strategy our manipulation may not have reached the most medial or ventral parts of the LHA, while consistently reaching the central, lateral and dorsal LHA, as suggested by examination of India ink distribution in rat brains from this study as well as preliminary studies. Cannula implantation surgery was performed under ketamine/xylazine anesthesia. All rats were at least given one week to recover from surgery before experimental testing. Dental acrylic and jeweler's screws were used to secure the cannula and the incision was closed using an obturator as previously described [43]. Placement for ghrelin and Ghsr antagonist injections was confirmed by microinjection of India-ink post mortem at the same volume used throughout the study (0.5 μL). Representative image of intra-LHA injection is presented in Figure 1J. Only subjects with correctly placed cannulas were included in the data analysis. All ghrelin injections were unilateral; antagonist and AAV injections were bilateral. The latter were administered bilaterally since blocking unilaterally leaves the other side to transmit the signal, and therefore reducing the chances of detecting a behavioral impact of the blockade.
Figure 1.
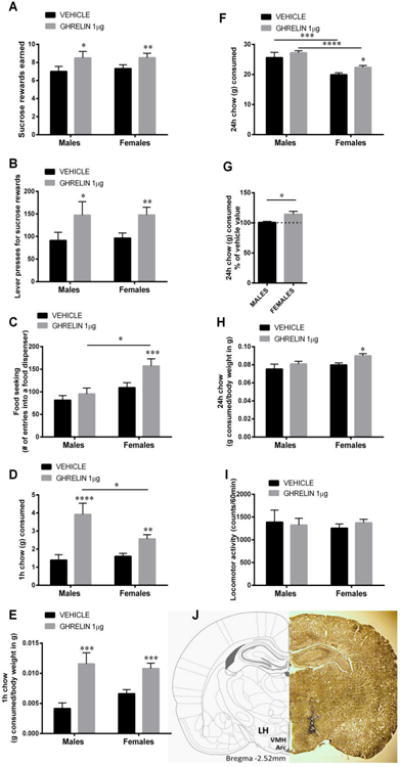
Ghrelin delivery to the lateral hypothalamic area (LHA) increases food reward and food intake in male and female rats. Intra-LHA ghrelin microinjection increases the amount of sucrose rewards earned (A) and the number of lever presses for the rewards (B) in a progressive ratio schedule in both male and female rats. Food-seeking behavior (C; number of head entries into the food dispenser) was not altered in male rats but was significantly increased in female rats after LH-targeting ghrelin injection. Chow intake was also increased at 1 h in both sexes; however, the response was more potent in males (D). In contrast, 24 h chow intake was elevated by the LH ghrelin treatment only in females (F). Since at 24 h chow intake differed significantly between males and females injected with vehicle, in order to directly compare response magnitude between the sexes data are also presented as % of vehicle intake, to account for the baseline differences (G). 1 and 24 h food intake data are also presented as g consumed per g of body weight (E, H). Locomotor activity was not affected by the treatment (I) Data are expressed as mean ±SEM. n=14-15 (males) and n=23-34 (females). Brain atlas and tissue section micrograph of a representative injection placement (J). LH, lateral hypothalamus; Arc, arcuate nucleus of the hypothalamus; VMH, ventromedial hypothalamus. *p < 0.05, ** p < 0.01,*** p < 0.005,**** p < 0.001 compared to vehicle (aCSF).
2.4 Operant conditioning
The operant lever press conditioning procedure is used to assess the motivation to obtain a food reward. Training and testing were conducted in rat conditioning chambers (Med-Associates, Georgia, VT, USA) as described previously [44, 45]. Training was conducted in four phases in ad libitum fed rats. Thus, rats were not food restricted at any time during operant training. Rats were first trained on the fixed ratio 1 (FR1) schedule in 30 min sessions (single press on the active lever resulted in the delivery of one sucrose pellet (45 mg)), followed by FR3 and FR5 (3 and 5 presses per pellet respectively), where a minimum of 30 responses on the active lever per session was required for advancement to the next schedule. Finally the rats were trained in progressive ratio (PR) conditioning sessions until stable responding was achieved. The response requirement increased according to the following equation: response ratio = [5e(0.2 × infusion number)] – 5 through the following series: 1, 2, 4, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328. Responding was considered stable when the number of pellets earned per session did not differ more than 15% between three consecutive sessions. All operant response testing was performed after the responses stabilized. Each PR session lasted for 60 min.
2.5 LHA-targeted ghrelin microinjection effects on food intake, body weight, locomotor activity andfood motivated behavior
To test the effects of ghrelin, ad libitum-fed male (n=14-15) or female (n=23-24) rats were injected with ghrelin (1.0 μg) or vehicle (aCSF) and operant conditioning responses were tested 10 min after injection. Food seeking was assessed as the number of head pokes into the feeding chamber during the 60 min operant session. Chow intake was measured 1 and 24 h after the operant testing sessions. Each treatment was counterbalanced where each condition was separated by a two-day period. Locomotor activity was measured using horizontal infrared beams in the operant chambers (Med-Associates).
2.6 Effects of pharmacological LHA-targeted blockade on food intake, body weight, locomotor activity and food motivated behavior
For experiments examining the effect of Ghsr blockade, rats were fasted overnight, and injected with YIL 781 (25 μg) or vehicle (aCSF) in the LHA at the start of the light cycle [n=5 (female rats) and n=9 (male rats)]. Behavior and body weight were measured as described above.
2.7 LHA dissections and gene expression
LHA tissue was collected by micro punch dissection of cryostat sections. Serial 80 μ m sections through figures 23–33 in the Paxinos and Watson rat brain atlas (second edition, 1986) were collected with the help of landmarks such as the fornix, optic tracts, and the third ventricle to identify LHA. RNA was isolated using the RNeasy Lipid Tissue Mini Kit (Quiagen) and gene expression levels were quantified by quantitative real-time PCR (qPCR) using Taqman gene expression kits from Life Technologies (primer information: Orx1 Rn00565995_ml; Ghsr1 Rn00821417_m1; actin beta Rn00667869_m1). Comparative threshold cycle method [46] was used to quantify relative mRNA expression.
2.8 Ghsr knockdown in female rats
To knockdown the expression of Ghsr in the LHA, an adeno-associated virus (AAV) containing short hairpin RNAs (shRNA) for RNA interference targeting the Ghsr transcript was used (AAV2-GFP-rGHSR-shRNA, Vector BioLabs). A scrambled shRNA expressing GFP was used as control (AAV2-GFP-U6-shRNA, Vector BioLabs). Female rats (n=12 per treatment group, each rat in this study received one microinjection of ghrelin and one of vehicle followed by a PR test one week prior to the AAV infusion) were trained in the operant conditioning test until stable responding was obtained on PR. Before surgery, rats were divided into two groups which were matched for body weight and PR-performance. After surgical implantation of bilateral LHA-directed guide cannulae (as described above), control (GFP AAV) or Ghsr-targeting (Ghsr AAV) AAV was infused bilaterally into the LHA (0.5 μl/hemisphere over a 5 min period). Microinjectors were left in place for an additional 10 min after infusion to allow for diffusion of injectate. Body weight and food intake were measured daily post AAV construct infusion, except for days where fasting or food restriction was applied. Motivation to self-administer sucrose was assessed using a PR schedule 3 and 7 days after AAV injections, since previous work indicated that RNAi-induced effects can emerge already one week after injections [47]. Before the PR test on day 14, rats were food restricted overnight since food deprivation has previously been shown to be effective in increasing circulating ghrelin levels in both male and female rats [53]. On days 17 and 20 additional PR tests were conducted following counterbalanced ghrelin or vehicle injections to confirm that the knockdown was functional i.e. sufficient to block or attenuate LHA-driven responses to ghrelin. Four weeks after the AAV infusion, rats received free choice of a high-fat/high-sugar diet (HFHS; which consisted of a choice of chow, water, lard or 30% sucrose water). Three weeks later, adipose tissue and brains were collected. The LHA was dissected as described above to assess Ghsr expression using qPCR. An LHA-containing coronal section (10 μm) was collected from each brain to verify correct placement of AAV injections.
2.9 Statistical analysis
All the data are presented as mean ± Standard Error of the Mean (SEM). Statistical significance was analyzed using t-test or one- or two-way ANOVA with Holm-Sidak's multiple comparison tests, when appropriate (GraphPad Software, Inc., San Diego, CA). P-values lower than 0.05 were considered statistically significant.
3. Results
3.1 Male and female rats respond differently to acute LHA-targeted ghrelin injections
Intra-LHA ghrelin microinjection induced a robust escalation in food reward behavior, as indicated by increased number of sucrose rewards earned in male and female rats (Fig 1A). Two-way ANOVA analysis indicates a significant effect of drug (F (1, 46) = 16.61, p<0.0005), but not sex (F (1, 46) = 0.06, p>0.05), and no interaction (F (1, 46) = 0.06, p>0.05). Likewise, increased number of lever presses emitted for the sucrose rewards were detected for both sexes (Fig 1B; two-way ANOVA analysis indicates a significant effect of drug (F (1, 46) = 14.34, p<0.0005), but not sex (F (1, 46) = 0.02, p>0.05), and no interaction (F (1, 46) = 0.03, p>0.05), without changes in food seeking (Fig 1C) in male rats. Unlike the response in males, the female rats also increased their food-seeking behavior (Fig 1C). Two-way ANOVA analysis indicates a significant effect of drug (F (1, 44) = 6.26, p<0.05), and sex (F (1, 44) = 4.92, p<0.05), but the interaction did not reach significance (F (1, 44) = 1.88, p=0.17). Locomotor activity was not affected by the treatment (Fig 1I): drug (F (1, 39) = 0.043, p>0.05), sex (F (1, 39) = 0.061, p>0.05), interaction (F (1, 39) = 0.47, p>0.05). Locomotor activity was measured in all females but only half of the males. The increase in 1 h food intake was more potent for male rats (Fig 1D,E), with ghrelin-injected male rats consuming more than three-fold the amount they ate while injected with vehicle, while female rats increased their intake an average of 50%. Two-way ANOVA analysis indicates a significant effect of drug (F (1, 45) = 34.45, p<0.0005), but not sex (F (1, 45) = 3.20, p=0.08), and a significant interaction (F (1, 45) = 7.0, p<0.05). At 24 h, food intake in males was not altered, but remained elevated 24h after the LHA-targeted ghrelin microinjection in female rats (Fig 1F,H). Two-way ANOVA analysis indicates a significant effect of drug (F (1, 35) = 6.443, p<0.05), and sex (F (1, 35) = 24.99, p<0.0005), but the drug × sex interaction did not reach significance (F (1, 35) = 0.26, p>0.05). Since 24h chow intake differed significantly between males and females injected with vehicle, in order to directly compare the response magnitude between the sexes data are also presented as % of vehicle intake, to account for the baseline differences, with this transformation it is clear that food intake is elevated above baseline in females but not males (Fig 1G). In line with the increased 24h food intake in females they also displayed increased body weight gain (1.04±1.48 for vehicle and 4.67±1.07 for ghrelin, p<0.05).
3.2 Ghsr and orexin expression in the LHA
Since a different pattern of response was found in male and female rats, we compared the mRNA levels of Ghsr in the LHA of male and female rats. We found that the female rats have approximately 30% more Ghsr mRNA compared to male rats (Fig 2A). Since little is known about the potential neurochemicals targeted by ghrelin in the LHA of female rats, we microinjected ghrelin into the LHA of the female rats and measured orexin gene expression. We found that orexin mRNA was increased after ghrelin treatment in female rats (Fig 2B).
Figure 2.
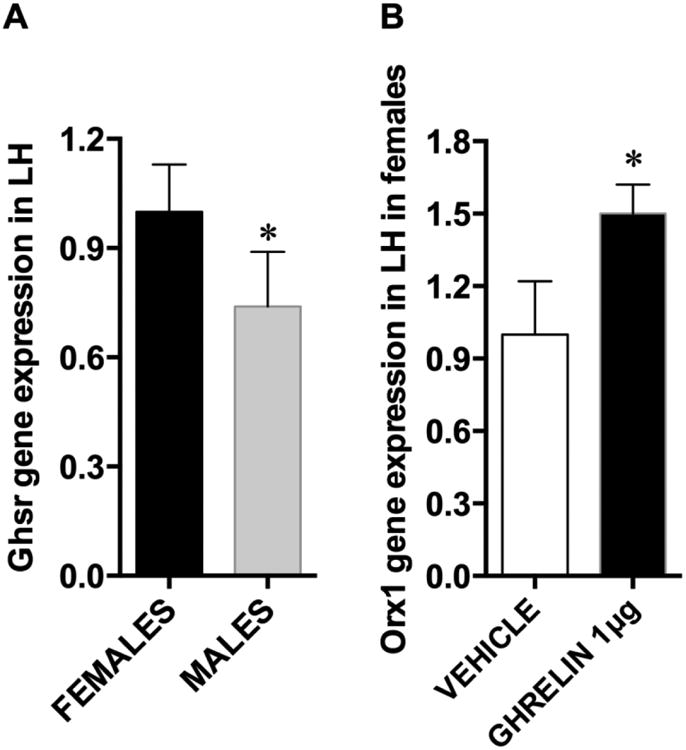
Ghsr and orexin 1 mRNA expression levels in the LHA. Expression of Ghsr differs in male and female rats; Ghsr mRNA was detected in both male and female LHA, but males showed significantly lower levels (A). The expression of the orexin 1 gene was increased by LHA-targeted ghrelin microinjection in females (B). Data are expressed as mean ±SEM. n=12, * p < 0.05.
3.3 Ghrelin receptor activation in the LHA is necessary for normal regulation of food reward and body weight in female but not male rats
Acute pharmacological blockade of LHA Ghsr by bilateral microinjection of YIL 781 led to reduced food reward (Fig 3A-B) but not food-seeking behaviors (Fig 3C) in female but not male rats. Two-way ANOVA analysis for food rewards earned indicates a significant effect of drug (F (1, 12) = 12.8, p<0.005), and sex (F (1, 12) = 7.3, p<0.05), interaction showed a strong trend to significance (F (1, 12) = 3.9, p=0.07). Two-way ANOVA analysis for the number of active lever presses indicates a significant effect of drug (F (1, 12) = 4.35, p<0.05), and sex (F (1, 12) = 4.38, p<0.05), but no significant interaction (F (1, 12) = 0.4157, p>0.05). Locomotor activity was not altered in either sex (Fig 3D; no significant effect of drug (F (1, 12) = 0.12, p>0.05), or sex (F (1, 12) = 0,123, p>0.05), and no significant interaction (F (1, 12) = 0.03, p>0.05). Food intake at 1 h was slightly reduced in females (Fig 3E,F), but this trend did not reach statistical significance. Two-way ANOVA analysis indicates no significant effect of drug (F (1, 11) = 0.10, p>0.05), but a significant effect of sex (F (1, 11) = 29.20, p<0.0005), the interaction did not reach significance (F (1, 11) = 1.04, p>0.05). At 24 h, food intake was reduced in female rats only (Fig 3G,H). Two-way ANOVA analysis on grams consumed during 24h indicates a significant effect of drug (F (1, 12) = 9.43, p<0.0005), and sex (F (1, 12) = 75.58, p<0.0005), and a significant interaction (F (1, 12) = 11.75, p<0.005). This sex difference remains significant when data are transformed as % of vehicle value in order to account for baseline intake differences in male and female rats (Fig 3L). For 24h body weight change 2-way ANOVA indicates a significant effect of drug (F (1, 12) = 7.1, p<0.05), a significant effect of sex (F (1, 12) = 9.78, p<0.05), but the interaction was not significant (F (1, 12) = 1.74, p=21); Fig 3I.
Figure 3.
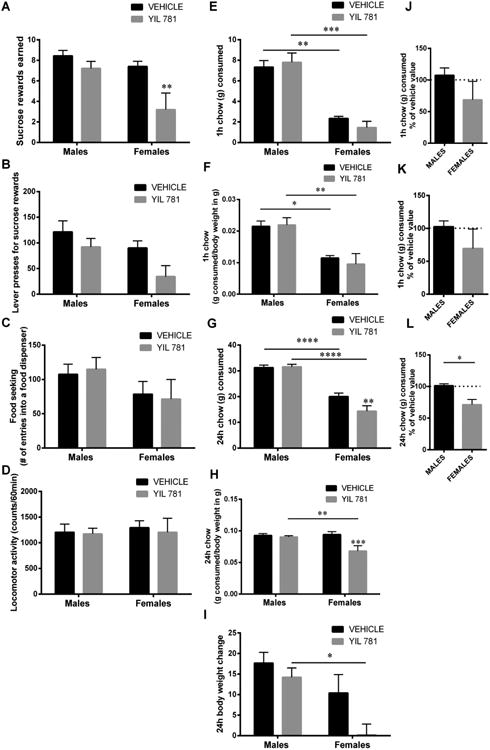
Acute pharmacological blockade of Ghsr decreases food reward and intake in females but not males. Intra-LHA microinjection of Ghsr antagonist, YIL 781 (25 ug) decreased the amount of sucrose rewards earned (A) and the number of lever presses for the rewards (B) in a progressive ratio schedule, without changing food seeking (C) or locomotor activity (D) in female rats. A non-significant trend for a reduced intake was detected at 1 h in females when expressed as g consumed (E) or grams consumed per grams of body weight (F). At 24 h food intake was significantly reduced in females when expressed as g consumed (G) or grams consumed per grams of body weight (H). Body weight gain was lower in female treated with the antagonist compared to antagonist treated males (I). Acute Ghsr blockade did not affect any of the parameters measured in male rats. Wherever vehicle-injected male and female behavior differed these parameters were also presented as % change from vehicle baseline (J-L). Data are expressed as mean ±SEM. n=5 (female rats) and n=9 (male rats). *p < 0.05, ** p < 0.01,*** p < 0.005,**** p < 0.001 compared to vehicle (aCSF).
3.4 Chronic LHA-targeted Ghsr knockdown in female rats does not alter reward or body weight
In order to follow up on the significant results of the acute Ghsr blockade in female rats and determine whether reward and ingestive behavior regulation is able to cope with a chronic reduction in LHA Ghsr, we bilaterally injected GFP AAV or Ghsr AAV into the LHA of female rats. Testing female rats was prioritized, over testing male rats, after the negative antagonist results in males. Experimental timeline (Fig 4) provides the timing of PR tests and maintenance diet changes relative to AAV infusions. Chow intake and body weight were measured daily for four weeks after the injection (Fig 4A-B); Ghsr knockdown did not alter the food intake or weight of the rats. Since no significant effect was found in rats fed a chow diet, rats were switched to a HFHS diet after four weeks, to determine whether this metabolic challenge could uncover the impact of chronic Ghsr reduction in the LHA. However, even under the HFHS diet conditions intake of chow (Fig 4D), lard (Fig 4E), sugar water (Fig 4F), and body weight (Fig 4C) were not altered by the Ghsr reduction in the LHA. Brown and white adipose tissue weight, measured at the termination of the experiment, was also not altered by the treatment (Fig 4G). LHA-targeted Ghsr AAV treatment reduced Ghsr mRNA by 25% in the LHA compared to the control group (Fig 4H). This reduction was sufficient to abolish the reward (Fig 5A-B) and food seeking (Fig 5C) responses to LHA-targeted ghrelin microinjections. Food intake actions of ghrelin, however, remained intact (Fig 5E). Locomotor activity was not altered (Fig 5D). Food reward responses in ad libitum-fed or food-restricted rats were not altered by the Ghsr AAV compared to control construct infused rats (Table 1). Because ghrelin was previously shown to play an important role in the potentiation of ingestive and motivated behavior by food restriction, overnight food-restricted rats were offered chow and chow intake was measured at 1 h. No significant differences were found between the control and Ghsr knockdown group (5.2±0.3 and 5.0±0.4 control and Ghsr knockdown group respectively).
Figure 4.
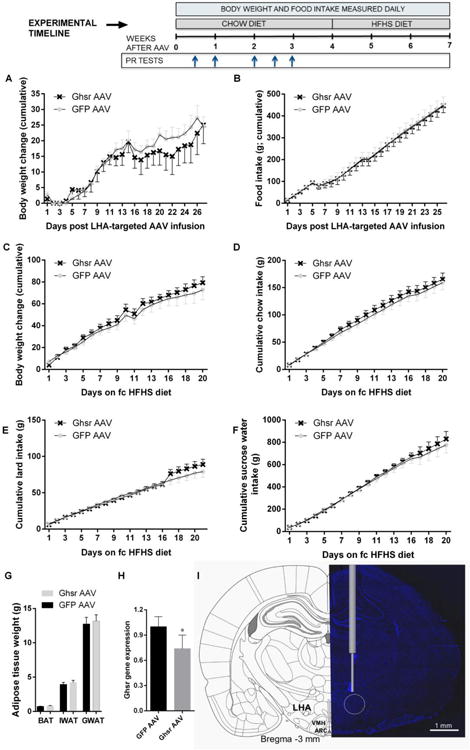
Chronic GHSR reduction in the LHA does not affect energy balance in female rats. Body weight gain (A) and chow intake (B) were not affected by the LHA-targeted Ghsr knockdown mediated by the Ghsr AAV injection. Similarly after HFHS diet challenge the body weight (C), chow (D), lard (E), and 30% sucrose water (F) intake were not altered by the knockdown. Fat mass in brown, gonadal and inguinal adipose tissues (BAT, GWAT and IWAT respectively; G) was also not altered. Seven weeks after AAV construct injections Ghsr mRNA expression was reduced by ∼25% in the LHA of rats treated with Ghsr AAV compared with controls (H). Representative AAV construct injection location (I). ARC: arcuate nucleus; VMH: ventromedial hypothalamus. Blue represents the DAPI nuclear stain. Timeline provides the timing of PR tests and maintenance diet changes relative to AAV infusions. Data are expressed as mean ±SEM. n=12 (female rats) per treatment group.
Figure 5.
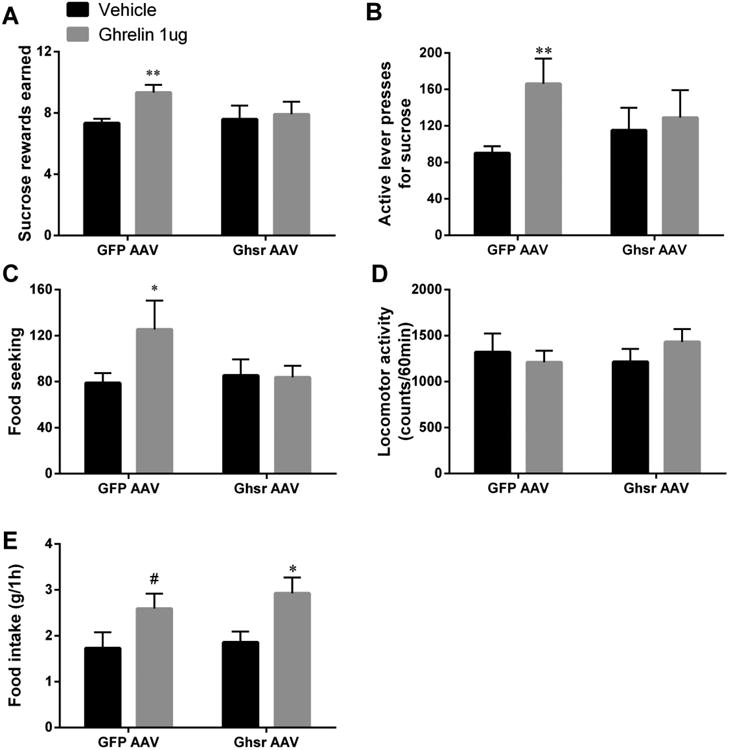
Ghsr knockdown in the LHA abolishes food reward impact of LHA-targeted ghrelin. Ghrelin increased the amount of sucrose rewards earned (A) and the number of active lever presses for sucrose (B) in control rats but not in rats with Ghsr knocked down. Similarly Ghsr knockdown was sufficient to abolish the food-seeking behavior potentiation induced by ghrelin (C). No changes in locomotor activity were detected (D). The knockdown was, however, not sufficient to attenuate the ghrelin-induced elevation in chow intake (E). Data are expressed as mean ±SEM. n=12 (female rats). * p < 0.05, ** p < 0.001, #p < 0.1.
Table 1.
Progressive ratio operant testing results from ghrelin receptor knockdown (Ghsr AAV) and control rats. On days 3 and 7 the rats were fed ad libitum. On day 14 the rats were food restricted overnight. n=12.
| DAY POST AAV INFUSIONS | MEASURED PARAMETER | CONTROL | Ghsr AAV | SIGNIFICANCE |
|---|---|---|---|---|
| DAY 3 | REWARDS EARNED | 7.2±0.5 | 6.7±1.1 | P=0.7; NS |
| ACTIVE LEVER | 85.8±14.3 | 106.7±33.4 | P=0.6; NS | |
| FOOD SEEKING | 88.3±14.5 | 106.6±24.7 | P=0.5; NS | |
| LOCOMOTOR ACTIVITY | 1384.3±155.9 | 1309.8±109.2 | P=0.7; NS | |
| DAY 7 | REWARDS EARNED | 8.3±0.7 | 8.0±0.7 | P=0.8; NS |
| ACTIVE LEVER | 134.2±23.0 | 116.3±21.1 | P=0.6; NS | |
| FOOD SEEKING | 114.3±15.4 | 122.8±18.1 | P=0.7; NS | |
| LOCOMOTOR ACTIVITY | 1098.0±131.3 | 891.3±137.5 | P=0.3; NS | |
| DAY 14 | REWARDS EARNED | 9.6±0.4 | 9.3±0.9 | P=0.8; NS |
| ACTIVE LEVER | 173.0±21.0 | 187.0±34.0 | P=0.7; NS | |
| FOOD SEEKING | 154.6±15.9 | 161.3±22.8 | P=0.8; NS | |
| LOCOMOTOR ACTIVITY | 1090.8±107.9 | 1135.6±124.1 | P=0.8; NS |
4. Discussion
The present study reveals that ghrelin potentiates ingestive and motivated food-driven behaviors via Ghsr activation in the LHA in both male and female rats. While the pharmacological effects were present in both males and females, the endogenous relevance (based on the antagonist data primarily, but also the AAV data) was only important in females. Female rats also expressed higher levels of Ghsr in their LHA. Collectively our results reveal sex differences in ghrelin's actions on the LHA. The revealed differences varied based on the behavioral parameter measured and the way ghrelin signaling was targeted.
Results show that ghrelin controls a wide array of feeding-associated behaviors by acting directly on the LHA, and that these effects were more pronounced in females than males. These data are in agreement with previous studies showing that LHA-directed ghrelin application increases ingestive behavior for chow in males [35-37]. The changes in food reward motivated behavior demonstrated in the present study are very much in line with previous data showing that intra-LHA ghrelin increases accumbal dopamine neurotransmission [35]. Since ghrelin has previously been shown to impact on other non-food associated behaviors, many of which are tightly linked to the mesolimbic dopamine signaling (e.g., impulsivity, novelty seeking, and reward-derived from substances of abuse [17, 48-50]), it is possible that some of these non-food-based behaviors are also controlled by Ghsr in the LHA, and also that this control may differ between the sexes.
Ghrelin's stimulatory effects on ingestive and food reward-based behaviors in the current study were far more robust in females than males. These results seemingly contrast with two previous studies which demonstrated that females are less sensitive to intraperitoneally or intraventricularly administered ghrelin's chow intake stimulatory effect [39, 51], an outcome shown to be dependent on estrogen. Interestingly, in the present study male rats displayed a more potent chow eating stimulatory effect one hour after injection, suggesting that short-term ingestive behavior may, in fact, be more sensitive to LHA-ghrelin treatment in males. However, only females responded with enhanced cued food-seeking behavior (increased activity around the food dispenser) after LHA-targeted ghrelin, suggesting that for some feeding-associated parameters, females display more sensitivity to ghrelin than males. Other than the different food-associated behavioral parameters evaluated, there are also other methodological differences between the current study and these previous studies. For example, ghrelin delivery was targeted to the LHA in the current study, whereas it was applied systemically or intracerebroventricularly (and therefore was able to reach many brain areas) in the previous work.
Whether LHA neurons co-express Ghsr and estrogen receptors remains unknown; other hypothalamic areas including ventromedial, arcuate, and preoptic hypothalamus harbor neurons that co-express both receptors [52]. Estrogen receptors are indeed expressed in the LHA, but whether they interact with Ghsr signaling is unknown. Nevertheless, the eating stimulatory effects of ghrelin microinjections targeting the arcuate and paraventricular nucleus did not find any sex differences [9]. Since this study did not follow the estrous cycle of their rats, it has been suggested that the cause for lack of reduced ghrelin-mediated behavioral effects in females was that these rats were acyclic [39, 51]. However, in the current study the rats had a normal estrous cycle, and each cycle phase was represented at the time of the injection, and thus reduced circulating estrogens are an unlikely explanation for the differential effects of ghrelin in female rats compared to males. Moreover, since female rats in our study did have intact circulating estrogens, it is possible that estrogen provided an inhibitory influence on ghrelin's effects in the LHA. Future investigations are needed to show whether the female LHA-ghrelin response can be augmented with ovariectomy.
To the best of our knowledge this is the first study to test the CNS delivery of a Ghsr antagonist in female rodents. When delivered to the LHA, the Ghsr antagonist was effective in reducing fasting-induced intake and fasting-potentiated food reward in females but not in males. In line with our results female Ghsr-null mice are more impacted by Ghsr deletion compared to male mice, demonstrated as considerably larger fat mass reduction and body weight compared to male Ghsr knockouts [40]. Collectively these data suggest that Ghsr signaling, at least at the level of the LHA, is more important in the regulation of feeding behavior and body weight regulation in females compared to males. Another potential explanation for the differential effects of acute Ghsr blockade in males and females could be that males may have lower levels (or insufficient amount to produce a response) of endogenous ghrelin reaching the LHA after the overnight fast. Indeed, intact female rats show higher levels of plasma ghrelin compared to males after overnight fast [53]. To which extent the increased plasma levels of ghrelin translate into distribution in the brain parenchyma is, however, unknown. Moreover, ghrelin receptor antagonists have been highly effective after intra-parenchymal (VTA) brain injection following an overnight fast in males [18], which indicates that overnight fasting can result in sufficient activity at the CNS Ghsr also in males. Thus, we consider that it is more likely that in males, activity at other Ghsr-expressing brain sites is sufficient to compensate for the lack of effect from the LHA. This interpretation is also in line with the fact that Ghsr are expressed in the LH, and injection of exogenous ghrelin is effective at altering food motivated behavior, as well as ingestive behavior, in males. In summary, in males Ghsr activation in the LH is sufficient but not necessary for feeding and food reward regulation. While in females LH Ghsr activation is both sufficient and necessary, for the latter at least in the acute setting, which highlights the importance of LH Ghsr signaling in females.
Ghrelin, at the level of the LHA, increased orexin gene expression in female rats. Thus, for both males and females, ghrelin may alter food-motivated behavior at the level of the LHA via orexin neurons [16, 20, 31, 33]. Orexin neurons project to the mesolimbic sites, including the VTA [34] and have been shown to increase motivated responding for sucrose in female rats. However, previous studies also indicate sexual dimorphisms in the orexin system. Female rats express higher levels of orexin receptors in the hypothalamus [54], and orexin up-regulation upon fasting is enhanced in females compared to male [55]. Moreover, orexin-driven regulation of food motivation differs in males compared to females. For example, CNS pharmacological blockade of orexin signaling does not alter food-motivated behavior in female rats (but it does reduce food-motivated behavior in male rats) [56, 57]. Thus, considering how important orexins are to ghrelin's orexigenic function, the sexually dimorphic responses to ghrelin revealed by the current data may be partly mediated by the differential sensitivity to orexin's actions. This hypothesis requires further investigation.
We did not find any changes in feeding, motivated responding for palatable food, or fat mass accumulation after chronic LHA Ghsr knockdown in female rats irrespective of the maintenance diet. In previous work, female Ghsr-null mice show reduced food intake, body weight, and adiposity when maintained on a high-fat diet [40] whereas they have only reduced body weight on a chow diet. One likely explanation for the different results is that Ghsr-expressing brain areas outside of the LHA compensate for the reduced Ghsr levels in the LHA, resulting in no net change in behavior or metabolism in rats. Another explanation could be that the reduction of Ghsr in the LHA in the current study is too small to affect normal physiology, since approximately 75% of Ghsr remain in the LHA after the knockdown. Our evidence supports the first option, however, as food reward effects of LHA-delivered ghrelin were abolished by the knockdown. Moreover, the lack of a difference in any parameters measured between vehicle-injected knockdown and control rats, combined with a completely abolished effect of intra-LH ghrelin in the same experiment suggests that the lack of effect of the knockdown on food reward behavior is unlikely to be due to the knockdown being insufficient to block ghrelin responses at the time of reward testing. Testing female rats after the Ghsr knockdown was prioritized over testing male rats in this study since male rat behavior was not altered by the antagonist treatment in the LH. With the current data, while we consider it unlikely that males would show an effect of the knockdown, we cannot eliminate this possibility.
Collectively data identify the LHA as an important locus for ghrelin-mediated control of food intake, as well as palatable food seeking and motivation. Importantly, our data also demonstrate that food motivation and food ingestion are differentially engaged by ghrelin in the LHA of male and female rats (with more pronounced effects observed in females), and highlight the importance of examining both sexes in food reward and intake studies.
Highlights.
Ghrelin stimulates food intake and food motivated behaviors by acting on the lateral hypothalamic area (LHA) in male and female rats.
Acute Ghsr blockade in the LHA reduces food intake, body weight, and motivated behavior for sucrose in female but not male rats.
Females have higher levels of Ghsr mRNA in the LHA compared to males.
Chronic LHA RNA inference-mediated Ghsr knockdown is not sufficient to affect baseline food intake or food reward responding in either sex.
Acknowledgments
This research was funded by the Swedish Research Council (2014-2945 to KPS and 2013–7107 to PR), Novo Nordisk Foundation Excellence project grant (to KPS), Ragnar Söderberg Foundation (KPS), Harald Jeanssons Stiftelse and Greta Jeanssons Stiftelse (KPS), and Magnus Bergvalls Stiftelse (KPS), and National Institute of Health (DK104897 to SEK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–45. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 2.Date Y, Murakami N, Kojima M, Kuroiwa T, Matsukura S, Kangawa K, et al. Central effects of a novel acylated peptide, ghrelin, on growth hormone release in rats. Biochemical and biophysical research communications. 2000;275:477–80. doi: 10.1006/bbrc.2000.3342. [DOI] [PubMed] [Google Scholar]
- 3.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 4.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–61. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 5.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. The Journal of clinical endocrinology and metabolism. 2001;86:4753–8. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 6.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 7.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 8.Asakawa A, Inui A, Kaga T, Katsuura G, Fujimiya M, Fujino MA, et al. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut. 2003;52:947–52. doi: 10.1136/gut.52.7.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Currie PJ, Mirza A, Fuld R, Park D, Vasselli JR. Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. Am J Physiol Regul Integr Comp Physiol. 2005;289:R353–R8. doi: 10.1152/ajpregu.00756.2004. [DOI] [PubMed] [Google Scholar]
- 10.Dickson SL, Leng G, Robinson IC. Systemic administration of growth hormone-releasing peptide activates hypothalamic arcuate neurons. Neuroscience. 1993;53:303–6. doi: 10.1016/0306-4522(93)90197-n. [DOI] [PubMed] [Google Scholar]
- 11.Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects ofbrainstem ghrelin administration. Diabetes. 2003;52:2260–5. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- 12.Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–7. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 13.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–39. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–9. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biological psychiatry. 2013;73:915–23. doi: 10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu TM, Hahn JD, Konanur VR, Noble EE, Suarez AN, Thai J, et al. Hippocampus ghrelin signaling mediates appetite through lateral hypothalamic orexin pathways. eLife. 2015:4. doi: 10.7554/eLife.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skibicka KP, Dickson SL. Ghrelin and food reward: the story of potential underlying substrates. Peptides. 2011;32:2265–73. doi: 10.1016/j.peptides.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–37. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Skibicka KP, Hansson C, Egecioglu E, Dickson SL. Role of ghrelin in food reward: impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addict Biol. 2012;17:95–107. doi: 10.1111/j.1369-1600.2010.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biological psychiatry. 2010;67:880–6. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinberg ZY, Nicholson ML, Currie PJ. 6-Hydroxydopamine lesions of the ventral tegmental area suppress ghrelin's ability to elicit food-reinforced behavior. Neuroscience letters. 2011;499:70–3. doi: 10.1016/j.neulet.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 22.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain research. Molecular brain research. 1997;48:23–9. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 23.Van den Pol AN. Lateral hypothalamic damage and body weight regulation: role of gender, diet, and lesion placement. The American journal of physiology. 1982;242:R265–74. doi: 10.1152/ajpregu.1982.242.3.R265. [DOI] [PubMed] [Google Scholar]
- 24.Anand BK, Brobeck JR. Localization of a “feeding center” in the hypothalamus of the rat. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 1951;77:323–4. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- 25.Morrison SD, Mayer J. Adipsia and aphagia in rats after lateral subthalamic lesions. The American journal of physiology. 1957;191:248–54. doi: 10.1152/ajplegacy.1957.191.2.248. [DOI] [PubMed] [Google Scholar]
- 26.Teitelbaum P, Stellar E. Recovery from the failure to eat produced by hypothalamic lesions. Science. 1954;120:894–5. doi: 10.1126/science.120.3126.894. [DOI] [PubMed] [Google Scholar]
- 27.Miller NE. Motivational effects of brain stimulation and drugs. Federation proceedings. 1960;19:846–54. [PubMed] [Google Scholar]
- 28.Olds J. Hypothalamic substrates of reward. Physiol Rev. 1962;42:554–604. doi: 10.1152/physrev.1962.42.4.554. [DOI] [PubMed] [Google Scholar]
- 29.Margules DL, Olds J. Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science. 1962;135:374–5. doi: 10.1126/science.135.3501.374. [DOI] [PubMed] [Google Scholar]
- 30.Hoebel BG, Teitelbaum P. Hypothalamic control of feeding and self-stimulation. Science. 1962;135:375–7. doi: 10.1126/science.135.3501.375. [DOI] [PubMed] [Google Scholar]
- 31.Olszewski PK, Li D, Grace MK, Billington CJ, Kotz CM, Levine AS. Neural basis of orexigenic effects of ghrelin acting within lateral hypothalamus. Peptides. 2003;24:597–602. doi: 10.1016/s0196-9781(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 32.Scott V, McDade DM, Luckman SM. Rapid changes in the sensitivity of arcuate nucleus neurons to central ghrelin in relation to feeding status. Physiol Behav. 2007;90:180–5. doi: 10.1016/j.physbeh.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 33.Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T, et al. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology. 2003;144:1506–12. doi: 10.1210/en.2002-220788. [DOI] [PubMed] [Google Scholar]
- 34.Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56(Suppl 1):112–21. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cone JJ, McCutcheon JE, Roitman MF. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci. 2014;34:4905–13. doi: 10.1523/JNEUROSCI.4404-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–7. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 37.Olszewski PK, Grace MK, Billington CJ, Levine AS. Hypothalamic paraventricular injections of ghrelin: effect on feeding and c-Fos immunoreactivity. Peptides. 2003;24:919–23. doi: 10.1016/s0196-9781(03)00159-1. [DOI] [PubMed] [Google Scholar]
- 38.Matsubara M, Sakata I, Wada R, Yamazaki M, Inoue K, Sakai T. Estrogen modulates ghrelin expression in the female rat stomach. Peptides. 2004;25:289–97. doi: 10.1016/j.peptides.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 39.Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, et al. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–8. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 40.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. The Journal of clinical investigation. 2005;115:3564–72. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esler WP, Rudolph J, Claus TH, Tang W, Barucci N, Brown SE, et al. Small-molecule ghrelin receptor antagonists improve glucose tolerance, suppress appetite, and promote weight loss. Endocrinology. 2007;148:5175–85. doi: 10.1210/en.2007-0239. [DOI] [PubMed] [Google Scholar]
- 42.Vogel H, Wolf S, Rabasa C, Rodriguez-Pacheco F, Babaei CS, Stober F, et al. GLP-1 and estrogen conjugate acts in the supramammillary nucleus to reduce food-reward and body weight. Neuropharmacology. 2016;110:396–406. doi: 10.1016/j.neuropharm.2016.07.039. [DOI] [PubMed] [Google Scholar]
- 43.Skibicka KP, Alhadeff AL, Grill HJ. Hindbrain cocaine- and amphetamine-regulated transcript induces hypothermia mediated by GLP-1 receptors. J Neurosci. 2009;29:6973–81. doi: 10.1523/JNEUROSCI.6144-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.la Fleur SE, Vanderschuren LJ, Luijendijk MC, Kloeze BM, Tiesjema B, Adan RA. A reciprocal interaction between food-motivated behavior and diet-induced obesity. Int J Obes (Lond) 2007;31:1286–94. doi: 10.1038/sj.ijo.0803570. [DOI] [PubMed] [Google Scholar]
- 45.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32:4812–20. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, et al. Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41:1917–28. doi: 10.1038/npp.2015.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansson C, Shirazi RH, Naslund J, Vogel H, Neuber C, Holm G, et al. Ghrelin influences novelty seeking behavior in rodents and men. PLoS One. 2012;7:e50409. doi: 10.1371/journal.pone.0050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderberg RH, Hansson C, Fenander M, Richard JE, Dickson SL, Nissbrandt H, et al. The Stomach-Derived Hormone Ghrelin Increases Impulsive Behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41:1199–209. doi: 10.1038/npp.2015.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Molecular and cellular endocrinology. 2011;340:80–7. doi: 10.1016/j.mce.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 51.Butera PC, Clough SJ, Bungo A. Cyclic estradiol treatment modulates the orexigenic effects of ghrelin in ovariectomized rats. Pharmacol Biochem Behav. 2014;124:356–60. doi: 10.1016/j.pbb.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frazao R, Dungan Lemko HM, da Silva RP, Ratra DV, Lee CE, Williams KW, et al. Estradiol modulates Kiss1 neuronal response to ghrelin. American journal of physiology. Endocrinology and metabolism. 2014;306:E606–14. doi: 10.1152/ajpendo.00211.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gayle DA, Desai M, Casillas E, Beloosesky R, Ross MG. Gender-specific orexigenic and anorexigenic mechanisms in rats. Life sciences. 2006;79:1531–6. doi: 10.1016/j.lfs.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 54.Johren O, Neidert SJ, Kummer M, Dominiak P. Sexually dimorphic expression of prepro-orexin mRNA in the rat hypothalamus. Peptides. 2002;23:1177–80. doi: 10.1016/s0196-9781(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 55.Funabashi T, Hagiwara H, Mogi K, Mitsushima D, Shinohara K, Kimura F. Sex differences in the responses of orexin neurons in the lateral hypothalamic area and feeding behavior to fasting. Neuroscience letters. 2009;463:31–4. doi: 10.1016/j.neulet.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 56.Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in female rats. Neuropharmacology. 2014;86:97–102. doi: 10.1016/j.neuropharm.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiology & behavior. 2010;100:419–28. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


