Abstract
Purpose
Recent evidence suggests that reaching the lowest achievable levels of testosterone with androgen deprivation therapy delays disease progression and increases overall survival in men with advanced prostate cancer. The aim of this analysis is to compare the post-treatment serum testosterone levels between patients undergoing subcapsular orchiectomy and patients treated with the luteinizing hormone-releasing hormone agonist triptorelin.
Material and Methods
In this randomized clinical trial, we included 58 consecutive hormone-naive men diagnosed with advanced prostate cancer at Herlev and Gentofte University Hospital, Denmark, from September 2013 to March 2015. Follow-up was 48 weeks. Participants were randomly assigned (1:1) to either subcapsular orchiectomy or triptorelin 22.5 mg given as 24 week depot injections. Androgen status was measured by liquid chromatography tandem mass spectrometry prior to treatment and after 12, 24 and 48 weeks. Between-group differences in achieved hormone levels were analyzed using longitudinal Tobit regressions.
Results
Triptorelin injections resulted in 29% (95% CI 17.2, 41.7) lower testosterone levels compared to subcapsular orchiectomy (p<.001). A significantly higher proportion of men receiving triptorelin had testosterone levels <20 ng/dL at 12 and 48 weeks compared to men undergoing orchiectomy (97% versus 79% and 100% versus 87%, p<.05). There was no detectable difference in adrenal androgen reductions between treatment groups.
Conclusions
The use of 24-week depot triptorelin injections results in significantly lower testosterone levels compared to subcapsular orchiectomy. This is the first randomized study to demonstrate a difference in treatment effect between surgical and medical castration on testosterone levels.
Keywords: Orchiectomy, Prostatic neoplasms, Testosterone, Triptorelin pamoate
INTRODUCTION
Androgen deprivation therapy (ADT) has been the key treatment modality for metastatic prostate cancer since its introduction in 1941. Methods of ADT include luteinizing hormone releasing hormone (LHRH) agonist and antagonist therapy as well as surgical treatment (bilateral orchiectomy or subcapsular orchiectomy). The aim of ADT is to alleviate prostate-cancer-related symptoms and reduce the tumor burden by reducing the serum testosterone to levels below 50 ng/dL.1
Recent studies have suggested that achieving testosterone levels lower than the commonly used threshold of 50 ng/dL delays cancer progression and increases both cancer-specific survival and overall survival.2–5 This has led to a new target level of total testosterone <20 ng/dL. Meanwhile, more accurate methods of testosterone level measurement, such as liquid chromatography – tandem mass spectrometry (LC-MS/MS)6, have become available, thus spurring a re-examination of the testosterone-lowering effect of different ADT methods. In 2012, Van der Sluis and colleagues published thought-provoking results showing that LHRH agonist injections lowered testosterone more than surgical castration did in 66 men.7 The study was limited by a cross-sectional design and a heterogeneous surgical intervention group that included both men undergoing sex-change operations and men with prostate cancer. Thus, further studies to retest and reproduce these findings are warranted.
This is our first planned publication from a randomized clinical trial comparing the use of LHRH agonist triptorelin with subcapsular orchiectomy, with a primary focus of comparing metabolic complications. The aim of the present analysis is to determine whether the post-treatment serum testosterone and adrenal androgen levels differ between the treatment modalities when measured using the accurate method of LC-MS/MS.
MATERIALS AND METHODS
Study Design and Participants
In this analysis from a 2-armed randomized clinical trial, the effect of triptorelin 22.5 mg (given as a 24-week depot injection) on the achieved serum testosterone levels with a 48-week follow-up is compared with the effect of subcapsular orchiectomy. Men under 90 years of age with an Eastern Cooperative Oncology Group performance status ≤ 2, a confirmed diagnosis of prostate cancer without curative treatment options, and an indication for receiving ADT were eligible for randomization. Exclusion criteria were prior androgen therapy, pharmacological treatment for osteoporosis, diabetes mellitus, and conditions (e.g., hemophilia) that substantially increase the risk of surgery (as decided by the treating physician). Allocated treatment began within 14 days of randomization. Randomization was conducted in a 1:1 allocation using a computer-generated randomization sequence blinded to the investigators. Sequentially numbered, sealed opaque envelopes made by a non-affiliated person were used for treatment allocation. Treatment was not blinded to the investigators after allocation. Participants allocated to the triptorelin arm were treated with the anti-androgen bicalutamide 50 mg daily for 30 days upon first injection. Participants received no other treatments during the trial that could interfere with the gonadal axis.
The trial was conducted in accordance with the Declaration of Helsinki and the legal regulations in Denmark. Permission was obtained from the Danish Medicines Agency (EudraCT 2013-002553-29; registered on www.clinicaltrialsregister.eu) and the Capital Regional Committee on Health Research Ethics in Denmark (H-2-2013-107). All patients gave oral and written consent prior to inclusion.
Data Collection
Participants were evaluated at baseline prior to treatment and at 12, 24 and 48 weeks after the first injection or surgery, respectively. Venous blood samples for hormonal measurements were all collected between 8 and 9 a.m. All participants fasted for a minimum of 8 hours prior to each visit. Body mass index was calculated from height (via mounted stadiometer) and weight (WB-110MA, TANITA, Tokyo, Japan).
Hormone Analyses
Serum total testosterone (normal reference range (NRR) 242 – 732 ng/dL), androstenedione (NRR 1.6 - 6.5 nmol/L), dehydroepiandrosterone sulfate (DHEAS) (NRR 700 – 7000 nmol/l) and 17-hydroxyprogesterone (NRR < 8 nmol/L) were measured by LC-MS/MS (Acquity UPLC Xevo™ TQ MS, Waters, USA). Testosterone levels were reported in nmol/L and converted for this manuscript to ng/dL by dividing by 0.0347. The inter-assay coefficient of variance (CV) for serum total testosterone was 6.7% at 24.7 ng/dL and 20.5% at 5.7 ng/dL. For testosterone, a lower reporting limit of detection (LRL) of 8.6 ng/dL was decided by the laboratory. The inter-assay CV for androstenedione, DHEAS, and 17-hydroxyprogesterone was 10.7% at 0.734 nmol/L, 9.1% at 298.7 nmol/L, 7.5% and 21.3% at 1.552 nmol/L and 0.367 nmol/L, respectively. The LRL for androstenedione, DHEAS, and 17-hydroxyprogesterone was 1.0 nmol/l, 100 nmol/L, and 1.0 nmol/L, respectively. Sex hormone binding globulin (SHBG) was analyzed by a sandwich chemiluminescence based immunoassay (Siemens, Munich, Germany), with a CV of < 7.0%. Estradiol (NRR < 0.146 nmol/L) was measured using a chemiluminescence based competitive immunoassay (ADVIA Centaur®, Siemens, Germany); the CV at 0.125 nmol/L was 12.0%. Luteinizing hormone (LH) (NRR 1.5–9.3 IU/L) and follicle stimulating hormone (FSH) (NRR 1.4–18.1 IU/L) were measured using a two-site sandwich chemiluminescence immunoassay (ADVIA Centaur®, Siemens, Germany). The intra-assay CV was 2.3% at 4.2 IU/L for LH and 2.9% at 6.9 IU/L for FSH. The LRL for LH was 0.3 IU/L.
Statistical Analyses
Statistical analyses were performed using the computing environment R (R Core Team (2016), Vienna, Austria. https://www.R-project.org/). Sample size determination was not based on power calculations for treatment differences in testosterone because this was a secondary endpoint. However, a post hoc estimate using a Monte Carlo analysis showed this study had 87% power to detect a baseline-adjusted difference in serum testosterone of 30%, based on 1,000 stratified-bootstrap samples.
Sex steroid hormone concentrations and SHBG were log-transformed to meet a normal distribution assumption. Pre-treatment versus post-treatment hormone values for each treatment group were analyzed using longitudinal Tobit regressions8 of the log hormone value against the post-treatment indicator. This model takes into account the floor effect caused by the LRLs of the hormone assays. Between-group differences in achieved hormone levels were analyzed using longitudinal Tobit regressions of post-treatment hormone values against a treatment factor (subcapsular orchiectomy/triptorelin). Two models were used for these analyses: (1) a model adjusting for the baseline values of the modeled hormone only and (2) a best-fit model adjusting for baseline values of the modeled hormone and forward selection. The variables included in the model were concurrent hormone values (excluding the one modeled), age and BMI (see supplements for final model selections). Plots of marginal residuals from the Tobit regressions were used to check model assumptions. A likelihood ratio test was used to determine if both treatments equally resulted in testosterone values < 20 ng/dL. All tests were two-sided, with statistical significance set at an α level of 0.05. All available data were used in the analyses.
RESULTS
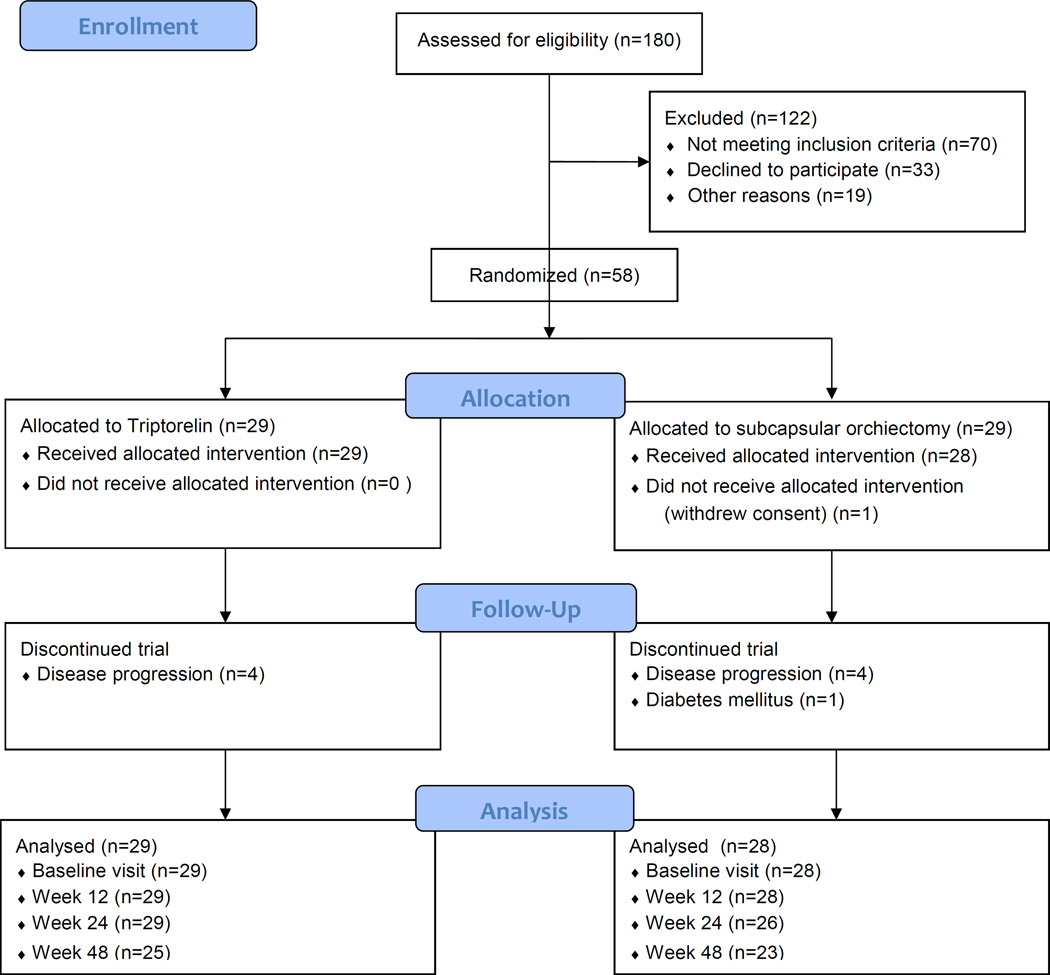
Fifty-eight men were enrolled between September 2013 and March 2015 from the Department of Urology, Herlev and Gentofte University Hospital. Figure 1 shows the CONSORT (Consolidated Standards of Reporting Trials) diagram for the trial. Participants were randomized to either subcapsular orchiectomy (n=29) or triptorelin (n=29). Baseline patient characteristics are shown in Table 1. One patient in the subcapsular orchiectomy group withdrew consent after randomization and before attending any study visits. Nine patients in total were excluded before the 48-week follow-up visit. Six of the 28 patients (21%) undergoing subcapsular orchiectomy reported postoperative scrotal swelling/hematoma not requiring surgical or pharmacological intervention. One patient was admitted with a post-operative, infected scrotal hematoma requiring surgical revision under general anesthesia. One of the 29 patients (3%) reported local muscle pain after the triptorelin injection, and one patient experienced transient pain in the hip opposite the site of injection, which was interpreted as a flare phenomenon.
Figure 1.
Consolidated Standards of Reporting Trials diagram of recruitment and loss to follow-up through the trial.
Table 1.
Baseline characteristics of participants
| Subcapsular orchiectomy group (n=28) |
Triptorelin group (n=29) |
|
|---|---|---|
| Age, years mean (SD) | 72 (8.8) | 75 (5.8) |
| Body mass index, kg/m2 mean (SD) | 27.0 (4.8) | 27.6 (3.5) |
| PSA, µg/L median (IQR) | 92 (45-352) | 61 (37-145) |
| Clinical T-stage No. (%) | ||
| T ≤ 2 | 7 (25) | 8 (28) |
| T ≥ 3 | 20 (71) | 20 (69) |
| Unknown | 1 (4) | 1 (3) |
|
Regional lymph node metastatic disease, No. (%) |
||
| Present | 3 (11) | 4 (14) |
| Unknown | 25 (89) | 25 (86) |
|
Bone and/or visceral metastatic disease, No. (%) |
24 (86) | 27 (93) |
| Gleason grading, No. (%) | ||
| < 7 | 6 (21) | 10 (34) |
| ≥ 8 | 21 (75) | 18 (62) |
| Unknown | 1 (4) | 1 (3) |
SD: Standard deviation; PSA: Prostate specific antigen; IQR: Interquartile range
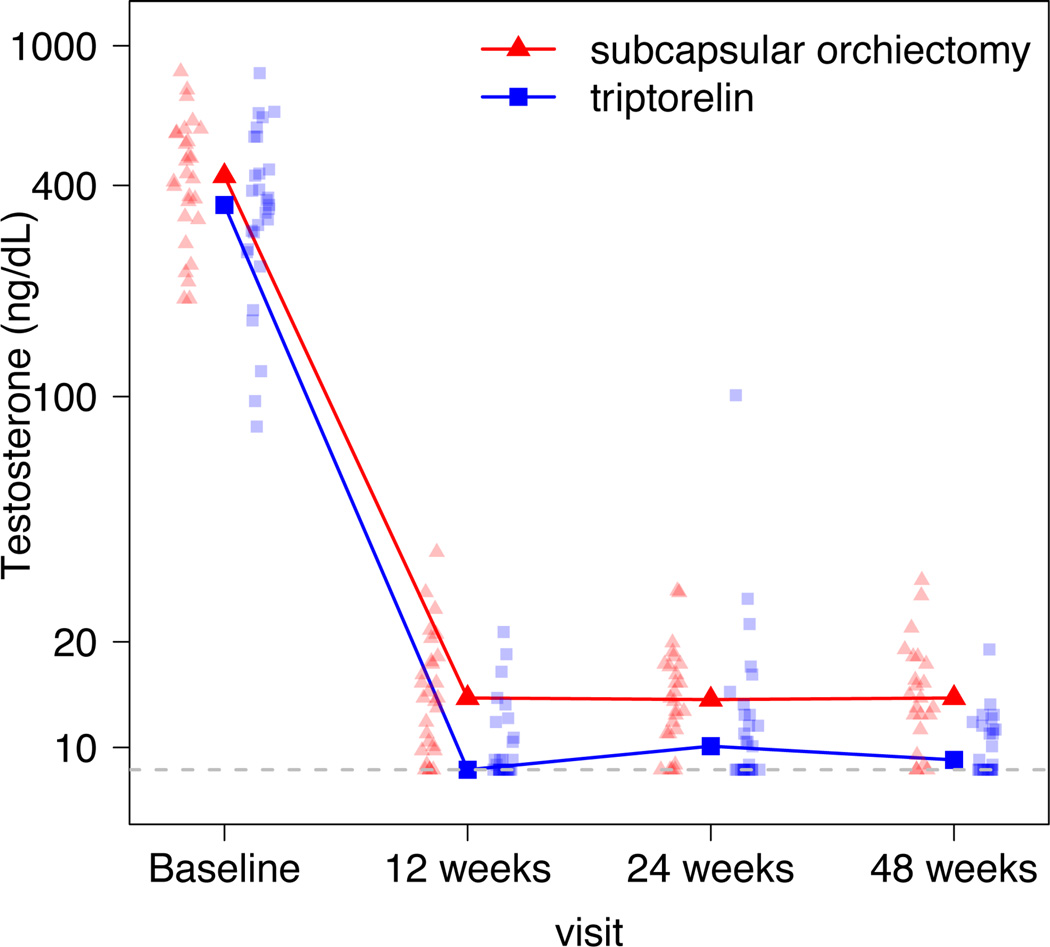
Serum testosterone levels over time are depicted in Figure 2 and the results of between-group analyses for testosterone, estradiol and adrenal androgens are shown in Table 2. Serum testosterone (adjusted for baseline testosterone, DHEAS and androstenedione) was 29% (95% CI 17.2, 41.7) lower after triptorelin therapy compared to subcapsular orchiectomy (p<.001). All patients achieved nadir testosterone levels <30 ng/dL after 48 weeks of follow-up. The proportion of patients achieving testosterone levels <20 ng/dL was 79% and 97% at 12 weeks (p<.05), 92% and 90% at 24 weeks (p=.73) and 87% and 100% at 48 weeks (p<.05) in the subcapsular orchiectomy and triptorelin groups, respectively. LH and FSH expectedly increased after subcapsular orchiectomy but were suppressed by triptorelin. The LH levels were below the LRL in the triptorelin group at all visits, except in 3 patients at the 24-week visit. One of these patients had a testosterone level that was above the castrate level (testosterone >50 ng/dL) at that same visit. The testosterone measurement in this patient at 48 weeks was back in the castrate level range. There were no significant differences in treatment effect for estradiol or adrenal androgens over time.
Figure 2.
Connected points depict median serum testosterone levels by treatment over time. The paler points (triangles and squares) represent individual testosterone measurements grouped by treatment. 8.6 ng/dL represents the lower reporting limit (LRL) of the assay.
Table 2.
Differences in serum hormone levels after triptorelin compared to subcapsular orchiectomy
| Only baseline level adjusted model† | Final adjusted model‡ | |||||
|---|---|---|---|---|---|---|
| Treatment difference (%)* |
95% confidence interval |
p- value |
Treatment difference (%)* |
95% confidence interval |
p- value |
|
| Testosterone | −31% | (−48.8, −13.0) | 0.005 | −29% | (−41.7, −17.2) | <.001 |
| Androstenedione | −2% | (−19.0, 15.7) | 0.852 | −1% | (−12.8, 11.5) | 0.915 |
| DHEAS | −5% | (−15.3, 6.1) | 0.408 | −5% | (−12.7, 3.5) | 0.276 |
|
17- hydroxyprogesterone |
Not available: data was too censored by the lower reporting limit of the assay | |||||
| SHBG | −7% | (−19.6, 5.2) | 0.377 | −9% | (−20.6, 2.1) | 0.127 |
| Estradiol | −11% | (−25.5, 4.1) | 0.180 | −9% | (−22.1, 3.6) | 0.180 |
Differences between treatment-groups estimated using Tobit regressions adjusted for baseline hormone levels, i.e. difference in reached testosterone values adjusted for baseline testosterone.
Differences between treatment groups estimated using Tobit regressions only adjusted for covariates (e.g. age, BMI, concurrent hormone values, baseline values of the modeled hormone) with effect modification. Final models are presented in supplements.
Triptorelin relative to subcapsular orchiectomy.
DHEAS: Dehydroepiandrosterone sulfate. SHBG: Sex hormone binding globulin.
The DHEAS, androstenedione, 17-hydroxyprogesterone and estradiol levels significantly decreased in both treatment arms (p<.001). The hormone values at each visit and hormone level reduction after treatment start are shown in Table 3. SHBG levels did not change in either treatment group.
Table 3.
Serum hormone levels at baseline and follow-up
| Medians (interquartile range) | |||||
|---|---|---|---|---|---|
| Baseline | 12 weeks | 24 weeks | 48 weeks | % Reduction (95% CI), p-valueƚ |
|
| Testosterone (ng/dL) | |||||
| Subcapsular orchiectomy | 425.1 (324.2,562.7) |
13.8 (10.0, 17.7) |
13.7 (11.0, 17.2) |
13.8 (11.8,17.7) |
97 (96.4, 97.2), p < .001 |
| Triptorelin | 351.6 (262.8,443.8) |
8.6* (8.6*, 10.7) |
10.1 (8.6*, 12.4) |
9.2 (8.6*, 11.5) |
97 (96.8, 98.0), p < .001 |
|
Androstenedione (nmol/L) |
|||||
| Subcapsular orchiectomy | 2.26 (1.81, 2.67) |
1.46 (1.05, 1.74) |
1.23 (1.00*, 1.67) |
1.46 (1.00*, 1.98) |
40 (33.1, 46.6), p < .001 |
| Triptorelin | 2.12 (1.61, 3.12) |
1.22 (1.00*, 1.69) |
1.12 (1.00*, 1.91) |
1.42 (1.00*, 1.97) |
40 (33.2, 47.5), p < .001 |
|
DHEAS (nmol/L) |
|||||
| Subcapsular orchiectomy | 1690 (1093, 2073) |
1370 (823, 1815) |
1200 (806, 2125) |
1370 (1085, 2160) |
17 (8.8, 24.8), p < .001 |
| Triptorelin | 1950 (1310, 2560) |
1290 (1000, 2130) |
1590 (800, 2290) |
1470 (886, 2540) |
22 (13.9, 30.6), p < .001 |
|
17-hydroxyprogesterone (nmol/L) |
|||||
| Subcapsular orchiectomy | 2.48 (1.61, 2.81) |
1.00* (1.00*, 1.05) |
1.00* (1.00*, 1.00*) |
1.00* (1.00*, 1.11) |
65 (58.5, 72.1), p < .001 |
| Triptorelin | 1.82 (1.50, 2.26) |
1.00* (1.00*, 1.00*) |
1.00* (1.00*, 1.05) |
1.00* (1.00*, 1.15) |
60 (50.1, 69.5), p < .001 |
| SHBG (nmol/L) | |||||
| Subcapsular orchiectomy | 53 (42, 72) | 57 (46, 67) | 57 (49, 74) | 56 (48, 75) | −3 (−13.5, 6.7), p = 0.502 |
| Triptorelin | 46 (34, 53) | 44 (33, 58) | 46 (38, 59) | 47 (35, 55) | 0 (−10.1, 10.7), p = 0.961 |
| Estradiol (nmol/L) | |||||
| Subcapsular orchiectomy | 0.11 (0.10, 0.13) |
0.05 (0.04*, 0.06) |
0.06 (0.05, 0.07) |
0.06 (0.04*, 0.08) |
54 (48.1, 60.4), p < .001 |
| Triptorelin | 0.10 (0.09, 0.11) |
0.04 (0.04*, 0.06) |
0.05 (0.04*, 0.07) |
0.05 (0.04*, 0.06) |
56 (49.0, 63.0), p < .001 |
| FSH (IU/L) | |||||
| Subcapsular orchiectomy | 9.2 (5.8, 13.5) |
55.3 (39.5, 64.8) |
60.4 (45.8, 71.4) |
Not measured |
−485 (−600.2, −370.2), p < .001 |
| Triptorelin | 9.4 (5.6, 13.0) |
4.3 (3.0, 5.6) |
4.6 (3.3, 5.4) |
Not measured |
55 (45.4, 64.3), p < .001 |
| LH (IU/L) | |||||
| Subcapsular orchiectomy | 5.0 (3.2, 7.5) |
31.3 (25.7, 34.4) |
32.3 (22.0, 37.8) |
Not measured |
−500 (−610.0, −389.4), p < .001 |
| Triptorelin | 4.0 (2.8, 6.1) |
0.3* (0.3*, 0.3*) |
0.3* (0.3*, 0.3*) |
Not measured |
98 (97.2, 99.6), p < .001 |
Within-group % reductions in hormone levels estimated by longitudinal Tobit regressions of log hormone levels versus post-treatment indicator.
Censored at lower reporting limit of the assay.
DHEAS: Dehydroepiandrosterone sulfate. SHBG: Sex hormone binding globulin. FSH: follicle stimulating hormone. LH: Luetinizing hormone.
DISCUSSION
This is the first randomized trial reporting lower testosterone levels after LHRH agonist therapy compared to surgical castration (29% reduction, p<.001). Previous randomized trials comparing LHRH agonists to orchiectomy did not find a difference in the efficacy of serum testosterone suppression.9, 10 However, the accuracy and precision of the immunoassays used at the time of these trials were insufficient to detect testosterone level differences at castrate range concentrations. Triptorelin was combined with bicalutamide for the first 30 days. Bicalutamide as monotherapy increases serum testosterone, but with a 2 months washout period before subsequent measurements, this is unlikely to have influenced the results.11
Orchiectomy increases LH and FSH through lack of negative feedback whereas triptorelin suppresses LH and FSH after an initial increase. LH receptors are present in prostate cancer cells, and the LH-LH receptor signaling pathway has been shown to both induce prostate cancer cell proliferation and increase steroidogenic gene expression and subsequent androgen production in vitro.12, 13 Thus, the differences in testosterone levels could reflect steroid production at the tumor level, independent of adrenal and testicular production.
LH suppression has also been hypothesized to decrease adrenal androgen production. This hypothesis originated after the detection of LH receptors in the adrenal cortex.14 This is intriguing because newer hormonal therapies that target adrenal androgen production through CYP17 inhibition have shown significant survival benefits in men with castrate-resistant prostate cancer.15 However, we could not show a treatment difference on adrenal androgens. In fact, we demonstrate a similar 20–60% reduction of so-called adrenal androgens after subcapsular orchiectomy.
Another possible explanation for our results is that testicular tissue is left behind using the subcapsular surgical approach. However, the median levels of testosterone in the present study are equivalent to the median testosterone levels (using best available measurements) after total orchiectomy (median 15 ng/dL) reported by Oefelein and colleagues16, which suggests that this explanation is not the case. However, a chemiluminescence immunoassay was used to obtain these data and as such the results cannot be directly compared. The subcapsular approach initially fell out of favor following the report of McDonald and Calams in 1958 showing the presence of leydig-like cells in the tunica albuginea and epididymis post-operatively.17 However, the method was reintroduced when subsequent smaller non-randomized studies (n<80) were unable to detect differences in testosterone levels after subcapsular and total orchiectomy.18, 19 Van der Sluis et al. also addressed the issue of residual testicular tissue after subcapsular orchiectomy by measuring inhibin b in their cross-sectional study showing lower testosterone levels after LHRH agonist therapy compared to surgical castration (subcapsular and total bilateral orchiectomy).7 Inhibin b was undetectable in all treated men and the authors concluded that differences in serum testosterone were not explained by residual testicular tissue. However, undetectable levels of inhibin b are also possible in cases with retained testosterone production.20 Therefore, residual Leydig cell function, in our opinion, cannot be ruled out as an explanatory mechanism for the lower levels of testosterone seen with LHRH agonist treatment.
The clinical implications of varying testosterone and gonadotropin levels need further evaluation. Earlier studies directly comparing orchiectomy with chemical ADT have not shown survival differences between the treatment modalities.9, 21, 22 However, definitive studies comparing surgical and chemical castration are lacking because these earlier trials were underpowered to detect small survival differences. Furthermore, LHRH agonists give rise to surges in testosterone at the end of a formulation’s duration in a small percentage of patients, which may affect cancer control. In our study, the LH levels were not fully suppressed in 3 of the 29 patients (10%) at 24 weeks, with one patient not reaching castrate levels of testosterone. This phenomenon appears to diminish with time because we did not detect a testosterone increase in these patients at 48 weeks. Morote et al. demonstrated, in a retrospective study of 73 men on ADT, that having breakthrough testosterone values above 32 ng/dL resulted in decreased progression-free survival.5
In addition, differences in the FSH levels could be clinically important. FSH receptors are present in prostate cancer cells23 and the vasculature of tumors and are thought to be involved in tumor angiogenesis and cancer progression.24 Studies have shown correlations between serum FSH and the pathological stage of prostate cancer23 and, more recently, time to castration resistant prostate cancer in a retrospective analysis.25
Moreover, a recent population-based study using data from 3295 men with prostate cancer undergoing ADT from the Surveillance, Epidemiology, and End Results (SEER) Medicare-linked database compared clinically relevant adverse effects between orchiectomy and LHRH agonist use. They found that LHRH agonist therapy significantly increased the risk of cardiovascular complications, diabetes mellitus and fracture compared to orchiectomy (p≤.01 for all conditions).26 Meanwhile, other studies have not found an association between ADT and cardiovascular events27, whereas still other studies have found an increased risk for cardiovascular disease for both orchiectomy and LHRH agonist therapy.28, 29 This suggestive evidence calls for a further randomized comparisons of ADT modalities. These trials should include LHRH antagonist therapy, which results in different FSH and LH profiles compared to LHRH agonists and orchiectomy.30
Some limitations of this study should be addressed. First, the evaluation of hormonal differences between the treatment modalities was a secondary endpoint of the trial. However, the highly significant statistical difference in testosterone levels along with the post hoc power analysis ascertains that this is not a chance finding and that the study was sufficiently powered. Another limitation is that the inter-assay CV for testosterone was higher in our study compared to previous studies7, resulting in a relatively high LRL for serum testosterone. This primarily affected the testosterone measurements in the triptorelin group because more men on triptorelin reached the LRL of testosterone; as such, these men may have had falsely high testosterone levels. Thus, the difference in testosterone levels between subcapsular orchiectomy and triptorelin may be larger than reported.
CONCLUSIONS
Significantly lower levels of testosterone within the castrate range were reached using the LHRH agonist triptorelin versus subcapsular orchiectomy. This is the first randomized study using the accurate method of LC-MS/MS to directly show differences in testosterone production after two forms of ADT and thereby supports the hypothesis that different ADT modalities can vary in both favorable and adverse clinical effects. Further studies are needed to fully elucidate the clinical implications of these treatment-related hormone differences.
Supplementary Material
Acknowledgments
We would like to thank the staff of the Endocrine Laboratory and the Urological Research Department at Herlev and Gentofte University Hospital for their highly skilled technical assistance and support during this trial.
Funding: This study was funded by the Research Foundation of Herlev and Gentofte Hospital (no specific grant number), Vissing Fonden (grant no. 54622) and Fabrikant Einar Willumsens Mindelegat (grant no. 6000073-000168). The funding supported data collection and hormone analyses. The financial sources had no role in the design or conduction of the study, the statistical analysis, writing of the manuscript or the decision to publish the final version of manuscript.
Standard abbreviations key
- ADT
Androgen deprivation therapy
- CV
Coefficient of variance
- DHEAS
Dehydroepiandrosterone sulfate
- FSH
Follicle stimulating hormone
- LC-MS/MS
Liquid chromatography – tandem mass spectrometry
- LH
Luteinizing hormone
- LHRH
Luteinizing hormone releasing hormone
- LRL
Lower reporting limit of detection
- NRR
Normal reference range
- SHBG
Sex hormone binding globulin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pagliarulo V, Bracarda S, Eisenberger MA, et al. Contemporary Role of Androgen Deprivation Therapy for Prostate Cancer. Eur. Urol. 2012;61:11–25. doi: 10.1016/j.eururo.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perachino M, Cavalli V, Bravi F. Testosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone-releasing hormone therapy: prognostic significance? BJU Int. 2010;105:648–651. doi: 10.1111/j.1464-410X.2009.08814.x. [DOI] [PubMed] [Google Scholar]
- 3.Bertaglia V, Tucci M, Fiori C, et al. Effects of serum testosterone levels after 6 months of androgen deprivation therapy on the outcome of patients with prostate cancer. Clin. Genitourin. Cancer. 2013;11:325–330.e1. doi: 10.1016/j.clgc.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Klotz L, O’Callaghan C, Ding K, et al. Nadir Testosterone Within First Year of Androgen-Deprivation Therapy (ADT) Predicts for Time to Castration-Resistant Progression: A Secondary Analysis of the PR-7 Trial of Intermittent Versus Continuous ADT. J. Clin. Oncol. 2015;33:1151–1156. doi: 10.1200/JCO.2014.58.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morote J, Orsola A, Planas J, et al. Redefining Clinically Significant Castration Levels in Patients With Prostate Cancer Receiving Continuous Androgen Deprivation Therapy. J. Urol. 2007;178:1290–1295. doi: 10.1016/j.juro.2007.05.129. [DOI] [PubMed] [Google Scholar]
- 6.Kulle aE, Riepe FG, Melchior D, et al. A novel ultrapressure liquid chromatography tandem mass spectrometry method for the simultaneous determination of androstenedione, testosterone, and dihydrotestosterone in pediatric blood samples: Age- and sex-specific reference data. J. Clin. Endocrinol. Metab. 2010;95:2399–2409. doi: 10.1210/jc.2009-1670. [DOI] [PubMed] [Google Scholar]
- 7.van der Sluis TM, Bui HN, Meuleman EJH, et al. Lower Testosterone Levels With Luteinizing Hormone-Releasing Hormone Agonist Therapy Than With Surgical Castration: New Insights Attained by Mass Spectrometry. J. Urol. 2012;187:1601–1607. doi: 10.1016/j.juro.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 8.Twisk J, Rijmen F. Longitudinal tobit regression: a new approach to analyze outcome variables with floor or ceiling effects. J. Clin. Epidemiol. 2009;62:953–958. doi: 10.1016/j.jclinepi.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Vogelzang N, Chodak G, Soloway M, et al. Goserelin versus orchiectomy in the treatment of advanced prostate cancer: final results of a randomized trial. Urology. 1995;46:220–226. doi: 10.1016/s0090-4295(99)80197-6. [DOI] [PubMed] [Google Scholar]
- 10.Parmar H, Phillips RH, Lightman SL, et al. Randomised controlled study of orchidectomy vs long-acting D-Trp-6-LHRH microcapsules in advanced prostatic carcinoma. Lancet (London, England) 1985;2:1201–1205. doi: 10.1016/s0140-6736(85)90739-1. [DOI] [PubMed] [Google Scholar]
- 11.Cockshott ID. Bicalutamide: clinical pharmacokinetics and metabolism. Clin. Pharmacokinet. 2004;43:855–878. doi: 10.2165/00003088-200443130-00003. [DOI] [PubMed] [Google Scholar]
- 12.Pinski J, Xiong S, Wang Q, et al. Effect of luteinizing hormone on the steroidogenic pathway in prostate cancer. Prostate. 2011;71:892–898. doi: 10.1002/pros.21305. [DOI] [PubMed] [Google Scholar]
- 13.Xiong S, Wang Q, Liu SV, et al. Effects of luteinizing hormone receptor signaling in prostate cancer cells. Prostate. 2015;75:141–150. doi: 10.1002/pros.22899. [DOI] [PubMed] [Google Scholar]
- 14.Nishii M, Nomura M, Sekine Y, et al. Luteinizing hormone (LH)-releasing hormone agonist reduces serum adrenal androgen levels in prostate cancer patients: implications for the effect of LH on the adrenal glands. J. Androl. 2012;33:1233–1238. doi: 10.2164/jandrol.112.016493. [DOI] [PubMed] [Google Scholar]
- 15.Yin L, Hu Q. CYP17 inhibitors—abiraterone, C17,20-lyase inhibitors and multi-targeting 14 agents. Nat. Rev. Urol. 2013;11:32–42. doi: 10.1038/nrurol.2013.274. [DOI] [PubMed] [Google Scholar]
- 16.Oefelein MG, Feng A, Scolieri MJ, et al. Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology. 2000;56:1021–1024. doi: 10.1016/s0090-4295(00)00793-7. [DOI] [PubMed] [Google Scholar]
- 17.McDonald JH, Calams JA, et al. Extraparenchymal Leydig-like cells: observations following subcapsular orchiectomy. J. Urol. 1959;82:145–147. doi: 10.1016/S0022-5347(17)65846-4. [DOI] [PubMed] [Google Scholar]
- 18.Chapman JP. Comparison of testosterone and LH values in subcapsular vs total orchiectomy patients. Urology. 1987;30:27–28. doi: 10.1016/0090-4295(87)90565-6. [DOI] [PubMed] [Google Scholar]
- 19.Zhang XZ, Donovan MP, Williams BT, et al. Comparison of subcapsular and total orchiectomy for treatment of metastatic prostate cancer. Urology. 1996;47:402–404. doi: 10.1016/S0090-4295(99)80460-9. [DOI] [PubMed] [Google Scholar]
- 20.Petersen PM, Andersson AM, Rørth M, et al. Undetectable inhibin B serum levels in men after testicular irradiation. J. Clin. Endocrinol. Metab. 1999;84:213–215. doi: 10.1210/jcem.84.1.5406. [DOI] [PubMed] [Google Scholar]
- 21.Peeling WB. Phase III studies to compare goserelin (zoladex) with orchiectomy and with diethylstilbestrol in treatment of prostatic carcinoma. Urology. 1989;33:45–52. doi: 10.1016/0090-4295(89)90106-4. [DOI] [PubMed] [Google Scholar]
- 22.Kaisary aV, Tyrrell CJ, Peeling WB, et al. Comparison of LHRH analogue (Zoladex) with orchiectomy in patients with metastatic prostatic carcinoma. Br. J. Urol. 1991;67:502–508. doi: 10.1111/j.1464-410x.1991.tb15195.x. [DOI] [PubMed] [Google Scholar]
- 23.Mariani S, Salvatori L, Basciani S, et al. Expression and Cellular Localization of Follicle-Stimulating Hormone Receptor in Normal Human Prostate, Benign Prostatic Hyperplasia and Prostate Cancer. J. Urol. 2006;175:2072–2077. doi: 10.1016/S0022-5347(06)00273-4. [DOI] [PubMed] [Google Scholar]
- 24.Radu A, Pichon C, Camparo P, et al. Expression of Follicle-Stimulating Hormone Receptor in Tumor Blood Vessels. N. Engl. J. Med. 2010;363:1621–1630. doi: 10.1056/NEJMoa1001283. [DOI] [PubMed] [Google Scholar]
- 25.Siemens DR, Hoare D, Skinner T, et al. Serum follicle-stimulating hormone levels predict time to development of castration resistant prostate cancer. Can. Urol. Assoc. J. 2015;9:122. doi: 10.5489/cuaj.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun M, Choueiri TK, Hamnvik O-PR, et al. Comparison of Gonadotropin-Releasing Hormone Agonists and Orchiectomy: Effects of Androgen-Deprivation Therapy. JAMA Oncol. 2016;2:500–507. doi: 10.1001/jamaoncol.2015.4917. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen P, Je Y, Schutz F, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: A Meta-analysis of Randomized Trials. JAMA. 2011;306:2359–2366. doi: 10.1001/jama.2011.1745. [DOI] [PubMed] [Google Scholar]
- 28.O’Farrell S, Garmo H, Holmberg L, et al. Risk and Timing of Cardiovascular Disease After Androgen-Deprivation Therapy in Men With Prostate Cancer. J. Clin. Oncol. 2015;33:1243–1251. doi: 10.1200/JCO.2014.59.1792. [DOI] [PubMed] [Google Scholar]
- 29.Bosco C, Bosnyak Z, Malmberg A, et al. Quantifying Observational Evidence for Risk of Fatal and Nonfatal Cardiovascular Disease Following Androgen Deprivation Therapy for Prostate Cancer: A Meta-analysis. Edited by Z Culig. Eur. Urol. 2015;68:386–396. doi: 10.1016/j.eururo.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 30.Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531–1538. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.