Abstract
Rationale
Metabotropic glutamate 2 and 3 receptors (mGluR2/3) are implicated in drug addiction as they limit excessive glutamate release during relapse. N-acetylaspartylglutamate (NAAG) is an endogenous mGluR2/3 agonist that is inactivated by the glutamate carboxypeptidase II (GCPII) enzyme. GCPII inhibitors, and NAAG itself, attenuate cocaine-seeking behaviors. However, their effects on the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) have not been examined.
Objectives
We determined whether withdrawal following repeated MDPV administration alters GCPII expression in corticolimbic regions. We also examined whether a GCPII inhibitor (2-(phosphonomethyl)-pentanedioic acid; 2-PMPA), and NAAG, reduces the rewarding and locomotor-stimulant effects of MDPV in rats.
Methods
GCPII was assessed following repeated MDPV exposure (7 days). The effects of 2-PMPA and NAAG on acute MDPV-induced hyperactivity were determined using a locomotor test. We also examined the inhibitory effects of 2-PMPA and NAAG on MDPV-induced place preference, and whether the mGluR2/3 antagonist LY341495 could prevent these effects.
Results
MDPV withdrawal reduced GCPII expression in the prefrontal cortex. Systemic injection of 2-PMPA (100 mg/kg) did not affect the hyperactivity produced by MDPV (0.5–3 mg/kg). However, nasal administration of NAAG did reduce MDPV-induced ambulation, but only at the highest dose (500 μg/10 μl). We also showed that 2-PMPA (10–30 mg/kg) and NAAG (10–500 μg/10 μl) dose-dependently attenuated MDPV place preference, and that the effect of NAAG was blocked by LY341495 (3 mg/kg).
Conclusions
These findings demonstrate that MDPV withdrawal produces dysregulation in the endogenous NAAG-GCPII signaling pathway in corticolimbic circuitry. Systemic administration of the GCPII inhibitor 2-PMPA, or NAAG, attenuates MDPV reward.
Keywords: MDPV, synthetic cathinone, glutamate, 2-PMPA, NAAG, reward
Introduction
Synthetic cathinones are a popular class of designer drugs that are structural derivatives of cathinone, the primary psychostimulant constituent of the khat plant (Glennon 2014). 3,4-Methylenedioxypyrovalerone (MDPV) is among the most popular synthetic cathinones. Similar to other amphetamine-like compounds (e.g. methamphetamine and cocaine), MDPV produces the desired effects of increased confidence, energy and alertness after acute administration (Karila et al. 2015). However, cases of hypertension, tachycardia, psychosis, suicidal ideation and violent behavior have been reported following repeated abuse (White 2016). Although MDPV was classified as a Schedule I drug in 2013, it is still available on the clandestine drug market, and has also served as a model for the new “second-generation” analogs with a similar pharmacological profile namely α-pyrrolidinovalerophenone (α-PVP), α-pyrrolidinobutiophenone (α-PBP) and α-pyrrolidinopropiophenone (α-PPP) (Marusich et al. 2014; Rickli et al. 2015).
MDPV acts on monoamine transporters to block dopamine (DA) and norepinephrine (NE) reuptake, with negligible effects on serotonin (5-HT), which results in elevated extracellular concentrations of DA and NE within brain reward pathways (Baumann et al. 2013; Simmler et al. 2013). The pharmacological action of MDPV is similar to that of cocaine; however, MDPV is 50- and 10-times more potent at the dopamine (DAT) and norepinephrine (NET) transporters, respectively (Baumann et al. 2013; Marusich et al. 2014; Simmler et al. 2013). Not surprisingly then, acute systemic administration of MDPV induces more robust ambulation and stereotypy than cocaine or methamphetamine (Aarde et al. 2013; Baumann et al. 2013; Gatch et al. 2013; Marusich et al. 2014), while repeated dosing of MDPV produces locomotor sensitization (Berquist et al. 2016). In abuse liability studies, MDPV produces more robust rewarding effects in the conditioned place preference (CPP) paradigm than amphetamine (Karlsson et al. 2014), and is self-administered at higher rates than cocaine or methamphetamine (Aarde et al. 2013; Watterson et al. 2014; Schindler et al. 2015). MDPV also strongly enhances brain-stimulation reward (BSR) in rats (Bonano et al. 2014; Watterson et al. 2014).
While effects of MDPV on monoamine systems have been well studied, far less is known about its effects on the glutamate (GLU) system. This lack of research is particularly pertinent since a central role for GLU dysregulation in psychostimulant reinforcement and relapse is well documented (for review, see Kalivas 2009; D’Souza 2015; Spencer et al. 2016). We recently showed that withdrawal following repeated MDPV administration disrupts GLU homeostasis by reducing glutamate transporter subtype 1 (GLT-1) levels in the nucleus accumbens (NAc) (Gregg et al. 2016). Furthermore, chronic treatment with the β-lactam antibiotic ceftriaxone (CTX), which upregulates GLT-1 expression, attenuates MDPV-induced locomotor sensitization and place preference (Gregg et al. 2016).
Psychostimulant addiction is also influenced by the Group II metabotropic glutamate 2 and 3 receptors (mGluR2/3), which act to inhibit excessive synaptic GLU release evoked by drug cues or stress that is thought to trigger relapse behavior (Baptista et al. 2004; Kalivas and Volkow 2005; Kalivas 2009; Liechti et al. 2007). The neuropeptide N-acetylaspartylglutamate (NAAG) functions as an endogenous mGluR2/3 agonist in the central nervous system (CNS) (Cartmell et al. 1998), and is hydrolyzed into N-acetylaspartate (NAA) and GLU by glutamate carboxypeptidase II (GCPII) (Tsukamoto et al. 2007). GCPII inhibitors, mGluR2/3 agonists and NAAG itself, have all been shown to inhibit cocaine-seeking behaviors in rodents. For example, the GCPII inhibitors 2-(phosphonomethyl)-pentanedioic acid (2-PMPA) and GPI-5693 attenuate cocaine-induced place preference (Slusher et al. 2001), and 2-PMPA also inhibits cocaine self-administration and reinstatement of cocaine-seeking behavior (Xi et al. 2010a, b). The mGluR2/3 agonist LY379268 reduces cocaine and nicotine self-administration and reinstatement of drug seeking (Baptista et al. 2004; Cannella et al. 2013; Liechti et al. 2007), and NAAG itself attenuates cocaine self-administration in rats maintained on a progressive ratio reinforcement schedule (Xi et al. 2010a).
Given the role of NAAG-GCPII interactions in psychostimulant reward, we first determined whether MDPV withdrawal alters GCPII expression in corticolimbic regions including the prefrontal cortex (PFC), NAc and striatum. Based on the previous findings with the GCPII inhibitor 2-PMPA, and NAAG itself, on rewarding effects of cocaine, we next examined whether these drugs could attenuate place preference and locomotor activation produced by MDPV. Lastly, we determined a possible mechanism underlying the effects of NAAG (and 2-PMPA) on MDPV-induced reward using the selective mGluR2/3 antagonist LY341495.
Materials and Methods
Animals
Experiments were conducted in male Sprague-Dawley rats purchased from Harlan Laboratories (Indianapolis, IN) that weighed between 250–275 g on arrival. Rats were pair-housed in a temperature- and humidity-controlled vivarium on a 12-h light/dark cycle (lights on at 07:00h). Food and water was available ad libitum, except during experimental procedures. All experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and Temple University’s Guidelines for the Care of Animals.
Drug preparation and administration
(±)-MDPV was synthesized following previously published methods (Abiedalla et al. 2012; Kolanos et al. 2015) by Dr. Allen Reitz and dissolved in physiological saline (0.9%). 2-PMPA was purchased from AstaTech, Inc. (Bristol, PA) and dissolved in a 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (in distilled water) vehicle. The selective mGluR2/3 antagonist LY341495 was obtained from Tocris BioScience (Minneapolis, MN) and dissolved in 10% dimethyl sulfoxide (DMSO), 10% Kolliphor EL and 80% physiological saline. MDPV, 2-PMPA and LY341495 were administered by intraperitoneal (i.p.) injection at a volume of 1 ml/kg. Equivalent injections of saline or vehicle were used for the control conditions. NAAG was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in saline. It was administered by nasal application according to a well-established protocol (Lukas and Neumann 2012) due to its poor blood-brain barrier (BBB) permeability. Controls were given an equivalent volume of saline (20 μl).
GCPII protein expression
The general procedure for Western blot protein analysis has been described previously (Gregg et al. 2016). After tissue sample preparation, equal amounts of protein (20 μg per loading sample, as determined using a Thermo Scientific NanoDrop 2000 spectrometer) were loaded onto 7.5% Mini Protean Precast gels (Bio-Rad, Hercules, CA) for separation and transferred onto nitrocellulose membranes (Life Technologies, Carlsbad, CA). Membranes were blocked for 1-h at room temperature in Odyssey Blocking Buffer (Li-Cor Biosciences, Lincoln, NE), followed by overnight incubation at 4°C with the GCPII primary antibody (1:5,000, mouse monoclonal; GeneTex, Irvine, CA). The next day, membranes were washed with Tween-Tris buffered saline (TTBS) 3 times for 8 min, followed by 90 min incubation with β-tubulin loading control primary antibody at room temperature (1:800,000, monoclonal mouse; Cell Signaling, Danvers, MA). Membranes were washed again with TTBS, and then incubated for 1-h at room temperature with IRDye 680-conjugated goat anti-mouse secondary antibody (1:10,000; Li-Cor Biosciences). Membranes were given 3 final TTBS washes, before proteins were detected and quantified using the Odyssey infrared imaging system (Li-Cor Biosciences). The relative density of each sample (GCPII/β-tubulin optical densities) were determined and averaged for each group.
Locomotor activity test
The locomotor activity tests were conducted according to the procedures described in detail in our previous publications (Gregg et al. 2013, 2015). Briefly, locomotor activity of individual rats was measured during the light cycle in activity chambers using a Digiscan DMicro System (Accuscan Inc., Columbus, OH). Two types of locomotor activity were automatically measured: 1) ambulatory activity produced by horizontal movements and 2) stereotypy resulting from recurring or focused movements.
Basal locomotor activity was measured for 30 min prior to rats being administered 2-PMPA or NAAG, or the corresponding control condition. This time also enabled rats to acclimate to the activity chambers. Thirty minutes later, rats were injected with saline or MDPV and locomotor activity recorded for 90 min. The exception to this was Experiment 4A where rats did not receive an injection of saline or MDPV.
Conditioned place preference test
CPP experiments were performed according to previously published methods described in detail elsewhere (Lisek et al. 2012; Gregg et al. 2015, 2016). The apparatus consisted of two equal-sized environmentally distinguishable compartments separated by a removable door. The CPP test followed a 4-day biased design and was carried out during the light cycle. Prior to conditioning, a ‘pre-test’ was conducted during which individual rats were allowed to explore both compartments for 30 min in a drug-free state. The time spent in each compartment was manually scored by a naïve experimenter. The compartment in which rats spent less time during the pre-test was designated their ‘less-preferred compartment’.
The 4-day conditioning phase began the day after the pre-test and consisted of two 30 min sessions each day conducted 4-h apart. In the first conditioning session, rats were injected with saline or MDPV before being placed in their initially less-preferred compartment. All treatment groups were administered saline in the second conditioning session and placed in their initially preferred compartment. We selected a dose of 2 mg/kg MDPV for all CPP experiments based on our recently published dose-response experiment (Gregg et al. 2016), and from previous work using a similar CPP paradigm (King et al. 2015a).
A ‘post-test’ was conducted on the day after the last conditioning day to determine the effects of 2-PMPA and NAAG on the expression of MDPV-induced place preference. Rats were injected with 2-PMPA or NAAG, or the corresponding control condition 30 min before being placed into the CPP apparatus, and then allowed 30 min to explore both compartments. LY341495 was administered 30 min prior to NAAG in Experiment 6. Rats were never given MDPV during the post-test. For graphical presentation and analysis, data was converted to a difference score (difference in time spent in the less-preferred compartment between post-test and pre-test).
Experiment 1: GCPII expression following MDPV withdrawal
Experiment 1 examined the effects of withdrawal following repeated administration of MDPV on GCPII protein expression. Following a previously published behavioral sensitization paradigm (Kalivas and Duffy 1998; Gregg et al. 2013, 2016), experimentally naïve rats (n = 6–7 per group) were injected with saline or MDPV (Days 1 and 7: 0.5 mg/kg and Days 2–6: 1 mg/kg) for 7 days. Rats were euthanized by CO2 asphyxiation and decapitated either 2 h (Withdrawal Day 0), 2 days (Withdrawal Day 2), 5 days (Withdrawal Day 5), or 10 days (Withdrawal Day 10) after the last drug injection. The brains were extracted and rapidly dissected on ice to isolate the PFC, NAc and striatum, and then processed for Western blotting.
Experiment 2: The effects of 2-PMPA on MDPV-induced ambulation and stereotypy
Experiment 2 determined the effects of 2-PMPA on the robust ambulation and stereotypy induced by MDPV. Experimentally naïve rats were randomly allocated to 4 treatment groups (n = 8 per group): 1) vehicle + saline, 2) vehicle + MDPV, 3) 2-PMPA + saline or 4) 2-PMPA + MDPV. 2-PMPA was administered at 100 mg/kg since this dose has been shown previously to inhibit cocaine self-administration and reinstatement of cocaine-seeking behavior (Xi et al. 2010a, b). MDPV was injected at 0.5, 1 and 3 mg/kg to determine its dose-response effects on locomotor activity. In a follow-up experiment (n = 8 per group), we tested a lower dose of 2-PMPA (30 mg/kg) that also previously reduced cocaine self-administration in rats (Xi et al. 2010a, b).
Experiment 3: The effects of 2-PMPA on the expression of MDPV-induced place preference
Experiment 3 examined the effects of 2-PMPA on the place preference induced by MDPV. Experimentally naïve rats were randomly allocated to receive saline or MDPV (2 mg/kg) during the conditioning phase, before being administered vehicle or 2-PMPA (30 mg/kg) prior to the post-test. Thus, the 4 treatment groups (n = 10–11 per group) were 1) saline + vehicle, 2) saline + 2-PMPA, 3) MDPV + vehicle and 4) MDPV + 2-PMPA. We decided not to test the higher dose of 2-PMPA (i.e. 100 mg/kg) in this experiment that was evaluated against cocaine by Xi and colleagues (2010a, b), as we observed significant locomotor side effects in Experiment 2. In a follow-up experiment (n = 8 per group), we also tested a lower dose of 2-PMPA (10 mg/kg) in animals conditioned with MDPV (2 mg/kg).
Experiments 4A and 4B: The locomotor effects of NAAG by itself, and in combination with MDPV
We first examined the intrinsic locomotor effects of NAAG by itself in Experiment 4A. Experimentally naïve rats (n = 7–8 per group) were randomly allocated to receive saline or one of 3 doses of NAAG (10, 100 and 500 μg/10 μl). Previous research shows that NAAG at 100 μg/10 μl attenuates cocaine-enhanced BSR, and tends to reduce the motivation of rats to self-administer cocaine (Xi et al. 2010a). Following a 1 week washout period, the same rats used in Experiment 4A were allocated to 4 treatment groups (Experiment 4B; n = 7–8 per group) to determine the dose-response effects of NAAG on MDPV-induced ambulation and stereotypy: 1) saline + MDPV, 2) 10 μg NAAG + MDPV, 3) 100 μg NAAG + MDPV or 4) 500 μg NAAG + MDPV. MDPV was administered at 2 mg/kg in Experiment 4B since this dose was used in all CPP experiments, and therefore we were most interested to see whether NAAG could antagonize its locomotor effects.
Experiment 5: The effects of NAAG on the place preference induced by MDPV
Experiment 5 determined the dose-response effects of NAAG on the expression of MDPV-induced place preference. Experimentally naïve rats were all conditioned with MDPV (2 mg/kg) prior to receiving saline, or one of 3 doses of NAAG (10, 100 or 500 μg/10 μl) (n = 10–15 per group) before the post-test. To ensure the effects of NAAG were not due to a non-specific aversive-like response, in a follow-up experiment we tested the 500 μg/10 μl dose of NAAG by itself (saline + NAAG) against a saline control group (saline + saline) in animals that were conditioned only with saline (n = 7–8 per group).
Experiment 6: The role of mGluR2/3 receptors in the inhibitory effects of NAAG on MDPV-induced place preference
Experiment 6 examined the extent to which mGluR2/3 receptors mediate the inhibitory effect of NAAG (500 μg/10 μl) on MDPV-induced CPP. In this experiment, we administered the selective mGluR2/3 antagonist LY341495 at a dose of 3 mg/kg based on previous evidence that it reverses anhedonia induced by chronic stress (Dwyer et al. 2013) and blocks the anti-reinstatement effect of modafinil in the CPP paradigm (Tahsili-Fahadan et al. 2010). Experimentally naïve rats were all conditioned with MDPV (2 mg/kg), before being randomly allocated to 4 treatment groups (n = 13–15 per group) prior to the post-test: 1) vehicle + saline, 2) vehicle + NAAG, 3) LY341495 + saline or 4) LY341495 + NAAG.
Statistical analysis
The relative sample densities of saline and MDPV-treated rats in the GCPII protein assays were expressed as a percentage of the saline control values, and then compared using a Student’s t-test. Data from Experiments 2, 3 and 6 were analyzed using a two-way ANOVA where the two between-subject factors were ‘pretreatment’ and ‘treatment’ (or ‘MDPV dose’ in Experiment 2). For the 2-PMPA dose-response CPP experiment, data were expressed as a percentage of the MDPV (2 mg/kg) values and analyzed with a one-way ANOVA with the between-subject factor of ‘2-PMPA dose’. Data from Experiments 4 and 5 were analyzed with a one-way ANOVA employing the between-subject factor of ‘NAAG dose’. The overall one- and two-way ANOVA’s were followed by Bonferroni post-hoc tests. For the follow-up CPP experiment with NAAG, the two treatment groups were compared using a Student’s t-test. All analyses were carried out using SPSS version 20 (SPSS Inc., IBM, Chicago, IL) with statistical significance set at p < 0.05.
Results
Experiment 1: GCPII expression is reduced in the PFC following MDPV withdrawal
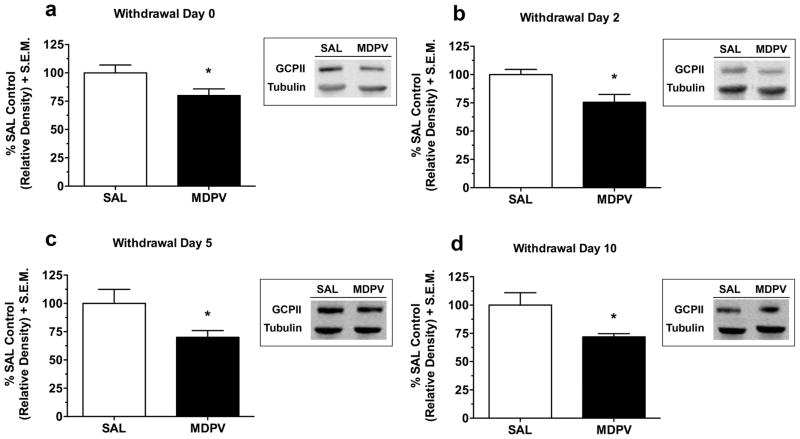
GCPII protein expression in the PFC at different time points during withdrawal following repeated MDPV administration is presented in Figure 1. GCPII expression was significantly reduced in the PFC at 2 h (Withdrawal Day 0 (Fig. 1a); t(12) = 2.22, p < 0.05), 2 days (Withdrawal Day 2 (Fig. 1b); t(11) = 2.83, p < 0.05), 5 days (Withdrawal Day 5 (Fig. 1c); t(10) = 2.46, p < 0.05), and 10 days (Withdrawal Day 10 (Fig. 1d); t(12) = 2.50, p < 0.05) after the last MDPV injection. In the NAc (see supplementary Figure S1), GCPII expression was only reduced 2 h into withdrawal (Withdrawal Day 0; t(12) = 2.86, p < 0.05), while in the striatum (see supplementary Figure S2), GCPII expression was not significantly reduced at any withdrawal time point (all p > 0.05) following repeated MDPV.
Fig. 1.
GCPII protein expression in the PFC during withdrawal from repeated MDPV administration. GCPII (95–110 kDa) expression was normalized to a β-tubulin (52 kDa) control protein to obtain a relative density. MDPV-treated rats showed reduced GCPII expression at 2 h (Withdrawal Day 0 (a); p < 0.05), 2 days (Withdrawal Day 2 (b); p < 0.05), 5 days (Withdrawal Day 5 (c); p < 0.05), and 10 days (Withdrawal Day 10 (d); p < 0.05) withdrawal. Data are presented as a percentage of the saline control values + S.E.M (n = 6–7 per group). The representative protein bands from the saline and MDPV treatment groups are shown in the corresponding boxes. *p < 0.05 relative to saline. SAL, saline
Experiment 2: MDPV-induced ambulation and stereotypy are not reduced by 2-PMPA
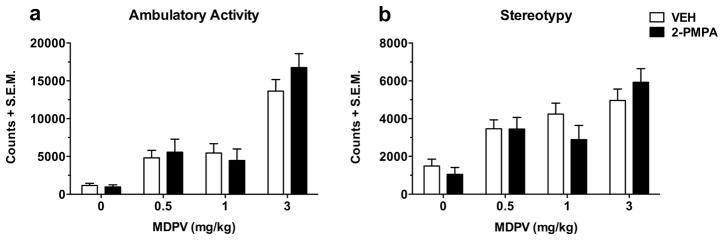
The effects of 2-PMPA (100 mg/kg) on the acute ambulatory activity and stereotypy produced by MDPV (0.5, 1 and 3 mg/kg) are shown in Figures 2a and 2b, respectively. Data were recorded as counts and summed over 90 min. MDPV dose-dependently increased ambulatory activity (MDPV dose effect: F(3,56) = 42.93, p < 0.001) and stereotypy (MDPV dose effect: F(3,56) = 17.52, p < 0.001). However, neither behavior was significantly affected by 2-PMPA (pretreatment effect on ambulatory activity: F(1,56) = 0.53, p > 0.05; pretreatment effect on stereotypy: F(1,56) = 0.27, p > 0.05) at any of the doses of MDPV (interaction effect on ambulatory activity: F(3,56) = 0.93, p > 0.05; interaction effect on stereotypy: F(3,56) = 1.40, p > 0.05). In a follow-up experiment, a lower dose of 2-PMPA (30 mg/kg) did not significantly affect MDPV-induced ambulation or stereotypy (both p > 0.05; data not shown).
Fig. 2.
The effects of 2-PMPA (100 mg/kg) on the acute ambulatory activity (a) and stereotypy (b) induced by increasing doses of MDPV (0.5, 1 and 3 mg/kg). Data were measured as counts and summed over 90 min. The dose-dependent increase in ambulatory activity and stereotypy produced by MDPV was not significantly affected by pretreatment with 2-PMPA (both p > 0.05). Data are shown as mean totals + S.E.M (n = 8 per group). VEH, vehicle
Experiment 3: 2-PMPA dose-dependently reduces the expression of MDPV-induced place preference
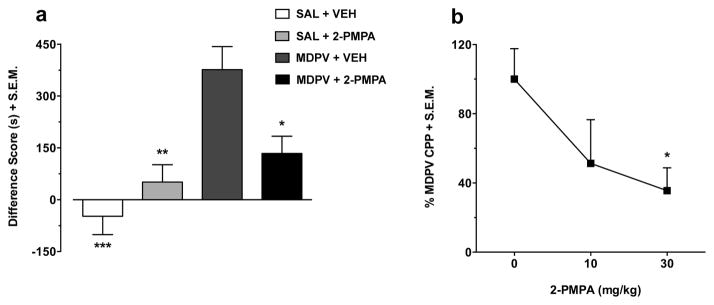
The effects of 2-PMPA (30 mg/kg) on the expression of MDPV (2 mg/kg) place preference are presented in Figure 3a. By itself, 2-PMPA had no significant effects in the CPP test (treatment effect: F(1,37) = 1.70, p > 0.05). MDPV produced a significant place preference (pretreatment effect: F(1,37) = 21.02, p < 0.001) independently of 2-PMPA treatment. However, the overall increase in place preference induced by MDPV differed depending on whether rats were treated with 2-PMPA or vehicle prior to the post-test (interaction effect: F(1,37) = 9.53, p < 0.01). Post-hoc tests showed that MDPV conditioned rats treated with vehicle displayed significantly increased place preference relative to saline conditioned animals given either vehicle (p < 0.001) or 2-PMPA (p < 0.01). The robust CPP in rats conditioned with MDPV was significantly reduced following treatment with 2-PMPA (p < 0.05). The dose-related effects of 2-PMPA (10 and 30 mg/kg) on the place preference produced by 2 mg/kg MDPV are depicted in Figure 3b. Overall, 2-PMPA dose-dependently decreased the robust MDPV-induced CPP (2-PMPA dose effect: F(2,26) = 3.61, p < 0.05), with a significant effect obtained at 30 mg/kg (p < 0.05), but not 10 mg/kg (p > 0.05).
Fig. 3.
The dose-related effects of 2-PMPA (10 and 30 mg/kg) on the place preference induced by MDPV (2 mg/kg). In Figure 3a, the data (n = 10–11 per group) are presented as a difference score (difference in time spent in the less-preferred compartment between post-test and pre-test). Results showed that the increase in place preference produced by MDPV was significantly reduced by 2-PMPA (p < 0.05). In Figure 3b, the data (n = 8 per group) are expressed as a percentage of the MDPV (2 mg/kg) values. 2-PMPA dose-dependently reduced MDPV-induced place preference with a significant effect obtained at 30 mg/kg (p < 0.05), but not 10 mg/kg (p > 0.05). Data are shown as mean + S.E.M. ***p < 0.001, **p < 0.01, *p < 0.05 relative to MDPV + vehicle. SAL, saline; VEH, vehicle
Experiments 4A and 4B: High dose NAAG attenuates MDPV-induced ambulation
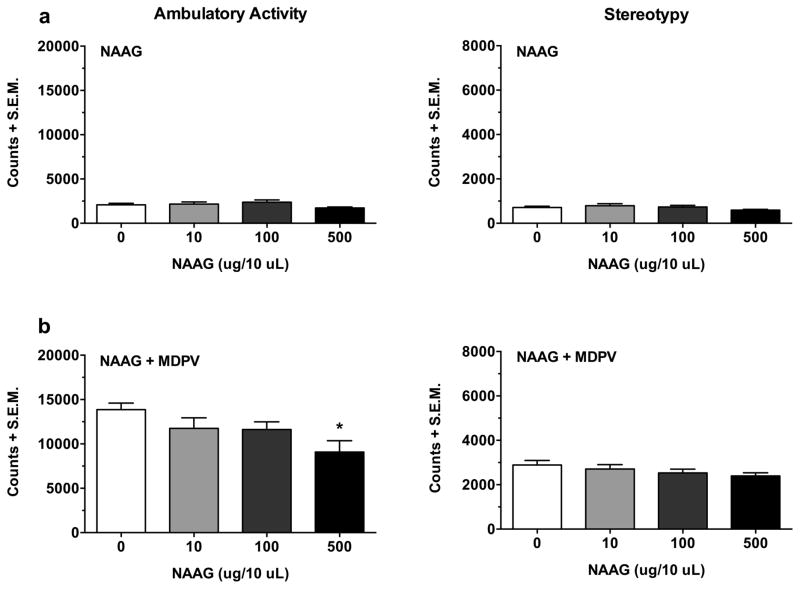
The intrinsic effects of NAAG (10, 100 and 500 μg/10 μl) by itself on ambulatory activity and stereotypy are shown in Figure 4a. Results indicated that NAAG, overall, had no significant effects on either behavior (NAAG dose effect on ambulatory activity: F(3,27) = 1.68, p > 0.05; NAAG dose effect on stereotypy: F(3,27) = 1.40, p > 0.05). The effects of NAAG (10, 100 and 500 μg/10 μl) on the acute increase in ambulatory activity and stereotypy produced by MDPV (2 mg/kg) are presented in Figure 4b. NAAG dose-dependently reduced the MDPV-induced increase in ambulatory activity (NAAG dose effect: F(3,26) = 3.49, p < 0.05), with a significant reduction at 500 μg/10 μl (p < 0.05), but not 10 or 100 μg/10 μl (both p > 0.05). Interestingly, NAAG did not significantly affect MDPV-induced stereotypy (NAAG dose effect: F(3,26) = 1.35, p > 0.05).
Fig. 4.
The effects of NAAG (10, 100 and 500 μg/10 μl) by itself (a), and in combination with MDPV (2 mg/kg) (b), on ambulatory activity and stereotypy. Data were measured as counts and summed over 90 min. NAAG by itself had no intrinsic effects on either behavior (Fig. 4a; both p > 0.05). However, NAAG did significantly attenuate the MDPV-induced increase in ambulatory activity, but only at the highest dose tested (i.e. 500 μg/10 μl) (Fig. 4b; p < 0.05). Data are presented as mean totals + S.E.M (n = 7–8 per group). *p < 0.05 compared to MDPV alone
Experiment 5: NAAG dose-dependently inhibits the expression of MDPV-induced place preference
The effects of NAAG (10, 100 and 500 μg/10 μl) on the place preference produced by MDPV (2 mg/kg) are presented in Figure 5. The expression of MDPV-induced CPP was dose-dependently attenuated by NAAG (NAAG dose effect: F(3,41) = 4.41, p < 0.01), with a significant reduction at 100 and 500 μg/10 μl (both p < 0.05), but not at the low dose of 10 μg/10 μl (p > 0.05). Treatment with the high dose of NAAG (i.e. 500 μg/10 μl) did not significantly affect place preference relative to saline, when rats were conditioned only with saline (t(13) = 0.31, p > 0.05; Figure 5 inset).
Fig. 5.
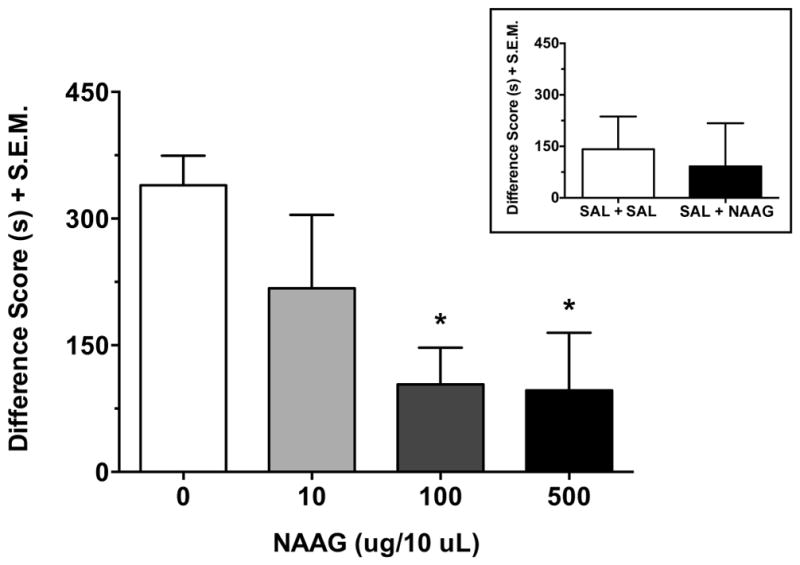
The dose-related effects of NAAG (10, 100 and 500 μg/10 μl) on the place preference produced by MDPV (2 mg/kg). Data (n = 10–15 per group) are presented as a difference score (difference in time spent in the less-preferred compartment between post-test and pre-test). NAAG dose-dependently reduced MDPV-induced CPP, with a significant effect observed at 100 and 500 μg/10 μl (both p < 0.05), but not at 10 μg/10 μl (p > 0.05). There was no significant effect of the high dose of NAAG (i.e. 500 μg/10 μl) on place preference relative to saline, when only saline was used during conditioning (n = 7–8 per group) (Figure 5 inset; p > 0.05). Data are shown as mean + S.E.M. *p < 0.05 relative to MDPV alone. SAL, saline
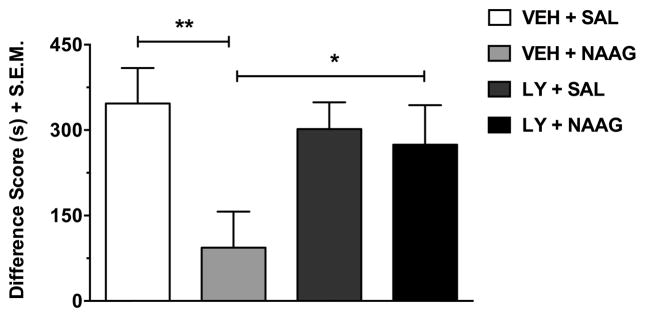
Experiment 6: The inhibitory effects of NAAG on MDPV-induced CPP are prevented by an mGluR2/3 antagonist
The effects of the mGluR2/3 antagonist LY341495 (3 mg/kg) in combination with NAAG (500 μg/10 μl) on MDPV (2 mg/kg) place preference are shown in Figure 6. Pretreatment with LY341495 by itself had no significant effects on MDPV-induced CPP (pretreatment effect: F(1,53) = 1.21, p > 0.05). However, there was a significant overall effect of NAAG on MDPV place preference (treatment effect: F(1,53) = 5.17, p < 0.05) that tended to differ depending on whether rats were pretreated with vehicle or LY341495 (interaction effect: F(1,53) = 3.33, p = 0.074). Post-hoc tests revealed that NAAG significantly reduced MDPV place preference in vehicle-pretreated rats (p < 0.01), but this effect was blocked when rats were pretreated with LY341495 (p < 0.05). There was no significant difference in CPP between rats administered LY341495 + saline and those in the vehicle + saline control group (p > 0.05).
Fig. 6.
The effects of the mGluR2/3 antagonist LY341495 (3 mg/kg) in combination with NAAG (500 μg/10 μl) on MDPV (2 mg/kg) place preference. Data (n = 13–15 per group) are shown as a difference score (difference in time spent in the less-preferred compartment between post-test and pre-test). The significant inhibitory action of NAAG on MDPV-induced CPP (p < 0.01) was blocked by pretreatment with LY341495 (p < 0.05). Data are presented as mean + S.E.M. **p < 0.01, *p < 0.05. SAL, saline; VEH, vehicle; LY, LY341495
Discussion
In the current study, we examined the effect of withdrawal following repeated MDPV exposure on GCPII expression in brain reward substrates. We also determined whether a GCPII inhibitor 2-PMPA, or NAAG, an endogenous mGluR2/3 agonist, could attenuate the potent locomotor-stimulant and rewarding effects of MDPV. Results showed that MDPV withdrawal decreased GCPII expression in the PFC, but not in the NAc or striatum, and this effect lasted for up to 10 days following withdrawal. NAAG at the highest dose tested attenuated the increase in ambulatory activity produced by MDPV, an effect not observed with 2-PMPA. However, both 2-PMPA and NAAG dose-dependently reduced the expression of MDPV-induced place preference in the CPP paradigm. The selective mGluR2/3 antagonist LY341495 prevented the inhibitory effect of NAAG on the robust place preference produced by MDPV.
Withdrawal following 7 days of MDPV exposure led to a significant reduction in GCPII expression in the PFC, but not in the NAc (albeit only 2 h into withdrawal) or the striatum. Interestingly, GCPII expression in the PFC was reduced at all withdrawal time points examined including 2 h after the last MDPV injection. Therefore, it could be argued that withdrawal was not necessarily a causative factor for the reduction in GCPII, but rather this was a lasting effect of MDPV itself. A decrease in the membrane-bound protein GCPII that is expressed primarily on glial cells, may result in lower extrasynaptic GLU levels through reduced metabolic degradation of NAAG (Tsukamoto et al. 2007). This may partly explain the reduction in extracellular GLU concentration in the PFC that occurs during withdrawal from psychostimulant exposure (Ben-Shahar et al. 2012; Parsegian and See 2014). However, a secondary consequence of this reduction in extracellular GLU may be reduced endogenous tone at presynaptic mGluR2/3 receptors located on GLUergic terminals in the PFC, which leads to enhanced excitatory output to the NAc and VTA where GLU release is increased following acute drug exposure (D’Souza 2015), and during reinstatement of drug-seeking behavior (Moussawi and Kalivas 2010). The current results support previous findings that repeated exposure to MDPV (Gregg et al. 2016), and other drugs of abuse (Kalivas 2009; Liechti et al. 2007), induces neuroadaptations in GLU signaling in corticolimbic regions that influence drug-seeking behaviors.
Consistent with past research (Aarde et al. 2013; Baumann et al. 2013; Fantegrossi et al. 2013; Gatch et al. 2013; Novellas et al. 2015; Gregg et al. 2016), acute injection of MDPV increased ambulatory and stereotypic behavior. Neither effect was reduced by pretreatment with 2-PMPA, which is similar to previous results in which 2-PMPA did not affect locomotor activation produced by an acute cocaine challenge (Shippenberg et al. 2000). In contrast, the increased ambulatory activity produced by MDPV was reduced by nasal administration of NAAG, but only at the highest dose (i.e. 500 μg/10 μl) that had no intrinsic locomotor effects when tested alone. The divergent results obtained with NAAG and 2-PMPA may be due to the direct stimulation of mGluR2/3 receptors by NAAG, compared with indirect stimulation by 2-PMPA through inhibition of GCPII activity that leads to an increase in endogenous NAAG levels (Nagel et al. 2006). On the other hand, the greater efficacy of NAAG may stem from improved brain penetration achieved with nasal versus intraperitoneal drug administration (Lochhead and Thorne 2012). Indeed, Rais and colleagues (2015) recently showed that nasal delivery of 2-PMPA resulted in significantly higher concentrations of the drug in brain tissue compared to an equivalent dose given by intraperitoneal injection. Certainly, it would be of interest to determine whether nasal relative to intraperitoneal administration of 2-PMPA has greater efficacy against the behavioral effects of MDPV, as well as other psychostimulants.
It is interesting to note that while NAAG did inhibit the increase in ambulatory activity produced by MDPV, it did not significantly attenuate MDPV-induced stereotypy. Shippenberg and colleagues (2000) observed a similar distinction with 2-PMPA that reduced sensitization of cocaine-induced ambulation, but not stereotypy. In addition, both the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline (NBQX) (Li et al. 1997) and neurotensin (Jolicoeur et al. 1983) have distinct effects on ambulatory versus stereotypic effects of psychostimulants, which has been suggested to indicate these two behaviors are governed by discrete neural pathways in the brain (Kelly et al. 1975). Overall, the lack of effect of 2-PMPA, and the relatively subtle attenuation of MDPV-induced hyperactivity by NAAG, suggest that both drugs do not produce strong DAergic effects, and instead act primarily on the GLUergic system.
MDPV produced a significant place preference in the CPP paradigm that supports a number of previous findings (Karlsson et al. 2014; King et al. 2015a, b; Gregg et al. 2016). Similar to cocaine-induced place preference (Slusher et al. 2001), treatment with 2-PMPA or NAAG dose-dependently reduced the expression of MDPV-induced CPP, suggesting both drugs attenuated the strong rewarding properties of MDPV. Importantly, these effects were not mediated by an aversive-like response, as treatment with either 2-PMPA or NAAG alone did not alter place preference relative to the respective control conditions. Systemic injection of LY341495 blocked the inhibitory effect of NAAG on MDPV-induced CPP, thereby indicating that NAAG (and possibly 2-PMPA) reduced MDPV reward via an mGluR2/3-mediated mechanism. The effects of 2-PMPA and NAAG on MDPV place preference may have resulted from increased endogenous tone at presynaptic mGluR2/3 receptors that inhibited the enhanced synaptic GLU released upon reintroduction to the MDPV-associated compartment following conditioning. However, it is possible that both drugs attenuated MDPV-induced CPP via activation of mGluR3 receptors expressed by glial cells, which modulate extrasynaptic GLU levels through enhanced GLU uptake (Yao et al. 2005). This is consistent with our recent study that showed CTX, an activator of the astrocytic protein GLT-1 that is responsible for the majority of GLU uptake in the brain, attenuated the rewarding effects of MDPV in the CPP paradigm (Gregg et al. 2016).
The current CPP results demonstrate an inhibitory effect of 2-PMPA and NAAG on MDPV reward; however, the relevant brain region(s) that were involved in these effects are not well understood. One region likely to be involved is the PFC since it was the only area in which we observed a significant reduction in GCPII expression following MDPV withdrawal. However, the NAc may also be involved as direct infusion of 2-PMPA or NAAG into this region inhibited cocaine-primed reinstatement in the intravenous self-administration (IVSA) model, which was prevented by intra-NAc injection of LY341495 (Xi et al. 2010b). Liechti and colleagues (2007) found local administration of the mGluR2/3 agonist LY379268 in the NAc attenuated nicotine self-administration and reinstatement of nicotine-seeking behavior. Interestingly, these authors noted a similar reduction in the reinforcing effects of nicotine following direct infusion of LY379268 into the VTA (Liechti et al. 2007). The VTA receives dense GLUergic inputs from a number of brain regions including the PFC, which modulate the burst firing of VTA DAergic neurons that are implicated in acute drug-induced reward (reviewed in Geisler and Wise 2008). Given these findings, further investigation is necessary to determine if 2-PMPA and NAAG acted directly in the PFC, and/or in projection sites such as the VTA and NAc, to attenuate MDPV reward.
Although inhibition of synaptic GLU release by 2-PMPA and NAAG is the most comprehensively studied mechanism by which these drugs reduce rewarding effects of psychostimulants, an alternative explanation for the current findings may be that 2-PMPA and NAAG normalized disruptions in DA signaling caused by MDPV exposure. Indeed, it has been shown that presynaptic mGluR2/3 receptors are located on DA as well as GLU neurons, which when stimulated inhibit synaptic DA release in corticolimbic brain regions (Xi et al. 2010b). A large body of research shows that DA plays a central role in the acute rewarding properties of drugs of abuse (reviewed in Volkow and Morales 2015), and therefore, it is plausible that the reduction in MDPV-induced place preference by 2-PMPA and NAAG in the current study was mediated in part by changes in DA signaling. Future experiments would benefit from examining the relative contribution of DA and GLU modulation to the effects of GCPII inhibitors and mGluR2/3 receptor modulators on the rewarding and reinforcing properties of MDPV and other psychostimulants.
In summary, we provide the first evidence that a major pathway of GLU metabolism (GCPII) in the PFC is dysregulated following withdrawal from repeated MDPV exposure. These effects may contribute to downstream disturbances in GLU homeostasis in the NAc that underlie drug-seeking behavior. We also show for the first time that a GCPII inhibitor (2-PMPA), and the endogenous mGluR2/3 agonist NAAG, attenuate the strong rewarding properties of MDPV likely through direct (or indirect) stimulation of mGluR2/3 receptors.
Supplementary Material
Fig. S1 GCPII protein expression in the nucleus accumbens (NAc) during withdrawal following repeated MDPV administration. GCPII (95–110 kDa) expression was normalized to a β-tubulin (52 kDa) control protein to obtain a relative density. GCPII expression was reduced 2 h (Withdrawal Day 0 (a); p < 0.05) after repeated MDPV exposure, but not 2 days (Withdrawal Day 2 (b); p > 0.05), 5 days (Withdrawal Day 5 (c); p > 0.05), or 10 days (Withdrawal Day 10 (d); p > 0.05) following treatment. Data are depicted as a percentage of the saline control values + S.E.M (n = 6–7 per group). The representative protein bands from the saline and MDPV treatment groups are presented in the corresponding boxes. *p < 0.05 relative to saline. SAL, saline
Fig. S2 GCPII protein expression in the striatum during withdrawal following repeated MDPV administration. GCPII (95–110 kDa) expression was normalized to a β-tubulin (52 kDa) control protein to obtain a relative density. GCPII expression was not significantly reduced at any withdrawal time point (Withdrawal Day 0 (a), Withdrawal Day 2 (b), Withdrawal Day 5 (c) and Withdrawal Day 10 (d); all p > 0.05) following repeated MDPV. Data are presented as a percentage of the saline control values + S.E.M (n = 6–7 per group). The representative protein bands from the saline and MDPV treatment groups are provided in the corresponding boxes. SAL, saline
Acknowledgments
Research was funded by National Institute on Drug Abuse grants R01DA039139 and P30DA013429 to SMR, and T32DA007237 to CH.
Footnotes
Electronic supplementary material: The online version of this article contains supplementary material, which is available to authorized users.
Funding and Disclosure: The authors declare no conflict of interest.
Contributor Information
Callum Hicks, Department of Pharmacology, Temple University School of Medicine, Philadelphia, PA. Center for Substance Abuse Research, Temple University School of Medicine, Philadelphia, PA.
Ryan A. Gregg, Department of Pharmacology, Temple University School of Medicine, Philadelphia, PA. Center for Substance Abuse Research, Temple University School of Medicine, Philadelphia, PA
Sunil U. Nayak, Department of Pharmacology, Temple University School of Medicine, Philadelphia, PA. Center for Substance Abuse Research, Temple University School of Medicine, Philadelphia, PA
Lee Anne Cannella, Department of Pathology and Laboratory Medicine, Temple University School of Medicine, Philadelphia, PA.
Giana J. Schena, Department of Pharmacology, Temple University School of Medicine, Philadelphia, PA. Center for Substance Abuse Research, Temple University School of Medicine, Philadelphia, PA
Christopher S. Tallarida, Department of Pharmacology, Temple University School of Medicine, Philadelphia, PA. Center for Substance Abuse Research, Temple University School of Medicine, Philadelphia, PA
Allen B. Reitz, Fox Chase Chemical Diversity Center, Doylestown, PA
Garry R. Smith, Fox Chase Chemical Diversity Center, Doylestown, PA
Scott M. Rawls, Department of Pharmacology, Temple University School of Medicine, Philadelphia, PA. Center for Substance Abuse Research, Temple University School of Medicine, Philadelphia, PA
References
- Aarde S, Huang P, Creehan K, Dickerson T, Taffe M. The novel recreational drug 3, 4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abiedalla YFH, Abdel-Hay K, DeRuiter J, Clark CR. Synthesis and GC–MS analysis of a series of homologs and regioisomers of 3,4-methylenedioxypyrovalerone (MDPV) Forensic Sci Int. 2012;223:189–197. doi: 10.1016/j.forsciint.2012.08.040. [DOI] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24(20):4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar OM, Szumlinski KK, Lominac KD, Cohen A, Gordon E, Ploense KL, et al. Extended access to cocaine self-administration results in reduced glutamate function within the medial prefrontal cortex. Addict Biol. 2012;17:746–757. doi: 10.1111/j.1369-1600.2011.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquist MD, Traxler HK, Mahler AM, Baker LE. Sensitization to the locomotor stimulant effects of “bath salt” constituents, 4-methylmethcathinone (4-MMC) and 3,4-methylenedioxypyrovalerone (MDPV), in male Sprague-Dawley rats. Drug Alcohol Depend. 2016;164:128–134. doi: 10.1016/j.drugalcdep.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano J, Glennon R, De Felice L, Banks M, Negus S. Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology. 2014;231:199–207. doi: 10.1007/s00213-013-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella N, Halbout B, Uhrig S, Evrard L, Corsi M, Corti C, et al. The mGluR2/3 agonist LY379268 induced anti-reinstatement effects in rats exhibiting addiction-like behavior. Neuropsychopharmacology. 2013;38:2048–2056. doi: 10.1038/npp.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Adam G, Chaboz S, Henningsen R, Kemp JA, Klingelschmidt A, et al. Characterization of [3H]-(2S,2′R,3′R)-2-(2′,3′-dicarboxy-cyclopropyl) glycine ([3H]-DCG IV) binding to metabotropic mGlu2 receptor-transfected cell membranes. Br J Pharmacol. 1998;123:497–504. doi: 10.1038/sj.bjp.0701647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza MS. Glutamatergic transmission in drug reward: implications for drug addiction. Front Neurosci. 2015;9:404. doi: 10.3389/fnins.2015.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JM, Lepack AE, Duman RS. mGluR2/3 blockade produces rapid and long-lasting reversal of anhedonia caused by chronic stress exposure. J Mol Psychiatry. 2013;1:1. doi: 10.1186/2049-9256-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38:563–573. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of “bath salt” cathinones. Behav Pharmacol. 2013;24:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Wise RA. Functional implications of glutamatergic projections to the ventral tegmental area. Rev Neurosci. 2008;19:227–244. doi: 10.1515/revneuro.2008.19.4-5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA. Bath salts, mephedrone, and methylenedioxypyrovalerone as emerging illicit drugs that will need targeted therapeutic intervention. Adv Pharmacol. 2014;69:581–620. doi: 10.1016/B978-0-12-420118-7.00015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Tallarida CS, Reitz A, McCurdy C, Rawls SM. Mephedrone (4-methylmethcathinone), a principal constituent of psychoactive bath salts, produces behavioral sensitization in rats. Drug Alcohol Depend. 2013;133:746–750. doi: 10.1016/j.drugalcdep.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Hicks C, Nayak SU, Tallarida CS, Nucero P, Smith GR, et al. Synthetic cathinone MDPV downregulates glutamate transporter subtype I (GLT-1) and produces rewarding and locomotor-activating effects that are reduced by a GLT-1 activator. Neuropharmacology. 2016;108:111–119. doi: 10.1016/j.neuropharm.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Baumann MH, Partilla JS, Bonano JS, Vouga A, Tallarida CS, et al. Stereochemistry of mephedrone neuropharmacology: enantiomer-specific behavioural and neurochemical effects in rats. Br J Pharmacol. 2015;172:883–894. doi: 10.1111/bph.12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur F, De Michele G, Barbeau A, St-Pierre S. Neurotensin affects hyperactivity but not stereotypy induced by pre and post synaptic dopaminergic stimulation. Neurosci Biobehav Rev. 1983;7:385–390. doi: 10.1016/0149-7634(83)90043-x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J Neurochem. 1998;70:1497–1502. doi: 10.1046/j.1471-4159.1998.70041497.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Karila L, Megarbane B, Cottencin O, Lejoyeux M. Synthetic cathinones: a new public health problem. Curr Neuropharmacol. 2015;13:12–20. doi: 10.2174/1570159X13666141210224137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson L, Andersson M, Kronstrand R, Kugelberg FC. Mephedrone, methylone and 3,4-methylenedioxypyrovalerone (MDPV) induce conditioned place preference in mice. Basic Clin Pharmacol Toxicol. 2014;115:411–416. doi: 10.1111/bcpt.12253. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- King HE, Wetzell B, Rice KC, Riley AL. An assessment of MDPV-induced place preference in adult Sprague-Dawley rats. Drug Alcohol Depend. 2015a;146:116–119. doi: 10.1016/j.drugalcdep.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Wakeford A, Taylor W, Wetzell B, Rice KC, Riley AL. Sex differences in 3,4-methylenedioxypyrovalerone (MDPV)-induced taste avoidance and place preferences. Pharmacol Biochem Behav. 2015b;137:16–22. doi: 10.1016/j.pbb.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanos R, Partilla J, Baumann M, Hutsell B, Banks M, Negus S, et al. Stereoselective actions of methylenedioxypyrovalerone (MDPV) to inhibit dopamine and norepinephrine transporters and facilitate intracranial self-stimulation in rats. ACS Chem Neurosci. 2015;6:771–777. doi: 10.1021/acschemneuro.5b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Vartanian AJ, White FJ, Xue C-J, Wolf M. Effects of the AMPA receptor antagonist NBQX on the development and expression of behavioral sensitization to cocaine and amphetamine. Psychopharmacology. 1997;134:266–276. doi: 10.1007/s002130050449. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27(34):9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu Y-T, Reitz AB, Liu-Chen L-Y, et al. Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 2012;126:257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Lukas M, Neumann ID. Nasal application of neuropeptide S reduces anxiety and prolongs memory in rats: social versus non-social effects. Neuropharmacology. 2012;62:398–405. doi: 10.1016/j.neuropharm.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014;87:206–213. doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur J Pharmacol. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel J, Belozertseva I, Greco S, Kashkin V, Malyshkin A, Jirgensons A, et al. Effects of NAAG peptidase inhibitor 2-PMPA in model chronic pain–relation to brain concentration. Neuropharmacology. 2006;51:1163–1171. doi: 10.1016/j.neuropharm.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Novellas J, López-Arnau R, Pubill D, Camarasa J, Escubedo E. Concentrations of MDPV in rat striatum correlate with the psychostimulant effect. J Psychopharm. 2015;29:1209–1218. doi: 10.1177/0269881115598415. [DOI] [PubMed] [Google Scholar]
- Parsegian A, See RE. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology. 2014;39:811–822. doi: 10.1038/npp.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rais R, Wozniak K, Wu Y, Niwa M, Stathis M, Alt J, et al. Selective CNS uptake of the GCP-II inhibitor 2-PMPA following intranasal administration. PLoS ONE. 2015;10:e0131861. doi: 10.1371/journal.pone.0131861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickli A, Hoener MC, Liechti ME. Monoamine transporter and receptor interaction profiles of novel psychoactive substances: para-halogenated amphetamines and pyrovalerone cathinones. Eur Neuropsychopharmacol. 2015;25(3):365–376. doi: 10.1016/j.euroneuro.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, et al. Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology. 2015;233:1981–1990. doi: 10.1007/s00213-015-4057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Rea W, Slusher BS. Modulation of behavioral sensitization to cocaine by NAALADase inhibition. Synapse. 2000;38:161–166. doi: 10.1002/1098-2396(200011)38:2<161::AID-SYN7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Simmler L, Buser T, Donzelli M, Schramm Y, Dieu LH, Huwyler J, et al. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusher BS, Thomas A, Paul M, Schad CA, Ashby CR. Expression and acquisition of the conditioned place preference response to cocaine in rats is blocked by selective inhibitors of the enzyme N-acetylated-α-linked-acidic dipeptidase (NAALADASE) Synapse. 2001;41:22–28. doi: 10.1002/syn.1056. [DOI] [PubMed] [Google Scholar]
- Spencer S, Scofield M, Kalivas PW. The good and bad news about glutamate in drug addiction. J Psychopharm. 2016;30:1095–1098. doi: 10.1177/0269881116655248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahsili-Fahadan P, Carr GV, Harris GC, Aston-Jones G. Modafinil blocks reinstatement of extinguished opiate-seeking in rats: mediation by a glutamate mechanism. Neuropsychopharmacology. 2010;35:2203–2210. doi: 10.1038/npp.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T, Wozniak KM, Slusher BS. Progress in the discovery and development of glutamate carboxypeptidase II inhibitors. Drug Discov Today. 2007;12:767–776. doi: 10.1016/j.drudis.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Morales M. The brain on drugs: from reward to addiction. Cell. 2015;162:712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, et al. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2014;19:165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CM. Mephedrone and 3,4-methylenedioxypyrovalerone (MDPV): synthetic cathinones with serious health implications. J Clin Pharmacol. 2016;56:1319–1325. doi: 10.1002/jcph.742. [DOI] [PubMed] [Google Scholar]
- Xi Z-X, Kiyatkin M, Li X, Peng X-Q, Wiggins A, Spiller K, et al. N-acetylaspartylglutamate (NAAG) inhibits intravenous cocaine self-administration and cocaine-enhanced brain-stimulation reward in rats. Neuropharmacology. 2010a;58:304–313. doi: 10.1016/j.neuropharm.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Li X, Peng X-Q, Li J, Chun L, Gardner EL, et al. Inhibition of NAALADase by 2-PMPA attenuates cocaine-induced relapse in rats: a NAAG-mGluR2/3-mediated mechanism. J Neurochem. 2010b;112:564–576. doi: 10.1111/j.1471-4159.2009.06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H-H, Ding J-H, Zhou F, Wang F, Hu L-F, Sun T, et al. Enhancement of glutamate uptake mediates the neuroprotection exerted by activating group II or III metabotropic glutamate receptors on astrocytes. J Neurochem. 2005;92:948–961. doi: 10.1111/j.1471-4159.2004.02937.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 GCPII protein expression in the nucleus accumbens (NAc) during withdrawal following repeated MDPV administration. GCPII (95–110 kDa) expression was normalized to a β-tubulin (52 kDa) control protein to obtain a relative density. GCPII expression was reduced 2 h (Withdrawal Day 0 (a); p < 0.05) after repeated MDPV exposure, but not 2 days (Withdrawal Day 2 (b); p > 0.05), 5 days (Withdrawal Day 5 (c); p > 0.05), or 10 days (Withdrawal Day 10 (d); p > 0.05) following treatment. Data are depicted as a percentage of the saline control values + S.E.M (n = 6–7 per group). The representative protein bands from the saline and MDPV treatment groups are presented in the corresponding boxes. *p < 0.05 relative to saline. SAL, saline
Fig. S2 GCPII protein expression in the striatum during withdrawal following repeated MDPV administration. GCPII (95–110 kDa) expression was normalized to a β-tubulin (52 kDa) control protein to obtain a relative density. GCPII expression was not significantly reduced at any withdrawal time point (Withdrawal Day 0 (a), Withdrawal Day 2 (b), Withdrawal Day 5 (c) and Withdrawal Day 10 (d); all p > 0.05) following repeated MDPV. Data are presented as a percentage of the saline control values + S.E.M (n = 6–7 per group). The representative protein bands from the saline and MDPV treatment groups are provided in the corresponding boxes. SAL, saline