Abstract
Purpose
Squamous cell carcinoma of the anal canal (ASCC) accounts for 2-4% of gastrointestinal (GI) malignancies in the US and is increasing in incidence; however, genomic features of ASCC are incompletely characterized. Primary treatment of ASCC involves concurrent chemotherapy and radiation (CRT), but the mutational landscape of resistance to CRT is unknown. Here, we aim to compare mutational features of ASCC in the pre- and post-CRT setting.
Experimental Design
We perform whole exome sequencing of primary (n=31) and recurrent (n=30) ASCCs and correlate findings with clinical data. We compare genomic features of matched pre- and post-CRT tumors to identify genomic features of CRT response. Finally, we investigate the mutational underpinnings of an extraordinary ASCC response to immunotherapy.
Results
We find that both primary and recurrent ASCC tumors harbor mutations in genes such as PIK3CA and FBXW7 that are also mutated in other HPV-associated cancers. Overall mutational burden was not significantly different in pre- versus post-CRT tumors, and several examples of shared clonal driver mutations were identified. In two cases, clonally related pre- and post-CRT tumors harbored distinct oncogenic driver mutations in the same cancer gene (KRAS or FBXW7). A patient with recurrent disease achieved an exceptional response to anti-Programmed Death (PD-1) therapy, and genomic dissection revealed high mutational burden and predicted neoantigen load.
Conclusion
We perform comprehensive mutational analysis of ASCC and characterize mutational features associated with CRT. Although many primary and recurrent tumors share driver events, we identify several unique examples of clonal evolution in response to treatment.
Keywords: Anal cancer, squamous cell cancer, human papilloma virus (HPV), chemoradiotherapy, tumor evolution, genomics
Introduction
Anal squamous cell cancers (ASCCs) arise from the squamous lining of the terminal gastrointestinal tract and have distinct clinical and pathologic features from tumors that arise more proximally in the colon and rectum.[1, 2] Historically, radical surgery was the only curative treatment for ASCC; however, trials of pre-operative chemotherapy and radiation (CRT) in the 1970s revealed high tumor response rate, and CRT subsequently replaced surgery as the preferred treatment.[3-5] Recent updates of large randomized trials have confirmed that concurrent treatment with 5-fluorouracil, mitomycin C (or cisplatin), and ionizing radiation remains the standard of care.[6, 7] However, despite high rates of disease control and functional organ preservation, a subset of patients develop locally recurrent or metastatic disease for which treatment options are limited.
Analysis of several large cohorts of ASCCs have shown that the majority of ASCCs harbor human papillomavirus (HPV), and HPV associated ASCCs have increased sensitivity to CRT and improved disease outcomes compared to non-HPV associated tumors.[8, 9] In addition, epigenomic analysis has also shown differences in global methylation patterns in high- versus low-risk tumors.[10] Targeted sequencing of known cancer genes has revealed mutations in EGFR, KRAS, and PIK3CA, and a recent study using a combination of targeted sequencing and immunohistochemistry (IHC) revealed high levels of EGFR expression and frequent mutations in the PIK3CA/AKT pathway.[11-13] However, no exome-wide mutational studies are available and the impact of CRT on genomic evolution is unknown.
We hypothesized that comprehensive mutational profiling of a cohort of ASCC tumors may assist in defining the genomic landscape of this rare cancer and identify actionable therapeutic targets. Furthermore, comparison of paired primary and recurrent tumors may identify unique mutational features that drive resistance to standard treatment approaches and could thus inform the search for new therapeutic strategies. To this end, we performed whole exome sequencing (WES) of pre- and post-CRT ASCC cases in order to identify clinical-genomic correlates and characterize evolutionary patterns of treatment response.
Materials and Methods
Patients and Samples
All ASCC cases evaluated in the Department of Radiation Oncology at the Dana-Farber Cancer Institute/Brigham & Women’s Hospital between January 2005 and December 2013 were identified and reviewed following approval by the Institutional Review Board (IRB). Cases with sufficient tissue were reviewed by a gastrointestinal pathologist (J.L.H.) to confirm the diagnosis and identify regions of highest tumor density. Three 50 micron sections were cut from each available FFPE block and genomic DNA was purified using the QIAamp DNA FFPE extraction kit (Qiagen). Slides for p16 immunohistochemistry and HPV in situ hybridization were prepared from the same region of the FFPE tumor (see Supplementary Methods for additional details).
Data Processing and Analysis
Whole exome sequencing was performed at the Broad Institute, and data for both the pilot and extension cohorts were analyzed using established analytical pipelines (see Supplementary Methods for additional details). An additional novel filtering technique utilizing the COSMIC (version 75) database of somatic mutations in cancer[14] and the ExAC database of 60,000 germline samples[15] was applied to the extension cohort in an attempt to remove additional germline events (Supplementary Methods).
Cancer Cell Fraction, Neoantigen Prediction, and Mutation Signature Analysis
Probability distributions of possible cancer cell fractions (CCFs) of point mutations were calculated based on local copy-number and the estimated sample purity. Calculated CCFs were used to generate a sibling model for paired pre-treatment and post-treatment samples for patients in the pilot cohort (n=5). Neoantigen prediction was performed using POLYSOLVER and NetMHCpan in a similar method as described in Van Allen et al[16] (see Supplementary Methods for additional details). A Bayesian non-negative matrix factorization (NMF) approach was used to identify mutational signature contributions in the pilot cohort (n = 18 tumors). Three runs converged to three signature solution and seven runs converged to a four signature solution. Upon manual review, we selected the three signature solution for downstream analyses.[17]
Source Data and Code
Figures were created in Python-2.7 and are available on GitHub: https://github.com/vanallenlab/2016-Mouw_ASCC
Results
Clinical Characteristics of Pilot and Extension ASCC Cohorts
To investigate the genomic landscape of ASCC and identify features of treatment response, we reviewed ASCC cases treated in the primary and/or recurrent setting at our institution between 2005 and 2013. All cases with sufficient formalin-fixed, paraffin-embedded (FFPE) primary tumor tissue and germline DNA were included in the pilot cohort (n=13)(Table 1, Supplementary Fig. 1). Seven of the 13 patients in the pilot cohort had a complete clinical response to CRT and remained free of disease for at least two years (med. follow-up, 6.0 y; range, 2.1-9.8 y), whereas six patients developed a recurrence during follow-up (med. time to recurrence, 14.1 mo; range, 3.2-49.1 mo). There were no significant differences in clinical or tumor characteristics at diagnosis between patients who developed a recurrence and those who did not (Supplementary Table 1). As expected, nearly all tumors were HPV positive (12/13 primary tumors, 92%), and there was 100% concordance between p16 immunohistochemistry (IHC) and HPV in situ hybridization (Table 1; Supplementary Fig. 2). For each of the recurrent cases, the HPV status of the recurrent tumor was identical to the primary tumor.
Table 1.
Cohort Characteristics
|
All patients
(n=46) |
Pilot cohort
(n=13) |
Extension cohort
(n=33) |
p-value | |
|---|---|---|---|---|
| Median age, yrs (range) | 59.0 (33-83) | 58.0 (33-79) | 59.5 (44-83) | 0.66 |
| No. Female (%) | 33 (70.2%) | 6 (46%) | 26 (79%) | 0.030 |
| Tumor Stage | 0.58 | |||
| T2 | 19 | 6 | 13 | |
| T3 | 18 | 6 | 12 | |
| T4 | 5 | 1 | 4 | |
| Unknown | 4 | 0 | 4 | |
| Nodal Stage | 0.13 | |||
| N0 | 22 | 7 | 15 | |
| N1 | 12 | 4 | 8 | |
| N2 | 5 | 2 | 3 | |
| N3 | 5 | 0 | 5 | |
| Unknown | 2 | 0 | 2 | |
| AJCC Stage | 0.64 | |||
| II | 20 | 6 | 14 | |
| III | 24 | 7 | 17 | |
| IV | 0 | 0 | 0 | |
| Unknown | 2 | 0 | 2 | |
| HPV status of primary tumor | ||||
| HPV+ by p16 IHC (n=47) | 40/46 (87%) | 12/13 (92%) | 28/33 (85%) | 0.66 |
| HPV+ by ISH (n=12) | 11/12 (92%) | 11/12 (92%) | NA | |
| No. non-recurrent cases | 14 | 7 (54%) | 7 (21%) | 0.026 |
| Med. follow-up, yrs (range) | 6.0 (2.1-9.8) | 5.0 (1.0-6.1) | ||
| No. recurrent cases | 32 | 6 (46%) | 26 (79%) | 0.026 |
| Med. time to recurrence, mo. (range) |
14.1 (3.2-49.1) | 7.0 (2.2-257.6) |
To expand our findings from the pilot cohort, we identified an extension cohort consisting of an additional 33 ASCC cases (43 tumors). Cases in the extension cohort were identified from the same institutional pool as the pilot cohort, but lacked a source of matched germline DNA. Compared to the pilot cohort, there were more females and more recurrent cases in the extension cohort (Table 1). Seven of the 33 patients in the extension cohort had no disease recurrence during follow-up (med. follow-up, 5.0 y; range, 1.0-6.1 y), whereas 26 patients had disease recurrence (med. time to recurrence, 7.0 mo; range, 2.2-257.6 mo; Table 1). Of the 26 recurrent cases, paired primary and recurrent tumor(s) were available for 8 patients (17 tumors), primary tumor alone was available for 3 patients (3 tumors), and recurrent tumor alone was available for 15 patients (16 tumors; Supplementary Fig. 1). HPV status of the extension cohort was determined by p16 IHC, and the majority of cases were HPV positive (25/31, 81%). For cases with both primary and recurrent tumors, the HPV status was identical within each pair.
Genomic Landscape of Primary ASCC
For all cases in the pilot cohort, whole exome sequencing (WES) of the primary tumor and matched germline DNA was performed, and data were analyzed using published techniques (Methods). Mean target coverage was 107.3X for tumor and 108.4X for germline DNA (Supplementary Table 2). A total of 2980 somatic mutations, including 1875 missense, 170 nonsense, 30 frameshift, and 785 silent mutations were identified in the pilot cohort. The mean somatic mutational burden was 5.7 mutations/Mb (range, 0.8 to 35.5 mutations/Mb; Supplementary Table 3), similar to other HPV-associated squamous cell carcinomas.[18, 19]
Given the limited size of the cohort, we did not observe highly recurrent alterations in novel genes, and we therefore chose to focus our analyses on genes that are recurrently mutated in other HPV associated squamous cell cancers (Fig. 1). Among the thirteen primary tumors in the pilot cohort, four (31%) had a missense mutation in FBXW7, an E3 ubiquitin ligase that targets known oncoproteins such as c-Myc and cyclin E and is significantly mutated in multiple tumor types. All four FBXW7 missense mutations occurred at conserved arginines that play a role in substrate binding and are known mutational hotspots.[20]
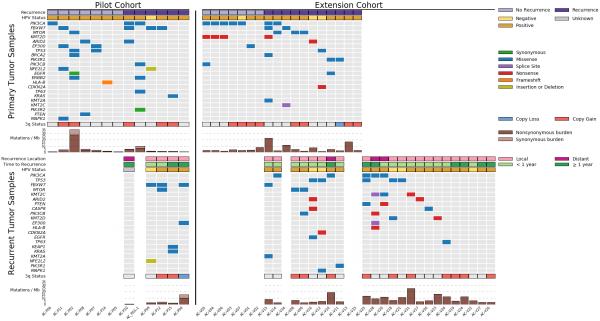
Figure 1. Overview of the mutational landscape of primary and recurrent ASCC.
Clinical characteristics, mutation and copy number data, and overall mutational burden are shown for the pilot cohort (left) and extension cohort (right). Primary tumors from each cohort are shown in the top half of the figure, and within each cohort, patients who were free from recurrence during follow-up are on the left (light purple) and patients who developed recurrent disease are on the right (dark purple). In the bottom half of the figure, similar data is displayed for recurrent tumors. Cases with both primary and recurrent tumors are vertically aligned. Copy number status for chromosome 3q is shown for ≥2-fold gain (red) and ≥2-fold loss (blue). Mutation burden is displayed as mutations/megabase (Mb). Recurrence location is annotated as local (defined as the primary tumor site or a pelvic/inguinal lymph node) or distant, and time to recurrence refers to the time interval between completion of chemoradiotherapy and biopsy-confirmed recurrent disease.
We also noted multiple events in other squamous cell carcinoma genes, including known activating mutations in PIK3CA, alterations in the KEAP-1 binding domain of NFE2L2, and mutations in TP63 and EP300, genes involved in squamous cell differentiation (Fig. 1).[21, 22] In addition, activating MAPK1 and inactivating PTEN mutations were noted in individual cases. Interestingly, the single HPV negative case did not have a TP53 mutation, whereas two of the 11 HPV positive cases harbored TP53 mutations.
WES was also performed for all primary tumors in the extension cohort, and mutations were nominated after removing putative germline variants (Methods; Supplementary Fig. 3). The most frequently mutated gene in the extension cohort was PIK3CA, which was mutated in 7 of 18 primary tumors (39%). In the combined (pilot plus extension) cohort, 10 of 31 tumors (32%) harbored a PIK3CA mutation, similar to the rate reported in recent targeted sequencing studies.[11, 12] Similarly, the overall mutation frequency of FBXW7 in the pooled cohort (6/31, 19%) is comparable to the rate described in other HPV-associated SCCs.[12]
We next compared the mutational characteristics of primary tumors that did not recur following CRT versus those that did. PIK3CA, the most commonly mutated gene among primary tumors, occurred at similar frequencies in the two groups (6/14 vs 4/17, p=0.44; Supplementary Fig. 4). There was a trend towards increased frequency of EP300 mutations in tumors that did not recur during follow-up (3/14 vs 0/17, p=0.08) and increased frequency of FBXW7 mutations in tumors that did recur (5/17 vs 1/14, p=0.18).
In addition to the mutational status of individual genes, we also sought to characterize the landscape of somatic copy number alterations. Analysis of both pilot and extension cohorts revealed frequent chromosome 3q amplification, an event that has previously been reported in HPV-associated malignancies and is associated with progression from dysplasia to invasive disease in both anal and cervical squamous cell carcinomas (Supplementary Fig. 5).[19, 23, 24] Chromosome 3q harbors several cancer-related genes including PIK3CA, SOX2, and TP63; however, the role of individual gene amplifications in ASCC tumorigenesis is unknown. There was no difference in chromosome 3q status in primary tumors that recurred following CRT versus those that did not.
Genomic Landscape of Recurrent ASCC
Although CRT is associated with high response rates, approximately one in five ASCC patients will develop recurrent disease. To better understand the genetic features of recurrent ASCC, we performed WES of 30 recurrent ASCC tumors from 28 patients (5 patients from pilot cohort, 23 patients from extension cohort; Fig. 1, Supplementary Fig. 1). Fourteen recurrent tumors (from 13 patients) were analyzed in parallel with the corresponding primary tumor from the same patients, whereas 16 recurrent tumors (from 15 patients) did not have an accompanying primary tumor. As in the primary tumor cohort, PIK3CA was the most commonly mutated gene among recurrent tumors (Fig. 1), followed by TP53 and FBXW7. Six of the 28 recurrent cases were HPV negative, and only one of the seven HPV negative cases in this study was not associated with recurrence. Four of the six recurrent HPV negative tumors harbored a TP53 mutation, whereas only one of the 22 HPV positive recurrent tumors had a TP53 mutation (p=0.003, Fisher’s exact test). One TP53-mutated tumor had a nonsense mutation in CDKN2A, an event frequently observed in TP53-mutated, HPV-negative head and neck SCC.[18]
Genomic evolution in matched primary and recurrent tumors
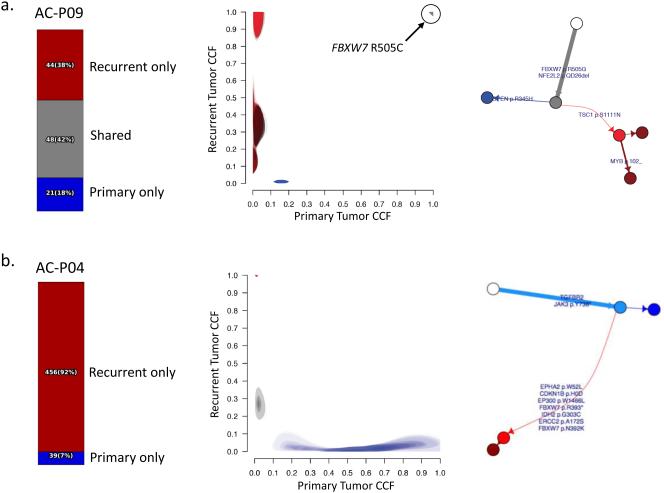
For thirteen patients, paired pre- and post-CRT tumors were available for analysis. In most cases, primary-recurrent pairs had mutations and copy number alterations that were shared as well as events that were unique to either the primary or recurrent tumor (Supplementary Fig. 6). However, in order to further characterize genomic evolution in the context of CRT, we used sample purity and local copy-number data from ABSOLUTE to calculate the cancer cell fraction (CCF) of point mutations and generate sibling models for patient-matched pre- and post-CRT tumors from the pilot cohort (n = 5; Methods).[25] In all but one case (AC-P10), we observed a clonal mutation (CCF = 1) in one or more putative driver cancer genes. For case AC-P10, the purity of the recurrent tumor at the time of sample acquisition was estimated to be less than 5%, possibly explaining the lack of any predicted clonal mutations in this sample.
In three of the remaining four cases, one or more clonal mutations were shared between primary and recurrent tumors. For example, in AC-P09, both the primary and recurrent tumors harbored a clonal FBXW7 R505C mutation (Fig. 2a). Interestingly, AC-P04 lacked any evidence of shared mutations between primary and recurrent tumors (Fig. 2b). In this case, the time interval between completion of CRT and recurrence was more than 4 years, raising the possibility that the disease recurrence may actually represent a second primary tumor. Further supporting this hypothesis, the invasive recurrence arose in the setting of diffuse HPV-associated anal intraepithelial neoplasia (AIN) in a patient with poorly-controlled HIV, underscoring the role of HPV and host immune status in ASCC risk and pathogenesis.[26]
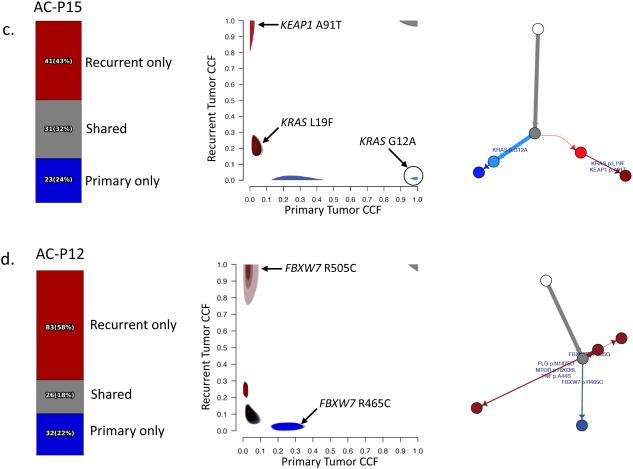
Figure 2. Genomic evolution in patient-matched pre- and post-CRT tumors.
For each case, the fraction of mutations unique to the primary (blue), unique to the recurrent tumor (red), or shared between primary and recurrent tumors (grey) is shown on the left. Cancer cell fractions (CCF) for mutations in the primary (x-axis) and recurrent (y-axis) tumors are compared, and the corresponding phylogenetic tree is shown on the right. Select cancer genes are annotated in the CCF plots and the phylogenetic trees.[37]
(a) The primary and recurrent tumors from case AC-P09 share many mutations, including a clonal FBXW7 R505C mutation.
(b) There are no shared mutations between the primary and recurrent tumor from case AC-P04, suggesting that the recurrent disease may actually represent a second primary tumor.
(c) In case AC-P15, the primary tumor has a clonal KRAS G12A mutation. The recurrent tumor shares several mutations with the primary tumor; however, the KRAS G12A mutation is absent while a distinct oncogenic KRAS mutation (L19F) is present. In addition, the recurrent tumor harbors a clonal mutation in KEAP1.
(d) In case AC-P12, the primary tumor has an oncogenic FBXW7 R465C mutation present in the primary tumor. The recurrent tumor shares several mutations with the primary tumor; however, the FBXW7 R465C mutation is absent from the recurrent tumor while a distinct oncogenic FBXW7 R505C mutation is present.
In two of the primary-recurrent pairs, we observed distinct mutations in a single cancer gene in phylogenetically-related primary and recurrent tumors. In one case (AC-P15), a known KRAS activating mutation (G12A) was clonal in the primary tumor (Fig. 2c). Despite sharing many other mutations with the primary tumor, the recurrent tumor lacked the KRAS G12A mutation and instead possessed a distinct KRAS L19F mutation. The KRAS L19F mutation has been observed previously in sporadic colorectal cancers and has an attenuated phenotype compared to the more common KRAS codon 12 mutations.[27, 28] Similarly, in another primary-recurrent tumor pair (AC-P12), one FBXW7 hotspot mutation (R465C) in the primary tumor was replaced by a different FBXW7 hotspot mutation (R505G) in the recurrent tumor (Fig. 2d). In both cases, the primary and recurrent tumors shared many passenger mutations, confirming their sibling relationship.
In addition, there were examples in which a recurrent tumor harbored a putative driver mutation in a gene that was not mutated in the primary tumor. For example, a novel KEAP1 mutation was observed in a recurrent KRAS mutated tumor (Fig. 2c). Activation of the NRF2 pathway by mutation of NFE2L2 or KEAP1 suppresses reactive oxygen species (ROS)-mediated cellular damage, particularly in KRAS-mutated tumors, and is associated with poor response to chemotherapy in lung squamous cell cancer.[21]
Effect of CRT on Mutational Signatures and Neoantigen Load
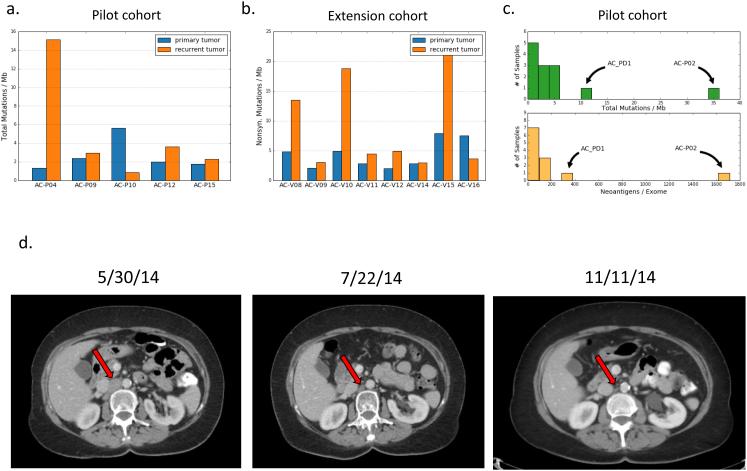
The standard treatment for locally advanced ASCC involves concurrent delivery of the multiple DNA damaging agents, and we sought to characterize the genomic consequences of DNA damage exposure by comparing mutational features of pre- and post-CRT tumors. Among patient-matched tumor pairs, there was a trend towards increased non-synonymous mutational burden in recurrent compared to primary tumors (7.1 vs 3.7 mutations/Mb, p=0.11, paired t-test; Fig. 3a, b).
Figure 3. Mutational burden, neoantigen load, and an exceptional response to immune checkpoint blockade in recurrent ASCC.
(a) Comparison of mutational burden in patient-matched pre- and post-CRT tumors from the pilot cohort. (b) Comparison of mutational burden in patient-matched pre- and post-CRT tumors from the extension cohort. (c) Mutational burden and predicted neoantigen burden in primary tumors (n = 13) from the pilot cohort. (d) Representative axial CT images showing a metastatic ASCC lesion at time of initiation of anti-PD-1 therapy (left), two months after initiation (center), and six months after initiation (right). The patient continues on anti-PD-1 therapy.
To further characterize the mutational effects of CRT on ASCC, we performed mutational signature analysis of pre- and post-CRT tumors using a novel non-negative matrix factorization (NMF) approach.[29] Analysis of all primary and recurrent tumors from the pilot cohort (n = 18) identified three operating mutational signatures (Supplementary Fig. 7a). Two of the signatures closely resembled previously described signatures – one attributed to activity of APOBEC enzymes and one attributed to aging-related accumulation of C>T mutations at CpG motifs (Supplementary Fig. 7b).[30] The third signature identified in the cohort is characterized primarily by C>A transversions and did not resemble any described signature. Interestingly, this signature was primarily active in a single recurrent tumor (AC-P04), but was absent in the primary tumor from the same patient (Supplementary Fig. 7c).
Given that DNA damage-induced somatic mutations can result in novel cancer-specific neoantigens, we used patient-specific non-synonymous mutations and HLA types to predict putative immunogenic neoantigens for matched primary and recurrent tumors from the pilot cohort (Methods, Supplementary Information).[16] As expected, the predicted tumor neoantigen load was closely correlated with the overall mutation burden (Fig. 3c). In four of five tumor pairs from the pilot cohort, the difference in neoantigen burden between primary and recurrent tumors was modest. However, in one case (AC-P-04), the recurrent tumor harbored significantly more mutations and neoantigens than the primary tumor, and this difference was contributed almost entirely by mutations attributed to the unique mutational signature (Supplementary Fig. 7c). Interestingly, this is the same case in which the recurrent tumor did not share mutations with the primary tumor (Fig. 2b).
Exceptional response to PD-1 blockade in ASCC
Recently, immune checkpoint blockade has emerged as an effective treatment strategy for subsets of patients with malignancies including ASCC.[31, 32] One patient in this study (AC_PD1) developed widespread lymph node metastases four months following completion of chemoradiotherapy for an HPV-associated ASCC. The patient began systemic therapy with 5-fluorouracil, oxaliplatin, and leucovorin, but computed tomography (CT) after six cycles demonstrated progressive disease, and the patient was subsequently enrolled on a clinical trial of the anti-PD-1 antibody pembrolizumab (Supplementary Methods). CT after six weeks of treatment with pembroluzimab showed shrinkage in multiple metastatic foci, and subsequent CTs have continued to demonstrate a durable response (tumor burden decreased 79% by RECIST criteria) that continues to persist 27 months following initiation of PD-1 blockade (Fig. 3d). Analysis of the primary tumor from the patient revealed missense mutations in PIK3CA, PIK3CB, and ERBB2 (Fig. 1). The overall mutational burden and predicted neoantigens were higher than all but one other tumor in the pilot cohort, but this difference was not statistically significant (Fig. 3c).
Discussion
Treatment of locally advanced ASCC involves concurrent chemotherapy and radiation using a treatment paradigm that has changed little in the past several decades. Although long-term disease control can be achieved in the majority of patients using this approach, treatment options for patients who develop recurrent disease are limited. In an attempt to characterize the mutational landscape of ASCC and understand genomic evolution through CRT, we performed WES of 31 primary and 30 recurrent ASCCs, including paired primary/recurrent samples from 13 patients. This study represents the first exome-wide mutational analysis in ASCC, and highlights the importance of genomic characterization of rare cancers for the purpose of translational discovery.
Several of the most frequently mutated genes in the cohort, including PIK3CA and FBXW7, have been previously identified in targeted ASCC sequencing studies and are significantly mutated in other HPV associated squamous cell cancers[18, 19], while mutations in other cancer genes such as NFE2L2 have not been previously reported in ASCC. TP53 mutations were significantly more common in HPV negative tumors, and HPV negative tumors were enriched among recurrent cases.[8]
Among primary tumors, EP300 mutations were more common in tumors that did not recur during follow-up, while FBXW7 mutations were more common in tumors that did recur; however, neither event reached statistical significance and additional studies in larger cohorts using a variety of genomic approaches including whole genome sequencing as well as transcriptomic, epigenetic, and proteomic analyses are warranted. Identifying predictive biomarkers of CRT response could allow providers to escalate therapy or incorporate novel agents for tumors harboring genomic predictors of increased recurrence risk, and thus remains an important challenge in ASCC and other tumors for which CRT is used in a curative approach.
Compared to the overall ASCC population, our cohort was enriched in recurrent cases. For many of the recurrent cases, both the primary and recurrent tumors were available, thus providing an opportunity to study the genomic evolution of ASCC through CRT. Despite antecedent exposure to DNA-damaging chemotherapy and ionizing radiation, the overall mutation burden was not significantly higher in recurrent versus primary tumors. Increased mutational burden associated with chemotherapy exposure has been described in settings such as relapsed ovarian cancer[33], while other studies have not noted marked differences in mutational load associated with exposure to chemotherapy and/or radiation.[33, 34]
Similarly, in most ASCC cases, the mutational signature composition did not vary markedly between primary and recurrent cases. One notable exception was AC-P04, a case in which the recurrent tumor had significantly higher mutational burden than the primary tumor and emergence of a novel mutational signature characterized by C>A transversions. Interestingly, C>A transversions are common in mutational signatures attributed to specific DNA damaging agents such as tobacco as well as in signatures associated with specific DNA repair defects such as homologous recombination deficiency.[30] Larger studies of pre- and post-CRT tumors, or whole genome analysis, may provide additional power to detect differences in mutational patterns that reflect the influence of genomic exposure to DNA damaging agents. Given the close association between somatic mutations and predicted neoantigens, understanding the impact of DNA damaging agents on the tumor mutational landscape may provide insights that allow immunotherapy agents to be incorporated more effectively with these agents.
In several cases, we observed interesting patterns of evolution among driver mutations in pre- and post-CRT tumors. In all but one case, the pre- and post-CRT tumors had shared mutations, suggesting a sibling relationship. In many cases, these shared mutations included putative clonal driver genes. In two cases, we observed distinct mutations in the same cancer gene (KRAS in AC-P15 and FBXW7 in AC-P12) in the primary versus recurrent tumor. This phenomenon has previously been described in pre- and post-treatment glioma samples as well as in biopsies from different regions of esophageal adenocarcinoma.[35, 36]
Many of the chemoradiotherapy regimens in current clinical use have evolved empirically, with agents and dosing schedules often selected based on historical or logistical factors rather than on a mechanistic understanding of potential interactions. Therefore, further work is needed to separate the contributions of chemotherapy- versus radiation-induced resistance mechanisms in recurrent tumors, and to find concurrent chemoradiotherapy regimens that maximize the potential for synergy while not exceeding normal tissue tolerance.
One patient who developed refractory metastatic disease after CRT subsequently achieved an exceptional response to anti-PD-1 therapy. Although elevated mutational burden has been associated with increased response to immune checkpoint blockade, the patient’s primary tumor did not have a statistically higher mutational burden than other tumors in the cohort. This case underscores the opportunity to use rare tumor types such as ASCC to elucidate novel mechanisms of drug response and also highlights the opportunity to broaden the scope of clinical development of new agents for rare cancers.
Supplementary Material
Statement of Translational Relevance.
Despite increasing incidence in the US, anal squamous cell carcinoma (ASCC) remains an understudied disease. The current standard therapy for ASCC involves concurrent chemotherapy and radiation (CRT), and although CRT is associated with high response rates, patients who fail CRT have limited treatment options and poor prognosis. The genomic landscape of ASCC is incompletely characterized, and little is known about the genetic features that drive response to CRT. To better understand mutational dynamics through CRT, we performed whole exome sequencing of 61 pre- and post-CRT tumors. We find several interesting examples of evolution in cancer driver genes, and perform the first genomic dissection of immunotherapy response in an ASCC patient who achieved an exceptional response to an anti-PD-1 agent. This study represents one of the most comprehensive genomic analyses performed in ASCC to date and lays the groundwork for future studies to identify predictive biomarkers and therapeutic targets in this disease.
Acknowledgments
Funding Support:
ASTRO Young Faculty Career Development Award (KWM), Dana Farber/Harvard Cancer Center Gastrointestinal Cancer SPORE Grant P50-CA127003 (KWM), Doris Duke Research Fellowship (JP), Damon Runyon Foundation Clinical Investigator Award (EMV), NIH K08 CA188615-02 (EMV), BroadNext10 (EMV), BroadIgnite (EMV), Ludwig Center at Harvard (ADD). The KEYNOTE-028 trial (NCT02054806) is funded by Merck.
Footnotes
No conflicts of interest to disclose.
References
- 1.Nelson RA, Levine AM, Bernstein L, Smith DD, Lai LL. Changing patterns of anal canal carcinoma in the United States. J Clin Oncol. 2013;31:1569–75. doi: 10.1200/JCO.2012.45.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Nigro ND, Vaitkevicius VK, Considine B., Jr. Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum. 1974;17:354–6. doi: 10.1007/BF02586980. [DOI] [PubMed] [Google Scholar]
- 4.Flam M, John M, Pajak TF, Petrelli N, Myerson R, Doggett S, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–39. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 5.Sischy B, Doggett RL, Krall JM, Taylor DG, Sause WT, Lipsett JA, et al. Definitive irradiation and chemotherapy for radiosensitization in management of anal carcinoma: interim report of Radiation Therapy Oncology Group study no. 8314. J Natl Cancer Inst. 1983;81:850–6. doi: 10.1093/jnci/81.11.850. [DOI] [PubMed] [Google Scholar]
- 6.Gunderson LL, Winter KA, Ajani JA, Pedersen JE, Moughan J, Benson AB, 3rd, et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol. 2012;30:4344–51. doi: 10.1200/JCO.2012.43.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James RD, Glynne-Jones R, Meadows HM, Cunningham D, Myint AS, Saunders MP, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 x 2 factorial trial. Lancet Oncol. 2013;14:516–24. doi: 10.1016/S1470-2045(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 8.Meulendijks D, Tomasoa NB, Dewit L, Smits PH, Bakker R, van Velthuysen ML, et al. HPV-negative squamous cell carcinoma of the anal canal is unresponsive to standard treatment and frequently carries disruptive mutations in TP53. Br J Cancer. 2015;112:1358–66. doi: 10.1038/bjc.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serup-Hansen E, Linnemann D, Skovrider-Ruminski W, Hogdall E, Geertsen PF, Havsteen H. Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American Joint Committee on Cancer stages I to III carcinoma of the anal canal. J Clin Oncol. 2014;32:1812–7. doi: 10.1200/JCO.2013.52.3464. [DOI] [PubMed] [Google Scholar]
- 10.Siegel EM, Eschrich S, Winter K, Riggs B, Berglund A, Ajidahun A, et al. Epigenomic characterization of locally advanced anal cancer: a radiation therapy oncology group 98-11 specimen study. Dis Colon Rectum. 2014;57:941–57. doi: 10.1097/DCR.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung JH, Sanford E, Johnson A, Klempner SJ, Schrock AB, Palma NA, et al. Comprehensive genomic profiling of anal squamous cell carcinoma reveals distinct genomically defined classes. Ann Oncol. 2016 doi: 10.1093/annonc/mdw152. [DOI] [PubMed] [Google Scholar]
- 12.Smaglo BG, Tesfaye A, Halfdanarson TR, Meyer JE, Wang J, Gatalica Z, et al. Comprehensive multiplatform biomarker analysis of 199 anal squamous cell carcinomas. Oncotarget. 2015 doi: 10.18632/oncotarget.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casadei Gardini A, Capelli L, Ulivi P, Giannini M, Freier E, Tamberi S, et al. KRAS, BRAF and PIK3CA status in squamous cell anal carcinoma (SCAC) PLoS One. 2014;9:e92071. doi: 10.1371/journal.pone.0092071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, et al. COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43:D805–11. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Exome Aggregation Consortium (ExAC) Cambridge, MA: Feb 12, 2016. Available from: http://exac.broadinstitute.org. [Google Scholar]
- 16.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–11. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasar S, Kim J, Improgo R, Tiao G, Polak P, Haradhvala N, et al. Whole-genome sequencing reveals activation-induced cytidine deaminase signatures during indolent chronic lymphocytic leukaemia evolution. Nat Commun. 2015;6:8866. doi: 10.1038/ncomms9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas, N Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–82. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ojesina AI, Lichtenstein L, Freeman SS, Pedamallu CS, Imaz-Rosshandler I, Pugh TJ, et al. Landscape of genomic alterations in cervical carcinomas. Nature. 2014;506:371–5. doi: 10.1038/nature12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis MA, Larimore EA, Fissel BM, Swanger J, Taatjes DJ, Clurman BE. The SCF-Fbw7 ubiquitin ligase degrades MED13 and MED13L and regulates CDK8 module association with Mediator. Genes Dev. 2013;27:151–6. doi: 10.1101/gad.207720.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cescon DW, She D, Sakashita S, Zhu CQ, Pintilie M, Shepherd FA, et al. NRF2 Pathway Activation and Adjuvant Chemotherapy Benefit in Lung Squamous Cell Carcinoma. Clin Cancer Res. 2015;21:2499–505. doi: 10.1158/1078-0432.CCR-14-2206. [DOI] [PubMed] [Google Scholar]
- 22.Rusan M, Li YY, Hammerman PS. Genomic landscape of human papillomavirus-associated cancers. Clin Cancer Res. 2015;21:2009–19. doi: 10.1158/1078-0432.CCR-14-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricciardi R, Burks E, Schoetz DJ, Verma Y, Kershnar E, Kilpatrick MW, et al. Is there a gain in chromosome 3q in the pathway to anal cancer? Dis Colon Rectum. 2014;57:1183–7. doi: 10.1097/DCR.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 24.Heselmeyer K, Schrock E, du Manoir S, Blegen H, Shah K, Steinbeck R, et al. Gain of chromosome 3q defines the transition from severe dysplasia to invasive carcinoma of the uterine cervix. Proc Natl Acad Sci U S A. 1996;93:479–84. doi: 10.1073/pnas.93.1.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter SL, Cibulskis K, Helman E, McKenna A, Shen H, Zack T, et al. Absolute quantification of somatic DNA alterations in human cancer. Nat Biotechnol. 2012;30:413–21. doi: 10.1038/nbt.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernardi MP, Ngan SY, Michael M, Lynch AC, Heriot AG, Ramsay RG, et al. Molecular biology of anal squamous cell carcinoma: implications for future research and clinical intervention. Lancet Oncol. 2015;16:e611–21. doi: 10.1016/S1470-2045(15)00292-2. [DOI] [PubMed] [Google Scholar]
- 27.Akagi K, Uchibori R, Yamaguchi K, Kurosawa K, Tanaka Y, Kozu T. Characterization of a novel oncogenic K-ras mutation in colon cancer. Biochem Biophys Res Commun. 2007;352:728–32. doi: 10.1016/j.bbrc.2006.11.091. [DOI] [PubMed] [Google Scholar]
- 28.Smith G, Bounds R, Wolf H, Steele RJ, Carey FA, Wolf CR. Activating K-Ras mutations outwith 'hotspot' codons in sporadic colorectal tumours - implications for personalised cancer medicine. Br J Cancer. 2010;102:693–703. doi: 10.1038/sj.bjc.6605534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J, Mouw KW, Polak P, Braunstein LZ, Kamburov A, Tiao G, et al. Somatic ERCC2 mutations are associated with a distinct genomic signature in urothelial tumors. Nat Genet. 2016;48:600–6. doi: 10.1038/ng.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 32.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–94. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 34.Hedberg ML, Goh G, Chiosea SI, Bauman JE, Freilino ML, Zeng Y, et al. Genetic landscape of metastatic and recurrent head and neck squamous cell carcinoma. J Clin Invest. 2016;126:169–80. doi: 10.1172/JCI82066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson BE, Mazor T, Hong C, Barnes M, Aihara K, McLean CY, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343:189–93. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murugaesu N, Wilson GA, Birkbak NJ, Watkins TB, McGranahan N, Kumar S, et al. Tracking the genomic evolution of esophageal adenocarcinoma through neoadjuvant chemotherapy. Cancer Discov. 2015;5:821–31. doi: 10.1158/2159-8290.CD-15-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.