Abstract
After publication of reports describing the presence of stem/progenitor cells among non-hormone-producing cells in the pituitary, the mechanism responsible for proliferation and differentiation generated considerable interest. Several studies have suggested that Notch signaling is involved. In the present study, we examined the histochemical relationship between Notch signaling molecules and the transcription factor SOX2 in rat pituitary. Combined in situ hybridization and immunohistochemistry showed that Notch2 mRNA and SOX2 were co-expressed at embryonic day 14.5 in most cells in the adenohypophyseal primordium. In adult rat pituitary, double immunohistochemistry showed that SOX2 and either Notch2 or the Notch signaling target HES1 were co-localized within cells with large oval nuclei in both the marginal cell layer and cell aggregates in the main part of the anterior lobe, which are believed to be stem cell niches. Furthermore, when the Notch signaling inhibitor DAPT was added to a primary culture of adult rat anterior pituitary cells, the proportion of SOX2-expressing cells within Notch2-positive cells was approximately 30% lower. These findings suggest that Notch signaling has a role in maintaining the stemness of precursor cells in the adult rat pituitary gland.
Keywords: Notch signaling, SOX2, rat, anterior pituitary, stem cell
I. Introduction
The rat anterior pituitary gland is composed of five types of hormone-producing cells plus non-hormone-producing cells. The non-hormone-producing cells comprise a heterogeneous population of cells that express S100b protein [1, 12]. These cells are thus referred to as S100b-positive cells [19]. Histologically defined folliculostellate cells and cells in the marginal cell layer belong to this cell population. The balance of cell types in the anterior pituitary is maintained by physiological demand throughout the embryonic and adult stages. Evidence from several studies suggests that a subset of S100b-positive cells expresses SOX2 and has characteristics of stem cells, namely, differentiation into any type of hormone-producing cell [2, 6, 8]. However, it remains to be determined how differentiation and self-renewal of stem/progenitor cells are controlled in tissue.
Notch signaling is a form of juxtacrine cell-to-cell communication and requires direct cell-to-cell attachment between signal-sending and -receiving cells. It regulates several genes associated with ontogenic cell differentiation and maintenance of adult tissue in a number of organs [14]. In the anterior pituitary gland, most mouse embryonic cells in the hypophyseal primordium (Rathke’s pouch) express Notch2 [15]. Chen et al. demonstrated the presence of the Notch signaling system in the postnatal mouse pituitary [5]. We recently reported that Notch signaling accelerates cell proliferation of S100b cells in adult rats [18]. More recently, Zhu et al. used a conditional knockout mouse model and found that SOX2+ stem cells are maintained by the canonical Notch signaling pathway [22]. These previous findings suggest that Notch signaling is associated with maintenance of stem/progenitor cells in the anterior pituitary gland. Unfortunately, our understanding of Notch signaling in SOX2-expressing stem cells is incomplete. The present study is the first to use histological techniques to prove that SOX2 expression is closely associated with Notch signaling in embryonic and adult rat anterior pituitary glands. In addition, the results of our related in vitro inhibition experiment suggest that SOX2 expression at least partly depends on Notch signaling.
II. Materials and Methods
Animals
Wistar rats were purchased from Japan SLC (Shizuoka, Japan). The rats were housed in a 12-hr light/dark cycle at 22 ± 1°C and given conventional food and water ad libitum. For the embryo experiment, the day on which spermatozoa were identified in a vaginal smear was designated embryonic day 0.5 (E0.5). All animal experiments were performed after receiving approval from the Institutional Animal Experiment Committee of Jichi Medical University and were conducted in accordance with the Institutional Regulations for Animal Experiments and Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
Tissue preparation
Pregnant rats were deeply anesthetized with pentobarbital sodium (25 mg per kg body weight, i.p., Kyoritsu Seiyaku, Tokyo, Japan) when their fetuses were at embryonic day 14.5 (E14.5), and the fetuses were removed. Male adult rats aged 8–10 weeks were perfused with 4% paraformaldehyde (PFA) in 50 mM phosphate buffer (PB) (pH 7.4) for 5 min through the left ventricle after deep anesthesia had been induced. The pituitary glands and heads of the fetuses were excised and immersed in 50 mM PB containing 4% PFA for 20–24 hr at 4°C. The tissues were transferred into 50 mM PB with 30% sucrose and immersed for 2 days at 4°C, after which they were embedded in Tissue-Tek OCT compound (Sakura FineTechnical, Tokyo, Japan) and frozen in liquid nitrogen. Frozen sections (3 μm-thick for adult tissue and 5 μm-thick for embryonic tissue) were obtained using a cryostat (CM3000; Leica Microsystems, Wetzlar, Germany) and mounted on glass slides.
Double immunohistochemistry for SOX2 and Notch2
Double fluorescent immunohistochemistry was used to examine the relation between SOX2 and Notch2 in adult tissue. Cryosections were blocked with 2% normal donkey serum for 1 hr at 30°C and incubated in phosphate-buffered saline (PBS) with a primary antibody against goat anti-SOX2 (1:250 dilution, Neuromics, Edina, MN, USA) overnight at 4°C. After washing with PBS, sections were incubated in PBS with a secondary fluorescent antibody, Alexa Fluor 568-conjugated donkey anti-goat IgG (1:200 dilution) (Thermo Fisher Scientific, Waltham, MA, USA), for 30 min at 30°C. After washing with PBS, sections were blocked with 10% normal goat serum and 3% BSA in PBS for 30 min at 30°C and incubated with goat anti-Notch2 antibody (20 μg/ml) (R&D Systems, Inc, Minneapolis, MN, USA) labeled with Alexa Fluor 488 by Zenon® Complex Formation/Labeling reagent (Thermo Fisher Scientific), according to the manufacturer’s instructions. Then, fluorescent signals were enhanced with rabbit anti-Alexa Fluor 488 antibody (1 μg/ml) and Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:200 dilution) (Thermo Fisher Scientific). Sections were cover-slipped with Vectashield HardSet mounting medium with 4',6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA, USA) and observed through an AX80 microscope equipped with a DP70 digital camera (Olympus, Tokyo, Japan).
In situ hybridization and immunohistochemistry
Suitable fixation conditions could not be developed for double immunohistochemistry, probably because fixatives separately affected the antigenicity of SOX2 and Notch2 in embryonic tissue. Thus, a combination of in situ hybridization for Notch2 mRNA and immunohistochemistry for SOX2 was used to examine their expressions during the embryonic stage. In situ hybridization was performed with digoxigenin (DIG)-labeled cRNA probes by using the method of Ramadhani et al. [16]. Probes were prepared by polymerase chain reaction, as reported in Tando et al. [18]. Visualization of mRNA was performed with alkaline phosphatase-conjugated anti-DIG antibody (Roche Diagnostics, Penzberg, Germany) by using 4-nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate (Roche Diagnostics). No signal was detected in sections processed with a DIG-labeled sense cRNA probe (the negative control).
For double-staining, after Notch2 mRNA was detected by in situ hybridization, the section was immunostained, as described by Azuma et al. [3]. The sections were incubated overnight at room temperature in PBS with the primary antibody against SOX2 (1:250 dilution). The ABC method (Vector Laboratories) was performed with 3,3'-diaminobenzidine (Dojindo Laboratories, Kumamoto, Japan) as substrate. The absence of an observable nonspecific immunoreaction was confirmed by incubating sections with normal serum from goat instead of the primary antibody, and then with the secondary antibody. Specimens were observed through an AX80 microscope equipped with a DP70 digital camera (Olympus).
Double immunohistochemistry for SOX2 and HES1
Cryosections (40 μm-thick) were cut and collected into PBS containing 0.1% Tween 20 (PBS-T). The sections were incubated with 5% skim milk in PBS-T for 60 min with gentle agitation at room temperature. Then, the specimens were incubated with the primary antibodies, rabbit anti-HES1 (1:400 dilution) (Cell Signaling Technology, Inc, Danvers, MA, USA) and goat polyclonal anti-SOX2, (1:500 dilution) overnight at room temperature. Finally, the sections were incubated with the secondary antibodies, Alexa Fluor 488-conjugated goat anti-rabbit IgG (Thermo Fisher Scientific) and Alexa Fluor 568-conjugated donkey anti-goat IgG antibodies (both 1:200 dilution) containing DAPI (0.5 μg/ml). The sections were subsequently observed with a confocal laser microscope (FV1000, Olympus). The absence of an observable nonspecific immunoreaction was confirmed by incubating sections with normal serum from rabbit or goat, instead of the primary antibody, and then with the secondary antibody.
Primary monolayer culture and immunocytochemistry
Rat anterior pituitary cells were dispersed as described previously [10]. Dispersed cells were re-suspended in Medium 199 (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (Moregate Biotech, Bulimba, Australia), 0.5 U/ml penicillin, and 0.5 μg/ml streptomycin (Thermo Fisher Scientific), plated on eight-well glass chamber slides (Nalge Nunc International, NY, USA) coated with laminin (1 μg/cm2, Merck Millipore, Darmstadt, Germany) at a density of 1 × 105 cells/400 μl/0.8 cm2/well, and cultured for 72 hr at 37°C in a humidified atmosphere of 5% CO2 and 95% air. The γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT; Wako, Osaka, Japan) was used to inhibit γ-secretase-dependent S3 cleavage of Notch, which releases the Notch intracellular domain [9]. DAPT dissolved in dimethyl sulfoxide (DMSO) or DMSO alone (as a control) was added to the medium during culture. The final concentration of DAPT was 50 μM, and the concentration of DMSO was 0.1%. After 72-hr culture, cells were fixed with 4% PFA in 25 mM PB for 30 min at room temperature and used for immunocytochemistry of SOX2 and Notch2. The immunocytochemistry procedures were the same as those described above. For cell counting, slides were randomly imaged by a confocal laser microscope (FV1000) with a 60-fold objective lens. An area of 0.026 mm2 was randomly imaged, and the percentage of SOX2-positive cells among Notch2-positive cells was determined. More than 500 cells were observed in each control and experimental group.
III. Results
Expression of Notch2 and SOX2 in rat embryonic pituitary gland
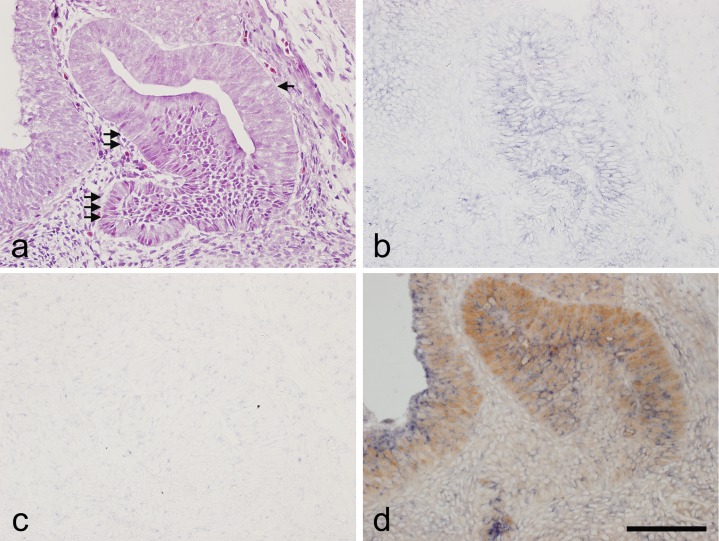
Expression of the Notch signaling receptor Notch2 was examined by in situ hybridization; SOX2 expression was determined by immunohistochemistry. Hematoxylin and eosin staining at E14.5 is shown in Figure 1a. As shown in Figure 1b, Notch2 mRNA was detected in the cytoplasm of almost all cells in the prospective pars distalis and pars intermedia. No signal was detected in the sense probe (Fig. 1c). The correlation between Notch2 expression and the stem/progenitor cell marker SOX2 was examined by the addition of SOX2 immunohistochemistry (Fig. 1d). SOX2 immunoreactivity was observed on the cell nucleus. All cells of the prospective pars intermedia expressed SOX2 and were accompanied by Notch2. In the prospective pars distalis, SOX2 expression was closely related to a positive Notch2 signal. In contrast, cells in the prospective pars tuberalis yielded negative results for both Notch2 and SOX2.
Fig. 1.
In situ hybridization of Notch2 and immunohistochemistry of SOX2 in a sagittal section of rat pituitary gland at embryonic day 14.5. a) Hematoxylin and eosin staining. b) In situ hybridization of Notch2. c) Negative control of in situ hybridization using a sense probe of Notch2. d) Double staining of Notch2 detected by in situ hybridization and SOX2 detected by immunohistochemistry. Immunoreactivity for SOX2 was revealed by 3,3'-diaminobenzidine (brown) and the Notch2 in situ hybridization signal with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (blue). Arrow: prospective pars intermedia, Double arrow: prospective pars distalis, Triple arrow: prospective pars tuberalis. Bar = 100 μm.
Co-localization of Notch2 and SOX2 in adult pituitary gland
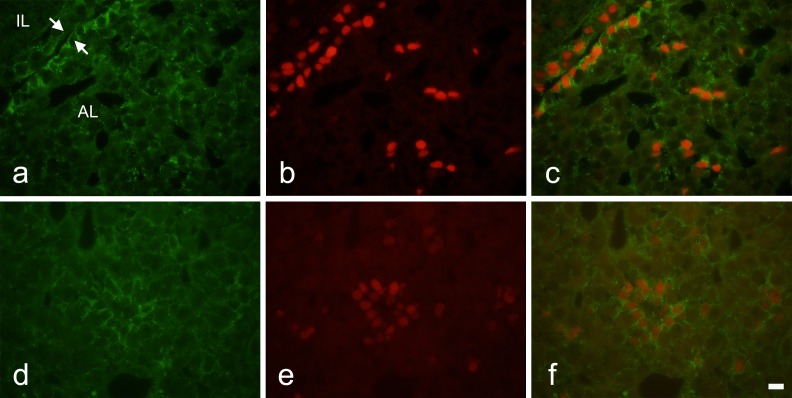
Next, we used double immunohistochemistry to examine distributions of Notch2 and SOX2 in adult rat pituitary. Notch2 immunoreactivity was noted in almost all cells of the marginal cell layer (Fig. 2a) and in some cells in the main part of the anterior lobe (Fig. 2d). In many cases, the Notch2 signal was strong around the entire circumference of the cells. Notch2-positive cells in the anterior lobe were clustered. These observations were consistent with those in our previous report [4]. SOX2 expression on the cell nucleus was diffuse and overlapped completely with Notch2-positive cells in the marginal cell layer (Fig. 2b, c). In the anterior lobe, SOX2 expression was limited to Notch2-positive cells (Fig. 2e, f). SOX2- and Notch2-positive cells had oval nuclei.
Fig. 2.
Double immunohistochemistry for Notch2 and SOX2 in adult rat pituitary gland. a–c) Frontal images around the marginal cell layers. d–f) Images of the main part of the anterior lobe. a and d) Notch2 immunoreactivity. b and e) SOX2 immunoreactivity. c and f) Merged images of a and b, and d and e, respectively. AL: anterior lobe, IL: intermediate lobe. Arrows: marginal cell layer. Bar = 10 μm.
Expression of SOX2 in cells expressing the Notch signaling target HES1
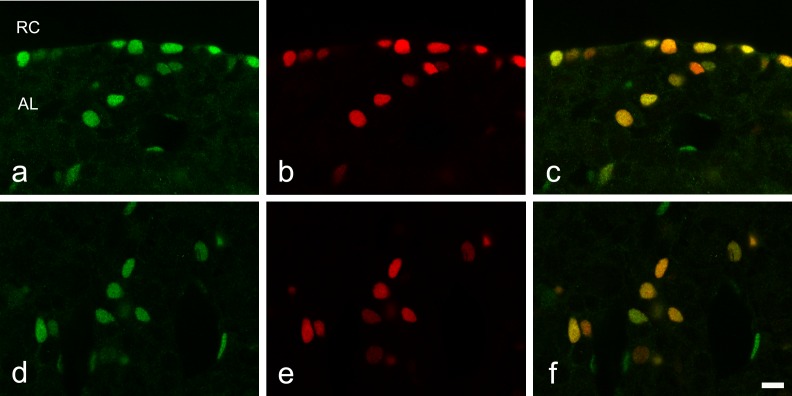
To identify whether Notch signaling is active in SOX2-expressing cells, we performed immunohistochemistry for HES1, the product of a representative Notch signaling target gene. Diffuse HES1 expression was detected in the nuclei of cells in the marginal cell layer and in part of the anterior lobe. SOX2 immunoreactivity was observed in the nuclei of all HES1-positive cells in the marginal cell layer (Fig. 3a–c). Likewise, in the anterior lobe, HES1 and SOX2 were co-expressed mostly in the same cells (Fig. 3d–f); however, some cells that were positive only for HES1 were noted. We previously reported that these single-positive cells were endothelial cells [4].
Fig. 3.
Double immunohistochemistry for SOX2 and the Notch target HES1 in the adult rat anterior pituitary gland. a–c) Frontal images around the marginal cell layer surrounding the residual lumen of Rathke’s pouch (Rathke’s Cleft, RC). d–f) Images from main part of the anterior lobe. a and d) HES1 immunoreactivity. b and e) SOX2 immunoreactivity. c and f) merged image of a and b, and d and e, respectively. AL: anterior lobe. Bar = 10 μm.
Effect of Notch signaling inhibition on SOX2 expression
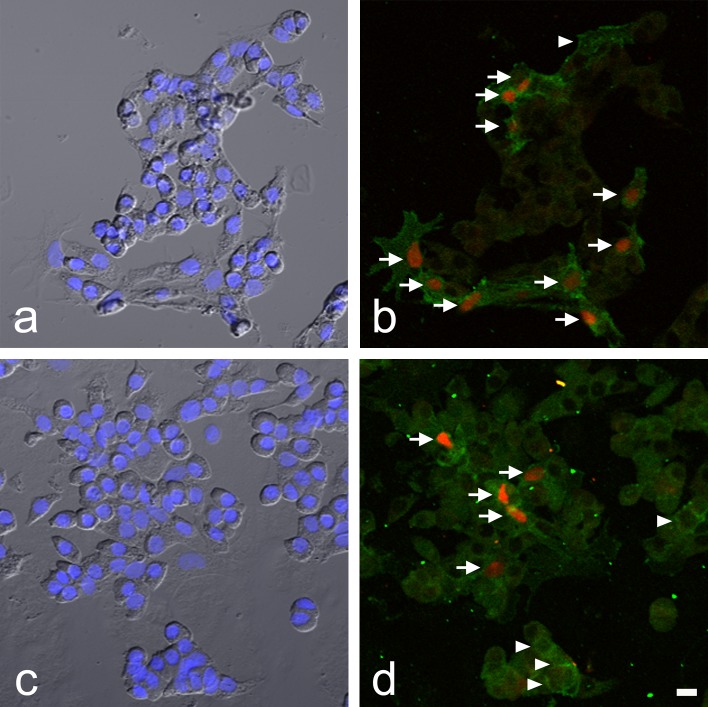
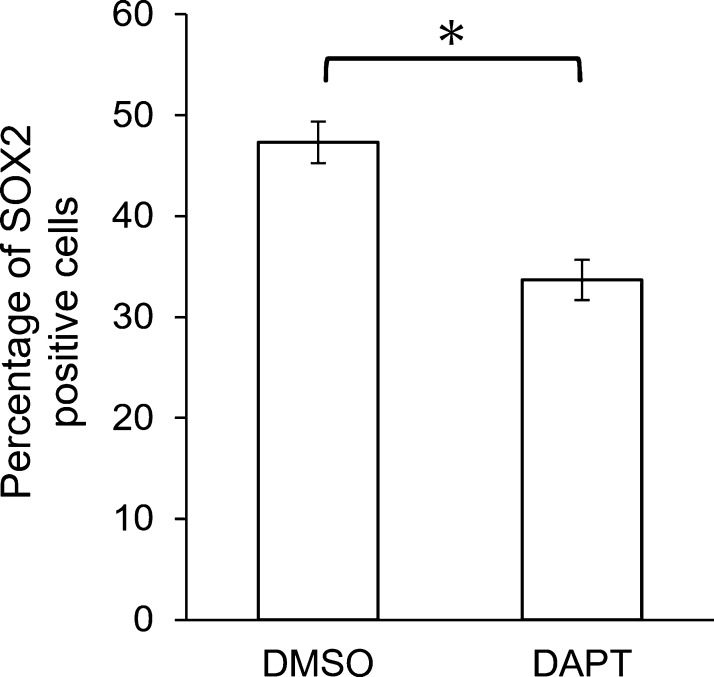
To examine the potential effect of Notch signaling on SOX2 expression, a primary monolayer culture of rat anterior lobe cells was treated with the Notch signaling inhibitor DAPT, and the number of SOX2-positive cells among Notch2-positive cells was determined by double immunocytochemistry (Fig. 4). Figure 4a and c are bright-field images showing DAPI staining of cultured cells in the control and DAPT treatment groups. We detected cells that expressed both SOX2 and Notch2 and cells that expressed only Notch2 (Fig. 4b, d). All SOX2-positive cells expressed Notch2. The number of SOX2-positive cells was clearly lower in the DAPT-treated group (Fig. 4d) than in the control group (Fig. 4b). The number of SOX2-positive cells among Notch2-positive cells was determined in each group (Fig. 5). As compared with the DAPT-treated group, the relative percentage of SOX2-positive cells within Notch2-positive cells was significantly lower, by approximately 30% (p < 0.01 by Mann-Whitney U-test). The difference in the intensity of SOX2 signals in the control and experimental cultures was undetectable.
Fig. 4.
SOX2-immunopositive cells in Notch2-immunopositive cells after treatment with the Notch signaling inhibitor N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT). a and c) Merged images of phase-contrast and 4',6-diamidino-2-phenylindole staining. b and d) Double immunocytochemical staining for SOX2 (red) and Notch2 (green). a and b) culture without DAPT (control). c and d) culture with DAPT. Arrows: double-positive cells. Arrowheads: Notch2 single positive-cells. Bar = 10 μm.
Fig. 5.
Percentage of SOX2-immunopositive cells in Notch2-immunopositive cells after treatment with the Notch signaling inhibitor DAPT (50 μM). After double staining of SOX2 and Notch2, the percentage of double-positive cells was determined. Mean ± SEM (n = 5). *Statistically significant by Mann-Whitney U-test (p < 0.01).
IV. Discussion
After reports described the presence of stem/progenitor cells in the pituitary, the mechanism responsible for proliferation and differentiation of these cells generated considerable interest [7, 19]. Several studies reported that Notch signaling is involved in maintenance and promotion of stem/progenitor cells [14, 15, 21]. Our research group showed that Notch signaling, which combines the Notch2 receptor and Jagged1 ligand, enhances the proliferative activity of S100b-positive cells in adult rats [18]. However, despite its importance, the relationship between Notch signaling and SOX2 expression has not yet been investigated with histological techniques, probably because of the technical difficulty of such studies.
In the present study, we used combined in situ hybridization and immunohistochemistry, or double immunohistochemistry, to observe the relation between SOX2 and Notch2 expression. As shown in Figure 1, at E14.5 Notch2 was expressed in most cells of the prospective pars intermedia and pars distalis but not in the prospective pars tuberalis—the so-called rostral tip—where cell differentiation is already advanced at this stage [13]. This observation supports the hypothesis that Notch signaling is associated with immature adenohypophyseal cells. The hypothesis is further strengthened by our finding that Notch2 expression was usually accompanied by nuclear SOX2. In addition, in the adult pituitary gland shown in Figure 2, the Notch2 molecule was observed on cells in the marginal cell layer and specific cell aggregates in the main part of the anterior lobe, and was accompanied by nuclear SOX2. Furthermore, double immunohistochemistry with SOX2 and HES1 (the product of a Notch signaling target gene) confirmed the relation between SOX2 expression and Notch signaling (Fig. 3). Except for HES1 expression in presumed endothelial cells (indicated by arrowheads) [4], SOX2 expression was always accompanied by HES1 expression. This suggests that SOX2 expression is maintained specifically in Notch-positive cells in the anterior pituitary gland throughout the embryonic and adult stages.
Next, we examined the effect of Notch signaling inhibition on SOX2 expression in primary culture. When the Notch signaling inhibitor DAPT was added to the culture medium, the number of SOX2-expressing cells within Notch2-expressing cells decreased significantly, while the intensity of SOX2 staining in positive cells was largely unchanged (Figs. 4 and 5). In conjunction with our previous report [18], which showed a relation between Notch signaling and cell proliferation, the present findings indicate that Notch signaling is associated with cell proliferation and maintenance of stemness in progenitor cells in the anterior pituitary gland.
In the putative stem cell niche, cell-to-cell signaling other than Notch signaling has been reported. Our group reported CXCL12–CXCR4 signaling [11], which is important in the regulation of hematopoietic stem cells [17], between S100b-positive cells. Recently, Yoshida et al. reported ephrin-B2 signaling in the same cell population [20]. A variety of signaling mechanisms may be required in order to maintain progenitor cells in the anterior pituitary progenitor niche. In addition to characterizing the intracellular signaling pathway of Notch, future studies should attempt to elucidate the crosstalk of these signaling pathways. Researchers should also investigate the conditions and extent of the contribution of SOX2-positive cells to maintenance of adult pituitary tissue.
V. Conflict of Interest
The authors have no conflict of interest that might prejudice the impartiality of this research.
VI. Acknowledgments
We thank Dr. Takehiro Tsukada for his superb guidance and experimental support. We also thank David Kipler, ELS for revising the language of the manuscript. This work was supported in part by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology of Japan to MK (25460275), by promotional funds for the Keirin Race of the Japan Keirin Association, and by a JMU Graduate Student Start-up Award from Jichi Medical University to KB.
VII. References
- 1.Allaerts W. and Vankelecom H. (2005) History and perspectives of pituitary folliculo-stellate cell research. Eur. J. Endocrinol. 153; 1–12. [DOI] [PubMed] [Google Scholar]
- 2.Andoniadou C. L., Matsushima D., Mousavy Gharavy S. N., Signore M., Mackintosh A. I., Schaeffer M., Gaston-Massuet C., Mollard P., Jacques T. S., Le Tissier P., Dattani M. T., Pevny L. H. and Martinez-Barbera J. P. (2013) Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell. 13; 433–445. [DOI] [PubMed] [Google Scholar]
- 3.Azuma M., Tofrizal A., Maliza R., Batchuluun K., Ramadhani D., Syaidah R., Tsukada T., Fujiwara K., Kikuchi M., Horiguchi K. and Yashiro T. (2015) Maintenance of the extracellular matrix in rat anterior pituitary gland: Identification of cells expressing tissue inhibitors of metalloproteinases. Acta Histochem. Cytochem. 48; 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batchuluun K., Azuma M., Yashiro T. and Kikuchi M (2016) Notch signaling-mediated cell-to-cell interaction is dependent on E-cadherin adhesion in adult rat anterior pituitary. Cell Tissue Res. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Chen J., Crabbe A., Van Duppen V. and Vankelecom H. (2006) The notch signaling system is present in the postnatal pituitary: marked expression and regulatory activity in the newly discovered side population. Mol. Endocrinol. 20; 3293–3307. [DOI] [PubMed] [Google Scholar]
- 6.Chen J., Gremeaux L., Fu Q., Liekens D., Van Laera S. and Vankelecom H. (2009) Pituitary progenitor cells tracked down by side population dissection. Stem Cells. 27; 1182–1195. [DOI] [PubMed] [Google Scholar]
- 7.Denef C. (2008) Paracrinicity: The story of 30 years of cellular pituitary crosstalk. J. Neuroendocrinol. 20; 1–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fauquier T., Rizzoti K., Dattani M., Lovell-Badge R. and Robinson I. C. A. F. (2008) SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc. Natl. Acad. Sci. U S A 105; 2907–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geling A., Steiner H., Willem M., Bally-Cuif L. and Haass C. (2002) A γ-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 3; 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horiguchi K., Kikuchi M., Kusumoto K., Fujiwara K., Kouki T., Kawanishi K. and Yashiro T. (2010) Living-cell imaging of transgenic rat anterior pituitary cells in primary culture reveals novel characteristics of folliculo-stellate cells. J. Endocrinol. 204; 115–123. [DOI] [PubMed] [Google Scholar]
- 11.Horiguchi K., Ilmiawati C., Fujiwara K., Tsukada T., Kikuchi M. and Yashiro T. (2012) Expression of chemokine CXCL12 and its receptor CXCR4 in folliculostellate (FS) cells of the rat anterior pituitary gland: the CXCL12/CXCR4 axis induces interconnection of FS cells. Endocrinology 153; 1717–1724. [DOI] [PubMed] [Google Scholar]
- 12.Inoue K., Couch E. F., Takano K. and Ogawa S. (1999) The structure and function of folliculo-stellate cells in the anterior pituitary gland. Arch. Histol. Cytol. 62; 205–218. [DOI] [PubMed] [Google Scholar]
- 13.Kikuchi M., Yatabe M., Fujiwara K., Horiguchi K., Kusumoto K., Kouki T., Sakamoto A. and Yashiro T. (2009) Spatio-temporal relation between cadherin switching and cytogenesis of hormone-producing cells in the developing rat adenohypophysis. Anat. Sci. Int. 84; 155–160. [DOI] [PubMed] [Google Scholar]
- 14.Koch U., Lehal R. and Radtke F. (2013) Stem cells living with a Notch. Development 140; 628–704. [DOI] [PubMed] [Google Scholar]
- 15.Raetzman L. T., Ross S. A., Cook S., Dunwoodie S. L., Camper S. A. and Thomas P. Q. (2004) Developmental regulation of Notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Dev. Biol. 265; 329–340. [DOI] [PubMed] [Google Scholar]
- 16.Ramadhani D., Tsukada T., Fujiwara K., Azuma M., Kikuchi M. and Yashiro T. (2014) Changes in laminin chain expression in pre- and postnatal rat pituitary gland. Acta Histochem. Cytochem. 47; 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugiyama T., Kohara H., Noda M. and Nagasawa T. (2006) Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25; 977–988. [DOI] [PubMed] [Google Scholar]
- 18.Tando Y., Fujiwara K., Yashiro T. and Kikuchi M. (2013) Localization of Notch signaling molecules and their effect on cellular proliferation in adult rat pituitary. Cell Tissue Res. 351; 511–519. [DOI] [PubMed] [Google Scholar]
- 19.Vankelecom H. and Chen J. (2014) Pituitary stem cells: Where do we stand? Mol. Cell. Endocrinol. 385; 2–17. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida S., Kato T., Higuchi M., Chen M., Ueharu H., Nishimura N. and Kato Y. (2015) Localization of juxtacrine factor ephrin-B2 in pituitary stem/progenitor cell niches throughout life. Cell Tissue Res. 359; 755–766. [DOI] [PubMed] [Google Scholar]
- 21.Zhu X., Zhang J., Tollkuhn J., Ohsawa R., Bresnick E. H., Guillemot F., Kageyama R. and Rosenfeld M. G. (2006) Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev. 20; 2739–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu X., Tolkuhn J., Taylor H. and Rosenfeld M. G. (2015) Notch-dependent pituitary SOX2+ stem cells exhibit a timed functional extinction in regulation of the postnatal gland. Stem Cell Reports 5; 1196–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]