Abstract
We report a case of primary central nervous system vasculitis (PCNSV) mimicking a cortical brain tumor. A 25-year-old woman presented with a 2-week history of headache and transient right hemiparesis. Brain magnetic resonance imaging (MRI) revealed a cortical-involving lesion on the left frontal lobe. The 6-cm sized lesion showed low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. The lesion had continual linear enhancement on the subcortical white matter and leptomeninges. There was no evidence of hemorrhage on susceptibility-weighted images and no diffusion restriction on diffusion-weighted images. The regional cerebral blood volume was decreased on the MR perfusion images, and spectroscopy showed increased lactate and lipid peaks. The symptoms were aggravated by fever and seizures. Biopsy was performed to rule out tumorous or inflammatory lesions. Pathologically, lymphocytes were infiltrated on the vessels, and the arachnoid membrane was thickened with inflammatory cells. The patient did not have any underlying diseases, including immune disorders. After high-dose steroid administration, her symptoms improved. Two months later, brain MRI showed a reduction in the infiltration of the T2 hyperintensity lesion with subtle subcortical enhancement. We present a case of PCNSV involving the left frontal lobe, showing vasogenic edema, mass effect, and subcortical linear contrast enhancement without hemorrhage or infarction.
Keywords: Central nervous system, Vasculitis, Radiology, Steroids
INTRODUCTION
Primary central nervous system vasculitis (PCNSV) is a rare disease affecting both medium- and small-sized vessels. PCNSV has an annual incidence rate of 2.4 cases per million personyears and causes significant morbidity and mortality [1,2,3]. Cerebral vasculitis causes various neurological symptoms such as headache, hemiparesis, and mental disturbances, and diagnosis can be difficult using magnetic resonance imaging (MRI) with conventional sequences [3,4,5]. The MRI features of cortical, subcortical, and deep white matter lesions, focal hemorrhage, and heterogeneous enhancement combined with clinical presentation suggest a diagnosis of PCNSV. Brain biopsy is necessary to rule out tumorous or other inflammatory lesions. Leptomeningeal cortical biopsy is the gold standard procedure for the diagnosis of PCNSV.
Differentiation between tumors and tumor-like lesions of the central nervous system is essential for planning adequate treatment, and for estimating outcomes and future prognosis [6]. PCNSV is frequently fatal, and early diagnosis and treatment could lead to a more favorable prognosis [7]. Treatment regimens for cerebral vasculitis are derived from therapeutic strategies used for other types of vasculitis. Early detection is important because corticosteroid treatment can often prevent serious outcomes [7,8].
We present a case of PCNSV mimicking a cortical brain tumor on neuroimaging that was treated with high-dose steroid therapy.
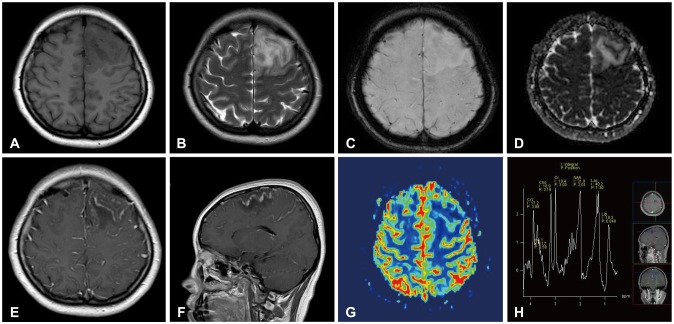
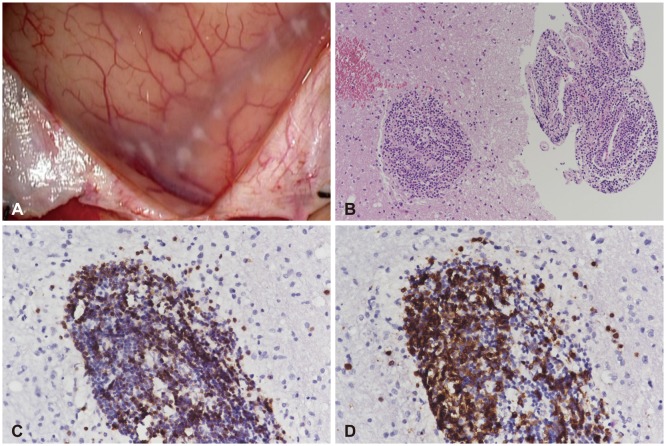
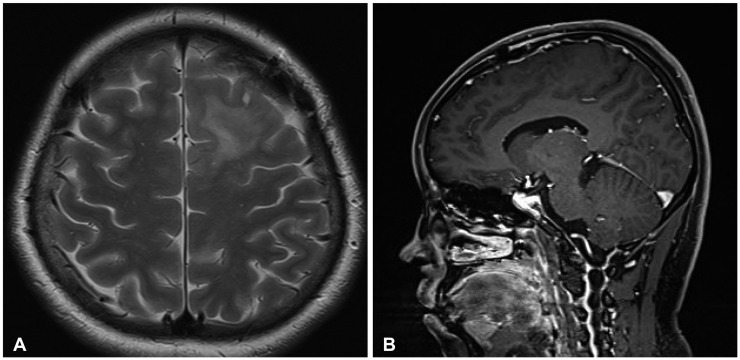
CASE REPORT
A 25-year-old woman presented with a 2-week history of headache and transient right hemiparesis. She did not have a history of hypertension, diabetes mellitus, or other diseases. Brain computed tomography (CT) showed a low-density lesion in the left frontal lobe, but CT-angiography showed no abnormal findings in the cerebral arteries. The 6-cm lesion involving the left frontal lobe showed low signal intensity on T1-weighted MRI and high signal intensity on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images (Fig. 1A, B). There was no evidence of hemorrhage on susceptibility-weighted images (Fig. 1C). The subcortical area of the lesion showed low signal intensity on diffusion-restriction images, and high signal intensity on an apparent diffusion coefficient map (Fig. 1D). The subcortical area and leptomeninges had continuous linear enhancement after gadolinium administration (Fig. 1E, F). The regional cerebral blood volume of the lesion was decreased on MR perfusion images, and the lactate peak was increased on MR spectroscopy (Fig. 1G, H). The symptoms were aggravated by fever and seizures. The results of laboratory testing showed that the white blood cell count was 14,200/mm3, with neutrophil dominance (82.3%). C-reactive protein was elevated to 1.15 mg/dL. For the frontal lobe-involving lesion with vasogenic edema, mass effect, and contrast enhancement, the provisional diagnosis was a cortical and subcortical brain tumor, such as glioma or lymphoma. Biopsy was performed to rule out tumorous or other inflammatory lesions. Intraoperatively, the arachnoid membrane was focally thickened with yellowish discoloration, and the cortical and subcortical areas appeared normal (Fig. 2A). The brain showed dominant lymphocytic infiltration in the small vessels (Fig. 2B), and the lymphocytes were immunopositive for CD3 and CD79a (Fig. 2C, D). The arachnoid membrane showed fibrotic changes. The patient did not have any underlying diseases, including immune diseases. The levels of antinuclear antibody, cyclic citrullinated peptide antibody, antineutrophil cytoplasmic antibody and rheumatoid factor were within the normal range. After high-dose steroid administration, the patient's symptoms improved. Two months later, follow-up brain MRI showed a reduction in the extent of the T2/FLAIR hyperintensity lesion, with decreased patchy subcortical enhancement in the left frontal lobe (Fig. 3).
Fig. 1. MR radiologic findings of primary central nervous system vasculitis. A and B: The left frontal lesion showed low signal intensity on T1-weighted MR images and high signal intensity on T2-weighted images. C: There was no hemorrhage on susceptibility-weighted images. D: The subcortical area of the lesion showed high signal intensity on an ADC map. E and F: The subcortical area and leptomeninges had linear and punctate enhancement. G and H: The regional cerebral blood volume of the lesion was decreased on MR perfusion images, and the lactate peak was increased on MR spectroscopy. ADC, apparent diffusion coefficient.
Fig. 2. Operative and pathologic findings of primary central nervous system vasculitis. A: The arachnoid membrane was focally thickened with yellowish discoloration, while the cortex showed a normal appearance. B: Vasculocentric lymphocytic infiltration (hematoxylin and eosin staining; original magnification, ×100). C: Immunopositivity for CD3 (original magnification, ×200). D: Immunopositivity for CD79a (original magnification, ×200).
Fig. 3. MR radiologic findings of improved primary central nervous system vasculitis. Two months later, follow-up brain MRI showed a reduction in the infiltration of the T2 hyperintensity lesion (A) without subcortical enhancement in the left frontal lobe (B).
DISCUSSION
The cause of PCNSV is unknown. However, infectious agents have been proposed as triggers because of the known association between cerebral vasculitis and infections [7]. The median age of PCNSV diagnosis is approximately 50 years, and most patients are diagnosed between 37 and 59 years [9]. Increased mortality during follow-up is related to cerebral infarctions and large vessel associations. The clinical manifestations are non-specific and many diverse symptoms can develop, including headache, cognitive impairment, ataxia, and seizures [7]. The onset of most symptoms is insidious.
The MRI findings of PCNSV can be variable and diagnosis can be difficult based on MRI findings. The common MRI features of PCNSV are multiple bilateral asymmetrical supratentorial lesions involving the gray and white matter, predominantly in the subcortical and deep white matter, the corpus callosum, and capsular tract. They are associated with minimal or no mass effect, focal hemorrhage, and heterogeneous parenchymal and leptomeningeal enhancement on normal angiography [5,10]. Intracerebral hemorrhage is present in 11-12% of cases [7]. The pattern of enhancement is variable. Irregular subcortical streaks, punctate enhancing areas, leptomeningeal enhancement, and focal cortical or diffuse parenchymal enhancement have been reported. Open brain biopsy is recommended for these patients. The lesions mimic de-myelinating disease, infarction, diffuse white matter disease, encephalitis, cerebral amyloid angiopathy, and tumor-like lesions such as glioma or lymphoma [5,10]. In this case, MRI showed a frontal lobe-involving lesion with vasogenic edema, mass effect, and subcortical linear contrast enhancement. The provisional diagnosis was a malignant cortical brain tumor such as glioma or lymphoma, and biopsy was necessary for differential diagnosis. Result of blood test in patients with PCNSV are usually normal, and consist of tests for acute-phase reactants, antinuclear antibodies, antineutrophil cytoplasm antibodies, and antiphospholipid antibodies [7]. In our case, white blood cell count was elevated with neutrophil dominance, and C-reactive protein was mildly elevated.
Calabrese and Mallek [11] proposed diagnostic criteria for PCNSV, including historical or clinical findings of an acquired neurological deficit of unknown origin after a thorough initial basic assessment; cerebral angiogram with classic features of vasculitis, or a CNS biopsy showing vasculitis; and no evidence of systemic vasculitis or any other disorder. However, cerebral angiograms showed a specificity as low as 30%, and biopsy is used for the definitive diagnosis of PCNSV [12,13]. The histopathologic findings of PCNSV showed 3 main patterns: granulomatous, lymphocytic, and necrotizing vasculitis [7]. Granulomatous vasculitis is the most common pattern (58%), with vasculocentric mononuclear and granulomatous inflammation. Lymphocytic vasculitis is the second most common pattern (28%), showing dominant lymphocytic inflammation associated with occasional plasma cell infiltration and vessel destruction. Lymphocytic pattern is typically reported in children with angiography-negative PCNSV. Necrotizing vasculitis is the least common pattern (14%), and is associated with transmural fibrinoid necrosis, which causes intracranial hemorrhage.
The outcome of PCNSV is frequently fatal, and early diagnosis and treatment could result in a more favorable prognosis [7,8,9]. Meningeal and parenchymal-enhancing lesions were associated with cognitive impairment, but had a good response to immunosuppressive treatment [7]. Rapidly progressing symptoms were often associated with a fatal outcome. Many bilateral large cerebral vessel lesions were shown using angiography; they were associated with multiple bilateral cerebral infarctions, which had pathologically granulomatous or necrotizing patterns, and the treatment response was poor. Approximately 4% of PCNSV cases involved a solitary tumor-like lesion [14]. Excision was curative in some cases, and high-dose steroids, cyclophosphamide, azathioprine and immunoglobulins resulted in a favorable outcome in others [1,2,5,7,9,11].
In conclusions, early diagnosis of tumor-like PCNSV could be essential because patient can be treated with high-dose steroid. We report a case of vasculitis involving the left frontal lobe, showing vasogenic edema, mass effect, and subcortical linear contrast enhancement without hemorrhage or infarction.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, & Future Planning (2014R1A1A1004469).
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Tanei T, Nakahara N, Takebayashi S, Ito M, Hashizume Y, Wakabayashi T. Primary angiitis of the central nervous system mimicking tumor-like lesion--case report. Neurol Med Chir (Tokyo) 2011;51:56–59. doi: 10.2176/nmc.51.56. [DOI] [PubMed] [Google Scholar]
- 2.Wiszniewska M, Szylberg T, Harat M. [Primary central nervous system vasculitis imitating a brain tumour--a case report] Neurol Neurochir Pol. 2008;42:358–361. [PubMed] [Google Scholar]
- 3.Berlit P. Diagnosis and treatment of cerebral vasculitis. Ther Adv Neurol Disord. 2010;3:29–42. doi: 10.1177/1756285609347123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leclercq D, Trunet S, Bertrand A, et al. Cerebral tumor or pseudotumor? Diagn Interv Imaging. 2014;95:906–916. doi: 10.1016/j.diii.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, John S, Joseph TP, Soloman T. Primary angiitis of the central nervous system: MRI features and clinical presentation. Australas Radiol. 2003;47:127–134. doi: 10.1046/j.0004-8461.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- 6.Huisman TA. Tumor-like lesions of the brain. Cancer Imaging. 2009;9 Spec No A:S10–S13. doi: 10.1102/1470-7330.2009.9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salvarani C, Brown RD, Jr, Hunder GG. Adult primary central nervous system vasculitis. Lancet. 2012;380:767–777. doi: 10.1016/S0140-6736(12)60069-5. [DOI] [PubMed] [Google Scholar]
- 8.Honda M, Koga M, Kanda T. [Treatment for central nervous system vasculitis] Brain Nerve. 2015;67:287–293. doi: 10.11477/mf.1416200133. [DOI] [PubMed] [Google Scholar]
- 9.Salvarani C, Brown RD, Jr, Calamia KT, et al. Primary central nervous system vasculitis: analysis of 101 patients. Ann Neurol. 2007;62:442–451. doi: 10.1002/ana.21226. [DOI] [PubMed] [Google Scholar]
- 10.Campi A, Benndorf G, Filippi M, Reganati P, Martinelli V, Terreni MR. Primary angiitis of the central nervous system: serial MRI of brain and spinal cord. Neuroradiology. 2001;43:599–607. doi: 10.1007/s002340100561. [DOI] [PubMed] [Google Scholar]
- 11.Calabrese LH, Mallek JA. Primary angiitis of the central nervous system. Report of 8 new cases, review of the literature, and proposal for diagnostic criteria. Medicine (Baltimore) 1988;67:20–39. doi: 10.1097/00005792-198801000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Duna GF, Calabrese LH. Limitations of invasive modalities in the diagnosis of primary angiitis of the central nervous system. J Rheumatol. 1995;22:662–667. [PubMed] [Google Scholar]
- 13.Birnbaum J, Hellmann DB. Primary angiitis of the central nervous system. Arch Neurol. 2009;66:704–709. doi: 10.1001/archneurol.2009.76. [DOI] [PubMed] [Google Scholar]
- 14.Molloy ES, Singhal AB, Calabrese LH. Tumour-like mass lesion: an under-recognised presentation of primary angiitis of the central nervous system. Ann Rheum Dis. 2008;67:1732–1735. doi: 10.1136/ard.2008.096800. [DOI] [PubMed] [Google Scholar]