Abstract
This study aims to evaluate the necessity of urgent neuroimaging for emergency admissions exhibiting symptomatology of profound hyponatremia. We retrospectively analyzed the medical records of all patients admitted to the emergency room of the University Hospital Münster from 2010 to 2014 with a serum sodium value < 125 mmol/L. From 52918 emergency admissions, 261 patients with profound hyponatremia were identified, of whom 140 (54%) had neurological symptoms. Unspecific weakness and confusion were the most prevalent of these symptoms (59%). Focal neurological signs [FNS] were present in 31% of cases and neuroimaging was performed in 68% (95/140) of symptomatic patients. Multiple logistic regression analysis identified FNS, seizures, altered consciousness and age as independent predictors for conducting neuroimaging (all p < 0.05). Significant pathological findings consistent with acute symptomatology were evident in 17 cases, all of whom had FNS. Recursive partitioning analyses confirmed FNS as the best predictor of neuroimaging pathology (p < 0.001). Absence of FNS had a negative predictive value of 100% [95% confidence interval: 93–100%] for excluding neuroimaging pathology. In conclusion, emergency patients with profound hyponatremia frequently show nonspecific-neurological symptoms and may undergo neuroimaging unnecessarily. The lack of FNS may serve as a valuable criterion for withholding neuroimaging until hyponatremia has been corrected.
Introduction
Hyponatremia is one of the most common disorders of body fluid and electrolyte balance encountered in clinical practice, and is primarily a disorder of water balance, with a relative excess of body water compared to total body solutes1–3. Profound hyponatremia (sodium <125 mmol/L) affects approximately 1% of all emergency admissions and is associated with more than twice the risk of in-hospital mortality1, 2, 4–6. It may cause cellular swelling with brain edema and subsequent dysfunction of the central nervous system1, 2, 7.
The clinical symptoms of hyponatremia vary according to the speed of onset and the severity of electrolyte imbalance. Symptoms can range from mild to severe, beginning with weakness/confusion and progressing to seizures and alteration of consciousness (e.g. somnolence, stupor, coma)2, 8.
Current management practices concerning initial neurological diagnostics and the use of neuroimaging in patients with profound hyponatremia remain unclear1. Previous clinical reviews and recent interdisciplinary European guidelines suggest immediate treatment with hypertonic saline in patients with profound hyponatremia and severe symptoms1, 2, 9. If neurological symptoms do not improve after saline infusion, these guidelines vaguely state that “…additional neurological investigations such as imaging may be helpful…”1. We noticed that there is a low threshold for conducting urgent neuroimaging in symptomatic emergency patients, possibly due to a lack of specific recommendations and a multitude of neurological symptoms, although hyponatremia could be a good explanation for the symptoms of such patients’.
Therefore, the aim of the present study was to identify a pattern of neurological symptoms in which urgent neuroimaging is required and to determine whether there is any symptomatology in which neuroimaging could be safely postponed.
Results
Study population
From 2010 to 2014, we identified 261 emergency department patients suffering from profound hypotonic hyponatremia (representing 0.49% of all emergency admissions during the study period). Following the exclusion of patients without neurological symptoms, the final dataset consisted of 140 patients with neurological symptoms consistent with symptomatic hyponatremia (53.6% of patients with profound hyponatremia) (Fig. 1). Patients were predominantly female (67.9%) and the median age was 66 years (range: 55–79 years). The mean serum sodium value was 120 mmol/L and mean plasma osmolality was 246 mmol/L (Table 1). Basic neurological assessment was documented in all patients by experienced emergency physicians. In addition, detailed neurological consultation reports were available for 75% of the patients with symptomatic hyponatremia.
Figure 1.
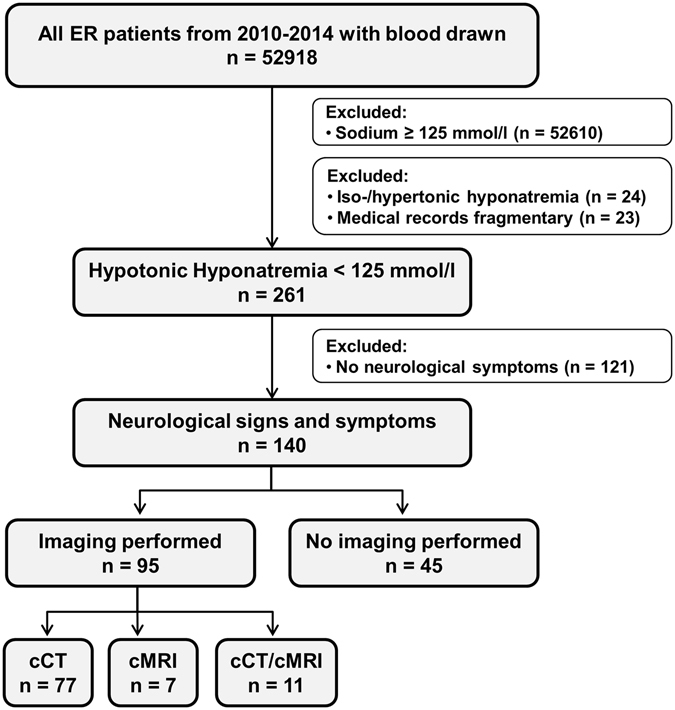
Participant flow chart of emergency admissions with profound symptomatic hyponatremia (University Hospital Münster, 2010–2014). ER, emergency room; cCT, cranial computed tomography; cMRI, cranial magnetic resonance tomography.
Table 1.
Characteristics of patients with severe hyponatremia and neurological symptoms.
| Variables | All patients | Neuroimaging performed | No neuroimaging performed | P-Value |
|---|---|---|---|---|
| (n = 140) | (n = 95) | (n = 45) | ||
| Age, in years | 66 (55–79) | 73 (57–81) | 61 (51–76) | 0.013 |
| Gender (female in %) | 95 (67.9) | 70 (73.7) | 25 (55.5) | 0.032 |
| Vital Signs | ||||
| Systolic blood pressure, mmHg | 130 (116–146) | 140 (126–160) | 120 (109–133) | 0.007 |
| Diastolic blood pressure, mmHg | 80 (70–90) | 80 (70–90) | 75 (60–84) | 0.115 |
| Heart frequency/min | 80 (67–90) | 84 (72–91) | 80 (66–90) | 0.254 |
| Laboratory Parameters, mmol/l | ||||
| Plasma sodium | 120 (116–122) | 121 (117–122) | 119 (116–121) | 0.134 |
| Plasma osmolality | 246 (236–257) | 245 (236–256) | 248 (237–261) | 0.512 |
| Urine sodium | 47 (17–90) | 62 (20–95) | 37 (15–70) | 0.032 |
| Urine osmolality | 294 (200–381) | 321 (223–430) | 252 (170–344) | 0.017 |
| Etiology of Hyponatremia, in % | ||||
| Diuretics | 23.6 | 23.1 | 24.4 | 0.867 |
| SIAD | 31.4 | 29.4 | 33.3 | 0.956 |
| Cortisol deficiency | 5.7 | 4.2 | 8.9 | 0.120 |
| Hypovolemia | 20.0 | 17.9 | 26.7 | 0.232 |
| Hypervolemia | 6.4 | 2.1 | 15.5 | 0.002 |
| Primary Polydipsia | 7.9 | 8.4 | 6.7 | 0.880 |
| Others | 1.4 | 1.1 | 2.2 | 0.586 |
| Neurological symptoms resolved upon hyponatremia treatment | 124 (88.6) | 79/95 (83.2) | 45/45 (100.0) | 0.003 |
| pathological neuroimaging* | 1/17 (5.9) | |||
| non-pathological neuroimaging# | 78/78 (100) | |||
| Door-to-imaging-time, minutes | 123 (61–238) | |||
Median and interquartile range reported for continuous variables and frequency; percentage reported for categorical variables. Differences between patients with/without neuroimaging were calculated using the Mann-Whitney U test for continuous variables and the Chi-square test for categorical variables. Two-sided p values < 0.05 were considered statistically significant. SIAD, syndrome of inappropriate antidiuresis (SIAD). *Pathological neuroimaging: neuroimaging showed findings related to acute symptomatology, e.g., ischemic stroke. #Non-pathological neuroimaging: neuroimaging was unremarkable (no incidental/pathological finding) or showed incidental findings not related to acute symptomatology, e.g., general cerebral atrophy, old ischemic stroke.
Neurological symptoms in hyponatremia
Consistent with the literature, nonspecific weakness and confusion were the most prevalent symptoms (59%), followed by focal neurological signs (FNS, 31%) including cranial nerve palsy, hemiparesis, gaze palsy and hemiataxia (Fig. 2A).
Figure 2.
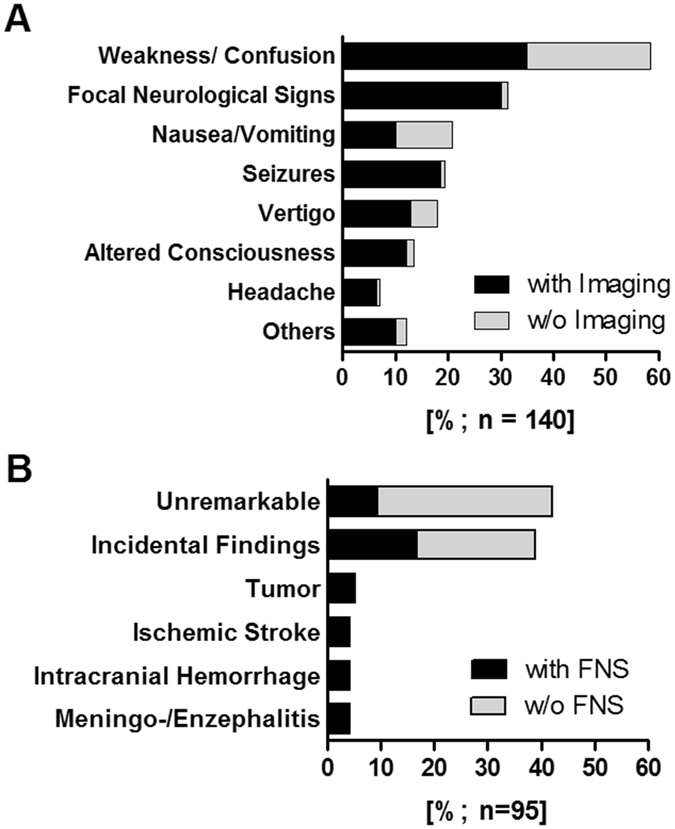
Neurological symptoms and imaging findings. (A) Prevalence of neurological symptoms (focal neurological signs [FNS] and signs of global brain dysfunction) in all patients with symptomatic hypotonic hyponatremia in our cohort (n = 140). (B) Results of neuroimaging studies (cCT and/or cMRI) in 95 of 140 patients (67.8%). Incidental findings (n = 38): imaging findings, which were not related to acute symptoms (multiple findings were permitted): general cerebral atrophy (n = 20), age-related periventricular white matter changes (n = 16), old ischemic stroke (n = 15), arteriosclerosis (n = 15), calcification of pineal gland/choroid plexus (n = 8), signs of former neurosurgical interventions (n = 3), signs of old fracture (n = 2).
Predictors for conducting neuroimaging
Neuroimaging was performed frequently in patients with severe symptoms (FNS, seizures and alteration of consciousness), whereas imaging was less frequently requested in other symptoms (Fig. 2A). Urgent neuroimaging studies (cCT and/or cMRI) were performed in 67.9% of patients (95/140). The majority of these (92.6%) were cCT scans performed at a median of 2 h after ER admission (Fig. 1). Using multivariate Cox regression analysis, we identified age and the presence of certain neurological symptoms (seizures, alteration of consciousness and FNS) as predictors for conducting neuroimaging studies (all p < 0.05) (Table 2).
Table 2.
Predictors for conducting neuroimaging (cCT/cMRI) using logistic regression analysis.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Plasma sodium level | 1.05 (0.98–1.13) | 0.161 | ||
| Gender (female) | 2.15 (1.02–4.51) | 0.042 | 2.49 (0.90–6.93) | 0.079 |
| Age | 1.31 (1.04–1.064) | 0.018 | 1.64 (1.18–2.28) | 0.003 |
| Neurological symptoms | ||||
| Weakness/Confusion | 0.37 (0.17–0.81) | 0.013 | 0.59 (0.21–1.69) | 0.325 |
| Nausea/Vomiting | 0.36 (0.15–0.83) | 0.016 | 0.67 (0.22–2.05) | 0.480 |
| Headache | 4.14 (0.50–34.12) | 0.187 | ||
| Vertigo | 1.30 (0.50–3.38) | 0.587 | ||
| Seizures | 17.0 (2.2–129.40) | 0.006 | 71.7 (7.33–700.6) | <0.001 |
| Altered consciousness | 4.80 (1.06–21.72) | 0.042 | 6.71 (1.14–39.46) | 0.035 |
| Focal neurological signs | 17.43 (4.0–76.11) | 0.001 | 32.95 (6.59–164.7) | <0.001 |
Odds ratios (OR), 95% confidence intervals (CI), and p-values were calculated using logistic regression analysis (backward elimination). Variables were selected a priori based upon theoretical considerations and existing literature. Factors statistically significant at the 10% level in our univariate analysis were then included in our multivariate model. Two-sided p values < 0.05 were considered statistically significant in the multivariate model.
Imaging findings in hyponatremia and predictors for neuroimaging pathology
Neuroimaging was unremarkable, or showed incidental findings not related to acute symptomatology, in 82.1% of all patients. In line with this, the symptoms of all these patients resolved upon hyponatremia treatment (Table 1). However, in 17 out of 95 patients (17.9%), neuroimaging revealed brain tumors (5.3%), ischemic stroke (4.2%), intracranial hemorrhage (4.2%) and meningo-/encephalitis (4.2%) (Fig. 2B). Nearly all of these patients failed to respond to hyponatremia treatment (94.1%; Table 1).
All 17 of these patients showed acute FNS (Fig. 2B; Supplementary Table S2), which highlighted the role of FNS as a strong predictor for neuroimaging pathology. Additional logistic regression analyses did not reveal any other variable which could predict pathological neuroimaging (Supplementary Table S1). Furthermore, regression tree analysis (recursive partitioning) confirmed FNS as the best predictor for neuroimaging pathology (p < 0.001; Supplementary Fig. S1). The presence of FNS predicted relevant neuroimaging findings with a sensitivity of 100% (95% confidence interval (CI): 79–100%), and a specificity of 68% (95% CI: 63–68%) (Chi-square test: p < 0.0001). The positive predictive value was 41% (95% CI: 32–41%). Accordingly, the absence of FNS had a negative predictive value of 100% (95% CI: 93–100%) for excluding significant neuroimaging pathology.
The number needed to test (NNT) for conducting neuroimaging with the aim of revealing pathological findings in patients with symptomatic hyponatremia was 5.6 in our original retrospective cohort. In other words, one out of six patients scanned had a pathological neuroimaging finding. Keeping in mind that the absence of FNS might serve as a criterion for withholding neuroimaging, the NNT for conducting neuroimaging could have been improved to 2.6 if scanning had been limited to the patients with FNS only.
Discussion
This retrospective study shows that in emergency admissions with profound hyponatremia, nonspecific-neurological symptoms are frequent and account for potentially unnecessary neuroimaging. A lack of specific recommendations in the current guidelines means that clinicians feel uncertain about the need for and timing of neuroimaging for symptomatic hyponatremia1, 2, 10–12. Since a high number (17.9%) of patients with profound hyponatremia in the present study showed neuroimaging abnormalities, we suggest careful consideration of neuroimaging for this group of patients (Fig. 2).
Importantly, we have identified focal neurological signs (FNS) as the best predictor for neuroimaging pathology with a negative predictive value of 100%. The absence of FNS might therefore serve as a criterion to rule out neuroimaging until hyponatremia has been corrected.
Our data reveal that the majority of patients with profound hyponatremia show non-specific symptoms such as weakness/confusion or nausea. However, only FNS was clearly associated with pathological neuroimaging findings, indicating that in patients with hyponatremia and FNS imaging is indispensable. This finding might be explained by differences in the pathophysiology of the signs and symptoms observed in such clinical situations. Hypotonic hyponatremia represents an excess of water relative to its solutes in the extracellular compartment. Because water can transfer freely from the extracellular to the intracellular compartment, hypotonic hyponatremia can lead to cellular swelling followed by brain edema. As this cellular swelling is not limited to one focal cerebral region, but affects the whole brain, it can result in generalized central nervous dysfunction1, 2, 7. In contrast to this, the presence of FNS indicates focal brain disturbances caused by various types of pathology (e.g. ischemic stroke)13–16. Accordingly virtually all of our patients with FNS and neuroimaging pathology did not respond to hyponatremia treatment.
Doctors, particularly in emergency settings require an easy-to-follow decision tree11, 12. Taking the absence of FNS as a simple rule for withholding neuroimaging in patients with profound and symptomatic hyponatremia would allow clinicians to focus on treating the hyponatremia1. However, patients with FNS should receive neuroimaging without delay, since in these patients an alternative etiology of symptoms other than hyponatremia is to be expected. If this rule had been applied in our cohort, only 44 imaging studies would have been performed instead of 95, and no relevant cerebral pathology would have been missed. Avoidance of unnecessary imaging would reduce radiation exposure, costs, and burden upon the emergency room and radiology resources. Potentially, we might have refused, or at least delayed, necessary neuroimaging in patients presenting without FNS, although this was not the case in our study. Of course, clinicians should consider the clinical context and may decide to proceed with direct imaging, or other diagnostic pathways as certain neurological conditions, including brain tumors, can have a prolonged course that may not lead to FNS evident on routine bedside examination. Furthermore, we recommend that neuroimaging should be carried out according to current guidelines in patients presenting with traumatic brain injury independent from hyponatremia.
Consistent with the literature, hyponatremia is frequent in patients suffering from any type of brain injury (e.g. traumatic brain injury or bleeding)17, 18. However, the pathophysiology of hyponatremia following brain injury is not completely understood, but is known to develop rapidly after brain injury and may often be caused by a sudden release of vasopressin [syndrome of inappropriate antidiuresis (SIAD)] and to a lesser extent by adrenocorticotropic hormone insufficiency or cerebral salt wasting syndrome 19–21. Besides acute brain injury, chronic pain can also be a strong stimulus for vasopressin1. We could therefore speculate that our patients with neuroimaging pathology developed hyponatremia secondary to the cerebral process encountered.
Our study has several limitations. Firstly, this was a retrospective, single-center study and our final data set only involved 140 patients. However, we collected data over a five year period and we were able to collate a homogenous and complete data set featuring a significant number of detailed neurological reports. Secondly, caution should be exercised when extrapolating our findings from the emergency room to the in-patient ward, although we expect the pathophysiological concept of FNS versus generalized brain edema to be valid in almost any clinical scenario. Thirdly, 45 of the 140 patients with symptomatic hyponatremia did not undergo neuroimaging, so we cannot exclude preselection bias. However, extensive evaluation of electronic chart records and discharge letters revealed that acute symptomatology resolved in all of these patients upon treatment for hyponatremia, almost excluding a causative role of any other (undiscovered) neurological diseases in this group. Fourthly, most imaging studies were performed despite the knowledge of profound hyponatremia; however in seven out of 95 patients, neuroimaging was performed immediately after admission before laboratory results became available. Fifthly, although we intended to only include patients with acute hyponatremia (duration <48 h), we cannot exclude that we may have included single cases with chronic hyponatremia (duration >48 h). However, the acute onset of symptoms was ascertained in all patients. Furthermore, all patients responded immediately and were provided standard treatment for acute hyponatremia. Finally, a significant difference in urine sodium and urine osmolality distribution in patients receiving, and not receiving neuroimaging was revealed. This was possibly driven by the different distribution of etiology in hyponatremia (e.g., significantly more cases of hypervolemic hyponatremia in the non-neuroimaging group).
Collectively, our data indicate that neurological symptoms in emergency patients with profound hyponatremia are relatively common and can lead to unnecessary neuroimaging. With due care, a lack of focal neurological signs might serve as a criterion for safely withholding neuroimaging until hyponatremia has been corrected. To strengthen these recommendations from our single-center study, further research is needed in future.
Methods
Study design and patients
This retrospective study was performed in the multidisciplinary emergency department of the University Hospital Münster, in which approximately 15.000 (mostly internal medicine and neurological) emergency room patients are treated each year. This study was approved by the Ethics Board of the Westphalian Wilhelm-University of Münster and the Medical Council of Westphalia-Lippe [Germany] and was performed in accordance with the Helsinki declaration. As approved by the Ethics Board, informed patient consent was not required for this study because of its retrospective design. Data from all patients ≥18 years of age who had a routine blood test in the emergency department between 2010 and 2014 were retrieved from the hospital information system. Next, patients with a serum sodium level ≥125 mmol/L were excluded (n = 52610). As we were interested in hypotonic hyponatremia, and because this is also what guidelines tend to focus upon, we excluded all cases with hypertonic or isotonic hyponatremia (serum osmolality ≥280 mmol/L or serum glucose ≥200 mg/dL; n = 24)1, 2 and those with incomplete medical records (n = 23). Clinical and laboratory data as well as neuroimaging reports (cranial computed tomography [cCT] and magnetic resonance imaging [cMRI]) were extracted from electronic medical records. A flow chart showing the inclusion and exclusion criteria for our patients is given in Fig. 1.
Neurological evaluation and definitions
Electronic chart records and discharge letters, including medical history, patient complaints, medication, detailed physical examination, laboratory parameters, diagnostics including imaging studies, and the time of hospital stay (cumulative period in the emergency department and hospital) were screened for neurological symptoms by the study team. Acute neurological symptoms preceding hospital admission (e.g., seizures, vomiting) and those presenting upon admission, were taken into account (multiple symptoms were permitted). We differentiated between focal neurological signs (FNS; e.g., cranial nerve palsy, hemiparesis, gaze palsy and hemiataxia) and signs of global brain dysfunction (e.g., alteration of consciousness, nausea/vomiting, weakness/confusion). Cases were thoroughly reviewed by at least two independent board certified investigators with longstanding clinical expertise in emergency medicine (AB, RD or PK) and neurological symptoms were only recorded, if a new onset of symptomatology could be ascertained, and/or symptoms were not already known for longer times to include only cases of acute hyponatremia. None of our patients had advanced underlying dementia, as ascertained by chart review.
Neuroimaging
cCT and cMRI were undertaken upon request by the treating physician and performed according to standardized radiology department procedures. Imaging reports were retrospectively classified as follows: unremarkable (no incidental/pathological finding), incidental (incidental finding not related to acute symptoms, e.g., general cerebral atrophy or old ischemic stroke) or pathological (findings related to acute symptomatology, e.g., ischemic stroke).
Statistical analysis
Data are presented as absolute values, percentages or medians with corresponding 25th and 75th percentiles (interquartile range; IQR) or 95% confidence intervals (CI), where stated. Differences between groups of patients were calculated using the Mann-Whitney U test for continuous variables and the Chi-square test for categorical variables. Predictors for conducting neuroimaging, and for the presence of neuroimaging pathology, were identified by logistic regression. Variables were selected a priori and were based upon theoretical considerations and existing literature2. Variables found to be statistically significant at the 10% level in our univariate analysis were then included in a multivariate model. Two-sided p values < 0.05 were considered statistically significant. In an exploratory approach, all variables incorporated in the multivariate cox logistic regression model were concurrently subjected to a decision tree analysis to recursively identify the best predictors and patient subgroups with different probabilities for neuroimaging. Data analysis was performed using IBM SPSS Statistics 22.0 (IBM Corp., Armonk, NY, USA). Contingency table-derived data and likelihood ratios were calculated using the StatPages website22. Figures were prepared using GraphPad Prism 5.02 (GraphPad Software, La Jolla, CA, USA).
Electronic supplementary material
Author Contributions
A.B. designed the study, analyzed the data and wrote the manuscript; R.D. analyzed and interpreted data and helped to write the manuscript; H.W. and W.S. interpreted data and helped to write the manuscript. P.B. provided access to patient medical records and analyzed data; P.K. and H.P. designed the study, analyzed the results and wrote the manuscript. All authors edited the scientific contents of the manuscript and gave approval of the final version prior to submission.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Philipp Kümpers and Hermann Pavenstädt contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-02030-6
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Spasovski G, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Intensive care medicine. 2014;40:320–331. doi: 10.1007/s00134-014-3210-2. [DOI] [PubMed] [Google Scholar]
- 2.Verbalis JG, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. The American journal of medicine. 2013;126:S1–42. doi: 10.1016/j.amjmed.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt BMW. The most frequent electrolyte disorders in the emergency department. Der Internist. 2015;56:753–759. doi: 10.1007/s00108-015-3670-7. [DOI] [PubMed] [Google Scholar]
- 4.Nigro N, et al. Symptoms and characteristics of individuals with profound hyponatremia: a prospective multicenter observational study. Journal of the American Geriatrics Society. 2015;63:470–475. doi: 10.1111/jgs.13325. [DOI] [PubMed] [Google Scholar]
- 5.Patel GP, Balk RA. Recognition and treatment of hyponatremia in acutely ill hospitalized patients. Clinical therapeutics. 2007;29:211–229. doi: 10.1016/j.clinthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Rudnay M, Lazurova I. [Prevalence of hyponatremia in patients on department of internal medicine] Vnitrni lekarstvi. 2013;59:876–879. [PubMed] [Google Scholar]
- 7.Adrogue HJ, Madias NE. Hyponatremia. The New England journal of medicine. 2000;342:1581–1589. doi: 10.1056/NEJM200005253422107. [DOI] [PubMed] [Google Scholar]
- 8.Haas CSH. Differenzialdiagnose und Therapie. Der Internist. 2014;55:1427–1442. doi: 10.1007/s00108-014-3609-4. [DOI] [PubMed] [Google Scholar]
- 9.Adrogue HJ, Madias NE. Diagnosis and treatment of hyponatremia. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2014;64:681–684. doi: 10.1053/j.ajkd.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. The American journal of medicine. 2006;119:S30–35. doi: 10.1016/j.amjmed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Fenske W, Maier SK, Blechschmidt A, Allolio B, Stork S. Utility and limitations of the traditional diagnostic approach to hyponatremia: a diagnostic study. The American journal of medicine. 2010;123:652–657. doi: 10.1016/j.amjmed.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Pfennig CL, Slovis CM. Sodium disorders in the emergency department: a review of hyponatremia and hypernatremia. Emergency medicine practice. 2012;14:1–26. [PubMed] [Google Scholar]
- 13.Wippold II F. Focal Neurologic Deficit. American College of Radiology. 2008;29:1998–2000. [PMC free article] [PubMed] [Google Scholar]
- 14.Daroff, R. B., Fenichel, G., Jankovic, J. & Maziotta, J. C. Bradley’s Neurology in Clinical Practice. Elsevier6 (2012).
- 15.Headache Classification Committee of the International Headache, S The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia: an international journal of headache. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Aranda F, et al. Pseudomigraine with temporary neurological symptoms and lymphocytic pleocytosis. A report of 50 cases. Brain: a journal of neurology. 1997;120(Pt 7):1105–1113. doi: 10.1093/brain/120.7.1105. [DOI] [PubMed] [Google Scholar]
- 17.Moro N, et al. Hyponatremia in patients with traumatic brain injury: incidence, mechanism, and response to sodium supplementation or retention therapy with hydrocortisone. Surgical neurology. 2007;68:387–393. doi: 10.1016/j.surneu.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 18.Sherlock M, et al. The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage. Clinical endocrinology. 2006;64:250–254. doi: 10.1111/j.1365-2265.2006.02432.x. [DOI] [PubMed] [Google Scholar]
- 19.Kleindienst A, Hannon MJ, Buchfelder M, Verbalis JG. Hyponatremia in Neurotrauma: The Role of Vasopressin. Journal of neurotrauma. 2016;33:615–624. doi: 10.1089/neu.2015.3981. [DOI] [PubMed] [Google Scholar]
- 20.Rabinstein AA, Wijdicks EF. Hyponatremia in critically ill neurological patients. The neurologist. 2003;9:290–300. doi: 10.1097/01.nrl.0000095258.07720.89. [DOI] [PubMed] [Google Scholar]
- 21.Kleindienst A, et al. Following brain trauma, copeptin, a stable peptide derived from the AVP precusor, does not reflect osmoregulation but correlates with injury severity. Acta neurochirurgica. Supplement. 2010;106:221–224. doi: 10.1007/978-3-211-98811-4_41. [DOI] [PubMed] [Google Scholar]
- 22.Pezzullo, C. Statpages: 2-way Contingency Table Analysis, http://www.statpages.info/ctab2x2.html (date of access: 30/03/2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


