Abstract
There is large variation in seed mass within P. oreoselinum (L.) Moench selected for the present study from two contrasting habitats: roadside and oak forest. Effect of seed position within a plant and of soil nutrients on seed mass, germination and seedlings growth were studied. Within an individual plant, seed mass decreased with umbel order and seeds from the central umbellet of the umbel were lighter than those from the outer edge, suggesting that variation in seed mass within an individual plant was due to the position effect. There was a significant relationship between seed mass and total germination. Covariate analysis showed the differences between sites in seed macronutrient contents were caused by respective differences in seed mass and soil macronutrients. This indicates substantial variation in the amount of reserves initially available for seedling growth. In conclusion, phenotypically-based variation in seed mass may arise from soil conditions, maternal traits or combination of the two. High variability in seed mass of P. oreoselinum favours its widespread geographic distribution. These results suggest that with respect to germination characteristics large seeds from primary order have a competitive advantage over small seeds produced on secondary umbels because they have higher overall germination.
Introduction
Seed size (mass) can affect germination time, germination percentage, dispersal, seed water relation, as well as seedling establishment, growth, survival, and thus it has important ecological consequences1–5. Seed mass within an individual species was considered for a long time to be relatively constant6. However, various studies have demonstrated that seed size may greaten vary in a given species among sites, among plants within given site, and even an individual plant7. The main reasons of seed mass variations are paternal genetic effect, timing of flowering and fecundation, brood size, sibling rivalry and position effect of seeds within a plant, or in inflorescence, and the position of a seed within fruit8.
Variation in seed mass has often been correlated with environmental factors7. The contents of various nutrients in plants vary with plant species and varieties and depend on the total nutrients supply in soils and on factors controlling their availability to plants. However, studies addressing the influence of environmental factors on seed mass in natural sites are rare. An increase in the macronutrient concentration in surrounding environment has often led to the production of seeds that are heavier and have greater quantities and concentrations of N (nitrogen) and P (phosphorus)9. This plastic response can have important fitness consequences for developing seedlings. The positive effects of seed size are often impossible to separate from those of seed mineral content, especially in wild habitats where variations in plant characteristics are great and not easily controlled10.
Variation in seedling size may by caused by differences in initial seed mass, microsite characteristics and/or genotypic variation6. For example, it is generally accepted that seedling size is usually directly related with food reserves and energy content of seeds6, 7. Since the inflorescence of the Apiaceae family consists of a series of sequentially formed umbels producing seeds, the position of a seed or fruit on a plant can affect seed mass, morphology, germination and dormancy characteristics11–14. Positional effect on seed mass was reported widely for many species. For example, Ojala15 observed that seed mass and germination percentage of Angelica archangelica were higher in seeds on the primary umbel than in those on secondary, tertiary, and quaternary umbels. Thomas16 and Thomas et al.17, 18 found reduced germination of Petroselinium crispum, Daucus carota and Apium graveolens seeds produced on secondary, tertiary, and quaternary umbels compared with those on primary umbels. Gray and Steckel19 found that umbel order affected germination of D. carota seeds, but on the other hand, in Heracleum mantegazzianum 13 and in Angelica acutiloba 14 germination percentage of the seeds obtained from different umbel orders and umbellet positions did not differ significantly.
Effects of seed position within a plant and of soil nutrients on seed mass, germination and seedlings growth were studied. Seeds harvested from primary and secondary umbels were examined. The objectives of this study were the following (1) to analyze the effect of the umbel order and umbellet position on seed mass and seed germination; (2) to quantify the degree of seed mass variation in P. oreoselinum among populations and plants within populations, and within individuals plants; (3) to determine possible relation between seed macronutrient content and respective soil macronutrients content; and (4) to examine the relationship between seed mass and seed macronutrient content.
Results
Soil nutrients
The Student’s t-test revealed statistically significant differences in soil nutrient contents between the studied habitats. Soils at the roadside contained lower concentrations of N (Student’s t-test, p-value = 0.0025), P (Student’s t-test, p-value = 0.0309), and K (Student’s t-test, p-value = 0.0445) than those in the forest (Table 1) probably reflecting their lower content in the parent rock.
Table 1.
Site characteristics and soil macronutrient (N, P or K) content (total N%, others mg/100 g).
| Site | Coordinates | Habitat | N (%) | P (mg/100 g) | K (mg/100 g) |
|---|---|---|---|---|---|
| Lipnica | 51.913690°N | roadside | 0.9 ± 0.1b | 7.32 ± 1.76b | 4.2 ± 0.2b |
| 18.868497°E | |||||
| Wilczków | 51.928406°N | forest | 2.6 ± 0.1a | 9.29 ± 1.23a | 5.4 ± 0.3a |
| 18.897422°N |
Statistical comparisons (Student’s t-test) within a column only; different letters beside means denote significant differences between habitats at p < 0.05. Data are means (±SD) from five samples at each habitat.
Seed mass
The mean mass of 25 seeds, pooled across plants and habitats, was 134 ± 19 mg, giving the mean mass of a single seed of 5.36 mg. Seed mass was variable: 1.7 fold between habitats, 1.6–1.9 fold within habitats, 1.4–1.6 fold within individuals, and 1.2–1.4 fold within umbels. Coefficients of variation between habitats averaged 14% (range 13–15) (Table 2). There was great difference in mean seed mass between roadside and forest (3.7 ± 0.3 mg and 6.9 ± 0.2 mg, respectively). The mean seed mass within a plant decreased significantly (p-value = 0.0241) from primary to secondary umbels in both habitats. Seeds produced by the primary umbels were significantly (p-value = 0.0217) heavier (5.8 ± 0.3 mg) than those from the secondary ones (4.8 ± 0.4 mg) (Fig. 1a). Seed mass was also very strongly influenced by the position within an umbel: the seeds from the outer edge of the umbel were heavier (5.9 ± 0.4 mg) than those from the center of the umbel (4.7 ± 0.3 mg) (Fig. 1b). Nested analysis of variance indicated that the percentage of variation in seed mass accounted for by plants and by umbels within plants was 25% and 21%, respectively (Table 3).
Table 2.
Variations in seed mass within sites, individuals, and umbel order.
| Habitat | Within habitat | Within plants | Within primary order | Within secondary order | ||
|---|---|---|---|---|---|---|
| Range in seed mass (mg) | Magnitude in individual seed mass variation (heaviest/lightest) | CV (%) | Mean seed mass variation (heaviest/lightest) | Mean seed mass variation (heaviest/lightest) | Mean seed mass variation (heaviest/lightest) | |
| Roadside | 3.5–6.5 | 1.9 | 15.4 | 1.6 | 1.2 | 1.3 |
| Forest | 4.4–7.2 | 1.6 | 13.0 | 1.4 | 1.4 | 1.2 |
| Total | 4.0–6.9 | 1.7 | 14.2 | |||
For the determination of average seed mass four replicates of 25 seeds each were weighed.
Figure 1.
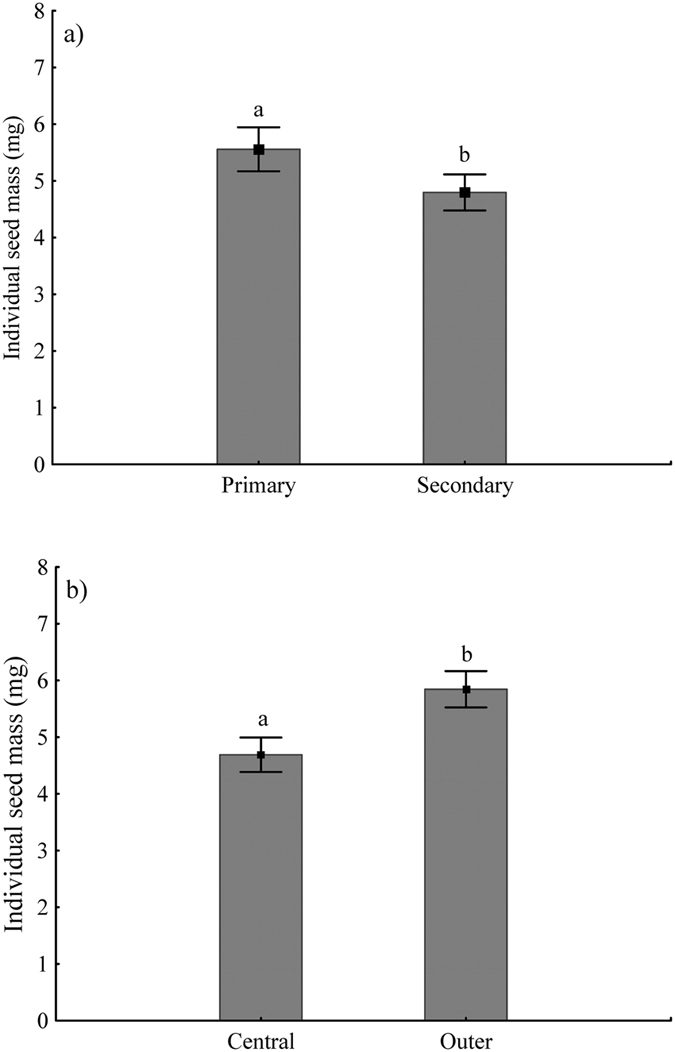
Differences in mean seed mass (±SD) between (a) umbel orders (primary and secondary) and (b) umbellet position (outer and central) in Peucedanum oreoselinum. Overall comparison between means was performed by Student’s t-test; different letters indicate significant (p < 0.05) differences between means.
Table 3.
Results of three-level nested analysis of variance testing for effect of habitat, individuals, and umbels on seed mass in Peucedanum oreoselinum.
| Source of variation | df | MS | Variance component | Proportion of variance explained |
|---|---|---|---|---|
| Habitat | 1 | 14.67*** | 0.034 | 29.1 |
| Plants within habitat | 126 | 0.56** | 0.012 | 25.6 |
| Umbels within plants | 967 | 0.14** | 0.019 | 21.2 |
| Error (within umbels) | 8872 | 0.03*** | 0.021 | 24.1 |
| Total | 9978 | 0.086 | 100 |
***p < 0.001, **p < 0.001 (Tukey’s test).
Effect of seed source on seed germination
Seeds collected from the forest plants germinated more rapidly than those from the roadside. In addition, the total percentage of germination differed significantly (p < 0.006) between habitats. A significantly greater (p-value = 0.0231) total percentage germination was observed for those seeds from forest compared to seeds from roadside (Table 4). ANOVAs (log-transformed data) revealed that umbel order, umbellet position, habitat, and their interaction were all of significant importance to both seed mass and percentage of germination, but there was no significant interaction effect on rate of germination (Table 5).
Table 4.
Seed mass, germination percentages and rate of germination (Timson’s Index) of seeds from differed umbel (primary and secondary) and umbellet position (outer and central).
| Habitat | Umbel order | Umbellet position | Individual seed mass (mg)* | Final germination (%)* | Rate of germination (%)* |
|---|---|---|---|---|---|
| Roadside | Primary | Outer | 5.6 ± 0.3a | 81 ± 3a | 58 ± 3a |
| Central | 4.6 ± 0.2b | 64 ± 4b | 56 ± 2a | ||
| Secondary | Outer | 4.9 ± 0.2ab | 66 ± 3b | 55 ± 4a | |
| Central | 3.7 ± 0.3c | 53 ± 5c | 52 ± 3a | ||
| Forest | Primary | Outer | 6.9 ± 0.2a | 86 ± 3a | 66 ± 6a |
| Central | 5.1 ± 0.5c | 69 ± 5b | 65 ± 4a | ||
| Secondary | Outer | 5.9 ± 0.5b | 79 ± 6b | 67 ± 4a | |
| Central | 5.0 ± 0.4c | 67 ± 2c | 64 ± 5a |
Germination data (mean ± SD) are from laboratory trials at 22 °C/10 °C with 14/10 h light treatment.
*The values followed by the same letter within a column do not differ significantly by ANOVA followed by Tukey’s test at p < 0.05 in the same habitat.
Table 5.
Tree-way ANOVA of effect of umbel order (primary vs secondary), umbellet position (central vs outer) in the umbel, habitat (roadside vs forest), and their interaction on seed mass, percentage of germination and rate of germination of Peucedanum oreoselinum.
| Dependent variable | Factor | df | MS | F-value | P-value |
|---|---|---|---|---|---|
| Seed mass | Umbel order (UO) | 1 | 0.76 | 12.34 | p = 0.0186 |
| Umbellet position (UP) | 1 | 0.85 | 10.56 | p = 0.0348 | |
| Habitat (H) | 1 | 0.64 | 7.98 | p = 0.0056 | |
| UO × UP | 1 | 1.87 | 5.78 | p = 0.0078 | |
| UO × H | 1 | 0.32 | 11.34 | p = 0.0095 | |
| UP × H | 1 | 0.36 | 7.94 | p = 0.018 | |
| UO × UP × H | 1 | 7.78 | 10.43 | p = 0.0134 | |
| Percentage germination | Umbel order (UO) | 1 | 0.34 | 1.23 | p = 0.1266 |
| Umbellet position (UP) | 1 | 1.28 | 1.07 | p = 0.1935 | |
| Habitat (H) | 1 | 0.72 | 4.32 | p = 0.0315 | |
| UO × UP | 1 | 1.68 | 12.34 | p = 0.0278 | |
| UO × H | 1 | 0.98 | 4.56 | p = 0.6546 | |
| UP × H | 1 | 1.86 | 5.99 | p = 0.3073 | |
| UO × UP × H | 1 | 3.34 | 9.67 | p = 0.0348 | |
| Rate of germination | Umbel order (UO) | 1 | 0.57 | 36.93 | p = 0.2605 |
| Umbellet position (UP) | 1 | 1.56 | 34.12 | p = 0.3225 | |
| Habitat (H) | 1 | 0.27 | 3.81 | p = 0.0296 | |
| UO × UP | 1 | 2.48 | 17.98 | p = 0.0757 | |
| UO × H | 1 | 0.65 | 2.89 | p = 0.1427 | |
| UP × H | 1 | 1.26 | 1.89 | p = 0.3038 | |
| UO × UP × H | 1 | 3.65 | 11.45 | p = 0.0412 |
Seedling growth
Overall, both shoot biomass (hereafter referred to as SB) and root biomass (hereafter referred to as RB) of the seedling from the seeds on the primary umbel were significantly higher than those of the seedlings from seeds on the secondary umbels (Table 6). These results suggests that the seedlings from seeds on the primary umbels were significantly more vigorous than those from seeds on the secondary umbels. Individual effects of umbel order, habitat, and their interaction on seedlings traits were significant. However, umbellet positions and interaction between umbel order and umbellet position had no effect on seedlings traits (Table 7). These results suggests that the seedlings from seeds from the primary umbels were more vigorous than those from the secondary umbels.
Table 6.
Shoot biomass (SB), root biomass (RB) and the percentage of biomass allocated to root (BAR) of seedlings of Peucedanum oreoselinum.
| Habitat | Umbel order | Umbellet position | SB (mg plant−1) | RB (mg plant−1) | BAR (%) |
|---|---|---|---|---|---|
| Roadside | Primary | Outer | 13.1 ± 0.7a | 4.3 ± 0.3a | 24.7 ± 0.5a |
| Central | 12.4 ± 0.5a | 4.1 ± 0.2a | 24.8 ± 0.6a | ||
| Secondary | Outer | 9.0 ± 0.4b | 2.7 ± 0.5b | 23.1 ± 0.9b | |
| Central | 8.8 ± 0.3b | 2.6 ± 0.4b | 22.8 ± 0.8b | ||
| Forest | Primary | Outer | 16.1 ± 0.6a | 5.9 ± 0.4a | 26.8 ± 0.6a |
| Central | 15.7 ± 0.5a | 5.8 ± 0.5a | 27.0 ± 0.7a | ||
| Secondary | Outer | 13.1 ± 0.5b | 3.0 ± 0.2b | 18.6 ± 0.3b | |
| Central | 13.0 ± 0.3b | 2.9 ± 0.1b | 18.2 ± 0.4b |
Values are means ± SD.
*The values followed by the same letter within a column do not differ significantly by ANOVA followed by Tukey’s test at p < 0.05 in the same habitat.
Table 7.
Tree-way ANOVA of effect of umbel order (primary vs secondary), umbellet position (central vs outer) in the umbel, habitat (roadside vs forest), and their interaction on seedling traits (shoot biomass [SB], root biomass [RB], and the percentage of biomass allocated to root [(BAR]) of Peucedanum oreoselinum.
| Dependent variable | Factor | df | MS | F-value | p-value |
|---|---|---|---|---|---|
| SB (mg plant−1) | Umbel order (UO) | 1 | 1.68 | 14.56 | p = 0.0255 |
| Umbellet position (UP) | 1 | 1.98 | 18.56 | p = 0.1267 | |
| Habitat (H) | 1 | 1.46 | 5.45 | p = 0.0079 | |
| UO × UP | 1 | 1.87 | 15.78 | p = 0.0786 | |
| UO × H | 1 | 1.23 | 2.56 | P = 0.0353 | |
| UP × H | 1 | 8.70 | 11.56 | p = 0.0155 | |
| UO × UP × H | 1 | 7.78 | 10.43 | p = 0.0134 | |
| RB (mg plant−1) | Umbel order (UO) | 1 | 1.65 | 1.76 | p = 0.0052 |
| Umbellet position (UP) | 1 | 3.08 | 12.02 | p = 0.2344 | |
| Habitat (H) | 1 | 1.34 | 3.98 | p = 0.0046 | |
| UO × UP | 1 | 1.87 | 15.34 | p = 0.0978 | |
| UO × H | 1 | 1.59 | 6.86 | p = 0.0042 | |
| UP × H | 1 | 8.60 | 5.99 | p = 0.0307 | |
| UO × UP × H | 1 | 3.34 | 9.67 | p = 0.0348 | |
| BAR (%) | Umbel order (UO) | 1 | 1.23 | 12.54 | p = 0.0077 |
| Umbellet position (UP) | 1 | 3.08 | 12.02 | p = 0.2347 | |
| Habitat (H) | 1 | 1.51 | 4.32 | p = 0.0026 | |
| UO × UP | 1 | 2.48 | 17.98 | p = 0.0657 | |
| UO × H | 1 | 1.63 | 3.27 | p = 0.0344 | |
| UP × H | 1 | 8.60 | 5.99 | p = 0.0307 | |
| UO × UP × H | 1 | 3.65 | 11.45 | p = 0.0412 |
Seed nutrient analyses
For all seed macronutrients, there were differences between habitats. The mean N, P, or K contents in seeds were significantly greater (N, p-value = 0.0234; P, p-value = 0.0365; K, p-value = 0.0216) for the forest than roadside seeds. Relationships between seed mass and seed nutrient contents differed between habitats, as indicated by significant habitat × seed mass interactions (Table 8). No significant differences were found for seed N:P ratio between habitats. The N, P and K contents of seeds (Fig. 2a–c) increased linearly with seed mass, as indicated by the slope (b) of the regression functions. The linear relationships between seed mass and seed content indicated that the increase in seed content per unit of seed mass was relatively uniform across seeds.
Table 8.
ANCOVAs of the effect of seed mass (covariate) and habitat (roadside vs forest) on the nitrogen (N), phosphorus (P) or potassium (K) content in seeds based on the data pooled across umbel orders.
| Nutrient | Seed mass | Habitat | Habitat × seed mass | Roadside (mg seed−1) | Forest (mg seed−1) |
|---|---|---|---|---|---|
| Nitrogen | 2789.28*** | 6.56* | 7.01* | 0.16 ± 0.004a | 0.21 ± 0.008b |
| Phosphorus | 1356.14*** | 5.44* | 6.12* | 0.03 ± 0.004a | 0.04 ± 0.004b |
| Potassium | 342.31*** | 6.34* | 5.98* | 0.07 ± 0.005a | 0.12 ± 0.006b |
| N:P | 45.32ns | 1.45ns | 3.32ns | 5.33 ± 0.24 | 5.25 ± 0.31 |
Total reserves of seeds (N, P or K mg seed−1) were calculated from the mass (mg dry weight [dry wt]) of each seed multiplied by the relative N, P or K concentration (% dry wt). Individual seed mass was obtained by taking the mean mass of sample of 100 seeds (N = 10 samples).
F-values for seed mass, habitat, and habitat × seed mass are given. *p < 0.05, **p < 0.01, ***p < 0.05. The values for seed mass followed by different letter in the same row are significantly different at p < 0.05 according Student’s t-test procedure.
Figure 2.
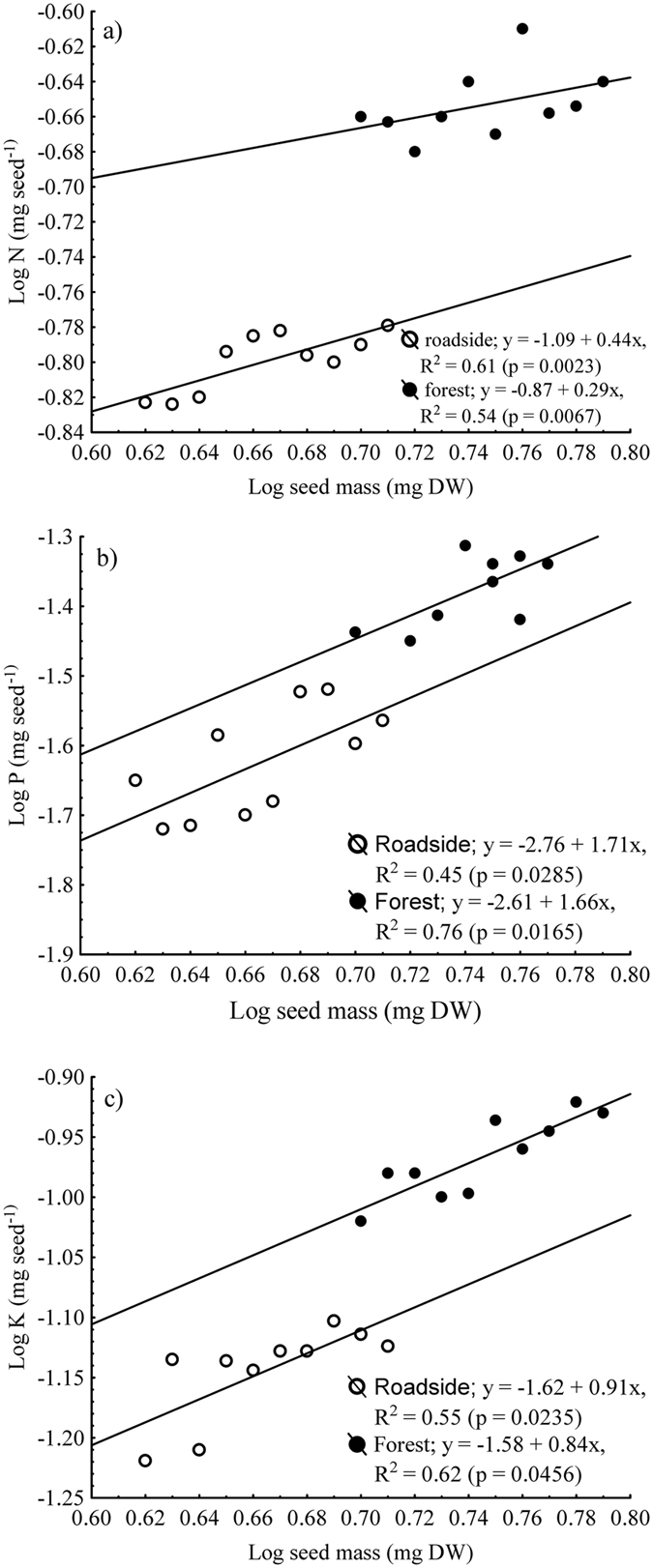
Relationships between seed mass (mg dry wt) and the (a) N, (b) P and (c) K content (mg dry wt seed−1) of seeds. Least-squares linear regression fitted to log10 transformed variables (solid lines). The coefficient of determination (R2) and the significance of the coefficient (P) are provided (n = 5). See Table 8 for analyses.
Regression of seed N, P, and K contents shows that seed N, P and K contents were positively related to soil macronutrients contents in each habitat (Fig. 3a–c).
Figure 3.
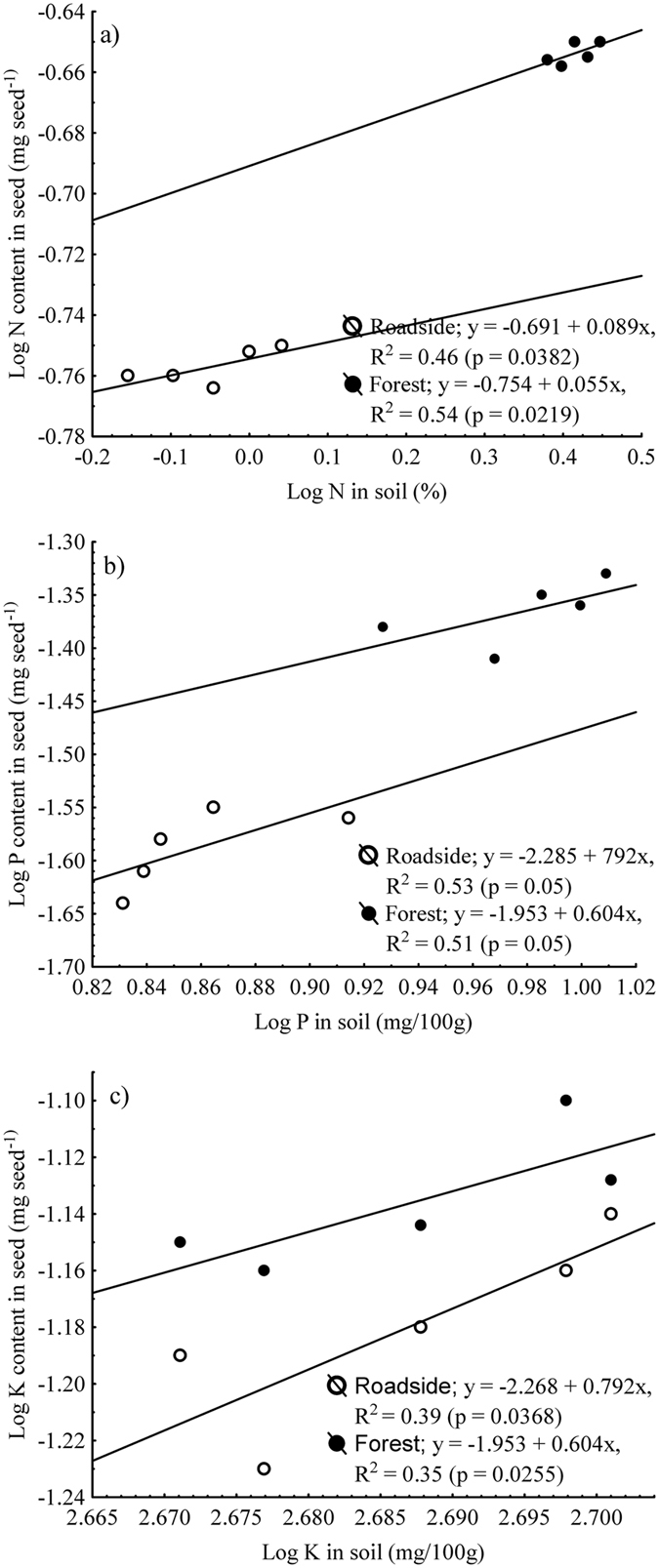
Regressions of macronutrient contents of seeds (y) on respective soil macronutrients (x): (a) nitrogen, (b) phosphorus and (c) potassium. Each point is the mean of 5 replicates of soils.
Discussion
In P. oreoselinum the seeds developed in the primary umbels were heavier than those produced by secondary umbels. In Lomatium grayi, 16% of the variance in seed mass within individual plants was explained by differences between umbels20.
The mean mass individual seed produced in forest site was striking by 2-fold greater than that in roadside site. A substantial portion of this variation is likely to have been the results of soil fertility. Soils at the roadside contained significantly lower concentrations of macronutrients than those in the forest; hence the levels of these nutrients in the seeds were different depending on the growth site. It is also likely that there is genetic differentiation between population for seed size. A similar result was already found for Stellaria media by Sobey21 and for Senecio jacobaea by Leiss and Müller-Schärer22.
This study also documents that the mass of the primary seeds was slightly more than twice that of the secondary seeds. This is consistent with the degree of variation reported for P. sativa 5. These differences are thought to be due mainly to variations in resources available to individual seeds. Hendrix5 suggested that the production of smaller seeds on later-developing umbels (e.g. secondary umbels) might be due to the decrease in the amounts of available resources over the growth season, which is disadvantageous for later-developing umbels as they compete for limited resources.
Variation of seed mass within umbels of P. oreoselinum was correlated with position: seeds from the outer edges of the umbels were heavier than those from their center. However, for seeds of Heracleum mantegazzianum the situation was opposite13, while for Angelica acutiloba no such differences were observed14.
A significantly greater total percentage germination was observed for those seeds from forest compared to seeds from roadside. Variations in seed germinability between different plant populations of a given species were previously documented. For example, Milberg et al.23 reported such a situation in Lithospermum arvense and Anchusa arvensis.
The present data show that the seed germination percentages were significantly different between the two umbel orders. This resulted from the fact that the seeds produced on the primary umbels were heavier. A positive relationship between seed mass and germination percentage has been found in Daucus carota 19, P. sativa 5, and Angelica acutiloba 14. These results are compatible with the view that rapid and better germination of seeds would result from larger food reserves and energy content in heavier seeds6. However, Hendrix5 reported a reversed situation in P. sativa: smaller seeds germinated more rapidly than larger ones.
Within P. oreoselinum there is considerable variation in mean seed mass between habitats. This difference might be caused by environmental factors. Several studies reported that an increase in the nutrient concentration in soil often leads to the production of heavier seeds that contain higher levels of mineral nutrients9. Plants of P. oreoselinum from the nutrient-rich habitat (oak forest) had heavier seeds compared to the nutrient-poor one (roadside). The relationship between seed mass and soil nutrient status has received little attention. In this study, regression of seed N, P, and K contents in P. oreoselinum in relation to the levels of respective elements in soil showed that seed and soil macronutrient levels were positively correlated. Similar relationships between seed mass, seed nutrient content, and soil fertility were reported between closely related species pairs in the family Proteaceae24. Aarssen and Burton25 observed the same effect in seeds of Senecio vulgaris. Plants of Senecio vulgaris were fertilized with high, medium and low levels of NPK (20–20–20), and the resulting seeds decreased in mass with the decrease in nutrients. Mass of individual Erigeron annuus achenes was 24.6 and 28.6 μg for plants that were grown under low and high levels of nutrients, respectively26. On the other hand, Lee and Fenner27 found that species in the grass genus Chionochloa from infertile soils had larger seeds with more seed nutrients but produced seedlings with smaller shoots than those from fertile sites. The availability of seed nutrient can have important consequences for the seedling vigor. Large seeds with greater amount of mineral nutrients tend to produce more vigorous seedlings when compared to small seeds with low levels of nutrients.
An increase in the nutrient concentration of the growing environment often leads to the production of heavier seeds that contain higher levels of mineral nutrients9. Thus, seedlings originating from larger seeds have both more time for development and nutrients available for growth. The results observed in this study support the conclusion that larger seeds produce seedlings with larger initial size as reported by others authors7, 9, 28. In this scenario it would be reasonable to think that larger seeds/seedlings have higher competitive ability relative to small seeds1, 4, 29. This is in contrast with other studies that detected negative30 or no relationship31 between seed size.
As noted above, for all macronutrients, there were significant differences between sites, and the N, P, and K contents of the seeds were 3%, 17%, and 4% greater for the forest than roadside seeds. For both habitats seed N:P ratios slightly exceed 5. The average seed N:P ratios reported for wild herbaceous plants is 1.5–15. Nitrogen and phosphorus concentrations in plant biomass, [N] and [P], are determined by the balance of N and P uptake, C assimilation, and the losses of N and P through turnover, leaching, exudation, herbivores and parasites. In an individual plant N:P ratio may depend additionally on internal nutrient translocation32. When comparing plants of the same species growing at different rates, Matzek and Vitousek33 found that faster-growing plants did not have consistently lower N:P ratios.
In summary, the results of this study indicated that umbel order and umbellet position had significant effect on the seed mass. In addition, mineral nutrient (N, P and K) contents in the seeds varied with habitat and depended on the total nutrient contents in soils and on factors controlling their availability to plants. Thus, phenotypically-based variation in seed mass may arise from variations in soil fertility or from a combination of environmental and maternal effect7. In the study reported here, it was also observed also that the umbel order affected the growth of the seedlings. The primary umbel produced the heaviest seeds and subsequently the most vigorous seedlings.
Methods
Studied plant
Peucedanum oreoselinum (L.) Moench, a member of the Apiaceae, is a temperate climate species, occurring in Europe, except for the islands. It is a perennial aromatic herb growing to 1.5 m. Lower leaves are up to 40 cm, 2- to 3-pinnate, triangular in outline; upper cauline leaves less are divided. Flowers are arranged in inflorescences - compound umbels of three orders. Peucedanum oreoselinum grows as an erect plant34. The monopodial main axis ends in a terminal primary (first-order) compound umbel. Branches, which are produced on the stem, terminate in secondary (second-order) umbels. Third-order umbels may arise on shoots branching from secondary shoots (Fig. 4). Third-order umbels usually consist only of male flowers and do not produce seeds. Umbels of the first order show the greatest number of umbellets and in the umbels of progressively higher order there is a gradual reduction in the number of umbellets. The plant is in flower from June to August, and the seeds ripen from July to September. As in most species of Apiaceae, P. oreoselinum is andromonoecious (male and hermaphrodite flowers on the same plant); flowers are pollinated by insects. Petals are white or pinkish, papillose. The fruit is 5–8 mm, broadly obovate. In this paper, the fruits of the P. oreoselinum are referred to as ‘seeds’ because they are functionally analogous to true seeds. Seeds ripen in late June through July and dispers slowly. This species is commonly found on sandy, loamy soils in mildly shaded sites such as road verges, in lowland coniferous forests and deciduous forests, but also in more open places such as meadows and riverbanks34.
Figure 4.
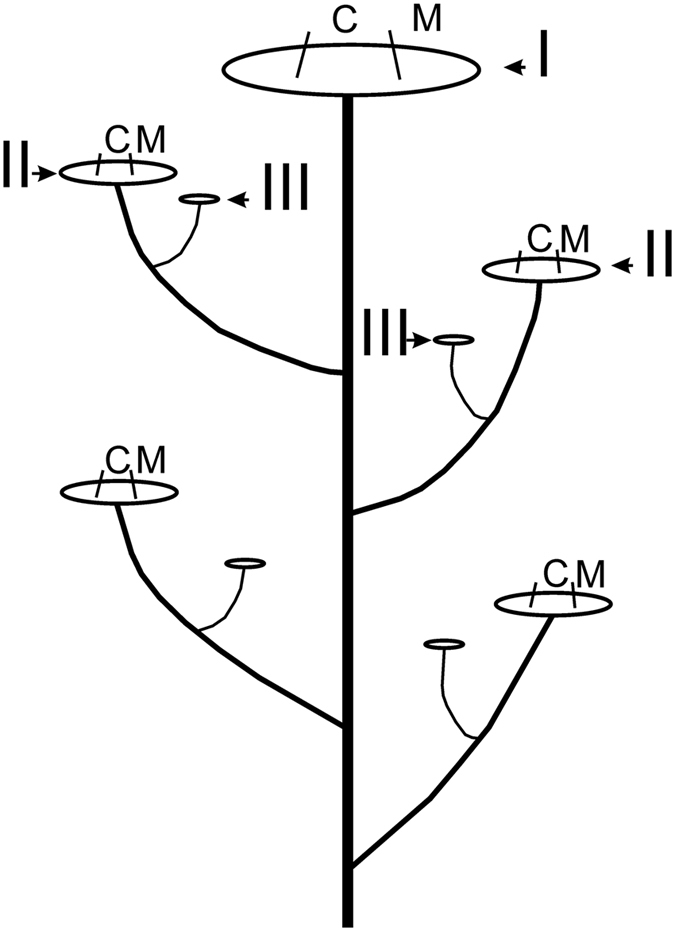
Diagram of three orders of umbels (I–III) of Peucedanum oreoselinum: the large first-order umbel (I), the succeeding second-order umbels (II) and the small third-order umbels (III).
Study sites
Two sites of P. oreoselinum were used. One site is located in Lipnica village, central Poland. At this site P. oreoselinum grow along roadside in the vicinity of agricultural fields. The other site is located near Wilczków city, 8 km from the Lipnica site. At this site, P. oreoselinum grow in the understory of an oak forest. The dominant tree is Quercus robur contributing 90% to the total ground cover. The undergrowth is dominated by Convallaria majalis which account for up to 40% of the ground cover. The following species were also recorded: Lathyrus niger, Melica nutans, Polygonatum odoratum, and Vicia cassubica. Total ground cover is c. 80%. The road verge site was significantly more arid than the forest site, all the plants grew in full sunlight. The site is characterized by the following combination of species: Alyssum alyssoides, Chondrilla juncea, Centaurea stoebe, Oenothera biennuis, Potentilla argentea, and Rubus plicatus. In August 2016, seeds were collected from randomly chosen mature large plants of similar size (basal stem diameter of the flowerings talk >5 mm) growing in each of two sites in central Poland, as summarized in Table 1. In each sampled individual (N = 10) the seeds were collected from the primary and secondary umbels.
Soil properties
In each location 5 core soil samples (2 cm diameter and 5 cm depth) were taken at random and put them in plastic bags. The samples were air-dried at 25 °C for 3 days and sieved through 2 mm mesh. The following parameters were determined in the soil samples (10 g dry soil): total nitrogen (N), phosphorus (P) and potassium (K) forms. Phosphorus was extracted with 0.5 sodium bicarbonate (NaHCO3), pH 8.5. and was measured colorimetrically by the molybdenum blue method. Potassium was extracted from soil with calcium lactate (C6H10CaO6) and determined with an atomic absorption spectrophotometer. Total nitrogen (N) was determined by the micro-Kjeldahl method by the wet oxidation of organic matter using sulfuric acid (H2SO4).
Plant material
Samples of >150 seeds were collected from each of 10 different P. oreoselinum plants separately in two natural sites. From each plant, the seeds were harvested from four different position. Two umbellet positions (central and outer termed) within each umbel order (primary and secondary) were selected (Fig. 4). The secondary umbels chosen for seed collection were 2nd from the top. The harvested seeds were air dried for 2 weeks at room temperature (±22 °C), Mean seed mass was determined by weighing 25 seeds (to the nearest 0.1 mg) per seed sample and dividing the obtained mass by 25.
Germination test
Large-scale screening studies revealed that many temperate-climate Apiaceae species have a chilling requirement12, so freshly matured seeds were not tested because they have poor germination. Experimental design consisted of four variants treatments (two umbel orders × two umbellet position) performed on ten different plants from two sites. There were four replicates of each treatment × plant × site combination, giving 80 samples of seeds (=4 × 10 × 2), each consisting of 25 randomly selected seeds. The seeds were placed on filter paper, wrapped in aluminium foil and placed in a refrigerator in the dark for 8 weeks at ±5 °C. This temperature is near-optimal for many seeds requiring low moisture and low temperature to break dormancy12. Following stratification treatment, seeds were allowed to imbibe for 24 hours in distilled water at room temperature (±22 °C). 9-cm diameter plastic Petri dishes were lined with two filter paper discs moistened with distilled water until saturated and 25 randomly chosen seeds were distributed in each dish. The seeds were incubated at 22/10 °C in light (14 h-photoperiod, 3.000 lux, Philips 35 W/33 lamps). Moisture was maintained with deionized water. Germinated seeds were counted and removed from the Petri dishes daily. Germination was defined as germinated seeds having at least 2 mm long radicle. After 23 days, the seeds were no longer germinating, so all germinated seedlings were removed. The rate of germination was estimated using a modified Timson’s index of germination velocity:
where G is seed germination percentage each day and t the total germination period35. Therefore if all of the seeds germinated in one day, the Timson’s index would be 100 (i.e., 2300/23). A higher value indicates more rapid germination.
Seedling growth
Following stratification, a total of 800 seeds, representing 25-seed replicates of two umbel orders × two umbellet position from different four plants of two sites, were randomly selected. The seeds were then imbibed in 9-cm Petri dishes lined with 2 layers of No. 1 Whatman filter paper moistened with 3 ml distilled water. The Petri dishes (each containing 25 seeds) were placed in growth room for 3 weeks. The germination conditions were the same as those described above in germination tests. At each day of incubation, 2 ml water was added to each Petri dish. As soon as the radicle appeared the newly germinated seedlings were transferred to plastic trays (20 × 15 cm) with standard soil, consisting of a substrate of 30% sand, 20% perlite, and 50% commercial peat. Each tray containing 25 seedlings served as replicate. The trays were placed in the growth room for 4 weeks. Deionized water was added as needed. The incubation conditions were the same as those described above. By the end of the cultivation period soil was washed from the roots. Then the shoots and roots of each seedling were dried at 70 °C for 48 h to quantify shoot biomass (SB) and root biomass (RB). The percentage of biomass allocated to root (BAR)30 was estimated using the following formula:
Seed macronutrient analysis
Ten samples of 100 seeds (10 seeds from each of 10 randomly chosen plants) was used for each site. The harvested seeds were pooled from different umbel order of each individual plant and mixed. The seeds were weighed and an average mass of a individual seed for each sample was calculated. Since the nutrient contents were expressed on a dry matter basis the samples at 105 °C for 5 h. The seed material was mineralized in a boiling mixture of 10 ml of HNO3 and 5 ml of H2SO4. Phosphorus was determined colourimetrically (see soil properties), potassium by atomic absorption spectrometry (as above), and the total nitrogen content by the micro-Kjeldahl procedure (as above). N:P ratio was calculated from these results as N/P.
Data analysis
The data for all statistical tests were log10 transformed before statistical analysis to ensure homogeneity of variance but non transformed data are shown in all figures and in tables. The data were tested for normality with the Kolmogorov-Smirnov test with the Lilliefors correction and homogeneity of variance with the Brown-Forsythe’s test. To examine variation in seed mass the coefficient of variation (CV = standard deviation/mean × 100) for seed dry mass was calculated. Mean seed mass variation was determined by dividing mass of the heaviest by mass of the lightest seeds. Significant differences in the soil macronutrient content and differences in the seed macronutrient content from different habitats were estimated using all-pair Student’s t-test comparisons. Student’s t-test was also performed to test for statistical differences in mean seed mass between umbel orders (primary vs secondary) and between umbellet position (outer vs central). One-way ANOVAs followed by Tukey’s post-hoc comparison tests were used to test for differences in seed mass, germination percentages and rate of germination (Timson’s Index) of seeds from differed umbels and umbellet positions collected for each habitat. One-way ANOVAs were also performed to test for differences in shoot biomass, root biomass and the percentage of biomass allocated to root. ANOVA was used to analyze the effect of umbel order, umbellet position in the umbel, habitat and their interaction on seedling traits (shoot biomass [SB], root biomass [RB], and the percentage of biomass allocated to root ([BAR]).
Analyses of covariance (ANCOVA) was performed to test the effect of seed mass (covariate) and habitat (roadside vs forest) on the nitrogen, phosphorus, and potassium content of seeds. Also ANCOVA was performed with seed mass as the covariate to test for significant differences between umbellet position with respect to the seedling traits indicated above. The effect of habitat, individuals, and umbels on the percentage of variation in seed mass was analyzed using a three-way nested ANOVA.
Regression analysis was carried out to assess the influence of the macronutrient content in the soil on their concentration in seed. The influence of seed mass on seed N, P or K content was also examined by lest-squares linear regression analyses. All statistical analyses were done using the Statistica 12.0 package36.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baker HG. Seed weight in relation to environmental conditions in California. Ecology. 1972;53:997–1010. doi: 10.2307/1935413. [DOI] [Google Scholar]
- 2.Silvertown JW. Seed size, life span, and germination date as coadapted features of plant life history. Am. Nat. 1981;118:860–864. doi: 10.1086/283876. [DOI] [Google Scholar]
- 3.Stanton ML. Seed variation in wild radish: effect of seed size on components of seedling and adult fitness. Ecology. 1984;65:1105–1112. doi: 10.2307/1938318. [DOI] [Google Scholar]
- 4.Gross KL. Effects of seed size and growth form on seedling establishment of six monocarpic perennial plants. J. Ecol. 1984;72:369–387. doi: 10.2307/2260053. [DOI] [Google Scholar]
- 5.Hendrix SD. Variation in seed weight and its effect on germination in Pastinaca sativa L. (Umbelliferae) Am. J. Bot. 1984;71:795–802. doi: 10.2307/2443470. [DOI] [Google Scholar]
- 6.Harper, J. L. Population biology of plants. (Academic, London 1977).
- 7.Wulff RD. Seed size variation in Desmodium paniculatum. I. Factors affecting seed size. J. Ecol. 1986;74:87–97. doi: 10.2307/2260350. [DOI] [Google Scholar]
- 8.Obeso JR. Mineral nutrient stoichiometric variability in Hedera helix (Araliaceae) seeds. Ann. Bot. 2012;109:801–806. doi: 10.1093/aob/mcr306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenner M. Environmental influences on seed size and composition. Hortic. Rev. 1992;74:385–392. [Google Scholar]
- 10.Krannitz PG. Variation in magnesium and nitrogen content in seeds of Antelope bitterbrush (Purshia tridentata, Rosaceae) Am. J. Bot. 1997;84:1738–1742. doi: 10.2307/2446473. [DOI] [PubMed] [Google Scholar]
- 11.Hassel RL, Kretchman DW. The effects of umbel order, soaking, and scarification on germination inhibiting substances in Petroselinum crispum L. and other Apiaceae seeds. HortScience. 1997;32:1227–1230. [Google Scholar]
- 12.Baskin, C. C. & Baskin, J. M. Seeds: ecology, biogeography, and evolution of dormancy and germination. (Academic Press, New York, USA 1998).
- 13.Moravcová L, Perglová I, Pyšek P, Jarošik V, Pergl J. Effects of fruit position on fruit mass and seed germination in the alien species Heracleum montegazzianum (Apiaceae) and the implications for its invasion. Acta Oecol. 2005;26:1–10. doi: 10.1016/j.actao.2005.01.004. [DOI] [Google Scholar]
- 14.Ninh TP, Hiroshi N, Toru T. 2007. Effect of umbel order and umbellet position on the production and characteristics of seeds and on the development and growth of seedlings in Angelica acutiloba Kitagawa. Jpn. J. Trop. Agr. 2007;51:46–53. [Google Scholar]
- 15.Ojala A. Seed dormancy and germination in Angelica archangelica subsp. archangelica (Apiaceae) Ann. Bot. Fennici. 1985;22:53–62. [Google Scholar]
- 16.Thomas TH. Relationships between position on the parent plant and germination characteristics of seeds of Parsley (Petroselinum crispum Nym.) Plant Growth Regul. 1996;18:175–18. doi: 10.1007/BF00024379. [DOI] [Google Scholar]
- 17.Thomas TH, Gray D, Biddington NL. The influence of the position of the seed on the mother plant on seed and seedling performance. Acta Hortic. 1978;83:57–66. doi: 10.17660/ActaHortic.1978.83.7. [DOI] [Google Scholar]
- 18.Thomas TH, Biddington NL, O’Toole DE. Relationship between position on the parent plant and dormancy characteristics of seeds of three cultivars of celery (Apium graveolens) Physiol. Plant. 1979;45:492–496. doi: 10.1111/j.1399-3054.1979.tb02620.x. [DOI] [Google Scholar]
- 19.Gray D, Steckel JRA. The effects of ripening and drying of carrot seed (Daucus carrota) crops on germination. Ann Appl. Biol. 1982;101:346–347. [Google Scholar]
- 20.Thompson JN. Variation among individual seed masses in Lomatium Grayi (Umbelliferae) under controlled conditions: magnitude and partitioning of the variance. Ecology. 1984;65:626–631. doi: 10.2307/1941425. [DOI] [Google Scholar]
- 21.Sobey DG. Differences in seed production between Stellaria media populations from different habitat types. Ann. Bot. 1987;59:543–549. doi: 10.1093/oxfordjournals.aob.a087348. [DOI] [Google Scholar]
- 22.Leiss K, Müller-Schärer H. Adaptation of Senecio vulgaris to ruderal and agricultural habitats. Am. J. Bot. 2001;88:1593–1599. doi: 10.2307/3558403. [DOI] [PubMed] [Google Scholar]
- 23.Milberg P, Andersson L, Elfverson C, Regnér S. Germination characteristics of seeds differing in mass. Seed Sci. Res. 1996;6:191–197. doi: 10.1017/S0960258500003251. [DOI] [Google Scholar]
- 24.Mustart PJ, Cowling RM. Seed size: phylogeny and adaptation in two closely related Proteaceae species-pairs. Oecologia. 1992;91:292–295. doi: 10.1007/BF00317799. [DOI] [PubMed] [Google Scholar]
- 25.Aarssen LW, Burton SM. Maternal effects at four levels in Senecio vulgaris (Asteraceae) grown on a soil nutrient gradient. Am. J. Bot. 1990;77:1231–1240. doi: 10.2307/2444634. [DOI] [Google Scholar]
- 26.Stratton DA. Competition prolongs expression of maternal effects in seedlings of Erigeron annuus (Asteraceae) Am. J. Bot. 1989;76:1646–1653. doi: 10.2307/2444402. [DOI] [Google Scholar]
- 27.Lee WG, Fenner M. Mineral nutrient allocation in seeds and shoots of twelve Chionochloa species in relation to soil fertility. J. Ecol. 1989;77:704–716. doi: 10.2307/2260980. [DOI] [Google Scholar]
- 28.Cordazzo CV. Effect of seed mass on germination and growth in three dominant species in southern Brazilian coastal dunes. Braz. J. Biol. 2002;62:427–435. doi: 10.1590/S1519-69842002000300005. [DOI] [PubMed] [Google Scholar]
- 29.Newbery DMcC, Newman EI. Competition between grassland plants of different initial sizes. Oecologia. 1978;633:361–380. doi: 10.1007/BF00348119. [DOI] [PubMed] [Google Scholar]
- 30.Zhang F, Yu SL, Wang JH. Studies on the photosynthetic characteristics and its relationship to yield in radix Cynanchum bungei Decne. J. Nuclear Agr. Sci. 2010;24:176–180. [Google Scholar]
- 31.Jurado E, Westoby M. Seedling growth in relation to seed size among species of arid. Aust. J. Ecol. 1992;80:407–416. doi: 10.2307/2260686. [DOI] [Google Scholar]
- 32.Güsewell S. N:P ratios in terrestrial plants: variation and functional significance. New Phytologist. 2004;164:243–266. doi: 10.1111/j.1469-8137.2004.01192.x. [DOI] [PubMed] [Google Scholar]
- 33.Matzek V, Vitousek PM. N:P stoichiometry and protein:RNA ratios in vascular plants: an evaluation of the growth rate hypothesis. Ecol. Lett. 2009;12:765–771. doi: 10.1111/j.1461-0248.2009.01310.x. [DOI] [PubMed] [Google Scholar]
- 34.Tutin, T. G. Peucedanum (L.) Moench. In Flora Europaea (eds Tutin, T. G. et al.), 360–364 (Cambridge University Press, Cambridge, UK 1968).
- 35.Khan MA, Ungar IA. Effect of thermoperiod on recovery of seed germination of halophytes from saline conditions. Am. J. Bot. 1997;84:279–283. doi: 10.2307/2446089. [DOI] [PubMed] [Google Scholar]
- 36.Stat-Soft Inc. Statistica for Windows. (Stat-soft, Inc., Tulsa 2016).


