Abstract
More extreme climatic events (ECEs) are among the most prominent consequences of climate change. Despite a long-standing recognition of the importance of ECEs by paleo-ecologists and macro-evolutionary biologists, ECEs have only recently received a strong interest in the wider ecological and evolutionary community. However, as with many rapidly expanding fields, it lacks structure and cohesiveness, which strongly limits scientific progress. Furthermore, due to the descriptive and anecdotal nature of many ECE studies it is still unclear what the most relevant questions and long-term consequences are of ECEs. To improve synthesis, we first discuss ways to define ECEs that facilitate comparison among studies. We then argue that biologists should adhere to more rigorous attribution and mechanistic methods to assess ECE impacts. Subsequently, we discuss conceptual and methodological links with climatology and disturbance-, tipping point- and paleo-ecology. These research fields have close linkages with ECE research, but differ in the identity and/or the relative severity of environmental factors. By summarizing the contributions to this theme issue we draw parallels between behavioural, ecological and evolutionary ECE studies, and suggest that an overarching challenge is that most empirical and theoretical evidence points towards responses being highly idiosyncratic, and thus predictability being low. Finally, we suggest a roadmap based on the proposition that an increased focus on the mechanisms behind the biological response function will be crucial for increased understanding and predictability of the impacts of ECE.
This article is part of the themed issue ‘Behavioural, ecological and evolutionary responses to extreme climatic events’.
Keywords: attribution, definition, idiosyncratic responses, climate variability, mechanism, biological response function
1. The need for more synthesis in extreme climatic event research
Extreme climatic events (ECEs) can have a dramatic impact on human society and biological systems. And while the extent to which a single ECE can be attributed to climate change is difficult to determine [1,2], it is clear that global climate change has led to an increased frequency, intensity and duration of ECEs [2,3]. As a result, ECEs are one of the most visible impacts of global change in our society and increasingly the focus of attention of the general public, policy makers, climatologists and also biologists [4]. But not all extreme weather and climate events have extreme impacts on specific systems [5], making the attribution of biological responses to climate extremes even more difficult [6].
There has been long-standing recognition of the importance of ECEs by particularly paleo-ecologists and macro-evolutionary biologists (e.g. [7], but see also [8,9]), but recently—in the face of anthropogenic climate change—ECEs have received much stronger interest in the wider ecological and evolutionary community. Consequently, the number of biological papers on ECEs is now increasing exponentially (e.g. [10]). However, as is the case for many rapidly developing fields, the emerging—or some might say reinvigorated [7–9]—field of ECEs lacks structure and cohesiveness, which limits scientific progress.
Meta-analyses and systematic reviews are much needed for synthesis of the field, but comparison among ECE studies has been limited because it is very challenging for various reasons. First, despite several reviews [3,10–15], there is no consensus on how to define an ECE within a biological context. Second, very few studies rigorously attribute the biological impacts to changes in climatic extremes and distinguish them from responses to concurrent other environmental changes (including changing climatic means and variability). Third, ECEs encompass a wide diversity of events (e.g. flood, heat wave, drought, hurricane) that act on very different spatio-temporal scales. Finally, ECEs are rare and thereby pose some particular practical and statistical challenges.
The rareness of ECEs also means that most studies on the impact of ECEs are anecdotal as they are based on non-experimental data (but see [12,16]) that only cover a single event [10,17]. Consequently, little progress is being made in our conceptual understanding of the impacts of and adaption to ECEs on longer—ecologically and evolutionary more relevant—timescales ([5,10], but see [12]). Finally, there is still relatively little synthesis across fields (evolution, ecology and behaviour) and levels of organization (individual, population and ecosystem; but see [18]).
This introduction and synthesis of the theme issue on ‘Behavioural, ecological and evolutionary responses to extreme climatic events’ will (i) provide some common terminology to define ECEs in a way that facilitates comparison among studies (§2) and make explicit the conceptual links between closely related disciplines (e.g. climatology, disturbance ecology; §§3–4), (ii) draw parallels between challenges in behavioural, ecological and evolutionary studies by summarizing the contributions to this theme issue (§5) and (iii) draw general conclusions leading to a roadmap for future research (§6).
2. Defining extreme climatic events
(a). What is an extreme climatic event?
Despite various attempts to define ECEs in a synthetic way [4,10–13,19,20], no universally accepted definition exists [10]. This lack of consistent terminology hampers the comparison across studies of the biological relevance of ECEs, since what one study considers to be an ECE is not necessarily considered an ECE by others. This problem is further exacerbated by many studies neglecting to clearly outline how they define an ECE in the first place [13].
Table 1 provides an overview of definitions of ECEs proposed in the literature. To better understand the challenges in defining the term ECE, it is helpful to first consider the type of phenomena people have included under the term ECE [4]. The term ECE has been used to describe meteorological phenomena, such as extreme high temperatures or rainfall [3]. In addition, some studies also consider ECEs to include consequential physical impacts—like flooding, hurricanes or wildfires—that are (at least partly) caused by meteorological phenomena [21]. Finally, some studies also include a spectrum of impacts for biological systems (or for economy or society in fields other than biology [22]), such as mass reproductive failure in shorebirds after flooding [23].
Table 1.
Overview of definitions of ECEs proposed in the literature. The column ‘type’ describes whether a definition takes a purely climatological perspective or also includes aspects of the impact of climate. The last column specifies whether a definition requires a climatic event to have a specific biological impact (see also §2c).
| source | definition | type | specifies impacts? |
|---|---|---|---|
| IPCC 2012 [4] | the occurrence of a value of a weather or climate variable above (or below) a threshold value near the upper (or lower) ends of the range of observed values of the variable (typically 5% or 10%) | climatological | no |
| NAS 2016 [1] | a weather or climate event that is rare at a particular place (and, sometimes, time of year). […] Definitions of rare vary, but an extreme weather event would normally be […] rarer than a particular percentile (e.g. 1st, 10th, 90th, 99th) of a probability density function estimated from observations expressed as departures from daily or monthly means | climatological | no |
| Jentsch et al. 2007 [12] | climatic extremes that have a strong abruptness (i.e. biological magnitude over biological duration) | impact-related | no |
| Bailey & van de Pol 2016 [10] | an episode where climate or climate-driven conditions trigger a negative threshold-like (nonlinear) biological response | impact-related | no |
| This study | climatic conditions that cause the (biological) response to be in the e.g. 5% of most extreme values of the (biological) response variable | impact-related | no |
| Smith 2011 [13] | an episode in which a statistically unusual or rare climatic period alters ecosystem structure and/or function well outside the bounds of what is considered typical or normal variability | impact-related | yes, ecosystem structure |
| Gutschik & BassiriRad 2003 [11] | an event during which the acclimatory capacity of an organism is substantially exceeded (i.e. a long return time or hysteresis) | impact-related | yes, hysteresis |
| Wingfield et al. 2017 [20] | climate causes the cumulative resources available to an individual to be exceeded by the sum of its energetic costs. This allostatic overload triggers the emergency life-history stage that temporarily allows the individual to cease regular activities in an attempt to survive the extreme conditions | impact-related | yes, allostatic overload |
(b). Climatological versus impact-related definitions of extreme climatic events
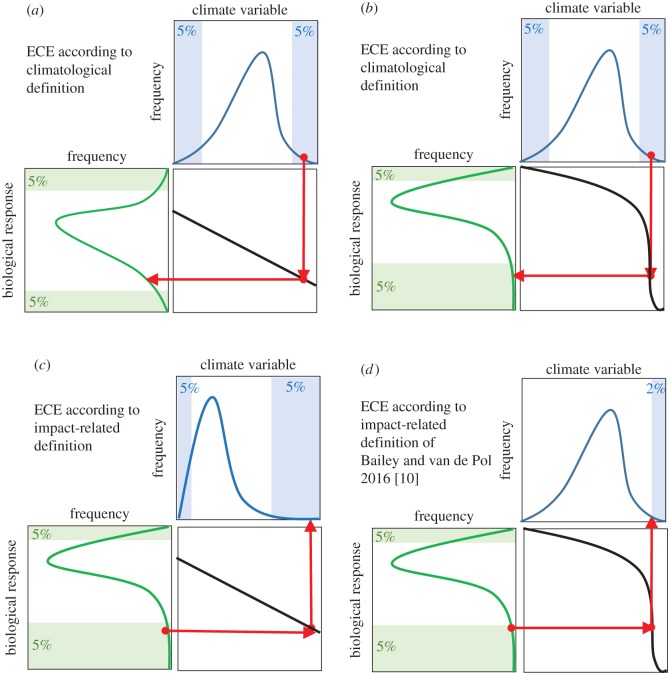
A first major difference among ECE definitions is thus whether or not they include the impact of the climate extreme in the definition [10]. Here, we therefore classify definitions as either ‘climatological’ or ‘impact-related’ (table 1). Climatological definitions only require the climate to be extreme, not the (biological) impact; by contrast, impact-related definitions typically require both the climate and (biological) impact to be extreme (figure 1a versus c). The use of a climatological-versus impact-related definition amounts to asking subtly different questions [10] and thus depends on whether one is interested in rare climate (‘What is the biological impact of this climate extreme?’) or if one is more focused on understanding rare biological extreme events, and the way climate extremes contribute to this (‘Which climate process drives this extreme biological event?’). However, even for climatological definitions, full separation of cause and impact can be difficult because the choice of meteorological phenomena and the way ECEs are quantified in a study typically depend on the event's biological relevance [4]. For example, in many countries the threshold for a heat wave is chosen based on its relevance for human health and societal impact.
Figure 1.
Schematic illustration of the difference between climatological and impact-related definitions. Climatological definitions only require the climate to be extreme (blue tails of the distribution) and there is no requirement that the biological impact be extreme (green tails of the distribution). Depending on an organism's or ecosystem's response curve (black line), a climatic extreme may not (a) or may (b) be associated with a biologically extreme response. It should be noted that extreme biological impacts are also caused by other non-climatic drivers, and that climate explains only part of the variation in the biological response [13]. (c) Impact-related definitions require both the climate and biological response to be extreme. Some impact-related definitions (d) do not a priori specify the threshold value beyond which climate is considered extreme, but instead use the nonlinearity of the biological response function to determine a climatic threshold (here upper 2%) that can be considered extreme from a biological point of view [10]. Red arrows depict the direction of approach, which reflects that the use of climatological and impact-related definitions involves asking different research questions (see §2b).
More generally, there is little consensus of a specific threshold value for extremeness. For climatological extremeness, a 10% frequency of occurrence over some historical period is most commonly used as a threshold (though 5% and 1% thresholds are also used [1]). However, climatic extremeness is not only described by its rate of occurrence [24], and little consensus exists on how to specify other attributes of extremeness in a comparable way [19], such as the magnitude, temporal duration, timing, spatial scale and multivariate dependency (particularly for compound events, e.g. drought). For the extremeness of the biological impact, even less specific descriptions are used: ‘strong magnitude’, ‘substantially exceeded’ and ‘well outside the bounds of what is considered typical or normal variability’ (table 1). No existing definition has set a specific threshold value for the biological response (e.g. the top 5% strongest biological responses) to be considered extreme, and for completeness we have added this more specific definition to table 1.
Most impact-related definitions only specify that the climatic event and its impact should be extreme, but do not specify the shape of the biological response function (table 1 and figure 1). By contrast, Bailey & van de Pol [10] suggest it only makes sense to study ECE when the biological response function is nonlinear (black line in figure 1d). The response function and its shape can be determined from observational data using either temporal (figure 2a) or spatial variation (figure 2b), from experimental manipulations at different climatic conditions in the lab (figure 2c), or from mechanistic models (figure 2d). Many mechanisms may cause nonlinearity—such as allostatic overload [20], hysteresis [11] and regime shifts [29]—and nonlinear responses are suggested to be a hallmark of ECE impacts [12,13]. Bailey & van de Pol argue that if there is a linear dependency between the climate and biological response, changes in climate will have the same impact regardless of whether they occur in ‘extreme’ or ‘non-extreme’ conditions and there is no reason to focus only on the tails of climate distributions when investigating the impacts of climate change. Furthermore, they suggest that the nonlinearity of the response function can be used as a biological context for deciding what is a meaningful threshold value of climatic extremeness [10]: the point where climate has a nonlinear impact on the biological response may be a less arbitrary threshold for climatic extremeness than an a priori chosen threshold of, for example, less than 5% (figure 1d).
Figure 2.

Four different ways to determine the biological response function: using (a) temporal or (b) spatial variation in observational studies, (c) experimental manipulation and (d) mechanistic modelling. The examples also highlight the diversity in response variables, from (a) phenological and (c) developmental phenotypic traits to (d) population and (b) ecosystem parameters. (a) Observational study relating temporal variation in the timing of egg laying (days since April 1) to annual variation in spring temperatures using linear regression on 47 years of data on wild-living British chaffinches (Fringilla coelebs) [25]. (b) Observational study relating spatial variation in annual primary plant productivity to spatial variation in precipitation using linear regression on data from 11 ecosystems [26]. (c) Experimental study determining the thermal performance curve for daily growth rates of hornworm larvae (Manduca sexta) using five different levels of experimentally manipulated rearing temperatures in the laboratory [27]. (d) Mechanistic study using a population matrix model parameterized with temperature-dependent demographic rates to calculate how the population growth rate of Daphnia lumholtzi depends on temperature [28]. Note that in (c,d) the climatological distribution can be derived from climatological time series (similar to blue panel of a), but that determining the distribution of biological response requires additional observations, as simply imposing the climate distribution to the response function ignores other sources of variation in biological response.
(c). The type of impact of an extreme climatic event
A second major difference among impact-related ECE definitions is whether they differentiate between the type or degree of impact. The definition of Smith 2011 ([13]; table 1) restricts the biological impact only to be extreme if it alters ecosystem structure and/or functioning, while individual- or population-level responses alone would not be considered extreme (figure 3a). Similarly, the definition of Gutschik & BassiriRad 2003 [11] restricts extreme biological responses to responses that have a long recovery/acclimation time (i.e. hysteresis, figure 3a). And sometimes climatic events are only considered to be extreme if they have large spatial impacts. Notwithstanding the fact that some impacts can be considered more ‘extreme’ than others, each study has its own research question and associated choice of biological response variable that already determines the spatio-temporal scale and level of organization at which something is considered meaningful. For example, evolutionary biologists typically consider a dramatic trait change to be extreme and don't get particularly excited by other non-genetic changes in ecosystem functioning, while an ecosystem ecologist would not be impressed by trait change unless it leads to altered ecosystem functioning. Consequently, including constraints on what type of response (either in spatio-temporal scale or level of organization; figure 3b) qualifies as extreme enough to be considered an ECE arguably does not contribute to the synthetic properties of ECE definition [10].
Figure 3.
(a) Scenarios that vary in the biological level of organization impacted (y-axis) by a climate extreme and in the temporal duration of impacts (x-axis; modified from [13]). (b) The three main axes that determine the type of impact of the biological response, illustrating the context-dependence of what is biologically extreme (though an impact at a higher biological level, larger spatial scale, and/or longer temporal scale is typically considered to be more catastrophic).
(d). Context-dependence of ‘events’
Finally, comparison among studies is also difficult due to the biological context-dependence of what constitutes an ‘event’ [12]. The word ‘event’ in the term ECE implies a short duration, abruptness and/or discreteness (fig. 4 in [12]). ECEs like extreme rainfall on a given day can rightfully be seen as events (sometimes called ‘simple events’ [19]). However, other ECEs such as drought or heat waves are caused by the compounding of outcomes from successive climate phenomena, for example, a succession of hot or dry days, or even years. Such ‘compound events’ can also be due to multiple compounding climate or physical variables (‘perfect storm’), which in themselves may not necessarily be extreme, but if they persist over time, their cumulative value is extreme (e.g. drought). Whether a compound event that spans a long period (e.g. Australia's ‘big dry’) should be considered an ECE according to impact-related definitions depends on the lifespan of the organism or the successional speed of the ecosystems in which it occurs [12]. Thus, this context-dependence on the model system allows for comparison between ECEs that last for days up to many years, but also implies that the same climatic event may be an ECE for one (long-lived) organism, but not for another (short-lived) organism (similar difficulties arise when comparing across ecosystems, locations and time periods [13]).
(e). A universal extreme climatic event definition?
To conclude, there is no universal definition of an ECE and achieving one is extremely challenging, which is exemplified by this theme issue as almost all definitions in table 1 were used in at least one contribution. However, we suggest that progress can be made to make ECE studies easier to compare. First, the usefulness of respectively a climatological or impact-related definition depends on the research question being: ‘What is the biological impact of this climate extreme?’ or ‘Which climate process drives this extreme biological event?’. Second, we think that for most biological studies it makes sense to use an impact-related definition, as biologists are ultimately interested in the biological response and generally their choice of climate variable to study is driven by its biological relevance anyway. Third, studies should more precisely define what threshold value of frequency, magnitude or duration they consider to be extreme, for example the 5% most extreme climatic and 5% most extreme biological response values observed, and provide the specific time and spatial scale of the ECE over a given reference time period (e.g. an extremely hot day in location X has a mean temperature over 30°C, as such value occurred less than 5% of the time from 1950–2010). Fourth, we agree with [10] that comparison across studies becomes more difficult if definitions consider impacts to be extreme only if they affect a higher organizational level or have a strong spatio-temporal impact (but studies should clearly specify what type of impact they are interested in; figure 3b). Finally, studies should not only provide a clear definition, but also use consistent terminology (climatological, impact-related, single versus compound events, etc.).
3. Detection and attribution of extreme climatic events in relation to other aspects of change
(a). Detection and attribution of extreme climatic events
To demonstrate that biological systems are impacted by climate change we need to identify an effect on the system and be able to attribute that effect to climate change [6,30]. In the context of ECEs this first requires detecting that the frequency of climate extremes has changed over time, and attribution of the observed changes in extremes to anthropogenic climate change, and not to some other meteorological process [2,31]. Second, it requires detecting that the climate extremes also have a biological impact, and that this impact cannot be attributed to other factors [4,6].
Climatologists perform the first step to detect and attribute changes in climatic extremes to global warming. Particularly, the attribution of climate extremes to global warming is challenging, as such rare events can also be part of the natural variability of the climate system or caused by other external factors, and thus requires an in-depth understanding of the underlying processes [2,32].
Biologists are tasked to perform the second step to detect and attribute the biological impacts of climate extremes. Similar to attribution by climatologists, biological attribution is complex, as the rareness of extreme events makes correlative approaches to attribution problematic [6]. Specifically, long time series and/or large impacts are needed to be able to show that the occurrence of extreme impacts are statistically associated with the occurrence of climate extremes ([6], but see [5]). A mechanistic understanding of the relationship between climate and the biological response (e.g. via models, or knowledge about how climate impacts the biological response over the full range of climate values, not only at the extremes) is extremely valuable as it not only increases the power to correctly attribute responses, but may even allow for reliably predicting biological impacts when few climate extremes are observed [6].
A second condition for a correct attribution to ECEs is that one should control for other factors that have an impact on the biological system of interest [4,6]. This is important because climate change is happening in a world undergoing many threats simultaneously (e.g. habitat destruction, invasive species). But even if the impact on the biological system can be attributed to climate, it is not necessarily impacted by the climate extreme alone. This difficulty has received particular attention among population ecologists, but is likely more widely relevant: other aspects of climate change beyond climate extremes, such as correlated changes in climate means and variability, may also affect biological responses.
Specifically, it has been argued that changes in climatic extremes are having a stronger impact on ecology [10] and evolution [11] than changes in climate means, and a similar discussion exists about the population dynamical impact of changes in climatic means and variability [28,33]. Such issues are important because the bulk of the biological research focuses on changes in climate means, while the effects of extremes and variability are often not studied, but could be crucial for making reliable predictions and an integrative understanding of impacts of climate change [33]. However, how does one separate the often concurrent impacts of changes in climatic means, variability and extremes [34]?
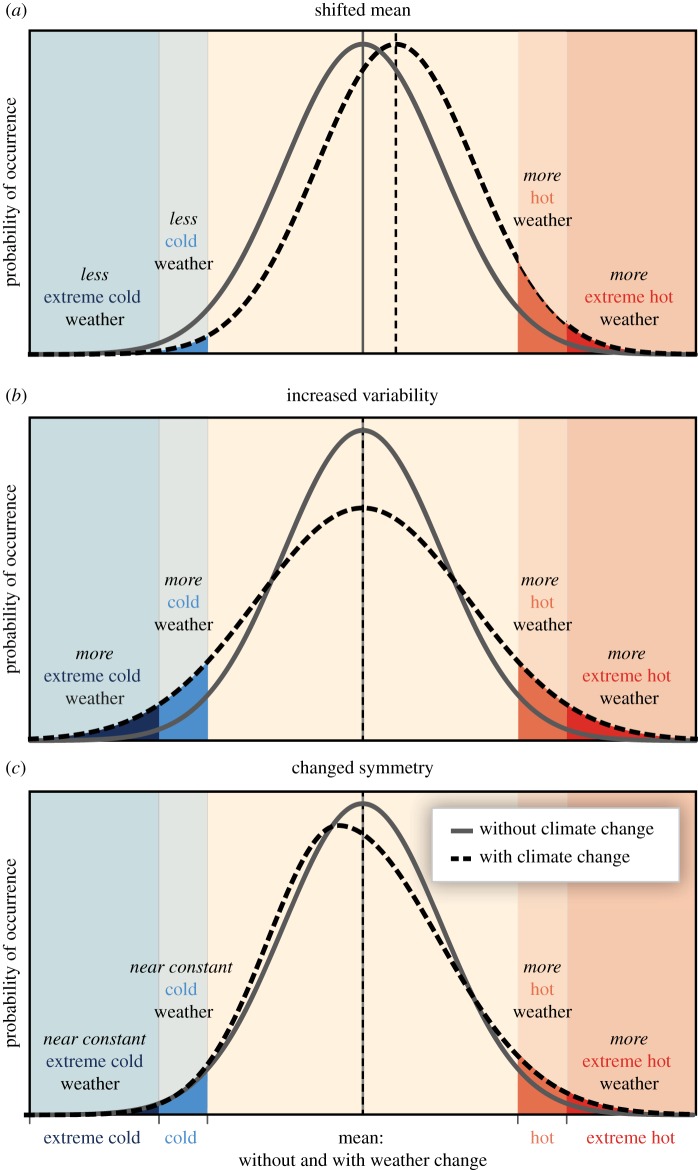
Problematically, increased climatic variability is often equated with more extremes in the literature, in the sense that studies on organisms' responses to variability are typically considered to also be on how they deal with extremes. However, increased climatic variability is only one cause of more climate extremes (figure 4b), but there are others (figure 4a,c; shift in mean or skew) (e.g. [2] and references therein). A clear distinction between the different aspects of climate change is also important because changes in climate extremes or variability can have distinct biological impacts, as they can act via different mechanisms. For example, climate extremes may cause adaptations in thermal tolerance to evolve (e.g. [35]), while climate variability may lead to the evolution of bet-hedging strategies. As another example, population biologists have long known that key metrics like the long-term population growth rate, extinction risk and fitness are affected by both the mean and variability in the annual performance [36,37]. Extremes can have a profound effect on the mean annual performance, while inter-annual climatic variability can affect the variability of fitness even when the mean annual performance is unaffected, meaning they both can affect long-term fitness, but independently via different mechanisms [10,34].
Figure 4.
The effect of changes in temperature distribution on extremes (from [4]). Different changes in temperature distributions between present and future climate and their effects on extreme values of the distributions: (a) effects of a shift of the entire distribution toward a warmer climate; (b) effects of an increase in temperature variability with no shift in the mean; (c) effects of an altered shape of the distribution, in this case a change in skew toward less cold and more hot days.
Theoretically it is possible to separate the effects of changes in means, variability and extremes, but only by choosing rather specific climate distributions in which the mean, variance and skew can be manipulated independently (either experimentally in the field, or via simulation in models; [33]). In such attribution approaches, changes in skew can mimic changes in extremes, while keeping the mean and variability of the climate distribution constant. In the field of population dynamics, the limited evidence from such attribution models suggests that changes in extremes are likely to be less important than changes in means and variability [28,33,34]. This tentative result sharply contrasts the many studies that show catastrophic impacts of climate extremes, but that do not consider the impacts of means and variability nor manipulate them independently (see reviews [10,17]).
Possibly these contrasting results can be (partly) reconciled: climatologists suggest that current changes in climate extremes are typically caused by changes in the mean climate (the entire distribution shifting; figure 4a) [2], and thus changes in extremes and means are often correlated. Studies that assess the impacts of extremes while not accounting for the confounding effects of changing climatic means may thus over-attribute impacts to extremes, while in fact they also should be partly attributed to correlated changes in mean (and the reverse holds for studies on changes in climate means that ignore correlated changes in extremes, or variability).
(b). An alternative view on attribution
An alternative view is to focus less on attributing impacts to changes in means, variability and extremes, but instead take a more holistic approach of climate change by modelling how the entire climatic distribution changes (ideally using IPCC-class models that account for model uncertainty [38]) and how this in turn affects biological systems. Such an approach does not allow for attributing impacts to climate extremes per se, but it does allow for attribution of impacts to climate change that simultaneously includes changes in climatic mean, variability and extremes (currently most studies narrowly focus on a single aspect—typically means).
Such a holistic approach also circumvents another problem of attribution, which is that the impact of one aspect of climate often depends on other aspects and thus full separation is always difficult (e.g. the impact of changes in variability depends on the (changes in) mean climatic conditions [28,33]). This not only holds for interactions among climatic drivers, but also for interactions between climatic and non-climatic drivers, as non-additive interactions among environmental drivers appear to be the norm rather than exception [39,40]. More generally, it has been argued that the emphasis on biological attribution might also be counterproductive [41]. Possibly we should focus more on understanding how different environmental drivers—of which extremes is only one—interact with each other to affect systems, as providing proof via attribution is distracting biologists from these pressing scientific questions that need to be addressed for making more reliable predictions and practical conservation measures [42].
4. Links to other scientific fields
For effectively delimiting and applying the biological science of ECEs, it is useful to briefly visit other research fields that also deal with abrupt and severe changes in nature, and to identify their commonalities and differences with ECE research. Here we discuss these other fields with reference to (i) the spatial and temporal scale of drivers and impact, (ii) whether the agents of change are principally climatological, physical or biological in nature (or a combination of these), and (iii) whether the biological impact versus the agent of change is ‘extreme’. Below we will give concrete examples from related research fields to illustrate how these three factors provide overlap with or deviations from the ECE research field.
(a). Spatial and temporal scale of drivers and impact
While this review, and ECE research in general, has focused mostly on extreme events and their impact at local to regional scales and at time scales from days to centuries, ECEs both overlap and differ from the planetary-scale extreme events (PEEs) or ‘catastrophes’ that have traditionally been the domain of paleo-ecology. While the agents of ECEs and PEEs are both abiotic in nature, they differ importantly in that ECEs are climate-induced while PEEs are principally physical, even though they generally lead to climatic or atmospheric regime shifts as well. An obvious example of an internal physical agent of a PEE is a chain of major volcanic eruptions, while major meteorite impact is due to an external physical agent. Such PEEs tend to occur not only on an enormous (i.e. global) spatial scale, but also at very long (i.e. geological) time intervals. They are also known for their enormity of biological impact, causing planetary environmental regime shifts (with biotic feedbacks possibly causing further drastic changes) and mass organismal extinctions, and driving macro-evolution [7].
(b). The nature of drivers of abrupt and severe change
(i). Fire
A special and, in the ECE and global change context, particularly important driver of abrupt and severe environmental change is fire. Fire has in common with ECEs that it is strongly (albeit not only; see below) linked to abiotic (i.e. climatic) drivers and that it can have very severe biological impact. Because of these commonalities, fire ecology, as an established research field, has supplied several methodological (including statistical) tools that are also proving useful for the biological study of ECEs. For instance, a demographic model originally developed for post-fire disturbance response has been applied to address the effect of extreme years (in terms of weather) on population growth of birds [38,43].
There are also important differences between ECE ecology and fire ecology. Crucially, while a wildfire itself can be very destructive and have major biological impact, it need not be the result of a climate extreme. For instance, the Fynbos in South Africa has frequent (less than 10-year interval) fire regimes under ‘normal climatic conditions’. ‘Extreme’ wildfires are most likely to occur in regions where fire regimes are very low and when several environmental drivers coincide. While prolonged drought is evidently a major driver of wildfire, even under certain milder-than-extreme environmental conditions in terms of drought and high temperatures, a very destructive wildfire with extreme biological impact can still occur if other biotic and abiotic drivers join in. In particular, the fuel quantity (i.e. accumulated living or dead organic matter on the surface, sometimes including peat layers) and fuel quality (especially physical structure enabling good ventilation for oxygen supply) are critical, while windy conditions help to ventilate and spread the fire [44]. Importantly also, no wildfire can start without an ignition source, be it of abiotic (lightning, volcanic eruption) or biotic nature, i.e. accidental or intentional ignition by people.
In practice, evidently, ECEs and extreme wildfires often go hand in hand. The great 2007 Anaktuvuk fire in the Alaskan tundra occurred after a prolonged period of very dry and hot summer weather and destroyed an area of approximately 1000 km2 and released almost 2 megatonnes of carbon in the process [45]. Thus, wildfires are a kind of abiotic intermediary to translate severe or even ECEs into biological impact via interaction with the local (remains of) organisms; and it is an intermediary of particular interest in view of global change and the growing and increasingly mobile world population, which together are set to induce stronger fire regimes in several biomes this century [46].
(ii). Biotic agents
While ECEs are ultimately always of abiotic origin and fire has both physical and biological components to its destructive force, some other agents of severe ecological impact are principally biological in nature. Herbivory, like fire, is often considered as a disturbance sensu Grime [47], i.e. as a process by which plant biomass (or sessile coral biomass) is killed, either entire individuals or parts of them. Herbivory can range in biological impact from mild to very severe. There are myriad examples of severe overgrazing leading to a major shift in ecosystem properties and often loss of function, for instance in the case of associated soil degradation and erosion [48]. Other examples include large-scale pine forest die-back due to beetle attack in Canada, which was shown to turn a large forested region from a carbon sink into a carbon source [49]. In such cases the biological impact may be of comparable magnitude (but not necessarily of comparable type) to that of an ECE, while the driver is totally different.
Some events combine the ECE concept and biologically driven disturbance impact. For instance, in subarctic Fennoscandia periodic outbreaks of the autumn moth, Epirrita autumnata, have been associated with winter climatic conditions. A few subsequent winters in which air temperatures never drop much below −30°C, i.e. ‘exceptionally warm’ winters, allow many eggs on the birch trees to survive. If favourable summer and autumn conditions for completion of the life cycle coincide with the moth population already being close to a major peak, they can lead to complete defoliation of virtually all birch trees by the Epirrita caterpillars over areas of hundreds of km2 [50]. When persisting for a few subsequent years, these outbreaks can turn birch forest into open heathland or tundra for several decades [51]. Thus the major biological impact in such a hybrid case is due to a combination of special climatic events coinciding with or triggering disturbance by (herbivorous) organisms (see also [13] for interactions of ECE and disturbance). As an extension of this concept, major biological impact can also be expected if an ECE leads to the loss [13] or abundant establishment of ecosystem engineers, as these organisms, by definition, have a disproportionate impact on their environment [52].
(c). Moderate drivers of change but severe impact: tipping points
Tipping point ecology centres on the concept ‘that gradual changes in temperature or other factors might have little effect until a threshold is reached at which a large shift occurs that might be difficult to reverse’ ([29], p. 648; see also [53,54]). This concept has in common with the ECE field that it deals with severe biological impact and it also stresses that the biological response function may be strongly nonlinear at its extremes (figure 1). Also, like ECE ecology, tipping point ecology has parallels with, and applications in, very different scientific and societal disciplines, e.g. medical [55] or financial [56]. However, there are two essential differences. Tipping points, by definition, define environmental regime shifts from one ‘stable’ state to an alternative one (figure 3a). In contrast, while ECEs can lead to such regime shifts, they do not necessarily have to, as they can also have major biological impact without overhauling the essential properties and functions of the ecosystem or the taxonomic and functional composition of organisms involved. Also, tipping points are often reached after a longer period of rather subtle, ‘trickle-wise’ changes in the environmental drivers or the ecosystem itself, i.e. without the explicit need for an ECE. These drivers need not be climatic but can also be physical–chemical or biotic in nature, for instance in the case of regime shifts in lakes subject to chronic nutrient input or introduction of alien fish species [29]. Since environmental thresholds leading to regime shifts, i.e. tipping points, can also be reached in response to extreme events, some environmental scenarios may fall under both concepts. Identifying explicitly for concrete scenarios where, over the trajectory of environmental change, both concepts overlap and where they diverge, will unify both research fields and help to predict environmental impact.
To summarize, ECE research features both important commonalities and differences with related fields such as paleo-ecology, tipping point ecology and disturbance ecology (including fire ecology) and these fields can learn from one another. Useful conceptual and methodological tools can be derived from these relatively established fields of study, while explicit comparison of theory and practice will also lead to ECE research feeding new insight into these related fields.
5. Parallels between behavioural, ecological and evolutionary extreme climatic event studies
(a). This theme issue
The 13 other contributions to this theme issue on Behavioural, ecological and evolutionary responses to extreme climatic events are structured in four parts. The first part focuses on general challenges to the field, such as those related to detection, attribution and thereby prediction of ECEs and their impact. The next three parts focus on the behavioural/plastic, ecological and evolutionary responses to ECEs. One goal of our theme issue was to invite contributions on topics that reflect important gaps in our knowledge (e.g. the evolution of plasticity in extreme environments [57]) or that represent controversial issues (e.g. the value of single event studies [17]). Another goal was to invite contributions from different fields that synthesize the ecological [2,18] and evolutionary literature on ECEs [7,35] and combine this with research papers that illustrate ways to make progress in answering important and interesting conceptual questions. The inclusion of contributions from such disparate fields as behavioural plasticity [23], community ecology [18] and evolution of thermal tolerance [35] was specifically chosen to highlight that these fields deal with similar challenges (e.g. they study events that are rare with respect to the duration of most studies in the wild), but also to illustrate that they can provide parallel insights (see later this section). Importantly, all empirical contributions use long time series (≥2 decades) from studied populations that included the occurrence of multiple ECEs.
(b). Part 1: Conceptual challenges and links to other fields
In the first part of the theme issue, Ummenhofer & Meehl [2] review our current understanding of climatological changes in ECEs and how they are assessed. They provide an overview of the existing evidence for change in climate extremes, focusing on climate variables relevant for both terrestrial and oceanic systems. By doing so they highlight that much progress has been made in assessing climate extremes and that further progress is expected due to the continued spatio-temporal downscaling of process-based climate models. Their discussion of the challenges in detecting and attributing climate extremes to climate change also provides an interesting parallel to the second contribution in this part by Solow [6]: climatologists have made important steps in attribution by developing a better understanding of the processes underlying their climate models, and Solow argues that for the question of attributing ecological responses to climate extremes, a good mechanistic understanding will also prove to be crucial. He illustrates this using a simple example that shows that it is hard to statistically attribute biological extremes to climate extremes and that this either requires very long time series and/or strong signals (see [5] for a real world example) to avoid low power. Subsequently, he shows how a mechanistic understanding will increase this power substantially, and even may allow for predicting biological impacts when few climate extremes are observed. The importance of such mechanistic understanding about how climate extremes affect organisms, population and ecosystems is repeatedly emphasized in the contributions of subsequent parts, although the starting point of studies can be very different (e.g. some studies take a known physiological mechanism as a starting point [20], while others use an exploratory correlative approach to focus the search for mechanisms [5]).
The first part concludes with an opinion piece by Altwegg et al. [17] discussing the controversial question: What can we learn from the many studies describing responses to a single ECE? A literature review shows that single event studies using experimental or opportunistic studies tend to be short-term, while only long-term observational studies that accidentally experienced an ECE investigated delayed responses. Moreover, besides the obvious difficulty of estimating the biological response from a single event, another limitation is that it prevents assessment on how any response depends on the state of the study system (see §3 this study, [35]). They propose a data- and theory-driven pathway for how single event studies may improve our understanding of ECEs, but for the former pathway the required information for meta-analysis is typically not reported, while for the latter pathway sufficient mechanistic understanding is lacking for most study systems.
(c). Part 2: Plastic responses to extreme climatic events
The second part of the theme issue deals with phenotypically plastic responses to ECEs. Chevin & Hoffmann [57] discuss how likely it is that species will adapt their phenotype adaptively under ECEs. They argue that there may often only be weak selection on plasticity at extreme conditions, as ECEs are rare or mainly affect low-quality habitats. A key process in shaping the phenotypes under ECEs is the genetic correlation across environments: if the response to mild values of the environmental variable correlates with the response for extreme values, and the optimum phenotype changes linearly from mild to extreme environments, there may be adaptive phenotypic plasticity for ECE. Wingfield et al. [20] use an interesting alternative approach to plasticity in response to ECEs. They do not consider how a phenotype is shaped under ECE compared to how it is shaped under non-extreme conditions but rather argue that there is an entire different phenotype that occurs during ECEs. They define ECEs as those conditions where an individual's available resources are not sufficient to match the sum of its energetic costs (called allostatic overload), which then triggers an emergency life-history stage when an individual ceases its regular activities in an attempt to survive the extreme conditions. Part two ends with Bailey et al. [23]'s study on a natural system where ECEs lead to flooding of shorebirds' nests. In their system, the frequency of extreme tidal floods has more than doubled. Despite this, they found no evidence of behavioural plasticity in nest elevation over a 20 year period, either as a response to two environmental cues or as a learned response to previous flooding experience. They discuss the lack of a plastic response in the context of the low predictability and detectability of ECEs and their potential cues.
(d). Part 3: Ecological responses to extreme climatic events
The theme issue's third part on the ecological responses to ECEs starts with a review by Felton & Smith [18] on another gap in our knowledge: How do impacts of ECEs cascade hierarchically from the individual to population to community and ultimately to the ecosystem level?, with a specific focus on arguably the best-studied ECE-model system: plants. They suggest that the scaling of individual responses to community or ecosystem responses is often predicated upon the functional identity of the species in the community, in particular the dominant species. Furthermore, the reported stability in ecosystem structure and functioning is often driven by processes at the community level, such as species niche partitioning and compensatory responses during or after the event.
The third part continues with three empirical papers investigating responses at either the species, population or individual level. Palmer et al. [5] use population time series of 238 British insect and bird species to address the question to what extent closely related species show temporal synchrony in population crashes or explosions and whether these can be attributed to specific climate extremes. It turns out that species generally do not agree on which years were extreme, and that responses (crashes outweighing explosions) were highly species-specific, also with respect to climatic drivers. Finally, ECEs did not predict long-term population trends, suggesting that ECEs were not driving these species’ historical declines. Pardo et al. [34] assessed the impact of changes in the mean, variability and extremes of sea surface temperatures on the demography of black-browed albatrosses (Thalassarche melanophris). They showed that a change in the mean of sea surface temperature had a positive effect on the population growth rate, despite causing more frequent and larger ECEs that negatively affect the growth rate. This in-depth study echoes the large-scale analysis of Palmer et al. [5], which concluded that the population trends of many species have not yet been dominated by ECEs.
Finally, Gardner et al. [58] study how ECEs affect individual fitness and demography in two Australian wrens. Interestingly, they do not only take the increase in extreme warm weather into account, but also the decrease in extreme cold winters. Similar to the avian population responses in Palmer et al. [5], demographic response of these two closely related similar-sized sympatric species was very different. In fairy-wrens (Malurus elegans) summer survival was higher in hot summers and after winters with few cold wet days, while in scrubwrens (Sericornis frontalis) winter survival was lower in cold wet winters. Unexpectedly, this did not result in an increased annual survival over time, but rather a decreasing survival in both species, suggesting other factors outweighed or prevented individual level impacts of ECE from cascading onto population demography.
(e). Part 4: Evolutionary responses to extreme climatic events
The final part of the theme issue focuses on evolutionary responses to ECEs. Grant et al. [7] discuss parallels between evolutionary processes acting on geological timescales and contemporary evolution in recent periods, by suggesting that ECEs are small-scale analogues of the dramatic changes documented in the fossil record. The review discusses a number of case studies on evolutionary responses in a wide variety of taxa to recent episodic and prolonged ECEs. They conclude that evolution in response to ECEs is likely to be widespread, as they set up strong selection pressures, particularly if ECEs alter community composition causing changes in species interactions. Kingsolver & Buckley [35] argue that to understand how ECEs affect selection and evolutionary responses, a better knowledge of the causal connections among climate conditions, phenotypes and fitness is needed. They use thermal biology (thermal performance curve and heat tolerance), in combination with extreme value theory (generalized extreme value distributions), as a quantitative framework for such a more mechanistic understanding. While this framework is useful, they explain that it is hampered by knowledge on the upper tails of performance curves (see also §6) and by the lack of incorporation of important effects of prior thermal history on performance and tolerance into models of climate change response. Finally, the last contribution of Marrot et al. [59] quantifies the effects of ECE on the fitness landscape (i.e. the linear selection gradients) for clutch size and egg-laying date in blue tits (Cyanistes caeruleus). For ECEs to affect the fitness landscape it is essential that the fitness of different phenotypes is differentially affected by ECEs, rather than that ECEs decrease fitness for all individuals in the population (see also [57]). There was no effect of ECEs on the strength of selection on clutch size but the strength of selection for earlier laying increased with the proportion of nests exposed to extreme hot days. Interestingly, the mean temperatures during the nestling period did not affect the strength of selection on laying date, suggesting that it is indeed the ECEs that cause the elevated selection.
(f). Parallels between behavioural, ecological and evolutionary extreme climatic event studies
We already highlighted the shared challenges that behavioural, ecological and evolutionary ECEs studies face in terms of deciding what an appropriate definition is (§2), and how to deal with the problem of attribution (§3). Another major parallel among studies in this theme issue appears to be that responses are highly idiosyncratic, indicating that generalization of ECE impacts is difficult and predictability low. For example, evolutionary responses in thermal tolerance in response to extremes can depend on the climatic history and amount of variability organisms previously experienced [35], and evolutionary change is often strongly mediated by changes in species interactions, which vary widely among ecosystems [7]. Behaviourally plastic responses may depend on the habitat type individuals live in [23] or on their energy reserves [20], while demographic responses can be highly age-dependent [34]. Potentially as a consequence, the responses of two populations of the same species have been shown to be as different as the responses of two different plant species to the same type of climatic extreme [60]. Thus to some extent, it may not be surprising that there is also very little consensus in responses to extremes when comparing closely related species [5], even if they live in the same area and have a similar ecology and body size [58]. These are not only empirical patterns (but see [61]), but there are also many theoretical reasons why one would expect a strong context-dependency at many levels of organization (e.g. [18]) or for evolutionary (e.g. [57]) and ecological processes (e.g. [28]).
Whether a strong context-dependence is a characteristic property of response to ECEs remains to be determined, as responses to other aspects of climate or environmental change are often also highly idiosyncratic [62]. McLean et al. [63] discuss four studies that have so far formally compared the amount of intraspecific and interspecific variation in climate sensitivities across a large group of species (all looking at phenotypic traits at the individual level). The only study on climate extremes showed that there was huge intraspecific variation and thus low predictability in plant biomass responses [60], while studies on responses to changes in climate means showed strong intraspecific consistency in phenological traits [64,65], but not for avian body mass [63]. A more direct avenue to explore this further would be to quantify if there is less consistency (more idiosyncrasy) in species responses to climate extremes than to, for example, climate mean on the same dataset, as could be done for the large comparative dataset analysed by Palmer et al. [5].
6. A roadmap for future research on extreme events
(a). Improving our approach of extreme climatic event studies
The previous sections already highlighted some important directions to make progress in our approach of ECE studies. Although a synthetic definition that will be universally useful may not be achievable, more specific definitions and using similar terminology will be key to facilitate meta-analyses and systematic reviews of ECE studies, which are crucial steps in the development of any research field. We should also make optimal use of the limited information we already have, which includes learning from the many anecdotal single events studies that currently dominate the literature [17].
Notwithstanding, it is clear that insights on the long-term ecological and evolutionary consequences of ECEs can only be derived from long-term studies [7,10,17]. To address this challenge, we may need to focus on model systems in which ECEs are becoming rapidly either more frequent and severe (e.g. heat waves [58], flooding [23]) or more infrequent and mild (e.g. cold spells, icesheet cover [10,66]). Moreover, we should make smart use of a combination of observational studies (using both temporal and spatial variation in ECEs), controlled experiments, biological and climatological modelling [10], as currently already attempted in the field of thermal ecology [35]. The field of ECE ecology is not unique in tackling such challenges and we can learn from related fields (climatology, disturbance and paleo-ecology) in terms of conceptual and methodological approaches as well as their historical development (see examples in §§3–4).
(b). Key conceptual challenges to improve our understanding of extreme climatic event impacts
Based on the insights from the papers in this theme issue and our assessment of the field, we outline five more conceptual objectives that we believe the field should aim to fulfil. These include (i) more focus on understanding of the biological response function, (ii) studies on the mechanisms underlying these response functions, (iii) the role of plasticity in the response to ECEs, (iv) understanding how effects of ECEs at the individual levels cascade up to the ecosystem level, and (v) understanding the role of ECEs in long-term evolution. We are aware that there are many more aspects of ECEs that are in need of a better understanding, but we think the five mentioned above and detailed below are on the forefront of where we should put our research efforts, as they will be key to further our understanding of the impact of ECEs.
(i). Understanding the biological response function
The key to understanding and predicting the ecological and evolutionary responses to ECEs is the shape of the biological response curve, as this ultimately translates changes in the climate distribution into changes in the distribution of biological responses (figure 1). An outstanding question is whether extreme biological responses to extreme climate are generally the result of a strong nonlinear biological response function (figure 2c,d) or that responses are typically more linear (figure 2a,b). A nonlinear response function may signify for example at the organismal level that thresholds for normal functioning are exceeded (e.g. an individual has to revert to an emergency ‘survival’ life-stage [20], or a different physiological mechanism is triggered [67]), and in such cases there is good reason to focus on the particular mechanisms by which organisms respond to extremes. By contrast, a more linear response function may signify that similar (e.g. physiological) mechanisms are involved in responses to changes in non-extreme climate values, and a specific focus on extremes may not be needed to improve our understanding and predictive ability [10].
All the observations of a biological system—not only those at the extreme tails—should be used to estimate the biological response function, which will also provide insights under which—not necessarily extreme—climatic conditions an extreme biological response will occur (e.g. figure 2a). Several challenges need to be tackled to obtain the response function. First, it requires longitudinal or large-scale spatial data obtained from long-term surveys or experimental settings to be able to robustly fit the biological response function (figure 2; [17,35]). Second, it is typically inevitable that some assumption has to be made about the shape of (parts of) the function, which requires a good understanding of the biological system [6]. Especially at the extremes of the distribution it will be impossible to estimate the shape reliably (due to the inherent rareness of extremes), but potentially very long-term studies and comparative analysis may provide insights into the general shape of response functions at the tails. Furthermore, specialized statistical methods and improved experimental design (e.g. more sampling at tails of thermal performance curves [35]) can help to more reliably assess the shape at both tails of the response function. Notwithstanding, even the many studies that have only experienced a single anecdotal ECE are valuable: a single ECE part of a longer time series of non-ECE years can still be used to determine whether the observed biological response to an ECE is what would be predicted from extrapolating the known relationship between biological and climatic variables from non-ECE conditions [17]. Finally, responses can be delayed [17] and may depend on the timing of the event relative to the life cycle of the organisms [58,59], an individual's state or habitat [20,23] and previous exposure to ECEs [35,57]. In the long run, mechanistic models will be needed rather than correlative models, particularly if we also want to predict how impacts depend on the timing or succession of ECEs.
(ii). Understanding the mechanisms underlying the response function
A mechanistic understanding of the relationship between climate and the biological response is extremely valuable as it not only increases the power to correctly attribute responses, but may even allow predicting biological impacts when few climate extremes are observed [6]. Models, experiments and observational studies (either over long periods or across spatial gradients) can all contribute to a mechanistic understanding of the ECE impact of ecological and evolutionary processes [10,17]. Bailey & van de Pol [10] discuss a case study illustrating that combining different approaches might be particularly valuable in ECE studies, and that the resulting mechanistic understanding can improve our predictive capabilities: a longitudinal study on a Dutch shorebird suggested that extremely cold winters can lead to mass mortality, but only appeared to do so in years with low food abundance [68]. Yet the relatively short study period (‘only 25 years with two extreme winters’) and limited geographical range made it difficult to attach confidence and generality to this conclusion. Future field studies in the region were able to corroborate this result in both Germany and elsewhere in the Netherlands [69,70], but work in the United Kingdom, where winters are milder, showed no such interaction between extremely cold winter temperatures and low food stocks [71]. The outcomes of many experiments and field studies on the feeding and distribution ecology and eco-physiological studies on the energetics of these shorebirds [72] were integrated into a mechanistic model, which helped explain these differences in survival patterns, concluding that mass mortality would only be likely to occur in the United Kingdom if winter severity were to increase in magnitude [73].
(iii). Understanding the role of plasticity in the response to extreme climatic events
An outstanding question is under what circumstances adaptive plasticity to ECEs may be able to evolve. The first theoretical ideas on this are now appearing [57], and they require empirical testing. It is important to know whether biological responses to extreme events and ordinary conditions are genetically correlated, as this may facilitate the evolution of plasticity and adaptation to ECEs [57]. A phenotypically plastic response before an ECE occurs requires a cue for organisms to respond to in order to mitigate the impact. A major unknown is whether predictable cues for ECEs exist, whether organisms are capable of detecting such cues [74] and whether their predictability is high enough for plasticity to evolve [23]. A phenotypically plastic response during or after an ECE provides an alternative mechanism to mitigate the impacts of ECE, and the existence of an emergency life-stage [20] implies that organisms have already evolved mechanisms to deal with ECEs. This should remind us that organisms have evolved on a planet that has previously undergone large shifts in climate, including changing extremes. Current global change differs from previous geological periods in the unprecedented rate of change and in that it occurs in a world already threatened by many other anthropogenic drivers. Such conditions not only are more likely to drive catastrophes such as the extinction crises that punctuate geological time [7], but also mean that plastic responses and evolutionary rescue require tackling multiple problems simultaneously.
(iv). Understanding how effects of extreme climatic events cascade across organizational levels
The effects of ECEs typically differ among individuals according to their behaviour, state, age, habitat or history [20,23,34,43,62]. However, we know little about how individual heterogeneity may buffer the effect of ECEs on population dynamics and whether it enhances future ECE tolerance by driving selective mortality and selecting for higher quality individuals. Comprehensive eco-evolutionary studies on how ECEs affect survival, mating success and reproduction as a function of various individual traits may help to improve our understanding of the importance of individual heterogeneity for population ecology [43] and for evolutionary responses to ECEs. The eco-evolutionary feedbacks between individual and population processes are only one example of how we need to improve our understanding of how effects of ECEs cascade across levels of biological organization.
Felton & Smith [18] argue that future research efforts to scale individual responses to community or ecosystem processes should focus on assessing the responses of functionally important species in the community, and relate these to the broader community context and ecosystem function. Prior research suggests that community-level properties and processes such as functional diversity, beneficial interactions and species invasions all have the potential to modify community and ecosystem resilience to ECEs. Thus, integrating population- and community-level processes into investigations of ECEs will be important in bridging individual to ecosystem responses.
However, we should not think about this topic in isolation, as the mechanisms that facilitate or prevent cascading effects of ECEs will often be shared mechanisms that also cause or buffer cascading responses to other sources of environmental change. Ultimately, a better understanding of the conditions that determine whether impacts cascade (or not) across hierarchical levels will be crucial for understanding the idiosyncrasy of responses at higher levels of organization (such as community and ecosystem responses, which are also of most concern from a conservation perspective).
(v). Understanding the role of extreme climatic events in long-term evolution
Gutschick & BassiriRad [11] posited that selective pressures imposed by ECEs may often be so strong that they outweigh the importance of selection acting throughout the many interspersed non-extreme normal years, and consequently that ECEs may be a major driver of evolutionary change. This idea is also important for understanding observed patterns of phenotypic change in longitudinal studies as it may also lead to trait variation in combination with long periods of directional selection, which is difficult to reconcile without taking the impacts of these rare ECEs into account [75]. However, we only have limited knowledge of whether ECEs are typically selective or instead reduce the fitness of all phenotypes [57]. Furthermore, a review of some of the most exemplary natural case studies on evolutionary responses in which ECEs have been suggested to have played a role concludes that few demonstrations of evolutionary change can so far be unambiguously tied to an ECE [7]. Yet, the same review also argues that there are many reasons why micro-evolutionary responses to ECEs are nonetheless likely to be widespread. In contradiction to the idea that ECEs dominate the fitness landscape, a recent meta-analysis of phenotypic selection in natural populations did not detect any association of heat waves or short-term droughts with spatio-temporal variation in selection; selection was instead associated with other aspects of climate such as mean precipitation [76]. Clearly, the role of ECEs in long-term evolution is still highly uncertain and we need more meta-analyses on the selective nature and strength of ECEs.
(c). In conclusion
The field of ECEs is undergoing rapid growth; this theme issue shows it's state of the art. It is too early to make strong generalizations, but we have mapped avenues along which the field can develop and learn from related fields. Understanding the behavioural, ecological and evolutionary impacts of ECEs is however crucial in a world where due to global climate change these ECEs will be rapidly increasing in frequency in the decades to come.
Acknowledgements
We thank Chris Thomas, Lauren Buckley and an anonymous reviewer for their constructive feedback, and M.v.d.P. would like to thank Liam Bailey for stimulating discussions.
Biographies
Guest editor's profiles
 Martijn van de Pol is a researcher at the Australian National University and Netherlands Institute of Ecology (NIOO-KNAW). He is an ecologist interested in the impact of the different aspects of climate change, particularly climate variability and extremes, and how they interact and accumulate with other aspects of environmental change and with individual heterogeneity. His works combines demographic and population modelling, developing statistical applications, and analyses of long-term field studies.
Martijn van de Pol is a researcher at the Australian National University and Netherlands Institute of Ecology (NIOO-KNAW). He is an ecologist interested in the impact of the different aspects of climate change, particularly climate variability and extremes, and how they interact and accumulate with other aspects of environmental change and with individual heterogeneity. His works combines demographic and population modelling, developing statistical applications, and analyses of long-term field studies.
 Stéphanie Jenouvrier is an associate scientist at the Woods Hole Oceanographic Institution (USA) and Centre d’études Biologiques de Chizé (France). She is an ecologist interested in understanding and predicting the effect of climate change on life history and population dynamics, especially for seabirds in the Southern Ocean. Her work combines long-term longitudinal data with demographic, statistical and mathematical models coupled with climates models participating in the Intergovernmental Panel on climate change assessment.
Stéphanie Jenouvrier is an associate scientist at the Woods Hole Oceanographic Institution (USA) and Centre d’études Biologiques de Chizé (France). She is an ecologist interested in understanding and predicting the effect of climate change on life history and population dynamics, especially for seabirds in the Southern Ocean. Her work combines long-term longitudinal data with demographic, statistical and mathematical models coupled with climates models participating in the Intergovernmental Panel on climate change assessment.
 Marcel E. Visser is head of department of the Animal Ecology at the Netherlands Institute of Ecology (NIOO-KNAW) and also professor on Seasonal Timing of Behaviour at Groningen University and on Ecological Genetics at Wageningen University. He is an evolutionary ecologist interested in the seasonal timing of reproduction and growth. As seasonal timing is strongly affected by global climate change he has been working on how species within the same food chain shift their timing differentially, whether natural selection will lead to adaptation to the changing environment and on the population consequences if this adaptation is not fast enough to keep up with climate change. His work combines long-term population studies on trees, insects and birds with laboratory experiments, genomic analysis and theoretical modelling.
Marcel E. Visser is head of department of the Animal Ecology at the Netherlands Institute of Ecology (NIOO-KNAW) and also professor on Seasonal Timing of Behaviour at Groningen University and on Ecological Genetics at Wageningen University. He is an evolutionary ecologist interested in the seasonal timing of reproduction and growth. As seasonal timing is strongly affected by global climate change he has been working on how species within the same food chain shift their timing differentially, whether natural selection will lead to adaptation to the changing environment and on the population consequences if this adaptation is not fast enough to keep up with climate change. His work combines long-term population studies on trees, insects and birds with laboratory experiments, genomic analysis and theoretical modelling.
Competing interests
We declare we have no competing interests.
Funding
M.v.d.P. was supported by an Australian Research Council Future fellowship (FT120100204); S.J. acknowledges support of NSF (award 1246407).
References
- 1.Committee on Extreme Weather Events and Climate Change Attribution, Board on Atmospheric Sciences and Climate, Division on Earth and Life Studies & National Academies of Sciences, Engineering, and Medicine. 2016. Attribution of extreme weather events in the context of climate change. Washington, DC: National Academies Press; [Accessed 20 June 2016.] [Google Scholar]
- 2.Ummenhofer CC, Meehl GA. 2017. Extreme weather and climate events with ecological relevance: a review. Phil. Trans. R. Soc. B 372, 20160135 ( 10.1098/rstb.2016.0135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO. 2001. Climate extremes: observations, modeling, and impacts. Science 289, 2068–2074. ( 10.1126/science.289.5487.2068) [DOI] [PubMed] [Google Scholar]
- 4.Managing the risks of extreme events and disasters to advance climate change adaptation. A special report of working groups I and II of the Intergovernmental Panel on Climate Change. 2012. IPCC. (eds CB Field et al.). Cambridge, UK and New York, NY: Cambridge University Press. See https://www.ipcc.ch/pdf/special-reports/srex/SREX_Full_Report.pdf. [DOI] [PubMed]
- 5.Palmer G, et al. 2017. Climate change, climatic variation and extreme biological responses. Phil. Trans. R. Soc. B 372, 20160144 ( 10.1098/rstb.2016.0144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solow AR. 2017. On detecting ecological impacts of extreme climate events and why it matters. Phil. Trans. R. Soc. B 372, 20160136 ( 10.1098/rstb.2016.0136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant PR, Grant BR, Huey RB, Johnson MTJ, Knoll AH, Schmitt J. 2017. Evolution caused by extreme events. Phil. Trans. R. Soc. B 372, 20160146 ( 10.1098/rstb.2016.0146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrewartha HG, Birch LC. 1954. The distribution and abundance of animals. Chicago, IL: University of Chicago Press. [Google Scholar]
- 9.Parsons PA. 1997. Extreme environmental change and evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 10.Bailey LD, van de Pol M. 2016. Tackling extremes: challenges for ecological and evolutionary research on extreme climatic events. J. Anim. Ecol. 85, 85–96. ( 10.1111/1365-2656.12451) [DOI] [PubMed] [Google Scholar]
- 11.Gutschick VP, BassiriRad H. 2003. Extreme events as shaping physiology, ecology, and evolution of plants: toward a unified definition and evaluation of their consequences. New Phytol. 160, 21–42. ( 10.1046/j.1469-8137.2003.00866.x) [DOI] [PubMed] [Google Scholar]
- 12.Jentsch A, Kreyling J, Beierkhunlein C. 2007. A new generation of climate-change experiments: events, not trends. Front. Ecol. Envrion. 5, 365–374. ( 10.1890/1540-9295(2007)5%5B365:ANGOCE%5D2.0.CO;2) [DOI] [Google Scholar]
- 13.Smith MD. 2011. An ecological perspective on extreme climatic events: a synthetic definition and framework to guide future research. J. Ecol. 99, 656–663. ( 10.1111/j.1365-2745.2011.01798.x) [DOI] [Google Scholar]
- 14.Moreno J, Moller AP. 2011. Extreme climatic events in relation to global change and their impact on life histories. Curr. Zool. 57, 375–389. ( 10.1093/czoolo/57.3.375) [DOI] [Google Scholar]
- 15.Niu S, Luo Y, Li D, Cao S, Xia J, Li J, Smith MD. 2014. Plant growth and mortality under climatic extremes: an overview. Environ. Exp. Bot. 98, 13–19. ( 10.1016/j.envexpbot.2013.10.004) [DOI] [Google Scholar]
- 16.Bokhorst S, Bjerke JW, Tømmervik H, Preece C, Phoenix GK. 2012. Ecosystem response to climatic change: the importance of the cold season. AMBIO 41, 246–255. ( 10.1007/s13280-012-0310-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altwegg R, Visser V, Bailey LD, Erni B. 2017. Learning from single extreme events. Phil. Trans. R. Soc. B 372, 20160141 ( 10.1098/rstb.2016.0141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felton AJ, Smith MD. 2017. Integrating plant ecological responses to climate extremes from individual to ecosystem levels. Phil. Trans. R. Soc. B 372, 20160142 ( 10.1098/rstb.2016.0142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephenson DB, Diaz H, Murnane R. 2008. Definition, diagnosis, and origin of extreme weather and climate events. New York, NY: Cambridge University Press. [Google Scholar]
- 20.Wingfield JC, Pérez JH, Krause JS, Word KR, González-Gómez PL, Lisovski S, Chmura HE. 2017. How birds cope physiologically and behaviourally with extreme climatic events. Phil. Trans. R. Soc. B 372, 20160140 ( 10.1098/rstb.2016.0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young PC. 2002. Advances in real-time flood forecasting. Phil. Trans. R. Soc. Lond. A 360, 1433–1450. ( 10.1098/rsta.2002.1008) [DOI] [PubMed] [Google Scholar]
- 22.Sarewitz D, Pielke R Jr. 2001. Extreme events: a research and policy framework for disasters in context. Int. Geol. Rev. 43, 406–418. ( 10.1080/00206810109465022) [DOI] [Google Scholar]
- 23.Bailey LD, Ens BJ, Both C, Heg D, Oosterbeek K, van de Pol M. 2017. No phenotypic plasticity in nest-site selection in response to extreme flooding events. Phil. Trans. R. Soc. B 372, 20160139 ( 10.1098/rstb.2016.0139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seneviratne SI, et al. 2012. Changes in climate extremes and their impacts on the natural physical environment. In Managing the risks of extreme events and disasters to advance climate change adaptation (eds Field CB, et al.), pp. 109–230. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 25.van de Pol M, Bailey LD, McLean N, Rijsdijk L, Lawson CR, Brouwer L. 2016. Identifying the best climatic predictors in ecology and evolution. Methods Ecol. Evol. 7, 1246–1257. ( 10.1111/2041-210X.12590) [DOI] [Google Scholar]
- 26.Knapp AK, Smith MD. 2001. Variation among biomes in temporal dynamics of aboveground primary production. Science 291, 481–484. ( 10.1126/science.291.5503.481) [DOI] [PubMed] [Google Scholar]
- 27.Kingsolver JG, Higgins JK, Augustine KE. 2015. Fluctuating temperatures and ectotherm growth: distinguishing non-linear and time-dependent effects. J. Exp. Biol. 218, 2218–2225. ( 10.1242/jeb.120733) [DOI] [PubMed] [Google Scholar]
- 28.Lawson CR, Vindenes Y, Bailey L, van de Pol M. 2015. Environmental variation and population responses to global change. Ecol. Lett. 18, 724–736. ( 10.1111/ele.12437) [DOI] [PubMed] [Google Scholar]
- 29.Scheffer M, Carpenter SR. 2003. Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol. Evol. 18, 648–656. ( 10.1016/j.tree.2003.09.002) [DOI] [Google Scholar]
- 30.Hegerl GC, Hoegh-Guldberg O, Casassa G, Hoerling MP, Kovats R, Parmesan C, Pierce DW, Stott PA. 2010. ‘Good practice guidance paper on detection and attribution related to anthropogenic climate change.’ Meeting report of the Intergovernmental Panel on Climate Change Expert Meeting on Detection and Attribution of Anthropogenic Climate Change, 14–16 September 2009 IPCC Working Group I Technical Support Unit, University of Bern, Bern, Switzerland: (See http://espace.library.uq.edu.au/view/UQ:236549.) [Google Scholar]
- 31.Bindoff NL, Stott PA, AchutaRao KM, Allen MR, Gutzler D, Hansingo K, Hegerl G et al. . 2013. Detection and attribution of climate change: from global to regional. In Climate change 2013: the physical science basis, Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds TF Stocker, D Qin, G-K Plattner, M Tignor, SK Allen, J Doschung, A Nauels, Y Xia, V Bex, PM Midgley), pp. 867–952. Cambridge, UK: Cambridge University Press. ( 10.1017/CBO9781107415324.022). [DOI]
- 32.Sarojini BB, Stott PA, Black E. 2016. Detection and attribution of human influence on regional precipitation. Nat. Clim. Change 6, 669–675. ( 10.1038/nclimate2976) [DOI] [Google Scholar]
- 33.Vasseur DA, DeLong JP, Gilbert B, Greig HS, Harley CDG, McCann KS, Savage V, Tunney TD, O'Connor MI. 2014. Increased temperature variation poses a greater risk to species than climate warming. Proc. R. Soc. B 281, 20132612 ( 10.1098/rspb.2013.2612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pardo D, Jenouvrier S, Weimerskirch H, Barbraud C. 2017. Effect of extreme sea surface temperature events on the demography of an age-structured albatross population. Phil. Trans. R. Soc. B 372, 20160143 ( 10.1098/rstb.2016.0143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kingsolver JG, Buckley LB. 2017. Quantifying thermal extremes and biological variation to predict evolutionary responses to changing climate. Phil. Trans. R. Soc. B 372, 20160147 ( 10.1098/rstb.2016.0147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewontin RC, Cohen D. 1969. On population growth in a randomly varying environment. Proc. Natl Acad. Sci. USA 62, 1056–1060. ( 10.1073/pnas.62.4.1056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyce MS, Haridas CV, Lee CT. 2006. Demography in an increasingly variable world. Trends Ecol. Evol. 21, 141–148. ( 10.1016/j.tree.2005.11.018) [DOI] [PubMed] [Google Scholar]
- 38.Jenouvrier S. 2013. Impacts of climate change on avian populations. Glob. Change Biol. 19, 2036–2057. ( 10.1111/gcb.12195) [DOI] [PubMed] [Google Scholar]
- 39.Crain CM, Kroeker K, Halpern BS. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315. ( 10.1111/j.1461-0248.2008.01253.x) [DOI] [PubMed] [Google Scholar]
- 40.Darling ES, Côté IM. 2008. Quantifying the evidence for ecological synergies. Ecol. Lett. 11, 1278–1286. ( 10.1111/j.1461-0248.2008.01243.x) [DOI] [PubMed] [Google Scholar]
- 41.Parmesan C, Burrows MT, Duarte CM, Poloczanska ES, Richardson AJ, Schoeman DS, Singer MC. 2013. Beyond climate change attribution in conservation and ecological research. Ecol. Lett. 16, 58–71. ( 10.1111/ele.12098) [DOI] [PubMed] [Google Scholar]
- 42.Parmesan C, Duarte C, Poloczanska E, Richardson AJ, Singer MC. 2011. Overstretching attribution. Nat. Clim. Change 1, 2–4. ( 10.1038/nclimate1056) [DOI] [Google Scholar]
- 43.Jenouvrier S, Péron C, Weimerskirch H. 2015. Extreme climate events and individual heterogeneity shape life-history traits and population dynamics. Ecol. Monogr. 85, 605–624. ( 10.1890/14-1834.1) [DOI] [Google Scholar]
- 44.Rothermel RC.1972. A mathematical model for predicting fire spread in wildland fuels. USDA Forest Service Research Paper INT-115. (See https://www.fs.fed.us/rm/pubs_int/int_rp115.pdf.)
- 45.Mack MC, Bret-Harte MS, Hollingsworth TN, Jandt RR, Schuur EA, Shaver GR, Verbyla DL. 2011. Carbon loss from an unprecedented Arctic tundra wildfire. Nature 475, 489–492. ( 10.1038/nature10283) [DOI] [PubMed] [Google Scholar]
- 46.Stocker T, et al. (eds.) 2013. Climate change 2013: the physical science basis, Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds TF Stocker, D Qin, G-K Plattner, M Tignor, SK Allen, J Doschung, A Nauels, Y Xia, V Bex, PM Midgley). Cambridge, UK: Cambridge University Press.
- 47.Grime JP. 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 111, 1169–1194. ( 10.1086/283244) [DOI] [Google Scholar]
- 48.Srivastava DS, Jefferies R. 1996. A positive feedback: herbivory, plant growth, salinity, and the desertification of an Arctic salt-marsh. J. Ecol. 84, 31–42. ( 10.2307/2261697) [DOI] [Google Scholar]
- 49.Kurz WA, Dymond C, Stinson G, Rampley G, Neilson E, Carroll A, Ebata T, Safranyik L. 2008. Mountain pine beetle and forest carbon feedback to climate change. Nature 452, 987–990. ( 10.1038/nature06777) [DOI] [PubMed] [Google Scholar]
- 50.Tenow O, Nilssen A. 1990. Egg cold hardiness and topoclimatic limitations to outbreaks of Epirrita autumnata in northern Fennoscandia. J. Appl. Ecol. 27, 723–734. ( 10.2307/2404314) [DOI] [Google Scholar]
- 51.Tenow O, Bylund H, Karlsson P, Hoogesteger J. 2004. Rejuvenation of a mountain birch forest by an Epirrita autumnata (Lepidoptera: Geometridae) outbreak. Acta Oecol. 25, 43–52. ( 10.1016/j.actao.2003.10.006) [DOI] [Google Scholar]
- 52.Jones CG, Lawton JH, Shachak M. 1997. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78, 1946–1957. ( 10.1890/0012-9658(1997)078%5B1946:PANEOO%5D2.0.CO;2) [DOI] [Google Scholar]
- 53.Rietkerk M, Dekker SC, de Ruiter PC, van de Koppel J. 2004. Self-organized patchiness and catastrophic shifts in ecosystems. Science 305, 1926–1929. ( 10.1126/science.1101867) [DOI] [PubMed] [Google Scholar]
- 54.Scheffer M, et al. 2009. Early-warning signals for critical transitions. Nature 461, 53–59. ( 10.1038/nature08227) [DOI] [PubMed] [Google Scholar]
- 55.Scheffer M, van den Berg A, Ferrari MD. 2013. Migraine strikes as neuronal excitability reaches a tipping point. PLoS ONE 8, e72514 ( 10.1371/journal.pone.0072514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Battiston S, et al. 2016. Complexity theory and financial regulation. Science 351, 818–819. ( 10.1126/science.aad0299) [DOI] [PubMed] [Google Scholar]
- 57.Chevin L-M, Hoffmann AA. 2017. Evolution of phenotypic plasticity in extreme environments. Phil. Trans. R. Soc. B 372, 20160138 ( 10.1098/rstb.2016.0138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gardner JL, Rowley E, de Rebeira P, de Rebeira A, Brouwer L. 2017. Effects of extreme weather on two sympatric Australian passerine bird species. Phil. Trans. R. Soc. B 372, 20160148 ( 10.1098/rstb.2016.0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marrot P, Garant D, Charmantier A. 2017. Multiple extreme climatic events strengthen selection for earlier breeding in a wild passerine. Phil. Trans. R. Soc. B 372, 20160372 ( 10.1098/rstb.2016.0372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malyshev AV, Arfin Khan MAS, Beierkuhnlein C, Steinbauer MJ, Henry HAL, Jentsch A, Dengler J, Willner E, Kreyling J. 2016. Plant responses to climatic extremes: within-species variation equals among-species variation. Glob. Change Biol. 22, 449–464. ( 10.1111/gcb.13114) [DOI] [PubMed] [Google Scholar]
- 61.Barbraud C, Delord K, Weimerskirch H. 2015. Extreme ecological response of a seabird community to unprecedented sea ice cover. R. Soc. open sci. 2, 140456 ( 10.1098/rsos.140456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buckley LB, Kingsolver JG. 2012. Functional and phylogenetic approaches to forecasting species’ responses to climate change. Annu. Rev. Ecol. Evol. Syst. 43, 205–226. ( 10.1146/annurev-ecolsys-110411-160516) [DOI] [Google Scholar]
- 63.McLean N, van der Jeugd H, van de Pol M. Submitted. Huge intra- and inter-specific variation in avian body mass responses to climate makes generalization difficult. J. Anim. Ecol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rubolini D, Møller AP, Rainio K, Lehikoinen E. 2007. Intraspecific consistency and geographic variability in temporal trends of spring migration phenology among European bird species. Clim. Res. 35, 135–146. ( 10.3354/cr00720) [DOI] [Google Scholar]
- 65.Thackeray SJ, et al. 2016. Phenological sensitivity to climate across taxa and trophic levels. Nature 535, 241–245. ( 10.1038/nature18608) [DOI] [PubMed] [Google Scholar]
- 66.van de Pol M, Brouwer L, Ens BJ, Oosterbeek K, Tinbergen JM. 2010. Fluctuating selection and the maintenance of individual and sex-specific diet specialization in free-living oystercatchers. Evolution 64, 836–851. ( 10.1111/j.1558-5646.2009.00859.x) [DOI] [PubMed] [Google Scholar]
- 67.Williams CM, Buckley LB, Sheldon KS, Vickers M, Pörtner H-O, Dowd WW, Gunderson AR, Marshall KE, Stillman JH. 2016. Biological impacts of thermal extremes: mechanisms and costs of functional responses matter. Integr. Comp. Biol. 56, 73–84. ( 10.1093/icb/icw013) [DOI] [PubMed] [Google Scholar]
- 68.Camphuysen CJ, Ens BJ, Heg D, Hulscher JB, van der Meer J, Smit CJ. 1996. Oystercatcher Haematopus ostralegus winter mortality in The Netherlands: the effect of severe weather and food supply. Ardea 84a, 469–492. [Google Scholar]
- 69.van de Pol M, Vindenes Y, Sæther B-E, Engen S, Ens BJ, Oosterbeek K, Tinbergen JM. 2010. Effects of climate change and variability on population dynamics in a long-lived shorebird. Ecology 91, 1192–1204. ( 10.1890/09-0410.1) [DOI] [PubMed] [Google Scholar]
- 70.Schwemmer P, Hälterlein B, Geiter O, Günther K, Corman VM, Garthe S. 2014. Weather-related winter mortality of Eurasian Oystercatchers (Haematopus ostralegus) in the Northeastern Wadden Sea. Waterbirds 37, 319–330. ( 10.1675/063.037.0310) [DOI] [Google Scholar]
- 71.Atkinson PW, Clark NA, Clark JA, Bell MC, Dare PJ, Ireland PL.2000. The effects of changes in shellfish stocks and winter weather on shorebird populations: results of a 30 year study on the Wash, England. 1–54. (See https://www.bto.org/research-data-services/publications/research-reports/2000/effects-changes-shellfish-stocks-and-winter.)
- 72.Ens BJ, van de Pol M, Goss-Custard JD. 2014. The study of career decisions: oystercatchers as social prisoners. In Advances in the study of behavior 46 (eds M Naguib, L Barrett, HJ Brockmann, S Healy, JC Mitani, TJ Roper, LW Simmons), pp. 343–420. Oxford, UK: Academic; [Accessed 2 December 2016] ( 10.1016/B978-0-12-800286-5.00008-0) [DOI] [Google Scholar]
- 73.Stillman RA, Goss-Custard JD, West AD, Le V.dit Durell SEA, Caldow RWG, McGrorty S, Clarke RT. 2000. Predicting mortality in novel environments: tests and sensitivity of a behaviour-based model. J. Appl. Ecol. 37, 564–588. ( 10.1046/j.1365-2664.2000.00506.x) [DOI] [Google Scholar]
- 74.Ainsworth TD, Heron SF, Ortiz JC, Mumby PJ, Grech A, Ogawa D, Eakin CM, Leggat W. 2016. Climate change disables coral bleaching protection on the Great Barrier Reef. Science 352, 338–342. ( 10.1126/science.aac7125) [DOI] [PubMed] [Google Scholar]
- 75.Brown CR, Brown MB. 1998. Intense natural selection on body size and wing and tail asymmetry in cliff swallows during severe weather. Evolution 52, 1461–1475. ( 10.2307/2411315) [DOI] [PubMed] [Google Scholar]
- 76.Siepielski AM, et al. 2017. Precipitation drives global variation in natural selection. Science 355, 959–962. ( 10.1126/science.aag2773) [DOI] [PubMed] [Google Scholar]