Abstract
Phenotypic plasticity, if adaptive, may allow species to counter the detrimental effects of extreme conditions, but the infrequent occurrence of extreme environments and/or their restriction to low-quality habitats within a species range means that they exert little direct selection on reaction norms. Plasticity could, therefore, be maladaptive under extreme environments, unless genetic correlations are strong between extreme and non-extreme environmental states, and the optimum phenotype changes smoothly with the environment. Empirical evidence suggests that populations and species from more variable environments show higher levels of plasticity that might preadapt them to extremes, but genetic variance for plastic responses can also be low, and genetic variation may not be expressed for some classes of traits under extreme conditions. Much of the empirical literature on plastic responses to extremes has not yet been linked to ecologically relevant conditions, such as asymmetrical fluctuations in the case of temperature extremes. Nevertheless, evolved plastic responses are likely to be important for natural and agricultural species increasingly exposed to climate extremes, and there is an urgent need to collect empirical information and link this to model predictions.
This article is part of the themed issue ‘Behavioural, ecological and evolutionary responses to extreme climatic events'.
Keywords: phenotypic plasticity, environmental tolerance, environmental stress, extreme environments, genetic constraints, adaptation
1. Introduction
Adaptive phenotypic plasticity and genetic adaptation are the main ways in which isolated populations can deal with environmental change, if they cannot track their most suitable habitats in space through dispersal because of natural barriers, habitat destruction/fragmentation and/or biological attributes limiting dispersal ability. Such phenotypic responses, and how they contribute to demography and extinction risk in a changing environment, are of interest to ecologists and evolutionists, because (i) current high rates of human-induced climate change are threatening many species of animals and plants, and (ii) it is now clearly established that environmental effects on demography are largely mediated by measurable phenotypes [1–5]. Furthermore, evidence is accumulating that the part of phenotypic change in the wild that is attributable to phenotypic plasticity is larger than was previously thought [6–8]. This has recently motivated a series of theoretical investigations of the interplay of phenotypic plasticity, evolution and population growth under different regimes of directional and random environmental change, aimed at identifying to what extent plasticity (and its evolution) may facilitate population persistence [9–14], as well as renewed interest in empirically characterizing limits to plastic responses within and between generations.
Climatic studies indicate that global change, beyond just consisting of trends in mean environmental variables, is also largely characterized by an increased frequency and severity of extreme events [15–17]. Biological responses to such extreme climatic events, including phenotypic plasticity, scale up from the individual to the ecosystem, as documented by considerable empirical literature on experimental exposure to strong environmental stress in the laboratory (reviewed in [18,19] and below), and ecological studies of natural populations [1,20,21]. In contrast with this abundance of biological data focusing specifically on extremes, theory on phenotypic plasticity and its evolution is mostly based on models that treat the environment—and biological responses to it—as a continuum, such that nothing specifically distinguishes extreme environments from others. How plasticity evolves in extreme environments, and how this affects population responses to environmental changes, are still relatively little understood. Our aim here is to refine theoretical questions and predictions about evolution of plasticity in extreme environments, and provide an overview of relevant empirical evidence, and further issues raised by this evidence.
In line with earlier work, we will use the term phenotypic plasticity to describe the influence of the environment on the phenotypic expression of individual traits other than fitness. Environmental effects on fitness itself, its life-history components (vital rates), or performance as a surrogate, are better described as environmental tolerance [22]. The relationship between both concepts is clear: environmental tolerance results from the plasticity (or absence thereof) of traits on which selection may change with the environment [11,23,24]. Towards extremes, environmental tolerance becomes stress resistance, and the underlying traits are resistance mechanisms. A large body of the literature on biological responses to extreme environments focuses on tolerance/resistance, where fitness/performance are the only measured traits. In such studies, the influence of past environments on fitness is described as acclimation, acclimatization or hardening, which implicitly involves phenotypic plasticity of underlying traits. Here, by contrast, we frame our theoretical arguments explicitly on plastic traits under selection, from which environmental effects on fitness emerge, following previous theory [11,23,24]. We then discuss empirical examples as much as possible in the light of these arguments.
2. Theoretical considerations
(a). What is an extreme environment?
From a purely probabilistic or statistical perspective, extremes simply correspond to the tails of the distribution of a random variable, which are rare by definition, and can be described by extreme value theory (as discussed in [25]). Rareness obviously depends on the time scale of observation [25,26], but for our purpose, extremes will mostly concern environments that have been rarely encountered in the recent evolutionary history of a species.
In a biological context, the definition of extreme environments also has to include an aspect of harshness or severity, defined by the response of organisms to these environments. This relates to the notion of environmental stress, which has slightly different meanings depending on the biological background ([18], pp. 1–20), but can generally be quantified by the incurred reduction of fitness. This biological component of the definition of extreme environments is to a large extent organism-specific, since environments that cause a stress in one species may be benign to another species. How extreme environments influence the evolution of plasticity depends on how the rareness and severity of these environments affect selection on reaction norms, and the genetic response to this selection, as we elaborate below.
(b). Environmental stress and selection in extreme environments
Empirical evidence about the contribution of extreme environments to natural selection has been reviewed elsewhere, including in this issue ([19], pp. 53–90, [25,27,28]), so we here focus more on theoretical and conceptual issues regarding natural selection in extreme environments.
The impact of environmental extremes on natural selection partly depends on what causes the stress-induced reduction of fitness. Evolutionary biologists often associate stress with maladaptation, whereby fitness is reduced because the phenotype departs from the best possible one in the stressful environment. This implies that an environment that is stressful for a given genotype/phenotype is not stressful for another, and that more stressful environments cause stronger selection [29,30]. Maladaptation is generally conceptualized (and modelled mathematically) as deviation of a focal phenotype from the local optimum phenotype set by the environment (figure 1a). If there is genetic variation, such stress-as-maladaptation can be overcome by adaptive evolution. Hence in that perspective, no environment is stressful per se, and the fundamental niche space is not fixed over the evolutionary history of a species [31]. Nevertheless, stress may persist at evolutionary equilibrium despite the presence of genetic variation if gene flow, directional mutation or other evolutionary forces cause systematic maladaptation in peripheral populations, thus restricting a species’ ecological niche and geographical range (e.g. [32,33]).
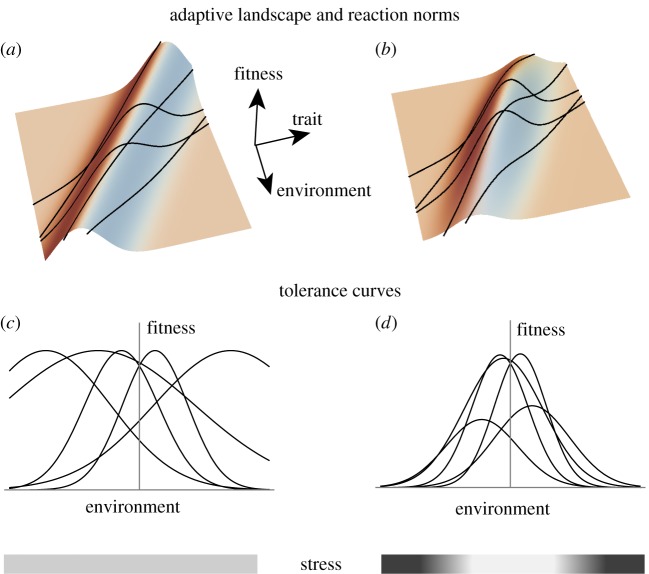
Figure 1.
Maladaptation, habitat quality and environmental stress. (a,b) Generalized adaptive landscapes, with fitness as a function of the trait and the environment (following Chevin et al. [11]). The ridge in the adaptive landscape marks the position of an optimum phenotype that changes with the environment. Projected onto this landscape are linear reaction norms (black lines) for different genotypes, with varying slopes and elevations (intercepts). (a) Maximum fitness is the same in all environments; (b) extreme environments are low-quality habitats where fitness is lower, even for the locally best phenotype. (c,d) The resulting tolerance curves, with fitness plotted against the environment. These are obtained for each genotype by ‘slicing’ through the above fitness landscape along the line given by the reaction norm. Without habitat quality effects (a), all fitness variation is caused by genotype-specific maladaptation, such that no environment is stressful per se for the whole species (c). By contrast, with differences in habitat quality (b), stress generally increases towards extreme environments, where the maximum fitness of all genotypes is lower, regardless of how well adapted they are relative to other genotypes (d).
But some environments may also be generally stressful to a species, such that the absolute fitness of all genotypes/phenotypes of that species, regardless of their variation within that environment, are overall lower there than in another environment. Figure 1b illustrates such a situation, where environmental extremes are stressful for all genotypes. This type of stress, which concerns the part of the fundamental niche space that is essentially non-evolvable over micro-evolutionary time scales (i.e. within species), relates to the ecological concept of habitat quality (as highlighted in [34]) and underlies phenomena such as source–sink population dynamics [35]. These two aspects of stress (maladaptation versus habitat quality) are often confounded in studies on environmental tolerance and stress resistance where fitness is the only measured trait (although they need not be [34]); however, under the more mechanistic view where environmental tolerance emerges from trait-fitness relationships that change with the environment (figure 1), it is important to distinguish these two sources of stress, as they have different implications for the evolution of plasticity in extreme environments.
For stress-as-maladaptation, extreme environments imply large maladaptation and hence the possibility of strong selection [29,30], including on the plasticity of a trait [36]. Conversely, for stress-as-low-habitat-quality, extreme environments correspond to patches with small demographic outputs, quantified for instance by their low reproductive values [37]. In a population that occupies a spatially or temporally heterogeneous environment, these low-productivity patches contribute comparatively less to selection on (and thus evolution of) reaction norms [38–40]. The rareness that also defines extreme environments has the same consequence as low-habitat quality: it reduces the contribution of these environments to evolution by natural selection.
(c). Genetic variance and constraints on plasticity
The assumption of relaxed (or weakened) selection in extreme environments has been used to argue that reaction norms should evolve essentially freely in these environments, and thus become maladaptive by deviating from the local optimum [41]. However, evolution of plasticity in extreme environments also depends on genetic (co)variances of reaction norms. Selection on a trait expressed in one environment causes a correlated response by the same trait expressed in another environment, whenever these two so-called ‘character states’ have additive genetic correlation [42,43]. Transposing this argument to a continuum of environments instead of discrete ones (e.g. temperature rather than host plants) by use of function-valued traits [44,45], the additive genetic covariance function of a trait across environments imposes constraints on reaction norm shape [46], especially if this covariance function is singular, with zero eigenvalues causing some reaction norm shapes to not be evolutionarily accessible [47]. Some models account for such constraints on reaction norm shape by assuming that the reaction norm is a specific function of the environment, such as a polynomial [48–50] or a sigmoid [51], with genetic variance affecting the parameters of this function. Which function is more relevant depends on the biological context: a linear reaction norm is generally a reasonable approximation for breeding time in birds [8], while sigmoid shapes are a good description of inducible defenses in water fleas [52], although both are simplified descriptions of the reaction norm that may be valid only over a given environmental range.
Constraints on reaction norm shape influence whether or not the reaction norm is likely to be adaptive in extreme environments (figure 2). The reaction norm does not necessarily evolve freely in extreme environments that contribute little to overall selection (because of their rarity or low reproductive output), because it can respond indirectly to directional selection on the trait expressed in more common environments. If the change in selective pressure (e.g. movement of an optimum phenotype) does not show any strong discontinuity from ordinary to extreme environments, then genetic correlations of traits across environments (and constraints on reaction norm shape) are likely to cause the reaction norm in extreme environments to be less maladaptive than expected by chance [53], challenging arguments about the evolution of maladaptive plasticity in extreme environments [41].
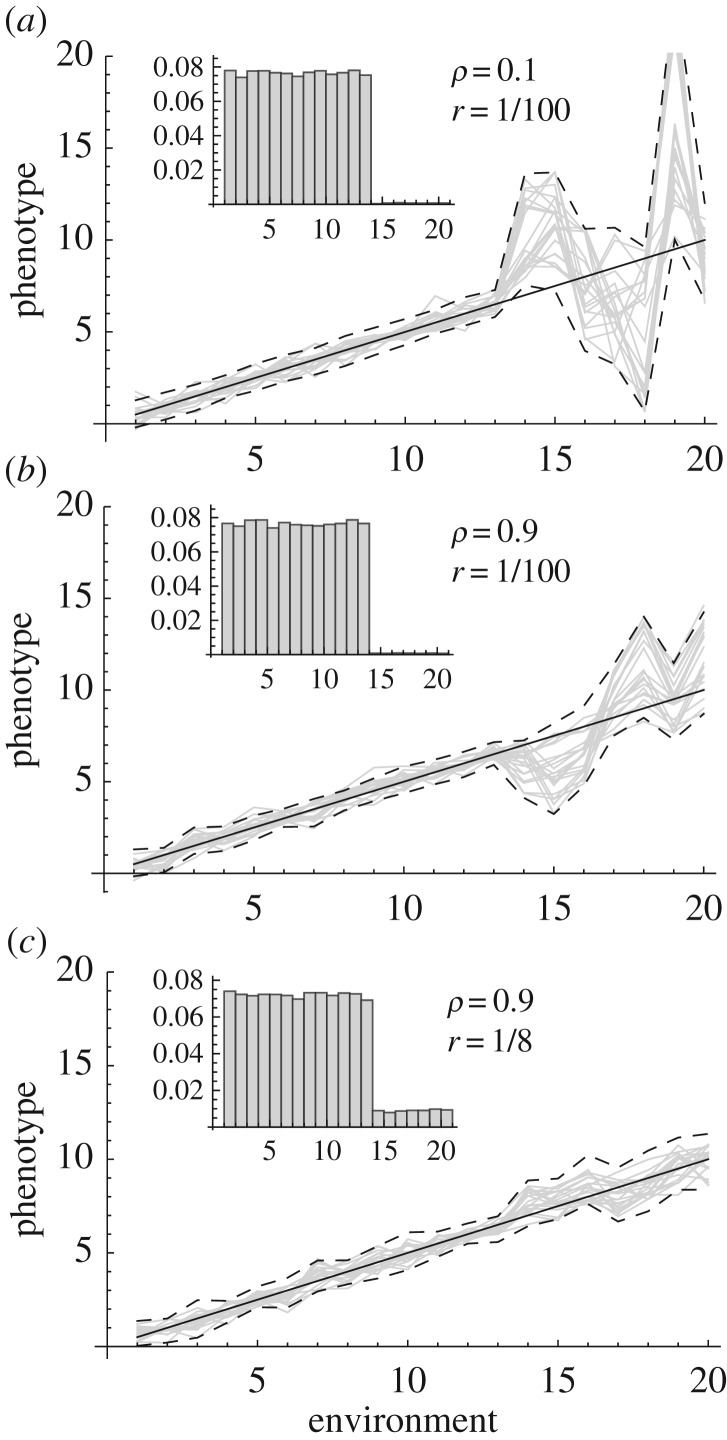
Figure 2.
Reaction norm evolution in extreme versus common environments. The evolution of reaction norms was simulated over a range of environments that differ in frequency, such that the higher third of the environmental range consists of extreme environments that are much rarer than the rest. The odds ratio r of the frequency of rare over common environments is indicated in each panel (a–c), and the distribution of these environments is illustrated graphically by the inset histograms. The phenotype expressed at each value of environmental unit was treated as a character state, with same additive genetic variance (G = 1) in all environments, and additive genetic correlation ρ|Δɛ| between character states expressed Δɛ environmental units apart. We simulated 20 000 generations of evolution under random genetic drift (with Ne = 1000) and natural selection caused by environmentally driven temporal fluctuations in an optimum phenotype, allowing for partial unpredictability of selection (see electronic supplementary material for more details on simulations). Grey lines show population mean reaction norms at 20 randomly picked generations, and dashed lines represent the 95% interval over all generations. The continuous line shows the expected optimum phenotype experienced by individuals developing in each of the environments.
This is illustrated in figure 2, which shows simulated reaction norms under random genetic drift and natural selection in a temporally fluctuating environment (details in the electronic supplementary material, appendix). In figure 2a, environments at the right end of the range are extremely rare, occurring 100 times less frequently than common environments on the left (inset histogram), and genetic correlations across environments are very weak, with ρ = 0.2 between phenotypes one environmental unit apart (e.g. temperatures that differ by 1°C), and essentially no correlation between environments more than 5 environmental units apart. In this context, phenotypes evolve mostly under random genetic drift in extreme environments, where the reaction norm can assume any shape, and this shape varies in time (broad dashed interval; also compare different grey lines), as suggested by Ghalambor et al. [41]. However, with genetic correlation across environments (figure 2b, ρ = 0.9 between adjacent environments, minimum correlation 0.13 between phenotypes expressed in environments 1 and 20), reaction norms depart less from their optimum in extreme environments, and their shape is also less erratic. This occurs because phenotypic evolution in extreme environments then becomes dominated by responses to selection in common environments, and the optimum changes linearly from common to extreme environments. Furthermore, if the frequency of extreme environments is higher (while remaining rare), reaction norm evolution becomes almost indistinguishable between extreme and common environments: in figure 2c, reaction norm shape remains linear and close to the optimum over the entire range, even though each extreme environment is eight times less likely to be encountered in a given generation than a common environment.
The results in figure 2 can be understood by noting that under the assumptions of the character-state model of quantitative genetics [42,43], the response to selection by the mean trait value expressed in an extreme environment is
| 2.1 |
with directional selection gradients denoted as β, additive genetic variances as G, additive genetic correlation between environments as ρ and subscripts e/c are for extreme/common environment. The first term on the right is the direct response to selection on the trait expressed in the extreme environment, while the second term is the indirect response caused by selection on the trait expressed in the common environment. Because selection in any generation only acts on the expressed trait value [43,46], the expected response to selection across generations is
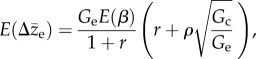 |
2.2 |
where E(β) is the expected selection gradient on the expressed trait value, and r is the odds ratio of frequencies of rare over common environments. Assuming similar genetic variances in extreme and common environments for simplicity, indirect responses to selection in common environments are stronger than direct response to selection in extreme environments whenever ρ > r, that is, when the genetic correlation between extreme and common environments is larger than their odds ratio (rare over common). The total expected response to selection in extreme environments is proportional to (ρ + r)/(1 + r), which is at least as large as ρ for very rare environments, and increases as the frequency of the extreme environment increases. Therefore, whenever ρ or r is large relative to the effective population size Ne [54], selection is a dominant force over random genetic drift for the evolution of reaction norms in extreme environments.
In practice, a majority of models use either the assumption of a constant reaction norm shape (strong genetic correlations across environments), or the converse one of a completely free shape (as implied in most optimization models searching for evolutionary stable strategies, e.g. [55,56]), which could both be argued to be somewhat unrealistic. However, they are good working hypotheses, as they lead to different predictions regarding how adaptive the reaction norm is likely to be in extreme environments. Ultimately, whether reaction norms are free to evolve or constrained in extreme environments is an empirical question.
(d). Fundamental constraints on plasticity in extreme environments?
A common verbal suggestion is that plasticity may allow populations to cope with environmental change over an ordinary range of environmental variation, beyond which ‘limits to plasticity’ are reached, such that populations in extreme environments have to rely more on evolution through genetic changes than on adaptive plasticity. However, the evolutionary foundations for such statements are not entirely clear.
Beyond arguments on relaxed selection described above, one important reason why plasticity may become inefficient to track an optimum phenotype (and thus become sub-optimal, or even maladaptive) in extreme environments is because a lack of genetic and mutational variance has prevented adaptive plasticity from evolving in those environments. But if so, the lack of genetic variation is most likely to also constrain evolution of the trait itself in these environments, and it is far from obvious that evolution can ‘take over’ where plasticity has failed. Here again, the answer has to be empirical: we need to evaluate whether the genetic and mutational variance for responding to extreme environments is lower than in ordinary environments, and whether this is truer for the plasticity of traits than for the traits themselves.
Another possible constraint on plasticity evolution in extreme environments concerns flexible or labile traits, which can change repeatedly through life. Extreme environments may be so stressful as to alter overall physiological functioning, thereby challenging phenotypic flexibility by making it too demanding for the organism. In terms of fitness, this can be thought of as a ‘production cost’ of plasticity (sensu [57]) that changes with the environment. Although theory has dealt with the cost of plasticity [10,58], including for labile traits [24,59], we are not aware of theoretical results about evolution of plasticity under environment-specific production costs for labile traits.
(e). Evolutionary predictions
Having reviewed some of the key elements for the theory of evolution of plasticity in extreme environments, we now place these elements in an ecological context, to highlight some of the major predictions that have emerged. It should be noted that few theoretical predictions exist about evolution of reaction norms specifically in extreme environments. Among those studies that did touch upon this issue, two types of predictions can be distinguished: those that focus on phenotypes expressed in extreme environments only, versus those about the whole reaction norm (expressed across environments) of genotypes evolving in patches with extreme environments.
A fairly large category of models assume that the reaction norm is constrained genetically to have a linear shape, with slope quantifying phenotypic plasticity, and that the optimum phenotype also changes linearly with the environment. In such models, if there is genetic variance in slopes, directional selection on a trait translates into stronger selection on its plasticity in extreme environments [36,49,60]. In this context, extreme environments are those that are far from the mean environment where reaction norms are assumed to have evolved to be canalized in the long run, such that their phenotypic variance is minimal [61,62], and their slopes are uncorrelated to their elevations [36,48,49,60]. With linear reaction norms and genetically variable slopes, extreme environments are thus associated with increased genetic variance for plastic traits, and play a crucial role in the evolution of plasticity. For instance, an abrupt environmental change towards a previously rare/extreme environment can cause the transient evolution of a more plastic reaction norm having a steeper slope over the whole range of environments [36]. As a consequence, such models predict that evolutionary rescue in a harsh environment should be mostly caused by the evolution of increased phenotypic plasticity [10]. A similar transient increase in plasticity occurs during rare incursions towards extremes in a stationary fluctuating environment with high autocorrelation [63]. And with gene flow in a heterogeneous environment causing continued directional selection in response to migration load, the same mechanism causes demes in patches with extreme environments to evolve reaction norms with steeper slopes [50,64].
The assumption of a linear reaction norm, despite being convenient for understanding simple principles about the evolution of plasticity, has to be violated ultimately, since no trait can tend to infinity as the environment increases. The linearity assumption can be relaxed by including higher-order terms in the polynomial that defines the reaction norm. For instance, de Jong [65] has shown that, even when the optimum phenotype changes linearly with the environment, the mean reaction norm over a population occupying a spatially heterogeneous environment can evolve to be curved, with steeper (or shallower) slopes towards extremes, if the joint distribution of environments of development and selection is asymmetric (i.e. skewed, with non-null third central moments). In models that fully relax assumptions on reaction norm shapes (ESS models, character-state models with weak genetic correlations across environments), plasticity in an extreme environment will essentially depend on selection in that environment, which will vary depending on the model, and few specific predictions emerge.
3. Empirical evidence
The theoretical treatment above outlined a number of key issues for the evolution of plasticity in extreme environments, thus identifying parameters that need to be measured empirically in order for alternative evolutionary hypotheses to be tested. We now review the empirical evidence on some of these questions. As measurements of the strength of selection in extreme versus ordinary environments have already been reviewed elsewhere [25,27,28], we mostly focus here on the genetic potential for and constraints on the evolution of plasticity in extreme environments. The questions we ask are, notably: To what extent can we detect genotypes that differ in plastic responses to extremes, and what is their level of heritable variation in plasticity within populations and species? Is there evidence that genetic correlations of trait values between extreme and ordinary environments influence the evolution of plasticity in extreme environments? Does genetic variance of traits vary across environments? And are costs of plasticity stronger in extreme environments?
(a). Plasticity in extreme environments
Many plastic traits increase the likelihood of organisms surviving extreme conditions (reviewed in [18]). For instance, survival at high temperature extremes is often linked to levels of heat-shock proteins (Hsps) expressed in cells, which provide protection for denatured proteins, and whose production is typically triggered by warm conditions that precede stressful temperatures. Plant survival and reproduction under dry conditions is affected by changes in traits affecting stomatal density, leaf shape and anatomy, growth rate, root density and length, xylem anatomy, etc. In insects, traits affecting drought stress might include metabolic rate, cuticular hydrocarbons, size, internal water storage and the ability to extract metabolic water from food sources.
There is evidence that environmental effects on these traits show geographical variation, reflecting past exposure to climate extremes. Levels of Hsp expression (and induction conditions) in insects vary among populations in patterns that are linked with the likelihood of stressful climatic conditions being encountered [66,67]. Geographical variation for traits underlying acclimation responses in damselflies undergoing range expansion also matches climatic conditions [68]. In plants, traits associated with drought resistance can be plastic and show latitudinal variation [69], as can thermal reaction norms of flowering time [70]. Genetic variation in phenological responses to climate has been documented in Arabidopsis thaliana [71], wild emmer [72], Aleppo pine seedlings [73] and many other species. However, related populations or species can vary markedly in both their geographical patterns of plastic responses [70] and inherent levels of plasticity [74,75], such that both fixed (non-plastic) and plastic responses to extremes are found [76], for reasons that are not well understood. For example, while Arabidopsis thaliana shows evidence of shifting patterns of plastic responses across its geographical range, drought adaptation across populations of Arabidopsis lyrata is based on fixed differences [77]. Similarly, Drosophila melanogaster shows fixed genetic differences across thermal gradients and little plasticity in resistance to short periods of heat [78].
Empirical evidence seems to point to a correlation of plasticity levels with environmental variability rather than with the overall level of stress, or exposure to stressful environments. For example, in a comparison of 14 pairs of congeneric plants from high and low elevations, Gugger et al. [74] found that species from mid-elevation locations (with intermediate but variable environments) showed a consistently stronger plastic phenological response to drought than those from high elevations (with less variable but on average harsher environments). A similar pattern is found in animals, where narrowly distributed species from stressful environments tend to show reduced levels of plastic responses, particularly in comparison with invasive species that are widely distributed across multiple variable environments and tend to have a high level of plasticity. Examples in animals include invasive prawns [79] and springtails [75], which may be more likely to cope with new conditions encountered under climate change. However, Drosophila populations and species that are more widespread and occupy more variable climates do not show higher levels of plasticity [80].
Rapid and recent evolutionary changes in plastic traits that allow populations to escape new stressful conditions associated with climate variation, as modelled by theory [10,36], have been documented empirically. Brassica rapa have evolved in response to drought stress by earlier (and longer) flowering and plant structural changes [81], and invasive grasses flower more rapidly and at a lower biomass in response to a shorter growing season [82]. The thermal reaction norms for size and development time has evolved over about a century in the cabbage white butterfly [83]. However, not all species have the ability to rapidly shift their plastic responses and adapt to climate change. In Concord Wood, many plant species that were unable to adjust their flowering time to altered thermal conditions have become locally extinct [84]. And in a great tit population in The Netherlands, plasticity of timing of reproduction is both heritable and under directional selection [85], yet the mismatch with the timing of maximum food abundance has increased over time [86,87], suggesting that plasticity evolution has not occurred fast enough.
(b). Genetic correlations between extreme and benign environments
As noted above, plastic responses to rare extreme conditions could evolve to be effectively adaptive by proxy, if there are genetic correlations between plastic responses under extreme and more benign conditions. This would favour genotypes with an ability to mount plastic responses that are adaptive beyond the environmental conditions they currently experience.
Patterns consistent with this hypothesis have been documented for D. melanogaster populations from warm and cooler areas [88,89]. Unfortunately, empirical research (particularly within populations) tends to characterize reaction norms under a relatively narrow range of conditions excluding extremes. Such analyses can detect heritable variation in plasticity [90], but not necessarily at the limits of the response curve. Nevertheless, some mechanisms might be expected to produce phenotypic correlations across environments. For instance, a heat-shock gene response that is triggered by non-extreme conditions could also have a benefit when thermal conditions become more extreme. In addition, Drosophila lines that have experimentally evolved at different temperatures develop altered acclimation responses, suggesting a genetic correlation between performance at non-stressful temperatures and thermal extremes [91].
In the agricultural literature, there is a wealth of information on genetic correlations across environments defined in terms of stress, as reflected in a loss of yield or production (i.e. performance traits). The cross-environment genetic correlation is widely used in livestock, where it indicates the extent to which livestock (or plant varietal) genotypes might perform across a range of environments that include challenges such as heat stress [92]. As extremes are encountered, the genetic correlation typically declines from 1 to 0 and sometimes reaches negative values [93,94].
Under the common assumption of linear reaction norms, the genetic correlation of character states across environments is a function of the environment and the variances in reaction norm elevation and slope [95]. Reaction norm slopes are often computed for varieties of crop plants exposed to a range of conditions (including drought and heat), and these typically detect differences in environmental slopes between varieties [96]. Breeds of livestock have also been shown to differ in terms of slopes across environmental conditions [97]. However, again genetic analyses of reaction norms rarely include extreme conditions, and hence do not allow investigating notably at which point the assumption of a linear reaction norm fails, even though this critically influences correlations of trait values between extreme and ordinary environments.
(c). Genetic variation in extreme environments
The theory reviewed above makes contrasted assumptions regarding genetic variance of plastic traits. Under linear reaction norms, genetic variance of the plastic trait is higher in extreme environments [36,49], while in the character-state approach the genetic variance can change arbitrarily between environments, but is often assumed constant for simplicity [43], as we did in figure 2.
For quantitative traits, it is well known that the expression of phenotypic variation (both VA and VE) depends critically on the environment [98]. For instance, the heritability of morphological (and to a lesser extent life-history) traits in wild animal populations tends to be lower under stressful conditions such as food limitation or parasite exposure [99]. One reason may be that ‘emergency’ responses affecting all genotypes result in slowed metabolism (or even dormancy, see below), causing reduced expression of genetic variation and low VA under stressful conditions. Another possibility discussed above is that plastic responses reach some limit, leading to a plateau in the reaction norm. On the other hand, evidence from laboratory experiments under strong physical stress (mostly on Drosophila) point towards the opposite pattern of increased phenotypic variance under extremes [98], caused by the expression of cryptic genetic variability resulting from trait ‘decanalization’ [100]. One possible solution to this apparent paradox (suggested in [99]) would be that genetic variance consistently increases in novel (i.e. previously rare) environments, and that favourable conditions (large food availability, low parasite load) are generally rare in nature.
Regarding plasticity per se, genetic variation in reaction norm slopes has been documented in a range of organisms and traits [101,102]. However, in some cases, heritability for plastic traits measured under field conditions can be low (does not differ from 0 for nesting time responses to temperature in flycatchers [103]). This may reflect an inability of the organism to sense the appropriate environmental cue and/or physiologically respond to it, and also often denotes our ignorance of the actual cue used by the organism. Heritable plasticity is also demonstrated indirectly by evidence for a genetic basis of between-population differences in plasticity. While related species can differ in reaction norms, particularly in their slopes and curvatures, populations tend to differ much less [104], suggesting limits to plasticity that act at the intraspecific level, but can be broken above the population level.
4. Ongoing and future challenges
We now highlight aspects of the evolution of plasticity in extreme environments that we think are currently underemphasized and deserve further investigation. These include features that are ubiquitous in experiments but little addressed by theory, as well as questions that are just beginning to receive attention in experimental work.
(a). Fine-grained timing of extreme environments
A challenge for considering extremes within a reaction norm approach is to correctly address the temporal aspect of phenotypic responses to the environment. The reaction norm is typically conceived as a set of character states across levels of an environment that is regarded as constant during a lifetime (as in figure 1). This is also reflected in many experiments where organisms are reared under a range of constant conditions to generate the temperature response curve. These experiments and their associated ‘static’ concept of reaction norm do not capture the important time dimension of the reaction norm in response to temporally fined-grained environments that vary on time scales below lifespans [34,105,106]. Moreover, it is not just the extreme values or duration of exposure that matter, but also how these develop. An extreme that is rapidly reached might be better (or less well) tolerated than one that is reached more slowly under a ramped change in the environment [107]. Constant conditions also do not capture the asymmetry often found for fluctuating conditions in the wild, where extremes on one end of the environmental range are experienced more often than on the other end. These types of fluctuations (and their asymmetry) are expected to increase under climate change, as evident already with respect to more rapid warming being experienced at night time compared with day time, with resulting reductions in temperature tolerance and fitness measures [108].
Another important temporal component is the age-dependency of reaction norms. The environment influences phenotypes during development for most morphological traits, so reaction norms often have an ontogenic component [34,109], and more generally plasticity is likely to change with life stages, as shown in many studies [110]. A reaction norm might be constructed for each life stage, but the challenge is then to integrate them into an overall response in temporally changing environments, accounting for the fact that conditions at one life stage might influence reaction norms at a subsequent life stage. Empirical studies have just started integrating temporal and ontogenic aspects in the study of reaction norms, notably for tolerance curves [34,106,111] and the evolution of age-dependent plasticity also is a relatively new topic for theory [112].
A final temporal issue is that the reaction norm is likely to depend on environments experienced in previous generations, through transgenerational plasticity [113,114]. This is thought to chiefly occur by adaptive epigenetics, whereby modifications to chromosomes triggered by environmental conditions are inherited and serve to increase fitness under similar or more extreme conditions in ensuing generations. But transgenerational effects are not necessarily beneficial [115], and their effects need to be taken into account when defining the nature of the plastic response to extreme environments. Only recently has evolutionary theory started to investigate how maternal effects and plasticity interact [116,117], and how epigenetic marks evolve [118] in changing environments, and further developments will probably be required to integrate theoretical predictions with empirical measurements.
(b). Correlates and costs of plasticity
Plasticity under extremes may depend on interactions with other traits, the indirect response to which may overcome the response to selection on the trait expressed in common environments. First, plasticity can be associated with trait differences within an environment (‘fixed’ differences). Under the assumption of linear reaction norms, such a genetic correlation between reaction norm slope—quantifying plasticity—and elevation (intercept in a given environment) necessarily occurs in most environments, whenever plasticity varies genetically [36,49]. In the context of acclimation, studies have investigated whether genotypes having a greater fixed level of resistance to an extreme exhibit reduced plasticity (lower acclimation response), with some supporting evidence in Drosophila [119], although this is not always the case [120]. There is also some mechanistic overlap between different forms of acclimation/hardening (short versus long-term plastic responses) and fixed differences in response to temperature in Drosophila [121]. Some species comparisons also point to interactions between fixed and plastic responses, including alpine plants from different elevations mentioned above [74], and comparisons of heat resistance and acclimation ability in Drosophila species [122].
Furthermore, phenotypic plasticity may be correlated with developmental instability, presumably because they are affected by common developmental processes. For instance, Arabidopsis genotypes with high levels of plasticity across environments also have higher developmental instability within environments [123]. This has been argued to be an indirect constitutive cost of plasticity, as developmental instability causes increased phenotypic variance within an environment, which is detrimental under stabilizing selection [123]. More generally, costs of plasticity, whose importance is debated in evolutionary biology [57,124,125], are likely to be more prominent in extreme environments. For instance, high expression of Hsps, as induced under extreme temperatures, has detrimental effects on life-history traits [126].
(c). Dormancy traits as specific responses to extremes
An important set of traits underlying plastic responses to extremes that differ qualitatively from most physiological or morphological responses described above are diapause, quiescence, hibernation and other forms of dormancy. Indeed, dormancy traits are escape strategies, whereby an organism avoids facing the detrimental effects of stress through a major slowdown in metabolism. This metabolic slowdown necessarily has cascading effects on many other traits, by reducing selection on them and limiting the expression of their genetic variation (as discussed above). Because metabolism is reactivated at a later time (under presumably more suitable environmental conditions), diapause and dormancy have often been interpreted conceptually as dispersal trough time [127].
Models have investigated the evolution of plastic dormancy, where an environmental cue predictive of extreme environments triggers the metabolic slowdown [128], and highlighted its interaction with dispersal through space [127,129]. Such interactions have been studied empirically by Fedorka et al. [130], who found that gene flow in crickets led to a maladaptive pattern along a climate gradient, whereby many crickets failed to enter diapause when it was clearly favoured by selection because of an influx of poorly adapted genotypes from areas where diapause was not favoured. This situation is expected to occur when a minority of individuals in a species range encounter stressful conditions.
Environmentally cued dormancy and diapause can be seen as extremes of a continuum that includes all plastic phenological traits, which are critically important under climate change [131]. Indeed, any adjustment of the timing of egg laying in birds [132] or bud burst in trees [133], for instance, involves mechanisms of slowed down metabolism, although only for a specific function rather than at the level of the whole organism. An interesting theoretical and empirical question is thus: what degree of extremeness in the environment is required to turn such phenotypically localized plastic slowdowns into whole organism dormancy or diapause, and why?
5. Applications
In the context of conservation biology under global change, plastic responses to extremes are important because environments that are now rare are likely to become increasingly common in the future. If genetic correlations of trait values across environments are such that plastic responses to rare extreme environments are already favoured under more common and moderately stressful conditions, then there may be a relatively high level of plasticity-based climate resilience in natural populations. In that case the conditions for persistence under climate change can be investigated and predicted to some extent by combining the current plasticity level with genetic evolution of the non-plastic component of traits [11,73,134–136]. On the other hand, if plastic responses are poorly correlated between extreme and favourable conditions, there may be value in the conservation of range edge populations which have been exposed more frequently to extremes [137], particularly given that these populations are likely to be highly threatened in many ecosystems by clearing and other disturbances.
The impact of plasticity on the performance of genotypes across environments is also relevant to maintaining agricultural and forestry productivity under extreme conditions encountered due to climate change [138]. There may be an opportunity to select specifically for increased or decreased plasticity, assuming that this will not cause undesirable responses to selection by ‘fixed’ trait values or performance in any environment. For instance, Aspinwall et al. [138] provide an example in cotton cultivars—based on published data [139]—of a negative association between yield and photosynthetic plasticity under drought versus well-watered conditions, suggesting that highly plastic genotypes have low average yields.
6. Conclusion
Despite relying on simple models, theory can start guiding our understanding of phenotypic plasticity and its evolution in extreme environments. Confronting theoretical predictions with empirical data is inherently difficult because the rareness of extremes in natural conditions and their severity (when tested in the laboratory where rareness is not an issue) makes this work challenging, necessarily decreasing productivity (i.e. more work is needed to produce the same amount of data). Nevertheless, the importance of plastic response to extreme environments for both basic science and applied fields make this effort worthwhile. We hope that the present paper contributes to fostering new studies of these processes by highlighting the most pressing questions. Beyond technical advances such as high-throughput phenotyping, progress will also require integrating novel questions about plasticity (age-dependency, transgenerational effects) with a more realistic account of the ecology of extreme events in nature, including how they unfold in time.
Supplementary Material
Acknowledgement
We thank Anne Charmantier, Joel Kingsolver, M van de Pol and an anonymous reviewer for useful feedback on this manuscript.
Competing interests
We have no competing interests.
Funding
L.-M. Chevin is supported by the European Research Council (grant ERC-2015-STG-678140-FluctEvol).
References
- 1.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/Annurev.Ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 2.Hoffmann AA, Sgro CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485. ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 3.Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson LE, Tuljapurkar S, Coulson T. 2010. Coupled dynamics of body mass and population growth in response to environmental change. Nature 466, 482–485. ( 10.1038/Nature09210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozgul A, Tuljapurkar S, Benton TG, Pemberton JM, Clutton-Brock TH, Coulson T. 2009. The dynamics of phenotypic change and the shrinking sheep of St. Kilda. Science 325, 464–467. ( 10.1126/Science.1173668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellner SP, Geber MA, Hairston NG Jr. 2011. Does rapid evolution matter? Measuring the rate of contemporary evolution and its impacts on ecological dynamics. Ecol. Lett. 14, 603–614. ( 10.1111/j.1461-0248.2011.01616.x) [DOI] [PubMed] [Google Scholar]
- 6.Merila J, Hendry AP. 2014. Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evol. Appl. 7, 1–14. ( 10.1111/eva.12137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gienapp P, Teplitsky C, Alho JS, Mills JA, Merila J. 2008. Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178. ( 10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- 8.Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LE, Sheldon BC. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803. ( 10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 9.Reed TE, Waples RS, Schindler DE, Hard JJ, Kinnison MT. 2010. Phenotypic plasticity and population viability: the importance of environmental predictability. Proc. R. Soc. B 277, 3391–3400. ( 10.1098/Rspb.2010.0771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chevin LM, Lande R. 2010. When do adaptive plasticity and genetic evolution prevent extinction of a density-regulated population? Evolution 64, 1143–1150. ( 10.1111/j.1558-5646.2009.00875.x) [DOI] [PubMed] [Google Scholar]
- 11.Chevin LM, Lande R, Mace GM. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357 ( 10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botero CA, Weissing FJ, Wright J, Rubenstein DR. 2015. Evolutionary tipping points in the capacity to adapt to environmental change. Proc. Natl Acad. Sci. USA 112, 184–189. ( 10.1073/pnas.1408589111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashander J, Chevin LM, Baskett M. 2016. Predicting rescue via evolving plasticity in stochastic environments. Proc. R. Soc. B 283, 20161690 ( 10.1098/rspb.2016.1690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovach-Orr C, Fussmann GF. 2013. Evolutionary and plastic rescue in multitrophic model communities. Phil. Trans. R. Soc. B 368, 20120084 ( 10.1098/rstb.2012.0084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO. 2000. Climate extremes: observations, modeling, and impacts. Science 289, 2068–2074. ( 10.1126/science.289.5487.2068) [DOI] [PubMed] [Google Scholar]
- 16.Stocker TF. 2014. Climate change 2013: the physical science basis: Working Group I contribution to the Fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 17.Ummenhofer CC, Meehl GA. 2017. Extreme weather and climate events with ecological relevance: a review. Phil. Trans. R. Soc. B 372, 20160135 ( 10.1098/rstb.2016.0135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann AA, Parsons PA. 1991. Evolutionary genetics and environmental stress. Oxford, UK: Oxford University Press. [Google Scholar]
- 19.Hoffmann AA, Parsons PA. 1997. Extreme environmental change and evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 20.Pardo D, Jenouvrier S, Weimerskirch H, Barbraud C. 2017. Effect of extreme sea surface temperature events on the demography of an age-structured albatross population. Phil. Trans. R. Soc. B 372, 20160143 ( 10.1098/rstb.2016.0143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey LD, Ens BJ, Both C, Heg D, Oosterbeek K, van de Pol M. 2017. No phenotypic plasticity in nest-site selection in response to extreme flooding events. Phil. Trans. R. Soc. B 372, 20160139 ( 10.1098/rstb.2016.0139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch M, Gabriel W. 1987. Environmental tolerance. Am. Nat. 129, 283–303. ( 10.1086/284635) [DOI] [Google Scholar]
- 23.Chevin LM, Gallet R, Gomulkiewicz R, Holt RD, Fellous S. 2013. Phenotypic plasticity in evolutionary rescue experiments. Phil. Trans. R. Soc. B 368, 20120089 ( 10.1098/rstb.2012.0089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lande R. 2014. Evolution of phenotypic plasticity and environmental tolerance of a labile quantitative character in a fluctuating environment. J. Evol. Biol. 27, 866–875. ( 10.1111/jeb.12360) [DOI] [PubMed] [Google Scholar]
- 25.Kingsolver JG, Buckley LB. 2017. Quantifying thermal extremes and biological variation to predict evolutionary responses to changing climate. Phil. Trans. R. Soc. B 372, 20160147 ( 10.1098/rstb.2016.0147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van de Pol M, Jenouvrier S, Cornelissen JHC, Visser ME. 2017. Behavioural, ecological and evolutionary responses to extreme climatic events: challenges and directions. Phil. Trans. R. Soc. B 372, 20160134 ( 10.1098/rstb.2016.0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant PR, Grant BR, Huey RB, Johnson MTJ, Knoll AH, Schmitt J. 2017. Evolution caused by extreme events. Phil. Trans. R. Soc. B 372, 20160146 ( 10.1098/rstb.2016.0146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marrot P, Garant D, Charmantier A. 2017. Multiple extreme climatic events strengthen selection for earlier breeding in a wild passerine. Phil. Trans. R. Soc. B 372, 20160372 ( 10.1098/rstb.2016.0372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agrawal AF, Whitlock MC. 2010. Environmental duress and epistasis: how does stress affect the strength of selection on new mutations? Trends Ecol. Evol. 25, 450–458. ( 10.1016/j.tree.2010.05.003) [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann AA, Hercus MJ. 2000. Environmental stress as an evolutionary force. Bioscience 50, 217–226. ( 10.1641/0006-3568(2000)050%5B0217:Esaaef%5D2.3.Co;2) [DOI] [Google Scholar]
- 31.Holt RD. 2009. Bringing the Hutchinsonian niche into the 21st century: ecological and evolutionary perspectives. Proc. Natl Acad. Sci. USA 106, 19 659–19 665. ( 10.1073/pnas.0905137106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-Ramos G, Kirkpatrick M. 1997. Genetic models of adaptation and gene flow in peripheral populations. Evolution 51, 21–28. ( 10.2307/2410956) [DOI] [PubMed] [Google Scholar]
- 33.Kirkpatrick M, Barton NH. 1997. Evolution of a species’ range. Am. Nat. 150, 1–23. ( 10.1086/286054) [DOI] [PubMed] [Google Scholar]
- 34.Nougué O, Svendsen N, Jabbour-Zahab R, Lenormand T, Chevin LM. 2016. The ontogeny of tolerance curves: habitat quality versus acclimation in a stressful environment. J. Anim. Ecol. 85, 1625–1635. ( 10.1111/1365-2656.12572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pulliam HR. 1988. Sources, sinks, and population regulation. Am. Nat. 132, 652–661. ( 10.1086/284880) [DOI] [Google Scholar]
- 36.Lande R. 2009. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435–1446. ( 10.1111/J.1420-9101.2009.01754.X). [DOI] [PubMed] [Google Scholar]
- 37.Kawecki T. 2004. Ecological and evolutionary consequences of source-sink population dynamics. In Ecology, genetics, and evolution of metapopulations (eds Hanski I, Gaggiotti O), pp. 387–414. Burlington, MA: Elsevier Academic Press. [Google Scholar]
- 38.Holt RD, Gaines MS. 1992. Analysis of adaptation in heterogeneous landscapes: implications for the evolution of fundamental niches. Evol. Ecol. 6, 433–447. ( 10.1007/BF02270702) [DOI] [Google Scholar]
- 39.Sasaki A, de Jong G. 1999. Density dependence and unpredictable selection in a heterogeneous environment: compromise and polymorphism in the ESS reaction norm. Evolution 53, 1329–1342. ( 10.2307/2640880) [DOI] [PubMed] [Google Scholar]
- 40.de Jong G, Behera N. 2002. The influence of life-history differences on the evolution of reaction norms. Evol. Ecol. Res. 4, 1–25. [Google Scholar]
- 41.Ghalambor C, McKay J, Carroll S, Reznick D. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407. ( 10.1111/j.1365-2435.2007.01283.x) [DOI] [Google Scholar]
- 42.Falconer DS. 1952. The problem of environment and selection. Am. Nat. 86, 293–298. ( 10.1086/281736) [DOI] [Google Scholar]
- 43.Via S, Lande R. 1985. Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39, 505–522. ( 10.2307/2408649) [DOI] [PubMed] [Google Scholar]
- 44.Kirkpatrick M, Lofsvold D. 1989. The evolution of growth trajectories and other complex quantitative characters. Genome 31, 778–783. ( 10.1139/g89-137) [DOI] [PubMed] [Google Scholar]
- 45.Stinchcombe JR, Kirkpatrick M, Function-valued Traits Working Group. 2012. Genetics and evolution of function-valued traits: understanding environmentally responsive phenotypes. Trends Ecol. Evol. 27, 637–647. ( 10.1016/j.tree.2012.07.002) [DOI] [PubMed] [Google Scholar]
- 46.Gomulkiewicz R, Kirkpatrick M. 1992. Quantitative genetics and the evolution of reaction norms. Evolution 46, 390–411. ( 10.2307/2409860) [DOI] [PubMed] [Google Scholar]
- 47.Kirkpatrick M, Lofsvold D. 1992. Measuring selection and constraint in the evolution of growth. Evolution 46, 954–971. ( 10.2307/2409749) [DOI] [PubMed] [Google Scholar]
- 48.de Jong G. 1990. Quantitative genetics of reaction norms. J. Evol. Biol. 3, 447–468. ( 10.1046/j.1420-9101.1990.3050447.x) [DOI] [Google Scholar]
- 49.Gavrilets S, Scheiner SM. 1993. The genetics of phenotypic plasticity. 5. Evolution of reaction norm shape. J. Evol. Biol. 6, 31–48. ( 10.1046/j.1420-9101.1993.6010031.x) [DOI] [Google Scholar]
- 50.Tufto J. 2000. The evolution of plasticity and nonplastic spatial and temporal adaptations in the presence of imperfect environmental cues. Am. Nat. 156, 121–130. ( 10.1086/303381) [DOI] [PubMed] [Google Scholar]
- 51.Chevin LM, Lande R. 2013. Evolution of discrete phenotypes from continuous norms of reaction. Am. Nat. 182, 13–27. ( 10.1086/670613) [DOI] [PubMed] [Google Scholar]
- 52.Hammill E, Rogers A, Beckerman A. 2008. Costs, benefits and the evolution of inducible defences: a case study with Daphnia pulex. J. Evol. Biol. 21, 705–715. ( 10.1111/j.1420-9101.2008.01520.x) [DOI] [PubMed] [Google Scholar]
- 53.de Jong G. 2005. Evolution of phenotypic plasticity: patterns of plasticity and the emergence of ecotypes. New Phytol. 166, 101–117. ( 10.1111/j.1469-8137.2005.01322.x) [DOI] [PubMed] [Google Scholar]
- 54.Lande R. 1976. Natural selection and random genetic drift in phenotypic evolution. Evolution 30, 314–334. ( 10.2307/2407703) [DOI] [PubMed] [Google Scholar]
- 55.Ernande B, Dieckmann U, Heino M. 2004. Adaptive changes in harvested populations: plasticity and evolution of age and size at maturation. Proc. R. Soc. Lond. B 271, 415–423. ( 10.1098/rspb.2003.2519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Svennungsen TO, Holen OH, Leimar O. 2011. Inducible defenses: continuous reaction norms or threshold traits? Am. Nat. 178, 397–410. ( 10.1086/661250) [DOI] [PubMed] [Google Scholar]
- 57.Dewitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81. ( 10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 58.Van Tienderen P. 1991. Evolution of generalists and specialists in spatially heterogeneous environments. Evolution 45, 1317–1331. ( 10.2307/2409882) [DOI] [PubMed] [Google Scholar]
- 59.Gabriel W. 2005. How stress selects for reversible phenotypic plasticity. J. Evol. Biol. 18, 873–883. ( 10.1111/j.1420-9101.2005.00959.x) [DOI] [PubMed] [Google Scholar]
- 60.Gavrilets S, Scheiner SM. 1993. The genetics of phenotypic plasticity. VI. Theoretical predictions for directional selection. J. Evol. Biol. 6, 49–68. ( 10.1046/j.1420-9101.1993.6010049.x) [DOI] [Google Scholar]
- 61.Bull JJ. 1987. Evolution of phenotypic variance. Evolution 41, 303–315. ( 10.2307/2409140) [DOI] [PubMed] [Google Scholar]
- 62.Kawecki TJ. 2000. The evolution of canalization under fluctuating selection. Evolution 54, 1–12. ( 10.1111/j.0014-3820.2000.tb00001.x) [DOI] [PubMed] [Google Scholar]
- 63.Tufto J. 2015. Genetic evolution, plasticity, and bet-hedging as adaptive responses to temporally autocorrelated fluctuating selection: a quantitative genetic model. Evolution 69, 2034–2049. ( 10.1111/evo.12716) [DOI] [PubMed] [Google Scholar]
- 64.Chevin LM, Lande R. 2011. Adaptation to marginal habitats by evolution of increased phenotypic plasticity. J. Evol. Biol. 24, 1462–1476. ( 10.1111/j.1420-9101.2011.02279.x) [DOI] [PubMed] [Google Scholar]
- 65.de Jong G. 1999. Unpredictable selection in a structured population leads to local genetic differentiation in evolved reaction norms. J. Evol. Biol. 12, 839–851. ( 10.1046/j.1420-9101.1999.00118.x) [DOI] [Google Scholar]
- 66.Narum SR, Campbell NR, Meyer KA, Miller MR, Hardy RW. 2013. Thermal adaptation and acclimation of ectotherms from differing aquatic climates. Mol. Ecol. 22, 3090–3097. ( 10.1111/mec.12240) [DOI] [PubMed] [Google Scholar]
- 67.Sorensen JG, Dahlgaard J, Loeschcke V. 2001. Genetic variation in thermal tolerance among natural populations of Drosophila buzzatii: down regulation of Hsp70 expression and variation in heat stress resistance traits. Funct. Ecol. 15, 289–296. ( 10.1046/j.1365-2435.2001.00525.x) [DOI] [Google Scholar]
- 68.Lancaster LT, Dudaniec RY, Hansson B, Svensson EI. 2015. Latitudinal shift in thermal niche breadth results from thermal release during a climate-mediated range expansion. J. Biogeogr. 42, 1953–1963. ( 10.1111/jbi.12553) [DOI] [Google Scholar]
- 69.Woods EC, Hastings AP, Turley NE, Heard SB, Agrawal AA. 2012. Adaptive geographical clines in the growth and defense of a native plant. Ecol. Monogr. 82, 149–168. ( 10.1890/11-1446.1) [DOI] [Google Scholar]
- 70.Toftegaard T, Posledovich D, Navarro-Cano JA, Wiklund C, Gotthard K, Ehrlen J. 2016. Variation in plant thermal reaction norms along a latitudinal gradient—more than adaptation to season length. Oikos 125, 622–628. ( 10.1111/oik.02323) [DOI] [Google Scholar]
- 71.Stinchcombe JR, Weinig C, Ungerer M, Olsen KM, Mays C, Halldorsdottir SS, Purugganan MD, Schmitt J. 2004. A latitudinal cline in flowering time in Arabidopsis thaliana modulated by the flowering time gene FRIGIDA. Proc. Natl Acad. Sci. USA 101, 4712–4717. ( 10.1073/pnas.0306401101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Volis S, Ormanbekova D, Yermekbayev K. 2015. Role of phenotypic plasticity and population differentiation in adaptation to novel environmental conditions. Ecol. Evol. 5, 3818–3829. ( 10.1002/ece3.1607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taibi K, del Campo AD, Aguado A, Mulet JM. 2015. The effect of genotype by environment interaction, phenotypic plasticity and adaptation on Pinus halepensis reforestation establishment under expected climate drifts. Ecol. Eng. 84, 218–228. ( 10.1016/j.ecoleng.2015.09.005) [DOI] [Google Scholar]
- 74.Gugger S, Kesselring H, Stocklin J, Hamann E. 2015. Lower plasticity exhibited by high- versus mid-elevation species in their phenological responses to manipulated temperature and drought. Ann. Bot. 116, 953–962. ( 10.1093/aob/mcv155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chown SL, Slabber S, McGeoch MA, Janion C, Leinaas HP. 2007. Phenotypic plasticity mediates climate change responses among invasive and indigenous arthropods. Proc. R. Soc. B 274, 2531–2537. ( 10.1098/rspb.2007.0772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maron JL, Elmendorf SC, Vila M. 2007. Contrasting plant physiological adaptation to climate in the native and introduced range of Hypericum perforatum. Evolution 61, 1912–1924. ( 10.1111/j.1558-5646.2007.00153.x) [DOI] [PubMed] [Google Scholar]
- 77.Paccard A, Fruleux A, Willi Y. 2014. Latitudinal trait variation and responses to drought in Arabidopsis lyrata. Oecologia 175, 577–587. ( 10.1007/s00442-014-2932-8) [DOI] [PubMed] [Google Scholar]
- 78.Sgro CM, Overgaard J, Kristensen TN, Mitchell KA, Cockerell FE, Hoffmann AA. 2010. A comprehensive assessment of geographic variation in heat tolerance and hardening capacity in populations of Drosophila melanogaster from eastern Australia. J. Evol. Biol. 23, 2484–2493. ( 10.1111/j.1420-9101.2010.02110.x) [DOI] [PubMed] [Google Scholar]
- 79.Magozzi S, Calosi P. 2015. Integrating metabolic performance, thermal tolerance, and plasticity enables for more accurate predictions on species vulnerability to acute and chronic effects of global warming. Glob. Change Biol. 21, 181–194. ( 10.1111/gcb.12695) [DOI] [PubMed] [Google Scholar]
- 80.Overgaard J, Kristensen TN, Mitchell KA, Hoffmann AA. 2011. Thermal tolerance in widespread and tropical Drosophila species: does phenotypic plasticity increase with latitude? Am. Nat. 178, S80–S96. ( 10.1086/661780) [DOI] [PubMed] [Google Scholar]
- 81.Franks SJ, Weis AE. 2008. A change in climate causes rapid evolution of multiple life-history traits and their interactions in an annual plant. J. Evol. Biol. 21, 1321–1334. ( 10.1111/j.1420-9101.2008.01566.x) [DOI] [PubMed] [Google Scholar]
- 82.Novy A, Flory SL, Hartman JM. 2013. Evidence for rapid evolution of phenology in an invasive grass. J. Evol. Biol. 26, 443–450. ( 10.1111/jeb.12047) [DOI] [PubMed] [Google Scholar]
- 83.Kingsolver J, Massie K, Ragland G, Smith M. 2007. Rapid population divergence in thermal reaction norms for an invading species: breaking the temperature–size rule. J. Evol. Biol. 20, 892–900. ( 10.1111/j.1420-9101.2007.01318.x) [DOI] [PubMed] [Google Scholar]
- 84.Willis CG, Ruhfel B, Primack RB, Miller-Rushing AJ, Davis CC. 2008. Phylogenetic patterns of species loss in Thoreau's woods are driven by climate change. Proc. Natl Acad. Sci. USA 105, 17 029–17 033. ( 10.1073/pnas.0806446105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nussey DH, Postma E, Gienapp P, Visser ME. 2005. Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306. ( 10.1126/Science.1117004) [DOI] [PubMed] [Google Scholar]
- 86.Reed TE, Jenouvrier S, Visser ME. 2013. Phenological mismatch strongly affects individual fitness but not population demography in a woodland passerine. J. Anim. Ecol. 82, 131–144. ( 10.1111/j.1365-2656.2012.02020.x) [DOI] [PubMed] [Google Scholar]
- 87.Chevin LM, Visser ME, Tufto J. 2015. Estimating the variation, autocorrelation, and environmental sensitivity of phenotypic selection. Evolution 69, 2319–2332. ( 10.1111/evo.12741) [DOI] [PubMed] [Google Scholar]
- 88.Morin JP, Moreteau B, Petavy G, David JR. 1999. Divergence of reaction norms of size characters between tropical and temperate populations of Drosophila melanogaster and D. simulans. J. Evol. Biol. 12, 329–339. ( 10.1046/j.1420-9101.1999.00038.x) [DOI] [Google Scholar]
- 89.Trotta V, Calboli FCF, Ziosi M, Guerra D, Pezzoli MC, David JR, Cavicchi S. 2006. Thermal plasticity in Drosophila melanogaster: a comparison of geographic populations. BMC Evol. Biol. 6, 13 ( 10.1186/1471-2148-6-67) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scheiner SM. 2002. Selection experiments and the study of phenotypic plasticity. J. Evol. Biol. 15, 889–898. ( 10.1046/j.1420-9101.2002.00468.x) [DOI] [Google Scholar]
- 91.Lerman DN, Feder ME. 2001. Laboratory selection at different temperatures modifies heat-shock transcription factor (HSF) activation in Drosophila melanogaster. J. Exp. Biol. 204, 315–323. [DOI] [PubMed] [Google Scholar]
- 92.Santana ML, Bignardi AB, Eler JP, Ferraz JBS. 2016. Genetic variation of the weaning weight of beef cattle as a function of accumulated heat stress. J. Anim. Breed. Genet. 133, 92–104. ( 10.1111/jbg.12169) [DOI] [PubMed] [Google Scholar]
- 93.Rose G, Kause A, Mulder HA, van der Werf JHJ, Thompson AN, Ferguson MB, van Arendonk JAM. 2013. Merino ewes can be bred for body weight change to be more tolerant to uncertain feed supply. J. Anim. Sci. 91, 2555–2565. ( 10.2527/jas.2012-5539) [DOI] [PubMed] [Google Scholar]
- 94.Mota RR, Tempelman RJ, Lopes PS, Aguilar I, Silva FF, Cardoso FF. 2016. Genotype by environment interaction for tick resistance of Hereford and Braford beef cattle using reaction norm models. Genet. Select. Evol. 48, 12 ( 10.1186/s12711-015-0178-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Jong G. 1990. Genotype-by-environment interaction and the genetic covariance between environments: multilocus genetics. Genetica 81, 171–177. ( 10.1007/BF00360862) [DOI] [Google Scholar]
- 96.Annicchiarico P, Mariani G. 1996. Prediction of adaptability and yield stability of durum wheat genotypes from yield response in normal and artificially drought-stressed conditions. Field Crops Res. 46, 71–80. ( 10.1016/0378-4290(95)00087-9) [DOI] [Google Scholar]
- 97.Li L, Hermesch S. 2016. Environmental variation and breed sensitivity for growth rate and backfat depth in pigs. Anim. Prod. Sci. 56, 61–69. ( 10.1071/an14066) [DOI] [PubMed] [Google Scholar]
- 98.Hoffmann AA, Merila J. 1999. Heritable variation and evolution under favourable and unfavourable conditions. Trends Ecol. Evol. 14, 96–101. ( 10.1016/S0169-5347(99)01595-5) [DOI] [PubMed] [Google Scholar]
- 99.Charmantier A, Garant D. 2005. Environmental quality and evolutionary potential: lessons from wild populations. Proc. R. Soc. B 272, 1415–1425. ( 10.1098/Rspb.2005.3117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paaby AB, Rockman MV. 2014. Cryptic genetic variation: evolution's hidden substrate. Nat. Rev. Genet. 15, 247–258. ( 10.1038/nrg3688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scheiner SM. 1993. Genetics and evolution of phenotypic plasticity. Annu. Rev. Ecol. Syst. 24, 35–68. ( 10.1146/annurev.es.24.110193.000343) [DOI] [Google Scholar]
- 102.Oomen RA, Hutchings JA. 2015. Genetic variability in reaction norms in fishes. Environ. Rev. 23, 353–366. ( 10.1139/Z86-007) [DOI] [Google Scholar]
- 103.Brommer JE, Merila J, Sheldon BC, Gustafsson L. 2005. Natural selection and genetic variation for reproductive reaction norms in a wild bird population. Evolution 59, 1362–1371. ( 10.1111/j.0014-3820.2005.tb01785.x) [DOI] [PubMed] [Google Scholar]
- 104.Murren CJ, et al. 2014. Evolutionary change in continuous reaction norms. Am. Nat. 183, 453–467. ( 10.1086/675302) [DOI] [PubMed] [Google Scholar]
- 105.Hoffmann AA. 2010. Physiological climatic limits in Drosophila: patterns and implications. J. Exp. Biol. 213, 870–880. ( 10.1242/jeb.037630) [DOI] [PubMed] [Google Scholar]
- 106.Kingsolver JG, Higgins JK, Augustine KE. 2015. Fluctuating temperatures and ectotherm growth: distinguishing non-linear and time-dependent effects. J. Exp. Biol. 218, 2218–2225. ( 10.1242/jeb.120733) [DOI] [PubMed] [Google Scholar]
- 107.Terblanche JS, Hoffmann AA, Mitchell KA, Rako L, le Roux PC, Chown SL. 2011. Ecologically relevant measures of tolerance to potentially lethal temperatures. J. Exp. Biol. 214, 3713–3725. ( 10.1242/jeb.061283) [DOI] [PubMed] [Google Scholar]
- 108.Zhao F, Zhang W, Hoffmann AA, Ma CS. 2014. Night warming on hot days produces novel impacts on development, survival and reproduction in a small arthropod. J. Anim. Ecol. 83, 769–778. ( 10.1111/1365-2656.12196) [DOI] [PubMed] [Google Scholar]
- 109.Schlichting CD, Pigliucci M. 1998. Phenotypic evolution: a reaction norm perspective. Sunderland, MA: Sinauer. [Google Scholar]
- 110.Telemeco RS. 2014. Immobile and mobile life-history stages have different thermal physiologies in a lizard. Physiol. Biochem. Zool. 87, 203–215. ( 10.1086/674959) [DOI] [PubMed] [Google Scholar]
- 111.Kingsolver JG, Woods HA. 2016. Beyond thermal performance curves: modeling time-dependent effects of thermal stress on ectotherm growth rates. Am. Nat. 187, 283–294. ( 10.1086/684786) [DOI] [PubMed] [Google Scholar]
- 112.Fischer B, van Doorn GS, Dieckmann U, Taborsky B. 2014. The evolution of age-dependent plasticity. Am. Nat. 183, 108–125. ( 10.1086/674008) [DOI] [PubMed] [Google Scholar]
- 113.Salinas S, Munch SB. 2012. Thermal legacies: transgenerational effects of temperature on growth in a vertebrate. Ecol. Lett. 15, 159–163. ( 10.1111/j.1461-0248.2011.01721.x) [DOI] [PubMed] [Google Scholar]
- 114.Walsh MR, Cooley F, Biles K, Munch SB. 2015. Predator-induced phenotypic plasticity within- and across-generations: a challenge for theory? Proc. R. Soc. B 282, 20142205 ( 10.1098/rspb.2014.2205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sheriff MJ, Love OP. 2013. Determining the adaptive potential of maternal stress. Ecol. Lett. 16, 271–280. ( 10.1111/ele.12042) [DOI] [PubMed] [Google Scholar]
- 116.Kuijper B, Hoyle RB. 2015. When to rely on maternal effects and when on phenotypic plasticity? Evolution 69, 950–968. ( 10.1111/evo.12635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ezard TH, Prizak R, Hoyle RB. 2014. The fitness costs of adaptation via phenotypic plasticity and maternal effects. Funct. Ecol. 28, 693–701. ( 10.1111/1365-2435.12207) [DOI] [Google Scholar]
- 118.Uller T, English S, Pen I. 2015. When is incomplete epigenetic resetting in germ cells favoured by natural selection? Proc. R. Soc. B 282, 20150682 ( 10.1098/rspb.2015.0682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoffmann AA, Sorensen JG, Loeschcke V. 2003. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 28, 175–216. ( 10.1016/S0306-4565(02)00057-8) [DOI] [Google Scholar]
- 120.Cooper BS, Tharp JM, Jernberg II, Angilletta MJ. 2012. Developmental plasticity of thermal tolerances in temperate and subtropical populations of Drosophila melanogaster. J. Therm. Biol. 37, 211–216. ( 10.1016/j.jtherbio.2012.01.001) [DOI] [Google Scholar]
- 121.Gerken AR, Eller OC, Hahn DA, Morgan TJ. 2015. Constraints, independence, and evolution of thermal plasticity: probing genetic architecture of long- and short-term thermal acclimation. Proc. Natl Acad. Sci. USA 112, 4399–4404. ( 10.1073/pnas.1503456112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kellett M, Hoffmann AA, McKechnie SW. 2005. Hardening capacity in the Drosophila melanogaster species group is constrained by basal thermotolerance. Funct. Ecol. 19, 853–858. ( 10.1111/j.1365-2435.2005.01025.x) [DOI] [Google Scholar]
- 123.Tonsor SJ, Elnaccash TW, Scheiner SM. 2013. Developmental instability is genetically correlated with phenotypic plasticity, constraining heritability, and fitness. Evolution 67, 2923–2935. ( 10.1111/evo.12175) [DOI] [PubMed] [Google Scholar]
- 124.Auld JR, Agrawal AA, Relyea RA. 2010. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B 277, 503–511. ( 10.1098/rspb.2009.1355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Van Buskirk J, Steiner UK. 2009. The fitness costs of developmental canalization and plasticity. J. Evol. Biol. 22, 852–860. ( 10.1111/j.1420-9101.2009.01685.x) [DOI] [PubMed] [Google Scholar]
- 126.Hoekstra LA, Montooth KL. 2013. Inducing extra copies of the Hsp70 gene in Drosophila melanogaster increases energetic demand. BMC Evol. Biol. 13, 11 ( 10.1186/1471-2148-13-68). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Venable DL, Lawlor L. 1980. Delayed germination and dispersal in desert annuals: escape in space and time. Oecologia 46, 272–282. ( 10.1007/BF00540137) [DOI] [PubMed] [Google Scholar]
- 128.Cohen D. 1967. Optimizing reproduction in a randomly varying environment when a correlation may exist between the conditions at the time a choice has to be made and the subsequent outcome. J. Theor. Biol. 16, 1–14. ( 10.1016/0022-5193(67)90050-1) [DOI] [PubMed] [Google Scholar]
- 129.Venable DL, Brown JS. 1988. The selective interactions of dispersal, dormancy, and seed size as adaptations for reducing risk in variable environments. Am. Nat. 131, 360–384. ( 10.1086/284795) [DOI] [Google Scholar]
- 130.Fedorka KM, Winterhalter WE, Shaw KL, Brogan WR, Mousseau TA. 2012. The role of gene flow asymmetry along an environmental gradient in constraining local adaptation and range expansion. J. Evol. Biol. 25, 1676–1685. ( 10.1111/j.1420-9101.2012.02552.x) [DOI] [PubMed] [Google Scholar]
- 131.Bradshaw WE, Holzapfel CM. 2008. Genetic response to rapid climate change: it's seasonal timing that matters. Mol. Ecol. 17, 157–166. ( 10.1111/J.1365-294x.2007.03509.X) [DOI] [PubMed] [Google Scholar]
- 132.Visser ME. 2008. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659. ( 10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chuine I. 2010. Why does phenology drive species distribution? Phil. Trans. R. Soc. B 365, 3149–3160. ( 10.1098/Rstb.2010.0142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vedder O, Bouwhuis S, Sheldon BC. 2013. Quantitative assessment of the importance of phenotypic plasticity in adaptation to climate change in wild bird populations. PLoS Biol. 11, e1001605 ( 10.1371/journal.pbio.1001605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gienapp P, Lof M, Reed TE, McNamara J, Verhulst S, Visser ME. 2013. Predicting demographically sustainable rates of adaptation: can great tit breeding time keep pace with climate change? Phil. Trans. R. Soc. B 368, 20120289 ( 10.1098/rstb.2012.0289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Phillimore AB, Leech DI, Pearce-Higgins JW, Hadfield JD. 2016. Passerines may be sufficiently plastic to track temperature-mediated shifts in optimum lay date. Glob. Change Biol. 22, 3259–3272. ( 10.1111/gcb.13302) [DOI] [PubMed] [Google Scholar]
- 137.Rehm EM, Olivas P, Stroud J, Feeley KJ. 2015. Losing your edge: climate change and the conservation value of range-edge populations. Ecol. Evol. 5, 4315–4326. ( 10.1002/ece3.1645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aspinwall MJ, Loik ME, de Dios VR, Tjoelker MG, Payton PR, Tissue DT. 2015. Utilizing intraspecific variation in phenotypic plasticity to bolster agricultural and forest productivity under climate change. Plant Cell Environ. 38, 1752–1764. ( 10.1111/pce.12424) [DOI] [PubMed] [Google Scholar]
- 139.Ullah I, Ashraf M, Zafar Y. 2008. Genotypic variation for drought tolerance in cotton (Gossypium hirsutum L.): leaf gas exchange and productivity. Flora 203, 105–115. ( 10.1016/j.flora.2007.12.001) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.