Abstract
Phenotypic plasticity is a crucial mechanism for responding to changes in climatic means, yet we know little about its role in responding to extreme climatic events (ECEs). ECEs may lack the reliable cues necessary for phenotypic plasticity to evolve; however, this has not been empirically tested. We investigated whether behavioural plasticity in nest-site selection allows a long-lived shorebird (Haematopus ostralegus) to respond to flooding. We collected longitudinal nest elevation data on individuals over two decades, during which time flooding events have become increasingly frequent. We found no evidence that individuals learn from flooding experiences, showing nest elevation change consistent with random nest-site selection. There was also no evidence of phenotypic plasticity in response to potential environmental cues (lunar nodal cycle and water height). A small number of individuals, those nesting near an artificial sea wall, did show an increase in nest elevation over time; however, there is no conclusive evidence this occurred in response to ECEs. Our study population showed no behavioural plasticity in response to changing ECE patterns. More research is needed to determine whether this pattern is consistent across species and types of ECEs. If so, ECEs may pose a major challenge to the resilience of wild populations.
This article is part of the themed issue ‘Behavioural, ecological and evolutionary responses to extreme climatic events’.
Keywords: climate change ecology, Haematopus ostralegus, extreme climatic event, learning, sea-level rise, environmental cues
1. Introduction
One of the most prominent aspects of global climate change is the more frequent occurrence of extreme climatic events (ECEs), such as droughts and floods [1,2]. ECEs can have consequences for biological systems at the population [3], community [4] and ecosystem level [5], but ultimately these impacts are driven by individual changes [6]. Organisms may respond to changing ECE frequencies through inter-generational micro-evolutionary change or intra-generational phenotypic plasticity in labile traits.
Phenotypic plasticity in response to gradual changes in mean climate is a well-studied topic [7,8], and has been suggested as the key mechanism through which organisms can respond to recent climate change [8]. By contrast, consideration of phenotypic plasticity as a response to ECEs has been mostly theoretical [9–12]. Phenotypic plasticity may allow individuals to respond pre-emptively to upcoming ECEs or adapt once the extreme conditions arise through behavioural or physiological change [12–14]. To pre-empt future ECEs, individuals must possess a reliable cue to predict future conditions [15]. When a reliable cue is absent, or the reliability of a cue deteriorates, phenotypic plasticity becomes maladaptive as individuals can ‘overshoot’ optimal conditions [15–17]. ECEs are often considered unpredictable in the biological literature, but it is possible that ECEs may simply be rare rather than unpredictable [10]. In this case there may still be reliable cues available to individuals that will allow them to predict oncoming ECEs and respond accordingly. Climatological work has highlighted the potential for ECEs to show predictability under certain circumstances [18]; but whether these reliable cues are detectable by organisms and will facilitate responses to ECEs is still unknown.
Even without reliable cues, individuals may exhibit plasticity through learning, where an individual remembers previously experienced conditions and adjusts its future response. For example, nesting blue tits (Cyanistes caeruleus) learnt to adjust their laying date when they experienced a mismatch with their food source in the previous spring [19]. Unlike other types of plasticity, which will generally require exposure to environmental conditions across multiple generations to evolve, learning allows individuals to generate responses to novel environmental conditions [20]. For example, Australian marbled frogs (Limnodynastes convexiusculus) learned to avoid toxic cane toads (Bufo marinus) following a first novel encounter [21]. For learning to occur however, environmental conditions must be stochastic enough to make learning worthwhile but not so much that the relationship between a stimulus and response are highly changeable [22]. Therefore, while climate change could select strongly for learning responses, such selection will require that conditions in one year are generally indicative of conditions in the next [23].
Learning is unlikely to evolve in response to ECEs if such events are infrequent compared to the lifespan of an organism; yet as climatic conditions change, learning responses to ECEs could become more common or previously evolved learning responses may be expressed. Learning responses to other types of stochastic events, such as predation, have previously been documented in birds [24,25], fish [26] and amphibians [27], and some studies in nesting birds have suggested that learning responses to ECEs can occur [28–30]. Yet, as with much of the ECE literature, these studies are often anecdotal (i.e. only consider a single ECE) making it difficult to generalize the results from one event to the next and reliably estimate responses [10]. Therefore, whether learning can act as a response to changing patterns of ECEs is still an open question.
In this study, we use detailed longitudinal behavioural information on individual Eurasian oystercatchers (Haematopus ostralegus) over two decades to test for behavioural plasticity in nest-site selection as a response to changing patterns of extreme flooding events. Since 1971 maximum high tides during the H. ostralegus breeding season have increased at twice the rate of mean high tides over the same period (0.8 versus 0.4 cm yr−1; electronic supplementary material, figure S1), driven by sea-level rise and changing wind and storm patterns [3]. Consequently, the frequency of historically rare extreme floods (defined using a biologically informed cut-off; see [10] and Methods for details) has more than doubled from once every 7.0 years, between 1971 and 1991, to once every 2.7 years since 1991.
These flooding events strongly impact H. ostralegus reproductive success, washing away eggs and drowning young chicks [3]. Over the two decades of this study, 51% of nests were located on sites that were inundated at some point during the breeding season. Although many vulnerable nests are predated or fledged before flooding can occur, on average 14% of nests are inundated annually during the incubation period. A large number of nests (42%) fail when covered by water, increasing to almost 60% once water is 15 cm or more above the nest. Even those nests that remain following flooding may fail to hatch after being submerged [31].
van de Pol et al. [3] outline three traits that may be used by H. ostralegus to reduce flooding risk. Increasing nest elevation will allow breeding pairs to reduce the chance that a nest will be inundated during a flooding event. Alternatively, as flooding risk is known to increase across the season, individuals may lessen flooding risk through laying date advancement. Finally, breeding pairs may shorten the incubation and early chick phase to reduce the length of time at which nests are at risk of flooding. Of these three behavioural traits, variation in nest elevation provides the most effective mechanism through which H. ostralegus pairs can mediate flooding risk [3]. For example, an increase in nest elevation of only 18 cm (equivalent to the median standard deviation of elevation within a territory; see Results) would completely alleviate H. ostralegus flooding risk in comparison to 1990–2008 levels. However, to achieve a similar reduction in flooding risk H. ostralegus would need to advance laying date by 34 days or reduce the length of the incubation and early chick phase by more than half (24 days) [3]. Importantly, H. ostralegus pairs also have plenty of opportunity to increase nest elevation, as chosen nesting sites are typically around half a metre lower than the highest available point within a territory (see Results).
Empirical field data further highlight the importance of nest elevation as a mediator of flooding risk. During flooding events, low nesting H. ostralegus experience greatly reduced nest success, in turn limiting the reproductive success of the breeding population as a whole. If the nesting behaviour of our population remains unchanged, increased frequency of such flooding events will pose a serious threat to population viability [3]. This is likely to be a common scenario among many coastal (beach and saltmarsh) nesting species [32–34]. However, it should not be assumed that nest elevation will remain static. As the frequency of flooding events increases we would expect directional selection to favour higher nest elevation, working via the fitness component of nest success. Encouragingly, our study population has shown an increase in mean nest elevation over time at half the rate of change seen in maximum high tide [35], providing evidence that changing nest elevation as a response to flooding may be possible.
Due to the long generation time of H. ostralegus (11–13 years; [36]), it is unlikely that micro-evolutionary changes in nest elevation will explain the rapid changes we observe in our population. Instead, phenotypic plasticity in nest-site selection provides a more plausible mechanism by which such changes may be explained, with individuals selecting higher elevation nest sites over their lifetimes. The current study system is perfectly suited to test for the presence of such phenotypic plasticity. Our extensive dataset on a long-lived species with high site fidelity includes many individuals that have been followed over multiple years, allowing us to detect small within-individual changes that might otherwise be missed. Importantly, by testing for the presence of phenotypic plasticity in H. ostralegus nest elevation we can also provide a test on the role of phenotypic plasticity as a response to ECEs more generally.
To assess the presence of phenotypic plasticity in nest-site selection we investigated three questions. First, do individuals exhibit a change in nest elevation over their lifetime? Any changes across our study period would provide initial evidence for phenotypic plasticity as a driver of nest elevation change.
Second, do reliable cues exist that predict extreme flooding events and do H. ostralegus adjust nest elevation in response to such environmental cues? If H. ostralegus exhibits phenotypic plasticity in response to changing flooding patterns then reliable cues are likely to exist that will allow for the prediction of future flooding risk. Both the lunar nodal cycle, a predictable 18.6 year cyclical pattern in water heights [37,38], and water levels in the preceding breeding season might act as such reliable cues. We predicted that individuals would increase nest elevation as lunar nodal cycle position advanced (i.e. as water heights increase) and as water heights in the preceding breeding season increased. Additionally, we tested the relationship between individual nest elevation and water heights in the upcoming breeding season to account for the presence of unknown reliable cues that may be correlated with flooding risk but were not directly measured.
Finally, do H. ostralegus demonstrate a learning response by changing their nest elevation following a flooding event? Even without the presence of reliable environmental cues, H. ostralegus may exhibit changes in nest elevation through learning. We predicted that individuals that experience a flooding event would increase the elevation of their next nest, with individuals whose nests were destroyed by flooding increasing more than those that survived a flood. Since random nest-site selection may also result in a positive change in nest elevation following flooding (see Methods), we specifically tested whether any increase in nest elevation was greater than that expected under random nest-site selection.
2. Methods
(a). Defining an extreme climatic event
To study ECEs it is important to define what conditions are classed as ‘extreme’. This definition will depend on the question of interest and the spatial, temporal and biological scale at which one works [39]. For this study, we are interested in investigating how climate-driven processes (i.e. tidal flooding) might impact individual behaviour, with a focus on the local spatial scale (a single study population) and short-term temporal scale (1–2 years). We use a ‘biological’ definition of ECEs [10]: ‘An episode where climate or climate-driven conditions trigger a negative threshold-like (nonlinear) biological response’. Setting an ECE baseline using a ‘biological’ definition allows us to document changes in the frequency of extreme conditions that are biologically meaningful, removing the need for definitions based purely on historical climatological frequency [10].
We used our current biological knowledge of the study system to select a standard water height over which we consider a flood to be ‘extreme’. Specifically, we considered data from 1995 and defined an extreme flooding event as water heights that exceeded 95% of all nests laid in this year (electronic supplementary material, figure S2), equivalent to 82 cm above 1971 mean high tide (here after MHT; source www.live.waterbase.nl). The year 1995 is the earliest in which both nest elevation and tidal data are available, providing us with a measure of tidal extremeness that pre-dates any phenotypic plasticity in nest elevation that may be present within our dataset.
(b). Study system
H. ostralegus is a ground-nesting shorebird that breeds on saltmarshes and beaches, close to the estuarine intertidal flats on which they feed. It is a long-lived species (generation time 11–13 years), and forms stable, long-term pair bonds [36]. Pairs have strong site fidelity, returning to the same territories year after year [40,41]. During the breeding season, males construct several nest cups within their territory, from which the female selects a suitable site for egg laying [42]. H. ostralegus are not a multi-brooding species, but often lay replacement clutches during a year following nest predation or flooding. As H. ostralegus do not construct nest mounds and use limited nesting material, nest elevation will be determined solely by nest-site selection.
Our study population, on the Dutch barrier island of Schiermonnikoog (53.4833°N, 6.1667°E), has been monitored since 1983 (see [43,44] for details). Numbers of H. ostralegus have declined sharply, with declines of 3% per annum in the region since 1991 [45]. Consequently, the area of the study was expanded over time to sample a similar number of breeding pairs. Most breeding birds (more than 90%) have been individually colour banded, allowing us to follow their behaviour over multiple years.
(c). Data collection
We monitored nesting activity from April to August annually, with most nests laid between May and June. Nests were located through systematic searching in the field every 2–3 days, with active nests revisited to determine nest fate. We identified nest parents by their colour bands. Nest elevation was recorded over a 20 year period (1995–2014; no data in 1997–1999), and measured in centimetres (±0.1 cm) above MHT.
We collected nest elevation data from 2912 nests of which 2250 had at least one banded parent. We had nest elevation data from 374 banded males (mean 5.7 nests over 4.2 years per individual) and 404 banded females (mean 4.9 nests over 3.7 years per individual). The methods used to measure nest elevation varied between years. In eight years (1995, 1996, 2008, and 2010–2014) nest elevation was measured directly in the field. Elevation was determined in situ using a water level device (1995–1996); laser machine control device (2008); and a differential GPS (2010–2014; ProMark 800 GNSS). All in situ methods provide measurement accuracy to within 2 cm, confirmed using existing calibration sites established by Rijkswaterstaat (Dutch Ministry for Infrastructure and Environment). For all other nests, elevation was determined by overlaying nest coordinates on a LiDAR digital elevation map (measured 2008; cell size 0.5 × 0.5 m; http://www.ahn.nl/index.html). This less precise ex situ method was used to collect data between 2000–2007 and 2009, as well as supplementing data in predominantly in situ measured years. These differences in precision between in situ and ex situ data collection are explicitly accounted for in all analyses (see below).
The lunar nodal cycle varies considerably in amplitude and phase globally [37], therefore we calculated the shape of the lunar nodal cycle specifically for our study site, employing methods outlined by Houston & Dean [38] and Baart et al. [37] with tidal data covering 1971 to 2015. On Schiermonnikoog, we identified a lunar nodal cycle with an amplitude of 2.22 cm and a phase of −0.76 radians (with 1970 set as year 0), representing fluctuations in water height of around 0.5 cm yr−1, a similar magnitude to the rate of sea-level rise recorded in our study region (electronic supplementary material, figure S3). To analyse lunar nodal cycle we gave each year a value based on the ‘position’ of the cycle, where −1 represents a trough in the cycle and 1 represents a peak.
Water height data were measured at our study site by Rijkswaterstaat (Dutch Ministry for Infrastructure and Environment). All data were collected at 10 min intervals providing an accurate measure of high tide values. To test the response of individuals to tidal patterns during both the preceding and current breeding season we calculated the mean high tide across May and June in all years, the point in time at which nesting activity is most frequent. To distinguish between the effects of lunar nodal cycle and seasonal differences in water height we then calculated a residual water height variable (the difference between actual water heights and water heights predicted based on the lunar nodal cycle).
To test for the presence of learning we first needed to determine the fate of each measured nest. We considered a nest site to be ‘flooded’ when recorded water levels exceeded nest elevation. Inspection of nests during and after flooding events showed that this was a suitable rule to determine flooding experience. We considered a nest to be active once the first egg was laid and inactive when no more eggs were present in the nest, either due to hatching, predation or flooding. The point at which a nest was considered inactive was determined as the mid-point between the penultimate and final nest check. We categorized nests into one of three different experiences: failed due to flooding, survived a flooding event, and unflooded. A nest was considered failed due to flooding when the inactive date of the nest was on the day of a flood. Nest checks were generally conducted immediately before and after a flooding tide, allowing us to more accurately estimate nest inactivity date and attribute nest failure due to flooding with high confidence. We then investigated the relationship between flooding experience and changes in nest elevation from one nest to the next. For this analysis we removed all individuals with only one nest, leaving 1508 nests.
As H. ostralegus territories vary widely in elevation, we expected that the characteristics of individual territories might be an important modulator of nest elevation. Specifically, we predicted that more variable territories (i.e. larger standard deviation of elevations within a territory; σE) would allow for stronger plastic responses in nest elevation, as such territories would provide more opportunities to nest higher. For each year we determined the known locations of individual pairs (nest locations and territory roosting locations), with which we generated polygons using a Voronoi algorithm in QGIS [46]. This procedure provided an estimate of territory location for each pair, with polygons covering an average of 6781 m2 (range: 2–94 537 m2). Territory polygons were overlaid on our LiDAR map to calculate σE. We had no records of in situ measured nests less than 20 cm above MHT; therefore, points below 20 cm were considered unviable nesting locations and were excluded from σE calculations.
The frequency of flooding events is also known to increase across the breeding season; therefore, the impact of nest elevation on flooding risk and the potential for phenotypic plasticity is likely to depend on egg laying date [3]. Laying date was incorporated into all analyses, defined as the date that the first egg of a clutch was laid, estimated with a precision of 1–2 days using methods described by van de Pol et al. [47].
(d). Geomorphological model
The elevation of nest sites on the salt marsh will increase naturally over time due to sedimentation (primarily driven by winter flooding) and glacial rebound [48]. To distinguish plastic changes in nest elevation from such geomorphological processes we used a geomorphological model designed and parameterized with field data from our study island to determine the annual rate of saltmarsh accretion as a function of elevation (see [3] for model details).
(e). Data analysis
Analyses were conducted using general linear mixed models with a Gaussian distribution (log identity), using the package nlme in R [49,50]. Akaike's Information Criterion, with a correction for small sample size (AICc), was used to conduct model selection [51], taking care to avoid the addition of ‘uninformative variables’ [52]. We carried out a model selection process with package MuMIn [53].
The best supported random effects structure was determined by comparing AICc values of models with varying random effects structures using maximum likelihood. Although H. ostralegus tend to form long-term pair bonds, pair divorce and widowing does occur (8% and 7% per annum respectively; [36,44]); therefore, we initially considered both male and female identity in our random effects structure. In the analysis of environmental cues, we found that male identity explained more of the variation in nest elevation than female identity (see Results); therefore, the rest of our study is focused on male behaviour. In the learning analysis, model comparisons showed no benefit of including any random effects, and all subsequent learning analysis was carried out using general linear (non-mixed) models (electronic supplementary material, table S1).
Over the course of the long-term study the size of the study area was expanded, with more high elevation locations included in later years. Therefore, we included a random intercepts term ‘AreaID’ to account for the potential influence of sub-area on nest elevation trends. We fitted our model error term as a function of ‘Method’ to account for differences in precision between in situ and ex situ collection methods.
In both the analysis of environmental cues and changes over time we carried out within-group centring of ‘MaleID’ [54]. This method allowed us to exclude any potential changes in nest elevation that may be driven by changes in population composition and focus specifically on phenotypic change within individual males over their lifetime. At the edge of our study site some males have the opportunity to nest on a large artificial sea wall (74 males; 20% of our study population). Nest elevation values from these pairs can lead to a violation of the assumption of normality within our data. Therefore, we conducted a Winsorisation procedure, in which nests high on the sea wall were capped at the value of the highest recorded non-sea wall nest (260 cm above MHT). In our analysis of within-individual change over time, those pairs with access to the sea wall were found to behave differently to others within the population (see Results). Variation in individual nest elevation was better explained by access to the sea wall than standard deviation of elevations within a territory (σE). As these two variables are strongly confounded, access to the sea wall (Sea wall) was used in place of σE for further analyses.
For the learning analysis, we determined the difference between the elevation of each nest and the elevation of the following nest belonging to the same male (ΔE). The following nest could be in either the same year or the following year and we included a categorical factor in our model to differentiate between these two scenarios (NextYear). This allows us to account for geomorphological processes that may influence nest elevation between seasons. Models were fitted with a fixed effects term for flooding experience (Exp; levels: failed due to flooding, survived a flood, unflooded), Seawall, LayDate, and NextYear. To account for differences in behaviour between birds with different territory characteristics an Exp * Seawall interaction was included. Furthermore, we included an Exp * LayDate interaction to account for potential seasonal variation in behavioural responses.
It is possible that a relationship could occur between flooding experience and nest elevation change (ΔE) even if individuals are selecting nest sites at random. This is due to the bounded nature of nest elevation data (i.e. individuals have a minimum and maximum potential elevation at which they can nest) [55]. For individuals nesting at the lower bounds of potential elevation the majority of alternative nest sites will be higher than their current site. These low nesting individuals are therefore likely to increase their nest elevation in their next nesting attempt, even if nest-site selection occurs at random. As flooded nests are likely to be much lower than unflooded nests this possibility may obscure any effect of flooding experience. We applied two approaches to account for this concern. Firstly, we quantified the relationship between flooding experience and elevation change in randomized data. We conducted 5000 iterations in which we randomized the order of nests for each male, maintaining the association between sea wall, laying date and nest elevation. We then recalculated values of ΔE using this randomly ordered data, estimating the change in nest elevation we would expect under a null hypothesis of random nest-site selection without learning. Additionally, we refitted our top learning model by replacing the experience term (Exp) with a term showing the difference between the elevation of each nest and the median elevation of our study area (140 cm above MHT). We compared our top model with this new model using AICc to test how well distance from the median explains change in nest elevation.
Due to the presence of replacement clutches our data included nests laid by individuals in the same year and across years. Lunar nodal cycle and mean water height measurements were measured at a yearly scale; therefore, analyses of environmental cues consider only changes in nest elevation between years. In comparison, learning analysis considers changes both within the same year and across years. In combination these analyses provide a test of nest elevation changes in H. ostralegus both within and between years.
3. Results
(a). Nest elevation consistency within males and females
Male identity better explained variation in nest elevation than female identity, suggesting that males are more likely to determine nest elevation. When both male and female identity were fitted as random effects, male explained 20% of variance in nest elevation while female only explained 5%. Importantly, when male identity was included alone it was able to explain all the variation previously explained by female (26%), but female was only able to explained 19% of variation when included without male. The long-term pair bonds formed in H. ostralegus mean that male and female identity will not be fully independent; therefore, in subsequent analyses we excluded female identity from our random effects structures.
(b). Nest and territory characteristics
Oystercatchers selected relatively high nest sites within their territory, although they did not nest at the highest sites available. On average, H. ostralegus laid nests higher than their mean territory elevation (5.7 cm, 95% CI = 4.1–7.2) but far below the maximum territory elevation (−59.4 cm, 95% CI = −56.6 to −62.1). The median mean elevation of H. ostralegus territories was 68.2 cm above MHT (range: 20.6–380.8 cm) and the median standard deviation of elevation within a territory was 18.0 cm (range: 0.4–188.4 cm).
(c). Phenotypic change over time
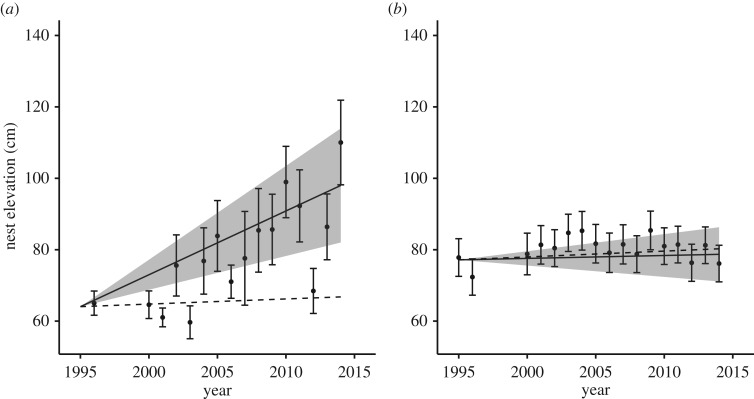
Individual H. ostralegus showed an increase in nest elevation over time; however, this change was restricted to those breeding birds with access to the artificial sea wall (74 pairs; 20% of the population). Birds with access to the sea wall showed a strong positive increase in nest elevation, more rapid than geomorphological processes would predict (figure 1a, table 1; βTIME = 1.66 cm yr−1, 95% CI = 0.88–2.44), while those birds without a sea-wall territory showed no nest elevation change over time (figure 1b, table 1; β = 0.07 cm/year, 95% CI = −0.26–0.40). The presence of the sea wall was able to explain variation in nest elevation more effectively than standard deviation of elevations within the territory (σE; table 1). Post hoc analysis including only those individuals with access to the sea wall showed a significant increase in sea-wall use over time (electronic supplementary material, figure S4); however, these sea-wall nests constituted only 9.2% of all nests laid by sea-wall birds and only 1.6% of all measured nests. Similar results were obtained when we considered changes in nest elevation of females instead of males (electronic supplementary material, table S3).
Figure 1.
(a) Within-individual change in nest elevation over time for H. ostralegus males with access to the artificial sea wall (1.66 ± 0.40 cm yr−1; solid line) and rate of nest elevation change predicted by sedimentation (0.13 cm yr−1; dashed line). Analysis based on 528 H. ostralegus nests from 74 individual males (mean 6.5 nests/individual). (b) Within-individual change in nest elevation over time for H. ostralegus males without access to the artificial sea wall (0.07 ± 0.17 cm yr−1; solid line) and rate of nest elevation change predicted by sedimentation (0.12 cm yr−1; dashed line). Analysis based on 2384 nests from 311 males (mean 5.3 nests/individual). For both plots, shaded region shows the 95% confidence interval for the slope of observed data.
Table 1.
Coefficients of models investigating within-individual change in Haematopus ostralegus nest elevation over time (cm ± s.e.). Models include Time, standard deviation of elevations within territories (σE) and egg laying date (LayDate). All models within the 95% confidence set are displayed plus the null model; for full model selection results see electronic supplementary material, table S2. Post hoc analysis includes a categorical term to account for the presence of an artificial sea wall (Seawall). Nests with a sea wall are used as the reference category. k denotes the number of parameters estimated in each model; wi denotes AICc model weights.
| ΔAICc | wi | k | intercept | time (years) | σE (cm) | time * σE | ||
|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.91 | 4 | 79.56 (±4.33) | 0.38 (±0.16) | 0.06 (±0.02) | 0.01 (±0.03e−1) | ||
| 5.91 | 0.05 | 2 | 79.58 (±4.37) | 0.32 (±0.16) | — | — | ||
| 6.09 | 0.04 | 1 | 79.52 (±4.38) | — | — | — | ||
| Post hoc comparison | ||||||||
| ΔAICc | wi | k | intercept | time (years) | σE (cm) | time * σE | seawall | time * seawall |
|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.99 | 4 | 81.75 (±9.51) | 1.66 (±0.40) | — | — | −2.41 (±10.80) | −1.59 (±0.43) |
| 10.20 | 0.01 | 4 | 79.56 (±4.33) | 0.38 (±0.16) | 0.06 (±0.02) | 0.01 (±0.03e−1) | — | — |
| 16.29 | 0.00 | 1 | 79.52 (±4.38) | — | — | — | — | — |
(d). Phenotypic plasticity in response to environmental cues
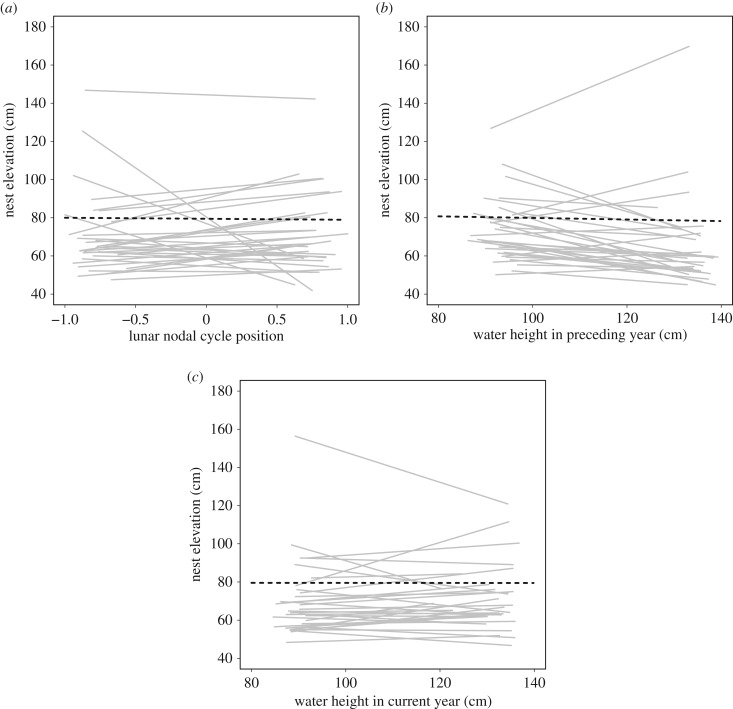
Both water levels during the preceding breeding season (May–June) and lunar nodal cycle position showed a positive correlation with water height during the current season (water height: Pearson's correlation 0.70, 95% CI = 0.51–0.83; lunar nodal cycle: Pearson's correlation 0.27, 95% CI = −0.02–0.53), suggesting that these could provide a reliable cue of future flooding risk. The influence of lunar nodal cycle position on nest elevation was included in the best supported model, but the direction of the effect was opposite to our prediction (figure 2a; table 2). Model selection provided little support for a positive relationship between water height in either the preceding or current breeding season and changes in individual nest elevation (figure 2b,c; electronic supplementary material, table S4). Frequency of sea-wall use also showed no change in response to any of the three tested environmental cues (electronic supplementary material, table S5). Similar results were obtained when we considered changes in nest elevation of females instead of males (electronic supplementary material, table S6).
Figure 2.
Relationship between Haematopus ostralegus nest elevation and (a) lunar nodal cycle position, where a value of 1 represents a water height peak and −1 represents a trough (dashed lines); (b) mean water height in the breeding season before nesting occurred (May–June); and (c) mean water height in the breeding season of nesting (May–June). Solid lines show nest elevation slopes for all male H. ostralegus with more than 10 years of data (n = 32). All elevation data measured in centimetres above mean high tide in 1971. Analysis conducted using 2912 nests from 1129 males.
Table 2.
Coefficients of models investigating the role of potential environmental cues on Haematopus ostralegus nest elevation (cm ± s.e.). Models include within-individual effects of lunar nodal cycle (LNC), water height in the current breeding season (May–June; Water), and sea-wall access (Seawall) where birds with the sea wall are used as the reference category. Top two models based on AICc are displayed plus the null model; for full model selection results see electronic supplementary material, table S4. k denotes the number of parameters estimated in each model; wi denotes AICc model weights.
| ΔAICc | wi | k | intercept | seawall | LNC | water (cm) | LNC * seawall | water * seawall |
|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.99 | 4 | 77.86 (±9.88) | 1.56 (±11.21) | −2.25 (±0.55) | — | 2.72 (±0.62) | — |
| 17.93 | <0.01 | 4 | 80.22 (±9.62) | −0.87 (±10.92) | — | −0.58 (±0.54) | — | 0.74 (±0.61) |
| 19.86 | <0.01 | 1 | 79.52 (±4.38) | — | — | — | — | — |
(e). Learning response
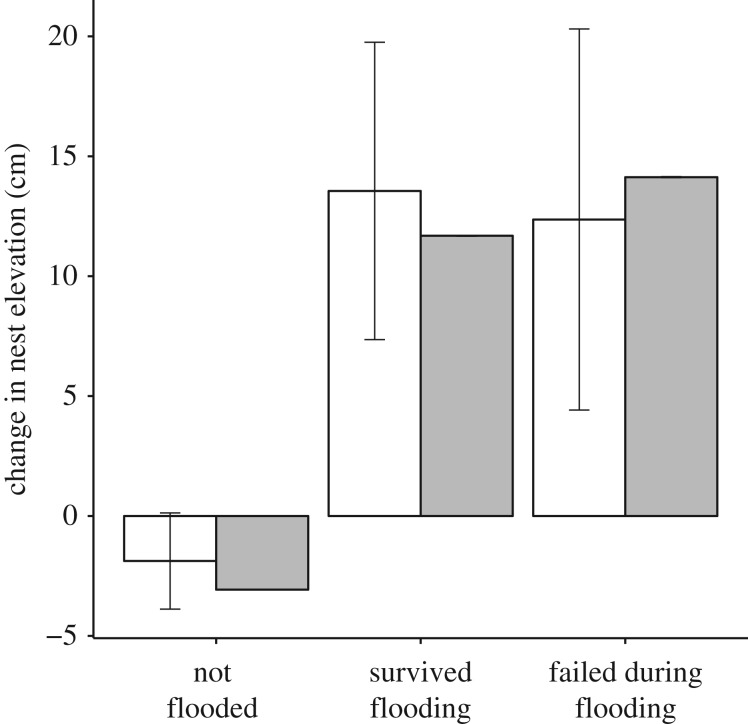
Flooding experience had a strong effect on nest elevation change. The top model contained flooding experience, access to the artificial sea wall (Seawall) and an interaction between the two (table 3). Males that experienced no flooding showed no change in nest elevation (figure 3; table 3, βUNFLOODED = −1.19 cm, 95% CI = −6.03–3.65). Males that experienced a flood increased their nest elevation more than those that had not experienced a flood, regardless of the ultimate fate of the flooded nest (figure 3; table 3, βFAILED = 21.24 cm, 95% CI = 5.19–37.29; βSURVIVED = 21.61 cm, 95% CI = 11.07–32.15). Whether the next nest was laid in the same year or the next year had no impact on the change in elevation (table 3, βNEXTYEAR = 1.02, 95% CI = −2.76–4.80). In post hoc analysis, we found that individuals that had previously experienced flooding, in breeding seasons before the current year, behaved the same as those individuals that had no earlier flooding experience (electronic supplementary material, table S8).
Table 3.
Coefficients of models investigating the role of flooding experience (Exp) on changes in Haematopus ostralegus nest elevation from one breeding attempt to the next (cm ± s.e.). Models include whether individuals have access to the artificial sea wall (Seawall) and a categorical variable specifying whether nest elevation change was measured within the same year or across two consecutive years (NextYear). Unflooded nests in the same year with access to the sea wall are used as the reference category. All models within the 95% confidence set are displayed plus the null model; for full model selection results see electronic supplementary material, table S7. k denotes the number of parameters estimated in each model; wi denotes AICc model weights.
| ΔAICc | wi | k | intercept | survived | failed | nextyear | seawall | survived * seawall | failed * seawall |
|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.65 | 7 | −1.08 (±2.31) |
21.61 (±5.38) |
21.24 (±8.19) |
1.02 (±1.93) |
−1.19 (±2.47) |
−10.82 (±6.74) |
−11.60 (±9.91) |
| 1.40 | 0.32 | 6 | −0.71 (±2.19) |
21.40 (±5.37) |
21.35 (±8.19) |
— | −1.14 (±2.47) |
−10.62 (±6.73) |
−11.72 (±9.90) |
| 50.07 | <0.01 | 1 | 0.42 (±0.95) |
— | — | — | — | — | — |
Figure 3.
Impact of flooding experience on Haematopus ostralegus nest elevation change in both observed data (white) and randomized data reflecting random nest-site selection (grey; see electronic supplementary material, figure S5). Error bars represent 95% confidence intervals for observed data. Analysis conducted using 1508 nests from 297 males.
However, a randomization procedure showed that the differences in nest elevation change between flooded and unflooded nests were of the same magnitude as we would expect from birds exhibiting random nest-site selection without learning (figure 3; electronic supplementary material, figure S5). Furthermore, a model including the difference between nest elevation and the median elevation in our study site was able to explain observed changes in nest elevation more effectively than flooding experience (electronic supplementary material, table S9). The differences we observed between flooded and unflooded nests were likely driven by differences in nest elevation between these two groups (electronic supplementary material, figure S6; βUNFLOODED = 27.47 cm, 95% CI = 22.56–32.40). Similar results were obtained when we considered changes in nest elevation of females instead of males (electronic supplementary material, table S10).
(f). Fecundity selection landscape
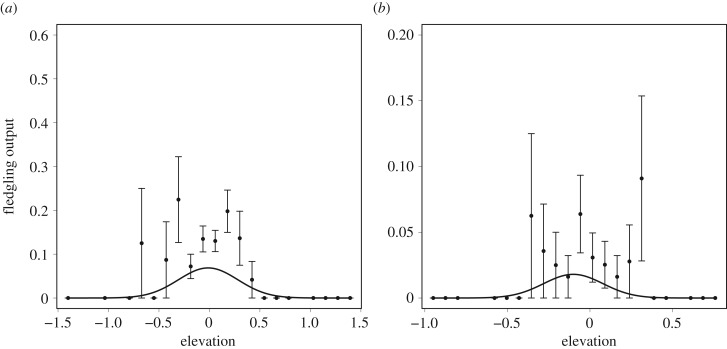
We expected increased flooding risk to impose directional selection on nest elevation via the fitness component of nest success. However, as our results showed no evidence of phenotypic plasticity in H. ostralegus nest elevation we went on to specifically test the relationship between nest elevation and annual reproductive success (fledgling production). This post hoc analysis provided no evidence for directional selection on H. ostralegus nest elevation but did show evidence for stabilizing selection in this trait (figure 4; electronic supplementary material, table S11).
Figure 4.
Quadratic relationship between Haematopus ostralegus nest elevation (within territories) and reproductive success (fledgling output ± s.e.) in (a) coastal and (b) non-coastal territories. x axis represents relative change in elevation within territories. See electronic supplementary material, Appendix B, for detailed methods.
This analysis accounts for the impact of flooding events on H. ostralegus nest success [3] as well as selective pressures imposed via other fitness components that may vary with nest elevation (e.g. chick mortality or nest predation). We measured within-territory changes in nest elevation to avoid any potential differences in reproductive success that may be driven by environmental differences between territories. In this way, we considered the impact of relative changes in nest elevation (within a territory) on H. ostralegus reproductive success. We also included terms to account for variation in reproductive success that may be caused by differences in social status (whether a territory has access to the coast) and flooding risk between territories (mean territory height). We considered both a linear and quadratic relationship between elevation and reproductive success to test for directional and stabilizing selection respectively. Models were compared using AICc. The model structure is discussed in greater detail in supplementary material (see electronic supplementary material, Appendix B).
4. Discussion
This study used an extensive longitudinal behavioural dataset, collected over two decades, to investigate behavioural plasticity in response to increasingly frequent extreme climatic events (ECEs). Despite increases in the mean nest elevation of our study population we were unable to show evidence of phenotypic plasticity in H. ostralegus nest elevation in response to extreme flooding events. We documented within-individual increases in nest elevation over time in a limited subset of our study population; however, we found no evidence that this phenotypic change had occurred in response to flooding. There was no evidence of behavioural plasticity in H. ostralegus nest elevation in response to environmental cues or as a learned response to previous flooding experience. Although flooding events disproportionately impact the reproductive success of low nesting birds, post hoc evidence of stabilizing selection in the fecundity selection landscape suggests that the general absence of behavioural plasticity may be a consequence of counter-acting selective pressures that disfavour increased nest elevation.
(a). Nest elevation as a repeatable trait
Interestingly, we found that male identity better explained variation in nest elevation than female identity, suggesting that site choice by males during nest cup building may be a more important mediator of nest elevation than the female decision of which nest cup to use for egg laying. Repeatability of nest elevation within males (26% of variance explained) highlights the potential for heritability to occur in this trait. This repeatability may be driven at least partly by territorial and physical constraints (i.e. males do not have access to nest sites at all potential nest elevations); however, our results suggested that males often have access to a range of elevations within their territory. Encouragingly, all our results were consistent using both males and females showing that our conclusions are not driven by our focus on male identity.
(b). Does phenotypic plasticity occur in response to extreme flooding events?
There was no evidence of increased nest elevation in response to either of our tested environmental cues. Nest elevation showed no positive relationship with either the lunar nodal cycle phase or water heights in the preceding breeding season, irrespective of whether individuals had access to the artificial sea wall. It may be possible that H. ostralegus is able to use an alternative environmental cue to track changes in flooding patterns; however, our analyses also showed no relationship between nest elevation and water height measured in the same year, which seems to discount this possibility.
There was also no evidence of learning in response to flooding experience. Although increases in nest elevation following a flooding experience could be interpreted as evidence of learning in H. ostralegus, the differences we observed between flooded and unflooded nests were no larger than expected due to random nest-site selection (figure 3; electronic supplementary material, table S9). This strongly suggests that the apparent learning result is due to the fact that flooded nests are found at lower elevations, rather than providing evidence of individual learning. It is worth considering that random nest-site selection could help explain previous reports of learning in response to flooding events [28–30], and will be important to consider in future studies that test changes in bounded data over time.
The lack of both phenotypic plasticity in response to measured environmental cues and lack of learning may be a reflection of the stochastic and unpredictable nature of extreme floods. Although water heights in the previous breeding season and lunar nodal cycle position were correlated with mean breeding season water height (0.70 and 0.27 respectively), it is possible that these correlations may not be sufficient to act as reliable cues. Theoretical work by Reed et al. [15] found that phenotypic plasticity would be maladaptive to population viability where environmental cues showed a reliability (i.e. correlation between a cue and the environmental optimum) of less than 0.5, with this threshold increasing further as environments become more stochastic. Similarly, learning is only likely to evolve when conditions in one year are indicative of conditions in the next [23]. While climate change has driven an increase in mean water heights, whether an extreme flooding event will occur in a given year greatly depends on both wind speed and direction as well as monthly tidal cycles (i.e. spring tides) [3]. Although there is a correlation between mean breeding season water heights (0.70), the correlation between the occurrences of extreme floods from one breeding season to the next between 1971 and 2015 is much smaller (Pearson's correlation 0.38, 95% CI = 0.03–0.56). Indeed, in comparison to other threats, flooding risk is considered to be fairly unpredictable [30]. Therefore, the lack of evidence for both learning and phenotypic plasticity in response to environmental cues potentially supports the idea that ECEs are simply too unpredictable to facilitate the use of phenotypic plasticity.
(c). Counter-acting selection pressures
Although we found no evidence of learning or phenotypic plasticity in response to our tested environmental cues, we did observe an increase in nest elevation over time in those males with territorial access to the artificial sea wall (74 pairs; 20% of our study population). The explanation for this trend is not immediately clear. There is a possibility that such a result stems from geomorphological processes. Sea-wall territories typically encompass habitat in both low-elevation saltmarsh and on the high-elevation sea wall. Over time, territory boundaries are likely to have changed to incorporate a larger proportion of sea-wall habitat, driven by both the disappearance of other pairs that compete for sea-wall space as the population has declined [45] and the loss of available saltmarsh habitat due to coastal erosion (LD Bailey 2014, personal observation). Population declines and saltmarsh erosion will likely lead to greater utilization of sea-wall nest sites without any need for behavioural plasticity in nest elevation, with individuals selecting sea-wall nest sites more often by chance. However, as the frequency of flooding events, the rate of saltmarsh loss, and reduction in population size have changed concurrently over the study period our ability to distinguish between this possibility and other behavioural mechanisms is limited.
Alternatively, the phenotypic change we observe in sea-wall birds may be evidence of phenotypic plasticity in response to environmental cues unrelated to flooding frequency. What such an alternative cue might be is unclear. It is also not immediately clear why we might observe marked differences in phenotypic change between sea-wall and non-sea-wall birds within our study population. One potential explanation may be environmental differences between sea-wall and non-sea-wall areas. The sea-wall represents a unique nesting habitat for H. ostralegus, as it provides high elevation nest sites but is covered in short vegetation, due to both sheep and geese grazing, rather than the tall perennial grasses (e.g. Elymus athericus) that dominate natural high-elevation locations [56,57]. H. ostralegus are generally known to select nest sites with relatively low vegetation [42], a pattern that has been observed directly in our study population (electronic supplementary material, Appendix C). This preference for low vegetation may be driven by mortality selection, as areas with low vegetation provide incubating adults with good visibility to spot approaching threats [58]. In long-lived species like H. ostralegus a preference for increased adult survival at the expense of nest survival, which often decreases in lower vegetation, would be expected [59]. Therefore, the disparity we see between sea-wall and non-sea-wall birds may present evidence for competing fecundity and mortality selection on H. ostralegus nest-site selection behaviour. Further study on H. ostralegus nest preference may allow for a better understanding of nest elevation responses in sea-wall birds. For example, future studies could consider artificially reducing vegetation height in high elevation areas to observe H. ostralegus nesting responses.
In addition to the importance of vegetation height, alternative selection pressures may also exist that will influence H. ostralegus nest elevation decisions. It is possible that high-elevation sites may make nests more obvious to the avian nest predators that dominate this study system. Similarly, vegetation type may play an important role, with nesting individuals potentially showing a preference for specific ground cover so as to increase nest camouflage [60]. As vegetation type covaries strongly with elevation, due to differences in winter flooding frequency [56,57], it is possible that increasing nest elevation may necessitate nesting in non-preferred vegetation. Therefore, changes in nest-site characteristics at higher elevations may reduce reproductive success through increased predation.
The presence of counter-acting selective pressures on various components of reproductive success would help explain the lack of evidence for directional fecundity selection in nest elevation and thus help explain why the majority of our studied individuals show no change in nest elevation. Although flooding events are known to cause high reproductive failure in H. ostralegus [3] alternative selective pressures, such as those discussed above, may be inhibiting any plastic nest elevation response. This may be further exacerbated by the infrequent nature of ECEs, with higher elevation nest sites potentially favoured in flooding years but selected against in non-flooding years [10,30,35]. Although there is currently no clear selection for higher nests, as flooding events become more frequent the selective landscape may begin to change. It will be imperative to consider the possibility of counter-acting selective pressures in other cases where we seek to understand responses to ECEs. While the detrimental impacts of ECEs are clear during extreme years, we must also incorporate factors that drive organismal responses in the more frequent benign conditions [61]. This raises the importance of long-term datasets for studying ECEs as they will provide insights into the responses of organisms in both extreme and non-extreme years.
(d). Alternatives to phenotypic plasticity
As H. ostralegus is a long-lived species, we initially hypothesized that phenotypic plasticity in nest-site selection would provide the most likely explanation for the increasing mean nest elevation observed within our population [35]; however, our current analyses provide no evidence to support this prediction, compelling us to consider alternative mechanisms. Micro-evolution in nest elevation is possible, although the long generation time of our study species makes this explanation unlikely. Selective appearance, where new breeders settle disproportionately in higher elevation areas, or selective disappearance, where individuals in low elevation areas leave the population more often, may provide more likely alternative explanations. Importantly, any of these mechanisms would explain observed increases in the population without the need for phenotypic plasticity over the lifetime of an individual [54]. It seems plausible that individuals leaving the population (selective disappearance) will be the more important modulator of population elevation due to the declines observed in this species, although whether this would be driven by adult mortality (i.e. higher mortality at low elevation sites) or dispersal (i.e. abandonment of low elevation territories by breeding individuals) is unclear. A general shift from coastal to inland breeding habitats has been observed in H. ostralegus across Europe over the past decades [62], suggesting that dispersal may play an important role; however, to disentangle these potential mechanisms effectively will require an analysis of settlement, mortality and dispersal patterns across both space and time.
(e). Consequences for shorebird conservation
From our results, it seems unlikely that phenotypic plasticity in nest elevation will provide an effective mechanism by which individuals can reduce their flooding risk. Consequently, the viability of our H. ostralegus population is likely to be seriously threatened by increasing sea levels and changing storm patterns [3]. Greater utilization of the sea wall by some individuals (74 pairs; 20% of the population) is unlikely to provide a solution. Although the number of nests laid directly on the sea wall has increased over time (electronic supplementary material, figure S4), the total number of nests on the sea wall is still limited (46 of 528 nests laid by birds with access to the sea wall) and none of these nests have produced fledglings. Greater utilization of the sea wall may therefore represent an example of maladaptive plasticity, providing further evidence that nesting at higher elevations entails potential costs.
The issue of coastal flooding may present a similar threat to other coastal nesting birds if plastic responses to flooding are generally uncommon; however, whether the results observed in H. ostralegus will be broadly applicable to other species is still an open question. Other coastal species may experience fewer reproductive detriments from nesting at higher elevations or in taller vegetation than observed in H. ostralegus, potentially facilitating greater nest-site movement [30,33,63]. It is also important to consider that responses to flooding risk may occur through other behavioural or physiological traits, such as egg laying date [3], flooding resilience of eggs [31], nest structure [64], or site fidelity [65]. While we focused specifically on nest elevation as the most effective mechanism for combating nest flooding, we cannot rule out the possibility that coastal bird species may respond to extreme flooding through other traits. Future studies that seek to investigate phenotypic plasticity as a response to ECEs should attempt to measure and compare a range of traits to overcome this limitation.
The lack of evidence for phenotypic plasticity in our study population in response to changing patterns of ECEs contrasts with the prevalence of phenotypic plasticity as a response to gradual changes in climatic means [8]. More studies are needed to see if this is a general pattern, or whether responses to ECEs will vary with the type of ECE (e.g. floods or fires) or the study system. Whether or not a population will respond to ECEs may depend on the frequency with which they occur and the presence of counter-acting forces that may select against organismal responses. Long-term, individual-based studies that encompass multiple ECEs, like ours, will be pivotal to improve our understanding of the impacts of future climatic change. If our result is broadly applicable, it is possible that many populations will be vulnerable to changing patterns of ECEs, with long-lived species, such as H. ostralegus, that lack the capacity for rapid inter-generational micro-evolutionary change, likely to be the most vulnerable.
Supplementary Material
Acknowledgements
Thank you to all the University of Groningen Animal Ecology students who made an invaluable contribution during data collection. Thanks also to the EEG journal club for great feedback on the manuscript during the writing process.
Ethics
No ethics approval was required for this project.
Data accessibility
Data available from the Dryad Digital Repository [66].
Authors' contributions
L.D.B. designed the study with the help of M.v.d.P.; L.D.B., K.O., M.v.d.P. and D.H. collected field data; L.D.B. carried out the statistical analysis; L.D.B., M.v.d.P., D.H., B.J.E. and C.B. helped draft the manuscript.
Competing interests
We have no competing interests.
Funding
For this project, L.D.B. was supported by an Australian Postgraduate Award scholarship and M.v.d.P. by the Australian Research Council (FT120100204).
References
- 1.Coumou D, Rahmstorf S. 2012. A decade of weather extremes. Nat. Clim. Change 2, 491–496. ( 10.1038/nclimate1452) [DOI] [Google Scholar]
- 2.IPCC. 2013. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.van de Pol M, et al. 2010. Do changes in the frequency, magnitude and timing of extreme climatic events threaten the population viability of coastal birds? J. Appl. Ecol. 47, 720–730. ( 10.1111/j.1365-2664.2010.01842.x) [DOI] [Google Scholar]
- 4.Harrison RD. 2000. Repercussions of El Niño: drought causes extinction and the breakdown of mutualism in Borneo. Proc. R. Soc. Lond. B 267, 911–915. ( 10.1098/rspb.2000.1089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rich PM, Breshears DD, White AB. 2008. Phenology of mixed woody-herbaceous ecosystems following extreme events: net and differential responses. Ecology 89, 342–352. ( 10.1890/06-2137.1) [DOI] [PubMed] [Google Scholar]
- 6.Felton AJ, Smith MD. 2017. Integrating plant ecological responses to climate extremes from individual to ecosystem levels. Phil. Trans. R. Soc. B 372, 20160142 ( 10.1098/rstb.2016.0142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LEB, Sheldon BC. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803. ( 10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 8.Gienapp P, Teplitsky C, Alho JS, Mills JA, Merilä J. 2008. Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178. ( 10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- 9.Moreno J, Møller AP. 2011. Extreme climatic events in relation to global change and their impact on life histories. Curr. Zool. 57, 375–389. ( 10.1093/czoolo/57.3.375) [DOI] [Google Scholar]
- 10.Bailey LD, van de Pol M. 2016. Tackling extremes: challenges for ecological and evolutionary research on extreme climatic events. J. Anim. Ecol. 85, 85–96. ( 10.1111/1365-2656.12451) [DOI] [PubMed] [Google Scholar]
- 11.Gimeno TE, Pías B, Lemos-Filho JP, Valladares F. 2009. Plasticity and stress tolerance override local adaptation in the responses of Mediterranean holm oak seedlings to drought and cold. Tree Physiol. 29, 87–98. ( 10.1093/treephys/tpn007) [DOI] [PubMed] [Google Scholar]
- 12.Gunderson AR, Stillman JH. 2015. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B 282, 20150401 ( 10.1098/rspb.2015.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bondarenco A, Körtner G, Geiser F. 2014. Hot bats: extreme thermal tolerance in a desert heat wave. Naturwissenschaften 101, 679–685. ( 10.1007/s00114-014-1202-2) [DOI] [PubMed] [Google Scholar]
- 14.Briscoe NJ, Handasyde KA, Griffiths SR, Porter WP, Krockenberger A, Kearney MR. 2014 doi: 10.1098/rsbl.2014.0235. Tree-hugging koalas demonstrate a novel thermoregulatory mechanism for arboreal mammals. Biol. Lett. 10 , 20140235. ( ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed TE, Waples RS, Schindler DE, Hard JJ, Kinnison MT. 2010. Phenotypic plasticity and population viability: the importance of environmental predictability. Proc. R. Soc. B 277, 3391–3400. ( 10.1098/rspb.2010.0771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed TE, Jenouvrier S, Visser ME. 2013. Phenological mismatch strongly affects individual fitness but not population demography in a woodland passerine. J. Anim. Ecol. 82, 131–144. ( 10.1111/j.1365-2656.2012.02020.x) [DOI] [PubMed] [Google Scholar]
- 17.Both C, van Asch M, Bijlsma RG, van den Burg AB, Visser ME. 2009. Climate change and unequal phenological changes across four trophic levels: constraints or adaptations? J. Anim. Ecol. 78, 73–83. ( 10.1111/j.1365-2656.2008.01458.x) [DOI] [PubMed] [Google Scholar]
- 18.Hallerberg S, Kantz H. 2008. Influence of the event magnitude on the predictability of an extreme event. Phys. Rev. E 77, 1–12. ( 10.1103/PhysRevE.77.011108) [DOI] [PubMed] [Google Scholar]
- 19.Grieco F, van Noordwijk AJ, Visser ME. 2002. Evidence for the effect of learning on timing of reproduction in blue tits. Science 296, 136–138. ( 10.1126/science.1068287) [DOI] [PubMed] [Google Scholar]
- 20.Dukas R. 2013. Effects of learning on evolution: robustness, innovation and speciation. Anim. Behav. 85, 1023–1030. ( 10.1016/j.anbehav.2012.12.030) [DOI] [Google Scholar]
- 21.Greenlees MJ, Phillips BL, Shine R. 2010. Adjusting to a toxic invader: native Australian frogs learn not to prey on cane toads. Behav. Ecol. 21, 966–971. ( 10.1093/beheco/arq095) [DOI] [Google Scholar]
- 22.Kerr B, Feldman MW. 2003. Carving the cognitive niche: optimal learning strategies in homogeneous and heterogeneous environments. J. Theor. Biol. 220, 169–188. ( 10.1006/jtbi.2003.3146) [DOI] [PubMed] [Google Scholar]
- 23.Visser ME. 2008. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659. ( 10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marzluff JM. 1988. Do pinyon jays alter nest placement based on prior experience? Anim. Behav. 36, 1–10. ( 10.1016/S0003-3472(88)80244-6) [DOI] [Google Scholar]
- 25.Wiebe KL, Martin K. 1998. Costs and benefits of nest cover for ptarmigan: changes within and between years. Anim. Behav. 56, 1137–1144. ( 10.1006/anbe.1998.0862) [DOI] [PubMed] [Google Scholar]
- 26.Ferrari MCO, Messier F, Chivers DP. 2007. First documentation of cultural transmission of predator recognition by larval amphibians. Ethology 113, 621–627. ( 10.1111/j.1439-0310.2007.01362.x) [DOI] [Google Scholar]
- 27.Polo-Cavia N, Gomez-Mestre I. 2014. Learned recognition of introduced predators determines survival of tadpole prey. Funct. Ecol. 28, 432–439. ( 10.1111/1365-2435.12175) [DOI] [Google Scholar]
- 28.Burger J, Shisler J. 1980. Colony and nest site selection in laughing gulls in response to tidal flooding. Condor 82, 251–258. ( 10.2307/1367389) [DOI] [Google Scholar]
- 29.Krol J, Hallmann C. 2011. Effect van bodemdaling op situering, hoogteligging en overstromingsrisico van broedkolonies op De Hon. In Monitoring effecten van bodemdaling op Ameland-Oost 2005–2010, pp. 93–124. Assen, The Netherlands: NAM. [Google Scholar]
- 30.Hunter EA, Nibbelink NP, Cooper RJ. 2016. Threat predictability influences seaside sparrow nest site selection when facing trade-offs from predation and flooding. Anim. Behav. 120, 135–142. ( 10.1016/j.anbehav.2016.08.001) [DOI] [Google Scholar]
- 31.Ward LD, Burger J. 1980. Survival of herring gull and domestic chicken embryos after simulated flooding. Condor 82, 142–148. ( 10.2307/1367466) [DOI] [Google Scholar]
- 32.Bayard TS, Elphick CS. 2011. Planning for sea-level rise: quantifying patterns of saltmarsh sparrow (Ammodramus caudacutus) nest flooding under current sea-level conditions. Auk 128, 393–403. ( 10.1525/auk.2011.10178) [DOI] [Google Scholar]
- 33.Valdes K, Kirstin V, Hunter EA, Nibbelink NP. 2016. Salt marsh elevation is a strong determinant of nest-site selection by Clapper Rails in Georgia, USA. J. Field Ornithol. 87, 65–73. ( 10.1111/jofo.12134) [DOI] [Google Scholar]
- 34.Pike DA, Stiner JC. 2007. Sea turtle species vary in their susceptibility to tropical cyclones. Oecologia 153, 471–478. ( 10.1007/s00442-007-0732-0) [DOI] [PubMed] [Google Scholar]
- 35.Bailey LD. 2016. Between the devil and the deep blue sea: consequences of extreme climatic events in the Eurasian oystercatcher (Haematopus ostralegus). PhD thesis, Australian National University, Canberra, Australia.
- 36.van de Pol M, Heg D, Bruinzeel LW, Kuijper B, Verhulst S. 2006. Experimental evidence for a causal effect of pair-bond duration on reproductive performance in oystercatchers (Haematopus ostralegus). Behav. Ecol. 17, 982–991. ( 10.1093/beheco/arl036) [DOI] [Google Scholar]
- 37.Baart F, van Gelder PHAJM, de Ronde J, van Koningsveld M, Wouters B. 2012. The effect of the 18.6-year lunar nodal cycle on regional sea-level rise estimates. J. Coast. Res. 28, 511–516. ( 10.2112/JCOASTRES-D-11-00169.1) [DOI] [Google Scholar]
- 38.Houston JR, Dean RG. 2011. Accounting for the nodal tide to improve estimates of sea level acceleration. J. Coast. Res. 27, 801–807. ( 10.2112/JCOASTRES-D-11-00045.1) [DOI] [Google Scholar]
- 39.van de Pol M, Jenouvrier S, Cornelissen JHC, Visser ME. 2017. Behavioural, ecological and evolutionary responses to extreme climatic events: challenges and directions. Phil. Trans. R. Soc. B 372, 20160134 ( 10.1098/rstb.2016.0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van de Pol M, Bruinzeel LW, Heg DI, Van der Jeugd HP, Verhulst S. 2006. A silver spoon for a golden future: long-term effects of natal origin on fitness prospects of oystercatchers (Haematopus ostralegus). J. Anim. Ecol. 75, 616–626. ( 10.1111/j.1365-2656.2006.01079.x) [DOI] [PubMed] [Google Scholar]
- 41.Ens BJ, van de Pol M, Goss-Custard JD. 2014. The study of career decisions: oystercatchers as social prisoners. In Advances in the study of behaviour (eds Naguib M, Mitani JC, Simmons LW, Brockmann HJ, Roper TJ, Barrett L, Healy S), pp. 343–410. Amsterdam, The Netherlands: Elsevier Science. [Google Scholar]
- 42.del Hoyo J, Elliott A, Sargatal J. 1992. Handbook of the Birds of the World. Barcelona, Spain: Lynx Edicions/Birdlife International. [Google Scholar]
- 43.Ens BJ, Kersten M, Brenninkmeijer A, Hulscher JB. 1992. Territory quality, parental effort and reproductive success of oystercatchers (Haematopus ostralegus). J. Anim. Ecol. 61, 703–715. ( 10.2307/5625) [DOI] [Google Scholar]
- 44.van de Pol M, Pen I, Heg D, Weissing FJ. 2007. Variation in habitat choice and delayed reproduction: adaptive queuing strategies or individual quality differences? Am. Nat. 170, 530–541. ( 10.1086/521237) [DOI] [PubMed] [Google Scholar]
- 45.van Roomen M, et al. 2012. Signals from the Wadden sea: population declines dominate among waterbirds depending on intertidal mudflats. Ocean Coast. Manage. 68, 79–88. ( 10.1016/j.ocecoaman.2012.04.004) [DOI] [Google Scholar]
- 46.QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. India: Free Software Foundation, http://qgis.osgeo.org. [Google Scholar]
- 47.van de Pol M, Bakker T, Saaltink D-J, Verhulst S. 2006. Rearing conditions determine offspring survival independent of egg quality: a cross-foster experiment with oystercatchers Haematopus ostralegus. Ibis 148, 203–210. ( 10.1111/j.1474-919X.2006.00479.x) [DOI] [Google Scholar]
- 48.van Wijnen HJ, Bakker JP. 2001. Long-term surface elevation change in salt marshes: a prediction of marsh response to future sea-level rise. Estuar. Coast. Shelf Sci. 52, 381–390. ( 10.1006/ecss.2000.0744) [DOI] [Google Scholar]
- 49.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. 2016. nlme: Linear and Nonlinear Mixed Effects Models, v.3. (http://CRAN.R-project.org/package=nlme) [Google Scholar]
- 50.R Core Team. 2016. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 51.Anderson DR, Burnham KP. 2002. Avoiding pitfalls when using information-theoretic methods. J. Wildl. Manage. 66, 912–918. ( 10.2307/3803155) [DOI] [Google Scholar]
- 52.Arnold TW. 2010. Uninformative parameters and model selection using Akaike's information criterion. J. Wildl. Manage. 74, 1175–1178. ( 10.1111/j.1937-2817.2010.tb01236.x) [DOI] [Google Scholar]
- 53.Bartoń K. 2013. MuMIn: multi-model inference. R package version 1 (http://cran.r-project.org/web/packages/MuMIn/index.html) [Google Scholar]
- 54.van de Pol M, Wright J. 2009. A simple method for distinguishing within- versus between-subject effects using mixed models. Anim. Behav. 77, 753–758. ( 10.1016/j.anbehav.2008.11.006) [DOI] [Google Scholar]
- 55.Freckleton RP, Watkinson AR, Green RE, Sutherland WJ. 2006. Census error and the detection of density dependence. J. Anim. Ecol. 75, 837–851. ( 10.1111/j.1365-2656.2006.01121.x) [DOI] [PubMed] [Google Scholar]
- 56.Olff H, Leeuw Jde, Bakker JP, Platerink RJ, van Wijnen HJ. 1997. Vegetation succession and herbivory in a salt marsh: changes induced by sea level rise and silt deposition along an elevational gradient. J. Ecol. 85, 799–814. ( 10.2307/2960603) [DOI] [Google Scholar]
- 57.Bockelmann A-C, Bakker JP, Neuhaus R, Lage J. 2002. The relation between vegetation zonation, elevation and inundation frequency in a Wadden Sea salt marsh. Aquat. Bot. 73, 211–221. ( 10.1016/S0304-3770(02)00022-0) [DOI] [Google Scholar]
- 58.Götmark F, Blomqvist D, Johansson OC, Bergkvist J. 1995. Nest site selection: a trade-off between concealment and view of the surroundings? J. Avian Biol. 26, 305–312. ( 10.2307/3677045) [DOI] [Google Scholar]
- 59.Ghalambor CK, Martin TE. 2001. Fecundity-survival trade-offs and parental risk-taking in birds. Science 292, 494–497. ( 10.1126/science.1059379) [DOI] [PubMed] [Google Scholar]
- 60.Troscianko J, Wilson-Aggarwal J, Stevens M, Spottiswoode CN. 2016. Camouflage predicts survival in ground-nesting birds. Sci. Rep. 6, 19966 ( 10.1038/srep19966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chevin L-M, Hoffmann AA. 2017. Evolution of phenotypic plasticity in extreme environments. Phil. Trans. R. Soc. B 372, 20160138 ( 10.1098/rstb.2016.0138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van de Pol M, et al. 2014. A global assessment of the conservation status of the nominate subspecies of Eurasian oystercatcher Haematopus ostralegus. Int. Wader Stud. 20, 47–61. [Google Scholar]
- 63.Gjerdrum C, Elphick CS, Rubega M. 2005. Nest site selection and nesting success in saltmarsh breeding sparrows: the importance of nest habitat, timing, and study site differences. Condor 107, 849–862. ( 10.1650/7723.1) [DOI] [Google Scholar]
- 64.Humphreys S, Elphick CS, Gjerdrum C, Rubega M. 2007. Testing the function of the domed nests of Saltmarsh Sharp-tailed Sparrows. J. Field Ornithol. 78, 152–158. ( 10.1111/j.1557-9263.2007.00098.x) [DOI] [Google Scholar]
- 65.Schmidt KA. 2001. Site fidelity in habitats with contrasting levels of nest predation and brood parasitism. Evol. Ecol. Res. 3, 553–565. [Google Scholar]
- 66.Bailey LD, Ens BJ, Both C, Heg D, Oosterbeek K, van de Pol M.. 2017. Data from: No phenotypic plasticity in nest-site selection in response to extreme flooding events. Dryad Digital Repository. ( 10.5061/dryad.np757) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository [66].