Abstract
Climate extremes will elicit responses from the individual to the ecosystem level. However, only recently have ecologists begun to synthetically assess responses to climate extremes across multiple levels of ecological organization. We review the literature to examine how plant responses vary and interact across levels of organization, focusing on how individual, population and community responses may inform ecosystem-level responses in herbaceous and forest plant communities. We report a high degree of variability at the individual level, and a consequential inconsistency in the translation of individual or population responses to directional changes in community- or ecosystem-level processes. The scaling of individual or population responses to community or ecosystem responses is often predicated upon the functional identity of the species in the community, in particular, the dominant species. Furthermore, the reported stability in plant community composition and functioning with respect to extremes is often driven by processes that operate at the community level, such as species niche partitioning and compensatory responses during or after the event. Future research efforts would benefit from assessing ecological responses across multiple levels of organization, as this will provide both a holistic and mechanistic understanding of ecosystem responses to increasing climatic variability.
This article is part of the themed issue ‘Behavioural, ecological and evolutionary responses to extreme climatic events’.
Keywords: climate change, climate extremes, ecosystem sensitivity, scale, ecological organization
1. Introduction
An emergent consequence of global climate change has been the increase in the frequency and severity of climate extremes [1]. Climate extremes, such as drought, heavy precipitation, heatwaves and cold snaps, have the potential to produce large impacts to ecosystem dynamics [1–3]. However, the type and magnitude of ecological impacts resulting from climate extremes, both within [4] and among [5] ecosystems are highly variable [6]. With regard to plant responses, the variation can range from changes to species population genetics [7], altered local species richness [8], rapid shifts in ecotone boundaries [9] to continental-scale reductions in gross primary production [10].
Implicit in these examples is the necessary consideration of the scale of the measurement. Ecologists have long recognized that the scale of an observation (e.g. temporal, spatial or level of organization) can significantly influence conclusions about the underlying processes determining a pattern [11]. It is also often the case that certain processes determine patterns observed at different scales [12], as ‘fast’ processes (e.g. respiration) at fine scales and ‘slow’ processes (e.g. succession) operating at broader scales can affect and feedback to each other [11,13]. These notions apply equally to ecosystem responses to climate extremes. For example, high sensitivity or alterations at fine scales, such as in plant physiology, can underlie and buffer impacts to broad scale processes, such as in net primary production [4,14]. Therefore, an understanding of the cross-scale interactions between different levels of ecological organization (e.g. individual, population or community) within an ecosystem may inform variability in ecosystem-level responses to climate extremes [6].
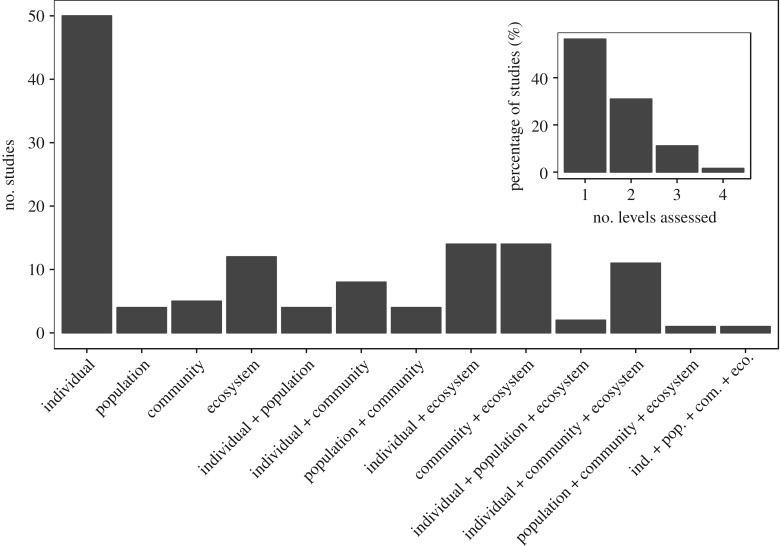
Prior efforts to scale from individual- to ecosystem-level processes have considered the metabolic rate [15], size and/or density [16] of organisms. Suding et al. [17] proposed that community dynamics often complicate scaling up from the individual level, and that plant community processes may be scaled to ecosystem productivity by relating species abundances with their functional traits. Only recently have ecologists begun to explicitly consider how responses to climate extremes at lower ecological levels, such as individual mortality, will scale to ecosystem-level processes, such as carbon and water cycling [18]. Nevertheless, despite calls in the literature as a research need [6], experimental or observational approaches that measure responses to climate extremes across multiple levels of ecological organization are relatively rare (figure 1).
Figure 1.
The number and type of study in terms of the ecological levels of organization that were assessed for the period 2000–2016. Inset is the data re-organized to more generally demonstrate the number of levels that studies have assessed. To date, ecological studies have typically focused assessment of responses to climate extremes at one ecological level, most notably the responses of individuals (e.g. physiology). What is clear is that studies that assess greater than two levels are comparatively rare, with the number of studies a decreasing function of the number of levels assessed. Studies were found and reviewed via a Web of Science and Google Scholar literature search utilizing the key words: climate extreme, plant, population, community, ecosystem. We then ran a separate search replacing climate extreme with drought, because this extreme has been of much focus in ecology. The list of studies and their DOI are provided in the electronic supplementary material.
Climate extremes will initially impact plant community and ecosystem processes via either physiological [19] or mechanical [20] impacts to individuals that produce the initiating conditions for responses [6,12] at the population or community level (figure 2). Smith [6] proposed that ecosystem responses to climate extremes consist of three integrative hierarchical (i.e. ordered) pathways: (i) the immediate physiological and growth impact to individuals, (ii) demographic changes to species abundances (community response), and (iii) mortality/loss of species and replacement with novel species. Polley et al. [21] extended this framework by proposing that climate change, here climate extremes, will impact ecosystem function as a consequence of the response and effect traits of individuals, and how the climatic conditions alter the relative abundance of these traits in the community (community effect).
Figure 2.

Conceptual diagram of how responses propagate across levels of ecological organization during and after a climate extreme. A climate extreme will initially impact individuals through physiological or mechanical damage that may impact growth and fitness. Consequent downstream impacts to demographic processes may produce changes in population sizes that will feed into community compositional changes, particularly if the species that is impacted is the dominant species or has a large initial population size. Abundance shifts or local extinction of species may be partly offset by community-level processes, such as niche partitioning or demographic compensation, that can then drive stability in the response or recovery of different ecosystem functions.
A response trait is considered to be a trait that may drive changes in the composition of species in the plant community, while an effect trait produces detectable feedbacks on ecosystem function [21]. For example, a trait that is highly responsive to drought stress, such as flowering in mesic grassland, may also have detectable feedback effects on ecosystem productivity due to the large investment of carbon in flowering stalks [22]. However, response and effect traits of plants may not necessarily be tightly coupled, and thus those traits responsible for driving plant community compositional change during or after an extreme may not translate to detectable impacts on ecosystem function, such as productivity [17,21]. For example, traits that are highly sensitive to stress, such as photosynthetic responses to drought, may buffer impacts to ecosystem productivity by increasing water-use efficiency of species in the community. Furthermore, if there is high intraspecific or interspecific variation among genotypes or species sensitivities to an extreme, the extant functional diversity within the community may also operate to stabilize ecosystem functions [23]. Thus, the fundamental links between processes occurring across levels of organization in an ecosystem suggest that their dynamics will be highly interactive both during and after periods of climatic extremity (figure 2).
In the following literature review, we assess how plant responses to climate extremes vary and potentially interact across levels of ecological organization. In particular, we focus on studies that have considered response and/or recovery dynamics from climate extremes across the individual, population, community and ecosystem level in herbaceous and forest plant communities. As a consequence, our review was not intended to assess how ecological responses to climate extremes differ across spatial [5] or temporal scales [24,25], studies of which are increasingly relevant and likely warrant their own independent reviews. We acknowledge that forest and herbaceous plant dynamics—which may include species turnover, productivity and sensitivity to global change drivers—can vary considerably between these ecosystem types as these dynamics operate on differential timescales. Rather, the focus of our review was to assess how information propagates across levels of organization within an ecosystem during and/or after periods of extreme climatic stress, and secondarily to see if the characteristics of these systems or the extremes may contribute to the observed dynamics.
While climate extremes are generally defined as statistically extreme or unusual climatic conditions (e.g. heatwaves or droughts), extreme climate events (ECEs) have been defined in a number of ways—both from climatic and ecological perspectives [6,26–29]. Indeed, these varying definitions, as well as the multiple different research approaches historically employed (e.g. observational versus experimental), underscore the challenges in attaining a general understanding of the ecological and evolutionary consequences of climate extremes [26]. For our purposes, we consider experimental, observational and opportunistic studies that assessed plant responses to climatically extreme conditions irrespective of the magnitude of the ecological responses. As a result, we do not limit our review to the climatic driver and ecological response definition proposed by Smith [6], or to an organismal focused definition, such as proposed by Gutschick & BassiriRad [27]. Instead, our approach was motivated towards improving an understanding of the ecological mechanisms that may underlie the variability in ecosystem resistance and resilience to periods of climatically extreme conditions. Thus for this review, we employ the climatological definition outlined in the 2016 Attribution of extreme weather events in the context of climate change [29] (table 1 from the Introduction and synthesis of this issue [26]).
Table 1.
Adapted from Hoover et al. [4,40], in which both a statistically extreme drought and heatwave were imposed on an intact grassland ecosystem over a 2-year period, and responses across multiple levels of ecological organization were assessed. Checked boxes indicate detectable impacts to that ecological level, while unchecked boxes signify no detectable effects. The experimental results demonstrate how responses can propagate across levels of organization during and after a climate extreme, and how both individual and community-level processes may both scale to the ecosystem level. Of equal importance is how two types of climate extremes yielded different dynamics; while responses cascaded across multiple levels of organization for extreme drought, the impacts from heatwave were not detectable beyond the physiological level. Such differences may very well be attributed to differential durations of the extremes, as the heatwaves were of much shorter durations than the drought. Nevertheless, the dynamics of how responses propagate across levels of organization within an ecosystem during or after an extreme are likely to differ depending on the type of climate extreme the system experiences, as well as the underlying characteristics of the extreme.
 |
Overall, our review has the underlying goal to synthesize past research findings and contribute to advancing a more integrative understanding about the role of responses at different levels of ecological organization in determining ecosystem response to and recovery from climate extremes. We sought to understand how the responses of processes occurring at lower levels, such as at the individual or population level, may inform higher order responses at the community and/or ecosystem level. Finally, another key objective of this review was to generate recommendations for researchers interested in mechanistically assessing ecosystem responses to climate extremes, and in particular the dynamics of ecosystem responses to climate extremes across levels of ecological organization.
2. Scaling individual plant responses to the population and community
The immediate impacts to ecosystem processes are likely to be driven by changes to physiological processes induced by the stress of a climate extreme [6]. Physiological adjustments in plants (e.g. rapid changes to stomatal aperture) operate to avoid the potentially irreversible functional damages a climatic stress can impose [19] and the associated fitness costs to the organism [27]. Physiological impacts to individuals will vary by the type of climate extreme experienced [19]. Force-driven mechanical damage is also of considerable importance in forest ecosystems exposed to high-energy storms [20], or as a result of secondary consequences of climate extremes such as fire or flooding. In general, the stresses induced by climate extremes tend to produce greater impacts on plant performance than gradual climate change [30] despite their shorter timescales. As a result, such events are posited to more likely reduce plant productivity and increase the probability of mortality [31].
Expectations that individual responses to climate extremes may scale to trajectories of plant population or community compositional change are based on the assumption that species and genotypes differ in their sensitivities to environmental changes. Indeed, a large body of the literature suggests that variability in the responses of key organismal traits associated with fitness, survival or the life histories of individuals will impact demographic and population-level dynamics, and that such links may occur through multiple pathways [32–34]. Moreover, the degree of change to plant community diversity (e.g. richness) or composition (e.g. species relative abundances) produced by a climate extreme should be an emergent property produced by differential responses and sensitivities of individual genotypes or species in the community, and the impact of the climate extreme on their relative abundances. Thus, genotypes within populations and species or functional groups within the community with differential sensitivities to a particular climate extreme presents a mechanistic pathway for directional changes to plant community diversity, composition and likely productivity.
Plant species in both forest and herbaceous ecosystems possess high variation in their physiological stress tolerances [9,35,36]. As a result, there is evidence of differential sensitivity among plant genotypes [37,38], species [9,39–41] and functional groups [4,42,43] to climate extremes. Liu et al. [44] observed large variation in long-term drought impacts to tree growth depending on the species considered in Mediterranean forest communities, with fruit production and growth largely impacted in certain species and others not affected. Similarly, Hoover et al. [4] observed differential sensitivity to repeated droughts and heatwaves between the C3 and C4 co-dominant plant species. This led to asymmetric impacts on species population sizes and thus a reordering of species abundances in the community following the extreme [4] (table 1). Interestingly, this variability also appears in other guilds besides plants. For example, Palmer et al. [45] reported that even closely related species within butterflies, moths and bird guilds varied significantly in what climatic conditions elicited extreme population responses. Thus, it has become increasingly clear that what is extreme for one species may not necessarily be extreme for another.
The observed variability in species sensitivity to climate extremes can be argued to underlie—and likely scale to—important plant community-level processes, such as niche complementarity. Through genotypes and/or species occupying different ecological niches, the temporal stability of ecosystem function is posited to be maintained in the face of environmental variation due to certain genotypes or species performing more optimally under different conditions [46]. Such a dynamic has been observed to decrease the temporal stability among species population dynamics within the community, yet increase the temporal stability of plant community productivity [47].
Yet despite evidence for differential sensitivities among plant genotypes and species to climate extremes and evidence for compositional changes [4], it is still rare that these observations scale to large community-level changes in species composition due to a single episode of climatic extremity (but see, [4,9]). Directional change to species composition that is driven by differential sensitivities among species may be most likely if a plant species' vulnerability to a climate extreme is matched with a low population size, as was observed in Minnesota grassland communities following drought [8]. However, non-random losses of species with low population sizes will likely not scale to large changes to community composition or productivity that is distinguishable from background variability [6,48]. It is often the case that there are reported physiological or phenotypic impacts to individuals that do not produce detectable impacts on plant population or community composition and ecosystem productivity [49,50]. For example, despite evidence of widespread tissue dieback in individuals, Kreyling et al. [49] observed plant community productivity to be unaffected by both drought and heavy precipitation events. Moreover, high phenotypic plasticity in key traits associated with demographic processes (e.g. seed size) may also partially buffer negative impacts at the population level during or after environmental stress [34].
There is also increasing evidence for high intraspecific variability in species responses to extremes, which has been observed to be of equal or even greater magnitude than interspecific variation [51]. High intraspecific variability may be influenced by ecotypic and genetic variation within a species ([37,52,53], but see [54]). High intraspecific trait variation has thus been posited to contribute to post-extreme shifts in community-weighted trait means irrespective of gains or losses of species from the community [55]. As a consequence, the impacts to individuals may not necessarily provide a single pathway for detecting impacts to population dynamics [33], and thus changes in community composition resulting from a climate extreme.
Variability in individual responses to climate extremes will be further driven by the ecological context in which the organism exists, in which plant responses may be modified by competitive interactions, soil mineral nutrient availability or trophic interactions such as herbivory [56]. Thus, the detection of individual or population responses to climate extremes must also consider co-occurring ecological drivers [57]. Moreover, plant communities also exhibit a certain degree of stochasticity in terms of the trajectories of species demography. For example, Kreyling et al. [58] reported that even when multiple identical plant communities were exposed to the European drought and heatwave of 2003, the successional development of the communities followed multiple different pathways. Therefore, it is clear that high variability at lower ecological levels of organization, such as individual or population responses, may further complicate efforts to scale-up these responses to community- and ecosystem-level responses to climate extremes.
(a). The role of species functional identity
Approaches designed to relate individual and/or population responses to alterations in community composition or ecosystem function must first consider both the population size and functional identity of a species in the community. This is so because a species' functional identity within the community is likely to modify the strength of interactions between individual and population responses to a climate extreme with community or ecosystem-level responses [59]. There is evidence to suggest that focusing assessment on the responses of functionally important species in the community can inform, at least in part, variability in community and ecosystem responses to climate extremes [4]. As terrestrial plant communities are commonly structured according to an abundance hierarchy [60], highly abundant or dominant species are hypothesized to drive community and ecosystem processes [61]. This perspective has been extended to ecosystem-level responses to climate extremes, as decreased performance or changes to the population size of dominant species are likely to produce detectable impacts on processes that occur at the community and ecosystem level [6,62].
Research in forest communities has demonstrated that climate extreme impacts on the dominant tree species can impact the diversity of forest plant communities and alter community-level processes [63]. Owing to the large influence of forests on the global carbon cycle [64], how climatically induced widespread tree mortality [65,66] will impact the composition and productivity of forest ecosystems is a topic of increasing interest [67,68]. Dominant tree species are an important structural component in forest ecosystems that significantly modify the physical environment. Consequently, widespread mortality, reduced growth or defoliation of a dominant tree species is expected to alter the ecology of the understory environment [69], and as a consequence likely impact the extant species in the community.
Defoliation or mortality of the dominant tree species due to climate extremes has been observed to impact plant community composition as a result of increased performance and richness of shade-intolerant understory species due to increased canopy openness and light availability [63,70–72]. This dynamic may scale to the ecosystem level if understory species are able to offset productivity declines of the dominant species. Differential sensitivities among co-dominant trees to a climate extreme [73] can alter the age structure and successional status of the ecosystem if one co-dominant species experiences a mortality threshold and the other does not [74]. Such changes to age structure or the successional state of vegetation may then impact the sensitivity of the ecosystem to future extremes [75]. Changes to dominance hierarchies due to differential sensitivities may further impact ecosystem productivity via competitive releases of a co-dominant [76], and may generate longer term impacts to the composition of the community [9]. Owing to the long temporal scales of forest ecosystem dynamics and tree life histories, climatically induced mortality events and loss of dominant trees may permanently alter the structure, distribution and function of the ecosystem. For example, a severe drought in the 1950s produced a rapid and seemingly permanent 2 km shift in the piñon–juniper woodland ecotone boundary within the Southwestern USA [9].
However, it is important to consider that the timescales of recovery of forest ecosystems from these mortality events may exceed the shorter timescales of many ecological studies, resulting in the perception of permanent change. This highlights the need to understand the timescales of extreme event impacts (e.g. shorter-term mortality events) versus the longer timescales of recovery dynamics in ecosystems with long-lived species. In other words, with short-term extreme events, such as drought, there is likely to be a mismatch in the timescale of dynamics driven by physiological (short-term growth) versus demographic responses (short to long-term re-growth and recruitment dynamics) and alterations in physical processes that may modify these responses over time. Indeed, there is extensive knowledge of shorter term responses of ecosystems to disturbances and climate extremes, as well as understanding of century-scale dynamics as observed from pollen records during glaciation cycles, but our understanding of dynamics at medium timescales and the mechanisms determining these dynamics remains limited.
In grasslands, dominant plant species are a common attribute of the ecosystem, and can drive ecosystem productivity irrespective of species richness [48]. For example, Arnone et al. [77] found grassland community production responses to heatwave to be driven primarily by the dominant C4 grass. Dominant species have also been observed to drive rapid recovery in ecosystem function following extreme drought [4,78]. Similarly, in arctic shrublands decreased shoot growth of the dominant shrubs due to an extreme heatwave was observed to be linked to decreases in gross primary production [79]. Gradual decreases in the performance of the dominant species in response to ‘press’ type climatic extremity may further impact community composition by gradually facilitating increased abundance of initially subordinate species, as was observed in the response of a semiarid shortgrass steppe to prolonged drought [80]. Thus, ecologically dominant species and their responses to climate extremes have the potential to influence the trajectories of community and ecosystem responses in both forest and herbaceous plant communities, despite the fact that these systems operate on differential temporal scales.
There is also emerging evidence that functionally distinct species, such as nitrogen-fixing legumes, can modify neighbouring species responses and potentially influence community-level processes despite their relatively low population sizes. For example, Khan et al. [81] found the presence of legumes to facilitate the performance of neighbouring species in the community under heavy precipitation, which in turn may have contributed to the stabilizing of above-ground productivity observed in the experiment. Similarly, the presence or the absence of legumes has been reported as a determinant in community resistance to the same type of climate extreme [82,83]. As such, functionally important species in the community that are not the dominant may also have the potential to impact individual, community and potentially ecosystem responses to climate extremes.
In total, there is evidence to suggest that ecologically and functionally dominant species can largely influence the response and/or recovery of community- and ecosystem-level processes to climate extremes. However, species with other functional roles in the community, despite their low population sizes, may also have the potential to impact community and ecosystem responses to an extreme. Owing to the directional nature of climate change and forecast increases in the magnitude of climate extremes, such as with global change-type droughts [65], declines in the performance or abundance of dominant species may occur as stress thresholds are more commonly experienced [84]. Declines in the performance or abundance of dominant species may be offset by the extant pool of species [80], and thus may portend a reordering of species abundances in the community and changes to ecosystem-level processes [85]. As such, reordering of species due to climate extremes, potentially driven by demographic responses of the dominant species (figure 2), may become an increasingly important pathway of change for plant community and ecosystem processes [86].
It must also be noted that plant communities exhibit varying degrees of species dominance [60]. Thus, differential mechanisms besides dominance may operate in communities where species abundances are more evenly distributed or species turnover is high. On this issue, the well-documented relationship of plant biodiversity with ecosystem functioning and stability [87–89] suggests that such dynamics are likely to operate in plant communities during and/or after periods of climatic stress. As a result, efforts that scale individual species responses to ecosystem responses to climate extremes will likely undermine the complexity of processes occurring at the community level.
3. Scaling community responses to ecosystem productivity
As climate change progresses, climate extremes are likely to become an increasingly important determinant in the structure (richness, diversity or composition) of plant communities. Indeed, there is evidence that climate extremes can impact the species diversity and composition of plant communities [8,9,70,90–93]. However, there is still little evidence that climate extremes often induce large changes to plant community composition [86], and thus large vegetation shifts following climate extremes are currently the exception rather than the norm (but see [9]). Although functional resistance to climate extremes is often low, rapid recovery and thus stability in ecosystem function is evident across systems ([4,82], but see [25]). The paucity in large compositional or functional changes appears to be often driven, in part, by context-dependent community-level processes that act to stabilize plant community structure and/or function in response to, or recovery from climate extremes [4,14,94].
The composition of interacting plant species within a community can greatly modify the response of both individual organisms and ecosystem productivity to a climate extreme [49,95–98]. While it is clear that ecologically dominant species can often drive trajectories of ecosystem response and recovery, a large body of evidence supports biodiversity as an ecological property of plant communities that increases their functional stability [87–89]. The diversity–stability hypothesis is rooted in the multifunctional advantage of niche partitioning among species, in which functional diversity among species is an emergent property of variability in the environment [46,99]. Indeed, these trade-offs in stress tolerance and responses to climatic extremes have been reported to increase both local [100] and regional diversity [90] patterns of plant communities within forest and herbaceous ecosystems. As alluded to earlier, stability in ecosystem productivity may also be driven by reduced stability at lower ecological levels, such as with species population dynamics [46,47]. Thus, the high variability that is evident at the individual or population level may provide a pathway to stabilize community composition [86] or ecosystem productivity [14] with respect to increases in climatic extremity and variability.
There is evidence to suggest that plant communities with greater species richness tend to be more functionally resistant to climate extremes [23,101–104], thus supporting the diversity–stability hypothesis. Community-level mechanisms of resistance appear to be, in part, driven by niche separation via differential functional responses among species to an extreme event. For example, Mariotte et al. [103] reported that even with declining performance of the dominant species under drought, subordinate species were able to maintain carbon uptake and therefore partially compensate for productivity declines. Niche partitioning has also been reported to occur due to morphological or temporal separations among species in soil-water resource acquisition under drought stress. Such differential acquisition strategies can partially reduce competition for soil moisture and stabilize carbon uptake [105]. Differential drought sensitivity of co-dominant trees may also relax competitive interactions between species, allowing compensatory growth of the less-sensitive species to occur that offsets growth reductions of the other species, as was observed within mixed stands of deciduous forest [76].
However, plant communities with greater species richness may also potentially have negative [106–109] effects on ecosystem stability under climatic extremity. Both the sampling and niche complementarity effect of biodiversity have been reported to decrease the resistance of ecosystem productivity to climate extremes. The sampling probability effect suggests that biodiversity and ecosystem function relationships may be often driven by the chance of a plant community containing a highly productive species [110]. Yet just as more species-rich communities may have a greater probability of containing highly productive species, such highly productive species may exhibit functional trade-offs and thus be highly sensitive to a particular climate extreme.
As a consequence, this dynamic has been observed to decrease the resistance of ecosystem productivity to an extreme [106]. Similarly, niche complementarity in soil-water resource use has been reported to produce a greater draw down in total soil water availability, and thus heighten interspecific competition, increase plant water stress and decrease the performance of species in the community [107]. However, these examples appear to currently be the exception rather than the rule. Interestingly, Lloret et al. [108] observed changes in the diversity–resistance relationship in response to drought in moving across climatic gradients, with positive relationships in water-limited sites, and more negative relationships in wetter sites. This result suggests a potentially key role for the climatic context in which species and communities have evolved for contributing to variability in the relationship between species richness and resistance to climate extremes.
Resilience, i.e. the rate and magnitude of recovery, in ecosystem productivity following climate extremes has been reported to be driven by compensatory and demographic responses of species in the community following the climate extreme [4]. As stated earlier, widespread mortality of dominant trees in forests due to extreme events can promote recruitment and growth of light-limited understory species in the community [41,70], which can partially offset reductions in ecosystem productivity while at the same time altering community composition. For example, Lloret et al. [41] observed mortality following a climate extreme to be positively correlated with seedling recruitment in Mediterranean shrublands. Thus, mortality or reduced performance of species in the community presents a potential pathway for other species in the community to compensate and offset productivity declines and drive ecosystem recovery, thereby enhancing stability in function.
By contrast, Isbell et al. [111] observed a lack of evidence for increased resilience in productivity with higher plant species diversity across grassland biodiversity experiments. Reductions in species richness also often do not preclude full recovery in productivity in native grassland plant communities [4,8]. However, diversity-dependant ecosystem recovery following extremes is also often reported [38]. Thus, there appears to be a lack of generality in the effect of plant diversity on ecosystem resilience to climate extremes. On this issue, ecosystem resilience to climate extremes may be a process in plant communities that is more strongly driven by both post-extreme abiotic conditions and the functional traits of the surviving species in the community, irrespective of species richness ([48] but see [112,113]). More specifically, resource availability following relaxation of the extreme and the capacity of the surviving species to respond to those conditions will likely drive ecosystem resilience [4,8]. Nevertheless, high resilience in community composition does not appear to be requisite for high resilience in ecosystem function following climate extremes, yet this may vary by case and warrants further exploration.
Another community-level process that may contribute to stability in ecosystem processes under climatic extremity is the beneficial interactions between species that can develop during a climate extreme, such as facilitation [114]. Shifts from competitive to facilitative interactions between species due to increases in abiotic stress underlie the stress gradient hypothesis [115]. Although there is evidence to suggest the existence of beneficial interactions between species under climatic extremity, these dynamics are ecologically context-dependent. For example, whether species interactions were facilitative, competitive or neutral in response to drought and heavy rainfall depended on community compositional context in European grassland [94]. Saccone et al. [116] found that facilitation of understory nurse seedlings during heatwave depended on soil moisture status, with facilitation disappearing under low soil moisture. Furthermore, the presence of legumes has been observed to benefit neighbouring plants under heavy rainfall via increased nitrogen availability, yet this effect may lessen or disappear under extreme drought [81]. Thus, it is still unclear the role that species interactions will play with respect to community and ecosystem responses to climate extremes.
(a). Species invasions and climate extremes
The entry of novel species into the community due to the community-level impacts of a climate extreme may become a stronger determinant of plant community change as the frequency and severity of extremes increases. As such, climate extremes have been posited to potentially facilitate species invasions via multiple mechanisms [117], of which there is some supporting evidence. Mortality of species in the community may produce ‘invasion windows’ that reset plant community development [118]. This resetting of community dynamics has been observed to facilitate the entry of novel species into the community, in large part due to space creation [119].
Mortality of individuals and space creation may also generate a pulse of resources during or after a climate extreme that facilitates the establishment of novel species into the community [120], as predicted by the fluctuating resource hypothesis [121]. Indeed, extreme wet years have been observed to potentially facilitate plant invasions [122], likely due to increased inorganic nitrogen availability via increased mineralization [92]. Extremes such as drought may also induce alterations to soil properties that promote exotic performance over natives [123]. Heightened performance of exotics under climatic extremity may then lead to community compositional changes, with consequences for ecosystem productivity [124].
However, plant communities may also possess a high degree of structural resistance to invasion during or following extremes, which varies depending on the degree to which the properties of the community (e.g. species richness) are altered. Invasion resistance appears to be predicated in part on both the species composition and richness of the community and the type of climate extreme. For example, Kreyling et al. [125] reported a nearly twofold increase in invasion under heavy rainfall as opposed to drought, yet with the degree of invasion generally reduced in more diverse communities. Similarly, Sheppard et al. [126] reported high variability in whether drought or heavy rainfall facilitated exotic performance. Establishment success of native versus exotic seedlings has also been demonstrated to depend on whether a temperature extreme was positive or negative [127], suggesting that there will be important interactions between the type of climate extreme and the traits of the invaders. Thus, whether or not climate extremes will facilitate plant invasions is likely to be contingent upon the type of extreme, how the extreme modifies the species composition and abiotic conditions of the community and the functional attributes of the invader.
4. Integrating responses across ecological scales
Studies that employ multi-scale approaches are valuable in demonstrating the importance of a holistic understanding of ecosystem responses to changing climatic variability [128] and extremity [4,14,129]. The magnitude of response to a climate extreme will likely vary with the ecological level of organization (e.g. ‘fast’ individual versus ‘slow’ community responses). Thus, an understanding of the relative sensitivities among scales may contribute insight towards the relation of each scale to one another, and contribute understanding towards the variability in ecosystem resistance and resilience to climate extremes. However, multi-scale studies employing greater than two ecological organizational levels are relatively rare (figure 1). The utility of multi-scale approaches in understanding ecosystem responses to climate extremes can be best demonstrated by those experiments that have assessed responses across multiple levels of organization, from the individual, population, community to the ecosystem level.
From a response perspective, Jentsch et al. [14] observed negligible effects of a statistically extreme 5-year growing season drought on both above- and below-ground ecosystem productivity in herbaceous plant communities. However, responses at the physiological were pervasive, which included reduced net photosynthesis, lower leaf water potentials and alterations to leaf C : N consistent with drought stress. At the plant community level, the experiment observed interspecific compensatory responses in species’ morphology, in particular tiller outputs, that contributed to the stabilization in community productivity. These dynamics occurred without large structural changes in plant community composition. Thus, this experiment supports the hypothesis of stabilizing mechanisms within plant communities under climatic extremity [86], and in particular the notion that processes that operate and interact across lower levels of organization may contribute to the stabilization of different ecosystem functions.
From a recovery perspective, Hoover et al. [4,40] (table 1) observed plant water status, net photosynthesis and productivity responses of the dominant grass species to generally correspond with large reductions in above-ground net primary productivity in response to extreme drought. However, despite low ecosystem resistance to climate extremes and near local extinction of the dominant forb species, demographic compensation of the dominant grass following the extreme drove full recovery in ecosystem productivity (table 1). Thus, while physiological and growth responses were partially linked with declines in ecosystem productivity and low resistance, it was community-level processes via demographic compensation of species following the extreme that largely drove full recovery and thus the stability of ecosystem production to drought. Furthermore, while drought responses interacted across scales in this ecosystem, heatwave impacts were not detectable beyond physiological responses, despite both events being statistically extreme with regard to the long-term climate record (Hoover et al. [4] for methods). Yet, importantly, the underlying characteristics of each extreme also differed, as the drought lasted the entirety of the growing seasons, while the heatwave occurred for two weeks during the middle of each growing season. Nevertheless, Jentsch et al. [14] and Hoover et al. [4,40] demonstrate that interactions between lower ecological levels can underlie and inform ecosystem-level responses to and recovery from climate extremes. Additionally, responses to climate extremes at lower levels, such as in physiology, may not always be detectable at the ecosystem level depending on the type of climate extreme the system was exposed to, and the underlying characteristics of the extreme (e.g. magnitude or duration). In total, these results suggest that the organizational dynamics of scale within an ecosystem are likely to differ depending on the type of climatic stress experienced by the system (table 1).
5. Synthesis, concluding remarks and recommendations for research
Research on the ecology of climate extremes has emerged as a frontier in climate change research. Although the number of ecological studies on climate extremes continues to grow [3,6,130] it is evident that both experimental and observational approaches often focus on one ecological level, and less often assess responses to an extreme climate period across multiple levels of ecological organization (figure 1). Our review suggests that an understanding of ecosystem responses to climate extremes will be heightened by consideration of cross-scale interactions. This is so because it is often the case that variability, sensitivity or changes observed at one level (e.g. physiology or population), can mechanistically act to reduce variability at other levels, such as ecosystem productivity [4,14,47], either during or after a climate extreme.
Within any given ecosystem, the variability of ecological responses is likely to decrease in moving from the individual, population or community to the ecosystem level. It also is clear that the efficacy of upscaling individual or population responses to the community or ecosystem level largely depends on the functional identity and/or population size of a species within the community. The literature to date has provided support for the notion that the responses of dominant species can feed into and impact plant community and ecosystem responses to climate extremes. Yet equally relevant are biodiversity-driven dynamics at the community level that may operate in concert with the responses of dominant species. Community-level processes such as niche partitioning (in resource acquisition or stress tolerance) and demographic compensation, both during and after extremes, are ecological mechanisms that can heighten the stability of plant community composition or function to climate extremes.
As a consequence of these dynamics, we contend that studies focused on responses at one ecological level do so at the potential risk of overlooking contributing drivers to the variability of the response at that level, at least from an organizational perspective. Variability in the response of ecosystem productivity to climate extremes is likely to, in part, be determined by how individual, population and community processes respond, interact and integrate during and/or after the extreme period. Indeed, extreme climate periods often do not elicit large ecosystem-level responses [6]. Yet, negligible impacts of a climate extreme on higher order ecosystem-level functions do not necessarily mean that the system has not been detectably impacted at lower levels of organization. More pronounced impacts at lower levels, such with individual plant physiology or morphology, may underlie or portend interactions between population and community-level processes. These dynamics can further add explanatory power to responses at the ecosystem level, such as net primary productivity [14]. However, we posit that different insights into the variability of ecosystem responses may be attained by scaling-down and decomposing ecosystem-level responses into its smaller components (top-down approach), versus scaling-up and integrating fine-scale responses to understand broader components (bottom-up approach), as has been the focus of this review. Thus, assessing how top-down versus bottom-up research approaches compare in terms of ecological response dynamics to climate extremes warrants further exploration.
Future research efforts focused on scaling individual responses to climate extremes to community or ecosystem processes ought to focus assessment on the responses of functionally distinct species in the community (e.g. dominant species), and relate those responses to the broader context of community and ecosystem responses. Thus, a deeper understanding of how current ecological dominants, i.e. those species with large population sizes, will respond to novel climatic stress may provide insight to the potential pathways and trajectories of change in community composition and/or productivity. Prior research also suggests that community-level properties and processes such as functional diversity, beneficial interactions and species invasions, all have the potential to modify community and ecosystem resistance and resilience to climate extremes.
Community ecology, in particular, is often described as having a ‘black box’ of complexity and contingency [131]. Indeed, such complexity appears to apply equally well to climate extremes. On this notion, we stress the need for deeper investigation into how processes and species interactions within (e.g. competition) and among (e.g. dispersal) communities may scale to impact the stability of community composition and ecosystem function, both locally and regionally, during and after climate extremes. This follows the concept that non-random community compositional changes are likely to be a mechanistic pathway for mediating changes to ecosystem function that operate in synergy with environmental change drivers [132], such as climate extremes. In addition, greater attention towards integrating population and community-level processes into impact-oriented investigations of ecosystem-level responses to climate extremes will be critical in bridging individual to ecosystem responses (figure 2). Indeed, while the complexities of studying the impacts of climate extremes across levels of organization underscore the challenges in studying their dynamics, such complexity also signifies the importance of a holistic approach in assessing their ecological consequences.
Supplementary Material
Supplementary Material
Acknowledgements
We thank M. van de Pol and two anonymous reviewers for their comments on a previous version of the manuscript.
Data accessibility
One data file containing studies and the study type used to generate figure 1 will be available in the electronic supplementary material. Figure 1 was generated using R statistical software environment (v. 3.2.1) accessible at https://www.rstudio.com/. The R code for this figure will also be provided in the electronic supplementary material.
Authors' contribution
A.J.F. and M.D.S. conceived the study and wrote the manuscript and contributed equally to generating all figures and tables. A.J.F. conducted the literature review.
Competing interests
The authors declare no competing interests.
Funding
M.D.S. was supported by the Drought-Net Research Coordination Network funded by the US National Science Foundation (NSF grant no. 1354732) and by the Macrosystems Biology/Emerging Frontiers Programs (NSF grant no. 1239559).
References
- 1.Seneviratne SI, et al. 2012. Changes in climate extremes and their impacts on the natural physical environment. In Managing the risks of extreme events and disasters to advance climate change adaptation (eds Field CB, et al.) A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change (IPCC), pp. 109–230. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Easterling D. 2000. Climate extremes: observations, modeling, and impacts. Science 289, 2068–2074. ( 10.1126/science.289.5487.2068) [DOI] [PubMed] [Google Scholar]
- 3.Ummenhofer CC, Meehl GA. 2017. Extreme weather and climate events with ecological relevance: a review. Phil. Trans. R. Soc. B 372, 20160135 ( 10.1098/rstb.2016.0135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoover D, Knapp A, Smith M. 2014. Resistance and resilience of a grassland ecosystem to climate extremes. Ecology 95, 2646–2656. ( 10.1890/13-2186.1) [DOI] [Google Scholar]
- 5.Knapp A, Carroll C, Denton E, La Pierre K, Collins S, Smith M. 2015. Differential sensitivity to regional-scale drought in six central US grasslands. Oecologia 177, 949–957. ( 10.1007/s00442-015-3233-6) [DOI] [PubMed] [Google Scholar]
- 6.Smith M. 2011. An ecological perspective on extreme climatic events: a synthetic definition and framework to guide future research. J. Ecol. 99, 656–663. ( 10.1111/j.1365-2745.2011.01798.x) [DOI] [Google Scholar]
- 7.Franks S, Sim S, Weis A. 2007. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc. Natl Acad. Sci. USA. 104, 1278–1282. ( 10.1073/pnas.0608379104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tilman D, El Haddi A. 1992. Drought and biodiversity in grasslands. Oecologia 89, 257–264. ( 10.1007/bf00317226) [DOI] [PubMed] [Google Scholar]
- 9.Allen CD, Breshears DD. 1998. Drought-induced shift of a forest-woodland ecotone: rapid landscape response to climate variation. Proc. Natl Acad. Sci. USA 95,14 839–14 842. ( 10.1073/pnas.95.25.14839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciais P, et al. 2005. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437, 529–533. ( 10.1038/nature03972) [DOI] [PubMed] [Google Scholar]
- 11.Levin S. 1992. The problem of pattern and scale in ecology: the Robert H. MacArthur award lecture . Ecology 73, 1943–1967. ( 10.2307/1941447) [DOI] [Google Scholar]
- 12.Wu J. 1999. Hierarchy and scaling: extrapolating information along a scaling ladder. Can. J. Remote Sens. 25, 367–380. ( 10.1080/07038992.1999.10874736) [DOI] [Google Scholar]
- 13.Holling CS. 1996. Engineering resilience versus ecological resilience. In Engineering within ecological constraints (ed. P Schulze), pp. 31–44. Washington, DC: National Academy of Sciences. [Google Scholar]
- 14.Jentsch A, et al. 2011. Climate extremes initiate ecosystem-regulating functions while maintaining productivity. J. Ecol. 99, 689–702. ( 10.1111/j.1365-2745.2011.01817.x) [DOI] [Google Scholar]
- 15.Allen A, Gillooly J, Brown J. 2005. Linking the global carbon cycle to individual metabolism. Funct. Ecol. 19, 202–213. ( 10.1111/j.1365-2435.2005.00952.x) [DOI] [Google Scholar]
- 16.Enquist B. 2002. Global allocation rules for patterns of biomass partitioning in seed plants. Science 295, 1517–1520. ( 10.1126/science.1066360) [DOI] [PubMed] [Google Scholar]
- 17.Suding K, et al. 2008. Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Glob. Change Biol. 14, 1125–1140. ( 10.1111/j.1365-2486.2008.01557.x) [DOI] [Google Scholar]
- 18.Anderegg W, et al. 2016. When a tree dies in the forest: scaling climate-driven tree mortality to ecosystem water and carbon fluxes. Ecosystems 19, 1133–1147. ( 10.1007/s10021-016-9982-1) [DOI] [Google Scholar]
- 19.Levitt J. 1972. Responses of plants to environmental stresses. New York, NY: Academic Press. [Google Scholar]
- 20.Laura Suarez M, Kitzberger T. 2010. Differential effects of climate variability on forest dynamics along a precipitation gradient in northern Patagonia. J. Ecol. 98, 1023–1034. ( 10.1111/j.1365-2745.2010.01698.x) [DOI] [Google Scholar]
- 21.Polley H, Derner J, Jackson R, Wilsey B, Fay P. 2014. Impacts of climate change drivers on C4 grassland productivity: scaling driver effects through the plant community. J. Exp. Bot. 65, 3415–3424. ( 10.1093/jxb/eru009) [DOI] [PubMed] [Google Scholar]
- 22.Dietrich J, Smith M. 2016. The effect of timing of growing season drought on flowering of a dominant C4 grass. Oecologia 181, 391–399. ( 10.1007/s00442-016-3579-4) [DOI] [PubMed] [Google Scholar]
- 23.Tilman D, Downing J. 1994. Biodiversity and stability in grasslands. Nature 367, 363–365. ( 10.1038/367363a0) [DOI] [Google Scholar]
- 24.Sala O, Gherardi L, Reichmann L, Jobbagy E, Peters D. 2012. Legacies of precipitation fluctuations on primary production: theory and data synthesis. Phil. Trans. R. Soc. B 367, 3135–3144. ( 10.1098/rstb.2011.0347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haddad N, Tilman D, Knops J. 2002. Long-term oscillations in grassland productivity induced by drought. Ecol. Lett. 5, 110–120. ( 10.1046/j.1461-0248.2002.00293.x) [DOI] [Google Scholar]
- 26.van de Pol M, Jenouvrier S, Cornelissen JHC, Visser ME. 2017. Behavioural, ecological and evolutionary responses to extreme climatic events: challenges and directions. Phil. Trans. R. Soc. B 372, 20160134 ( 10.1098/rstb.2016.0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutschick V, BassiriRad H. 2003. Extreme events as shaping physiology, ecology, and evolution of plants: toward a unified definition and evaluation of their consequences. New Phytol. 160, 21–42. ( 10.1046/j.1469-8137.2003.00866.x) [DOI] [PubMed] [Google Scholar]
- 28.Altwegg R, Visser V, Bailey LD, Erni B. 2017. Learning from single extreme events. Phil. Trans. R. Soc. B 372, 20160141 ( 10.1098/rstb.2016.0141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Committee on Extreme Weather Events and Climate Change Attribution, Board on Atmospheric Sciences and Climate, Division on Earth and Life Studies & National Academies of Sciences, Engineering, and Medicine. 2016. Attribution of extreme weather events in the context of climate change. Washington, DC: National Academies Press; (cited 20 June 2016). [Google Scholar]
- 30.Reyer C, et al. 2013. A plant's perspective of extremes: terrestrial plant responses to changing climatic variability. Glob. Change. Biol. 19, 75–89. ( 10.1111/gcb.12023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niu S, Luo Y, Li D, Cao S, Xia J, Li J, Smith M. 2014. Plant growth and mortality under climatic extremes: an overview. Environ. Exp. Bot. 98, 13–19. ( 10.1016/j.envexpbot.2013.10.004) [DOI] [Google Scholar]
- 32.van Tienderen P. 2000. Elasticities and the link between demographic and evolutionary dynamics. Ecology 81, 666–679. ( 10.1890/0012-9658(2000)081%5B0666:eatlbd%5D2.0.co;2) [DOI] [Google Scholar]
- 33.McLean N, Lawson C, Leech D, van de Pol M. 2016. Predicting when climate-driven phenotypic change affects population dynamics. Ecol. Lett. 19, 595–608. ( 10.1111/ele.12599) [DOI] [PubMed] [Google Scholar]
- 34.Jongejans E, Huber H, de Kroon H. 2010. Scaling up phenotypic plasticity with hierarchical population models. Evol. Ecol. 24, 585–599. ( 10.1007/s10682-009-9340-2) [DOI] [Google Scholar]
- 35.Craine J, Ocheltree T, Nippert J, Towne E, Skibbe A, Kembel S, Fargione J. 2012. Global diversity of drought tolerance and grassland climate-change resilience. Nat. Clim. Change 3, 63–67. ( 10.1038/nclimate1634) [DOI] [Google Scholar]
- 36.Maréchaux I, Bartlett M, Sack L, Baraloto C, Engel J, Joetzjer E, Chave J. 2015. Drought tolerance as predicted by leaf water potential at turgor loss point varies strongly across species within an Amazonian forest. Funct. Ecol. 29, 1268–1277. ( 10.1111/1365-2435.12452) [DOI] [Google Scholar]
- 37.Avolio M, Smith M. 2013. Mechanisms of selection: phenotypic differences among genotypes explain patterns of selection in a dominant species. Ecology 94, 953–965. ( 10.1890/12-1119.1) [DOI] [Google Scholar]
- 38.Reusch T, Ehlers A, Hammerli A, Worm B. 2005. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc. Natl Acad. Sci. USA 102, 2826–2831. ( 10.1073/pnas.0500008102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchand F, Verlinden M, Kockelbergh F, Graae B, Beyens L, Nijs I. 2006. Disentangling effects of an experimentally imposed extreme temperature event and naturally associated desiccation on Arctic tundra. Funct. Ecol. 20, 917–928. ( 10.1111/j.1365-2435.2006.01203.x) [DOI] [Google Scholar]
- 40.Hoover D, Knapp A, Smith M. 2014. Contrasting sensitivities of two dominant C4 grasses to heat waves and drought. Plant. Ecol. 215, 721–731. ( 10.1007/s11258-014-0345-8) [DOI] [Google Scholar]
- 41.Lloret F, de la Riva E, Pérez-Ramos I, Marañón T, Saura-Mas S, Díaz-Delgado R, Villar R. 2016. Climatic events inducing die-off in Mediterranean shrublands: are species’ responses related to their functional traits? Oecologia 180, 961–973. ( 10.1007/s00442-016-3550-4) [DOI] [PubMed] [Google Scholar]
- 42.Hoover D, Duniway M, Belnap J. 2015. Pulse-drought atop press-drought: unexpected plant responses and implications for dryland ecosystems. Oecologia 179, 1211–1221. ( 10.1007/s00442-015-3414-3) [DOI] [PubMed] [Google Scholar]
- 43.Debinski D, Wickham H, Kindscher K, Caruthers J, Germino M. 2010. Montane meadow change during drought varies with background hydrologic regime and plant functional group. Ecology 91, 1672–1681. ( 10.1890/09-0567.1) [DOI] [PubMed] [Google Scholar]
- 44.Liu D, Ogaya R, Barbeta A, Yang X, Peñuelas J. 2015. Contrasting impacts of continuous moderate drought and episodic severe droughts on the aboveground-biomass increment and litterfall of three coexisting Mediterranean woody species. Glob. Change Biol. 21, 4196–4209. ( 10.1111/gcb.13029) [DOI] [PubMed] [Google Scholar]
- 45.Palmer G, et al. 2017. Climate change, climatic variation and extreme biological responses. Phil. Trans. R. Soc. B 372, 20160144 ( 10.1098/rstb.2016.0144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tilman D. 1999. The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80, 1455 ( 10.2307/176540) [DOI] [Google Scholar]
- 47.Tilman D. 1996. Biodiversity: population versus ecosystem stability. Ecology 77, 350–363. ( 10.2307/2265614) [DOI] [Google Scholar]
- 48.Smith M, Knapp A. 2003. Dominant species maintain ecosystem function with non-random species loss. Ecol. Lett. 6, 509–517. ( 10.1046/j.1461-0248.2003.00454.x) [DOI] [Google Scholar]
- 49.Kreyling J, Wenigmann M, Beierkuhnlein C, Jentsch A. 2008. Effects of extreme weather events on plant productivity and tissue die-back are modified by community composition. Ecosystems 11, 752–763. ( 10.1007/s10021-008-9157-9) [DOI] [Google Scholar]
- 50.Fay P, Kaufman D, Nippert J, Carlisle J, Harper C. 2008. Changes in grassland ecosystem function due to extreme rainfall events: implications for responses to climate change. Glob. Change Biol. 14, 1600–1608. ( 10.1111/j.1365-2486.2008.01605.x) [DOI] [Google Scholar]
- 51.Malyshev A, Arfin Khan M, Beierkuhnlein C, Steinbauer M, Henry H, Jentsch A, Dengler J, Willner E, Kreyling J. 2016. Plant responses to climatic extremes: within-species variation equals among-species variation. Glob. Change Biol. 22, 449–464. ( 10.1111/gcb.13114) [DOI] [PubMed] [Google Scholar]
- 52.Matías L, González-Díaz P, Jump A. 2014. Larger investment in roots in southern range-edge populations of Scots pine is associated with increased growth and seedling resistance to extreme drought in response to simulated climate change. Environ. Exp. Bot. 105, 32–38. ( 10.1016/j.envexpbot.2014.04.003) [DOI] [Google Scholar]
- 53.Beierkuhnlein C, Thiel D, Jentsch A, Willner E, Kreyling J. 2011. Ecotypes of European grass species respond differently to warming and extreme drought. J. Ecol. 99, 703–713. ( 10.1111/j.1365-2745.2011.01809.x) [DOI] [Google Scholar]
- 54.Thiel D, Nagy L, Beierkuhnlein C, Huber G, Jentsch A, Konnert M, Kreyling J. 2012. Uniform drought and warming responses in Pinus nigra provenances despite specific overall performances. For. Ecol. Manage. 270, 200–208. ( 10.1016/j.foreco.2012.01.034) [DOI] [Google Scholar]
- 55.Jung V, Albert C, Violle C, Kunstler G, Loucougaray G, Spiegelberger T. 2014. Intraspecific trait variability mediates the response of subalpine grassland communities to extreme drought events. J. Ecol. 102, 45–53. ( 10.1111/1365-2745.12177) [DOI] [Google Scholar]
- 56.Valladares F, Gianoli E, Gómez J. 2007. Ecological limits to plant phenotypic plasticity. New Phytol. 176, 749–763. ( 10.1111/j.1469-8137.2007.02275.x) [DOI] [PubMed] [Google Scholar]
- 57.Solow AR. On detecting ecological impacts of extreme climate events and why it matters. Phil. Trans. R. Soc. B 372, 20160136 ( 10.1098/rstb.2016.0136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kreyling J, Jentsch A, Beierkuhnlein C. 2011. Stochastic trajectories of succession initiated by extreme climatic events. Ecol. Lett. 14, 758–764. ( 10.1111/j.1461-0248.2011.01637.x) [DOI] [PubMed] [Google Scholar]
- 59.White T, Campbell B, Kemp P, Hunt C. 2000. Sensitivity of three grassland communities to simulated extreme temperature and rainfall events. Glob. Change Biol. 6, 671–684. ( 10.1046/j.1365-2486.2000.00344.x) [DOI] [Google Scholar]
- 60.Whittaker R. 1965. Dominance and diversity in land plant communities: numerical relations of species express the importance of competition in community function and evolution. Science 147, 250–260. ( 10.1126/science.147.3655.250) [DOI] [PubMed] [Google Scholar]
- 61.Grime J. 1998. Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J. Ecol. 86, 902–910. ( 10.1046/j.1365-2745.1998.00306.x) [DOI] [Google Scholar]
- 62.Gitlin A, Sthultz C, Bowker M, Stumpf S, Paxton K, Kennedy K, Muñoz A, Bailey J, Whitham T. 2006. Mortality gradients within and among dominant plant populations as barometers of ecosystem change during extreme drought. Conserv. Biol. 20, 1477–1486. ( 10.1111/j.1523-1739.2006.00424.x) [DOI] [PubMed] [Google Scholar]
- 63.Kane J, Meinhardt K, Chang T, Cardall B, Michalet R, Whitham T. 2011. Drought-induced mortality of a foundation species (Juniperus monosperma) promotes positive afterlife effects in understory vegetation. Plant Ecol. 212, 733–741. ( 10.1007/s11258-010-9859-x) [DOI] [Google Scholar]
- 64.Pan Y, et al. 2011. A large and persistent carbon sink in the world's forests. Science 333, 988–993. ( 10.1126/science.1201609) [DOI] [PubMed] [Google Scholar]
- 65.Breshears D, et al. 2005. Regional vegetation die-off in response to global-change-type drought. Proc. Natl Acad. Sci. USA 102, 15 144–15 148. ( 10.1073/pnas.0505734102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bigler C, Gavin D, Gunning C, Veblen T. 2007. Drought induces lagged tree mortality in a subalpine forest in the Rocky Mountains. Oikos 116, 1983–1994. ( 10.1111/j.2007.0030-1299.16034.x) [DOI] [Google Scholar]
- 67.Anderegg W, Kane J, Anderegg L. 2012. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Change 3, 30–36. ( 10.1038/nclimate1635) [DOI] [Google Scholar]
- 68.Martinez-Vilalta J, Lloret F, Breshears D. 2012. Drought-induced forest decline: causes, scope and implications. Biol. Lett. 8, 689–691. ( 10.1098/rsbl.2011.1059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Royer P, Cobb N, Clifford M, Huang C, Breshears D, Adams H, Villegas J. 2011. Extreme climatic event-triggered overstorey vegetation loss increases understorey solar input regionally: primary and secondary ecological implications. J. Ecol. 99, 714–723. ( 10.1111/j.1365-2745.2011.01804.x) [DOI] [Google Scholar]
- 70.Saura-Mas S, Bonas A, Lloret F. 2015. Plant community response to drought-induced canopy defoliation in a Mediterranean Quercus ilex forest. Eur. J. For. Res. 134, 261–272. ( 10.1007/s10342-014-0848-9) [DOI] [Google Scholar]
- 71.Rich P, Breshears D, White A. 2008. Phenology of mixed woody–herbaceous ecosystems following extreme events: net and differential responses. Ecology 89, 342–352. ( 10.1890/06-2137.1) [DOI] [PubMed] [Google Scholar]
- 72.Suarez M, Sasal Y. 2012. Drought-induced mortality affects understory vegetation: release after death. Ecol. Res. 27, 715–724. ( 10.1007/s11284-012-0945-5) [DOI] [Google Scholar]
- 73.Barbeta A, Ogaya R, Peñuelas J. 2013. Dampening effects of long-term experimental drought on growth and mortality rates of a Holm oak forest. Glob. Change Biol. 19, 3133–3144. ( 10.1111/gcb.12269) [DOI] [PubMed] [Google Scholar]
- 74.Mueller R, Scudder C, Porter M, Talbot Trotter R, Gehring C, Whitham T. 2005. Differential tree mortality in response to severe drought: evidence for long-term vegetation shifts. J. Ecol. 93, 1085–1093. ( 10.1111/j.1365-2745.2005.01042.x) [DOI] [Google Scholar]
- 75.Kröel-Dulay G, et al. 2015. Increased sensitivity to climate change in disturbed ecosystems. Nat. Commun. 6, 6682 ( 10.1038/ncomms7682) [DOI] [PubMed] [Google Scholar]
- 76.Cavin L, Mountford E, Peterken G, Jump A. 2013. Extreme drought alters competitive dominance within and between tree species in a mixed forest stand. Funct. Ecol. 27, 1424–1435. ( 10.1111/1365-2435.12126) [DOI] [Google Scholar]
- 77.Arnone J, Jasoni R, Lucchesi A, Larsen J, Leger E, Sherry R, Luo Y, Schimel D, Verburg P. 2011. A climatically extreme year has large impacts on C4 species in tallgrass prairie ecosystems but only minor effects on species richness and other plant functional groups. J. Ecol. 99, 678–688. ( 10.1111/j.1365-2745.2011.01813.x) [DOI] [Google Scholar]
- 78.Weaver J. 1954. North American prairie. Lincoln, NE: Johnson Publishing. [Google Scholar]
- 79.Bokhorst S, Bjerke J, Street L, Callaghan T, Phoenix G. 2011. Impacts of multiple extreme winter warming events on sub-Arctic heathland: phenology, reproduction, growth, and CO2 flux responses. Glob. Change Biol. 17, 2817–2830. ( 10.1111/j.1365-2486.2011.02424.x) [DOI] [Google Scholar]
- 80.Evans S, Byrne K, Lauenroth W, Burke I. 2011. Defining the limit to resistance in a drought-tolerant grassland: long-term severe drought significantly reduces the dominant species and increases ruderals. J. Ecol. 99, 1500–1507. ( 10.1111/j.1365-2745.2011.01864.x) [DOI] [Google Scholar]
- 81.Khan MASA, Grant K, Beierkuhnlein C, Kreyling J, Jentsch A. 2014. Climatic extremes lead to species-specific legume facilitation in an experimental temperate grassland. Plant Soil 379, 161–175. ( 10.1007/s11104-014-2050-8) [DOI] [Google Scholar]
- 82.Dreesen F, De Boeck H, Janssens I, Nijs I. 2014. Do successive climate extremes weaken the resistance of plant communities? An experimental study using plant assemblages. Biogeosciences 11, 109–121. ( 10.5194/bg-11-109-2014) [DOI] [Google Scholar]
- 83.De Boeck H, Dreesen F, Janssens I, Nijs I. 2011. Whole-system responses of experimental plant communities to climate extremes imposed in different seasons. New Phytol. 189, 806–817. ( 10.1111/j.1469-8137.2010.03515.x) [DOI] [PubMed] [Google Scholar]
- 84.Mitchell P, O'Grady A, Hayes K, Pinkard E. 2014. Exposure of trees to drought-induced die-off is defined by a common climatic threshold across different vegetation types. Ecol. Evol. 4, 1088–1101. ( 10.1002/ece3.1008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith M, Knapp A, Collins S. 2009. A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology 90, 3279–3289. ( 10.1890/08-1815.1) [DOI] [PubMed] [Google Scholar]
- 86.Lloret F, Escudero A, Iriondo J, Martínez-Vilalta J, Valladares F. 2012. Extreme climatic events and vegetation: the role of stabilizing processes. Glob. Change Biol. 18, 797–805. ( 10.1111/j.1365-2486.2011.02624.x) [DOI] [Google Scholar]
- 87.Tilman D. 2001. Diversity and productivity in a long-term grassland experiment. Science 294, 843–845. ( 10.1126/science.1060391) [DOI] [PubMed] [Google Scholar]
- 88.Tilman D, Reich P, Knops J. 2006. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 441, 629–632. ( 10.1038/nature04742) [DOI] [PubMed] [Google Scholar]
- 89.Isbell F, et al. 2011. High plant diversity is needed to maintain ecosystem services. Nature 477, 199–202. ( 10.1038/nature10282) [DOI] [PubMed] [Google Scholar]
- 90.dos Santos A, Saraiva D, Müller S, Overbeck G. 2015. Interactive effects of environmental filtering predict beta-diversity patterns in a subtropical forest metacommunity. Perspect. Plant Eco. Evol. Syst. 17, 96–106. ( 10.1016/j.ppees.2015.01.002) [DOI] [Google Scholar]
- 91.Zeiter M, Schärrer S, Zweifel R, Newbery D, Stampfli A. 2016. Timing of extreme drought modifies reproductive output in semi-natural grassland. J. Veg. Sci. 27, 238–248. ( 10.1111/jvs.12362) [DOI] [Google Scholar]
- 92.Concilio A, Prevéy J, Omasta P, O'Connor J, Nippert J, Seastedt T. 2015. Response of a mixed grass prairie to an extreme precipitation event. Ecosphere 6, 1–12. art172 ( 10.1890/es15-00073.1) [DOI] [Google Scholar]
- 93.Smart S, Ellison A, Bunce R, Marrs R, Kirby K, Kimberley A, Scott A, Foster D. 2014. Quantifying the impact of an extreme climate event on species diversity in fragmented temperate forests: the effect of the October 1987 storm on British broadleaved woodlands. J. Ecol. 102, 1273–1287. ( 10.1111/1365-2745.12291) [DOI] [Google Scholar]
- 94.Grant K, Kreyling J, Heilmeier H, Beierkuhnlein C, Jentsch A. 2014. Extreme weather events and plant–plant interactions: shifts between competition and facilitation among grassland species in the face of drought and heavy rainfall. Ecol. Res. 29, 991–1001. ( 10.1007/s11284-014-1187-5) [DOI] [Google Scholar]
- 95.Fry E, Manning P, Allen D, Hurst A, Everwand G, Rimmler M, Power S. 2013. Plant functional group composition modifies the effects of precipitation change on grassland ecosystem function. PLoS ONE 8, e57027 ( 10.1371/journal.pone.0057027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gellesch E, Wellstein C, Beierkuhnlein C, Kreyling J, Walter J, Jentsch A. 2015. Plant community composition is a crucial factor for heath performance under precipitation extremes. J. Veg. Sci. 26, 975–984. ( 10.1111/jvs.12304) [DOI] [Google Scholar]
- 97.Urbina I, Sardans J, Beierkuhnlein C, Jentsch A, Backhaus S, Grant K, Kreyling J, Peñuelas J. 2015. Shifts in the elemental composition of plants during a very severe drought. Environ. Exp. Bot. 111, 63–73. ( 10.1016/j.envexpbot.2014.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arredondo T, Garcìa-Moya E, Huber-Sannwald E, Loescher H, Delgado-Balbuena J, Luna-Luna M. 2016. Drought manipulation and its direct and legacy effects on productivity of a monodominant and mixed-species semi-arid grassland. Agric. For. Meteorol. 223, 132–140. ( 10.1016/j.agrformet.2016.03.011) [DOI] [Google Scholar]
- 99.Tilman D. 1982. Resource competition and community structure. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 100.Silvertown J, Dodd M, Gowing D, Mountford J. 1999. Hydrologically defined niches reveal a basis for species richness in plant communities. Nature 400, 61–63. ( 10.1038/21877) [DOI] [Google Scholar]
- 101.Kahmen A, Perner J, Buchmann N. 2005. Diversity-dependent productivity in semi-natural grasslands following climate perturbations. Funct. Ecol. 19, 594–601. ( 10.1111/j.1365-2435.2005.01001.x) [DOI] [Google Scholar]
- 102.van Rooijen N, de Keersmaecker W, Ozinga W, Coppin P, Hennekens S, Schaminée J, Somers B, Honnay O. 2015. Plant species diversity mediates ecosystem stability of natural dune grasslands in response to drought. Ecosystems 18, 1383–1394. ( 10.1007/s10021-015-9905-6) [DOI] [Google Scholar]
- 103.Mariotte P, Vandenberghe C, Kardol P, Hagedorn F, Buttler A. 2013. Subordinate plant species enhance community resistance against drought in semi-natural grasslands. J. Ecol. 101, 763–773. ( 10.1111/1365-2745.12064) [DOI] [Google Scholar]
- 104.Bloor J, Bardgett R. 2012. Stability of above-ground and below-ground processes to extreme drought in model grassland ecosystems: interactions with plant species diversity and soil nitrogen availability. Perspect. Plant Ecol. Evol. Syst. 14, 193–204. ( 10.1016/j.ppees.2011.12.001) [DOI] [Google Scholar]
- 105.Lebourgeois F, Gomez N, Pinto P, Mérian P. 2013. Mixed stands reduce Abies alba tree-ring sensitivity to summer drought in the Vosges mountains, western Europe. For. Ecol. Manage. 303, 61–71. ( 10.1016/j.foreco.2013.04.003) [DOI] [Google Scholar]
- 106.Pfisterer A, Schmid B. 2002. Diversity-dependent production can decrease the stability of ecosystem functioning. Nature 416, 84–86. ( 10.1038/416084a) [DOI] [PubMed] [Google Scholar]
- 107.Van Peer L, Nijs I, Reheul D, De Cauwer B. 2004. Species richness and susceptibility to heat and drought extremes in synthesized grassland ecosystems: compositional vs physiological effects. Funct. Ecol. 18, 769–778. ( 10.1111/j.0269-8463.2004.00901.x) [DOI] [Google Scholar]
- 108.Lloret F, Lobo A, Estevan H, Maisongrande P, Vayreda J, Terradas J. 2007. Woody plant richness and NDVI response to drought events in Catalonian (Northeastern Spain) forests. Ecology 88, 2270–2279. ( 10.1890/06-1195.1) [DOI] [PubMed] [Google Scholar]
- 109.Fischer F, Wright A, Eisenhauer N, Ebeling A, Roscher C, Wagg C, Weigelt A, Weisser W, Pillar V. 2016. Plant species richness and functional traits affect community stability after a flood event. Phil. Trans. R. Soc. B 371, 20150276 ( 10.1098/rstb.2015.0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huston M. 1997. Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia 110, 449–460. ( 10.1007/s004420050180) [DOI] [PubMed] [Google Scholar]
- 111.Isbell F, et al. 2015. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 526, 574–577. ( 10.1038/nature15374) [DOI] [PubMed] [Google Scholar]
- 112.Van Ruijven J, Berendse F. 2010. Diversity enhances community recovery, but not resistance, after drought. J. Ecol. 98, 81–86. ( 10.1111/j.1365-2745.2009.01603.x) [DOI] [Google Scholar]
- 113.Tilman D. 1997. The influence of functional diversity and composition on ecosystem processes. Science 277, 1300–1302. ( 10.1126/science.277.5330.1300) [DOI] [Google Scholar]
- 114.Lloret F, Granzow-de la Cerda I. 2013. Plant competition and facilitation after extreme drought episodes in Mediterranean shrubland: does damage to vegetation cover trigger replacement by juniper woodland? J. Veg. Sci. 24, 1020–1032. ( 10.1111/jvs.12030) [DOI] [Google Scholar]
- 115.Michalet R, Le Bagousse-Pinguet Y, Maalouf J, Lortie C. 2013. Two alternatives to the stress-gradient hypothesis at the edge of life: the collapse of facilitation and the switch from facilitation to competition. J. Veg. Sci. 25, 609–613. ( 10.1111/jvs.12123) [DOI] [Google Scholar]
- 116.Saccone P, Delzon S, Pagès J, Brun J, Michalet R. 2009. The role of biotic interactions in altering tree seedling responses to an extreme climatic event. J. Veg. Sci. 20, 403–414. ( 10.1111/j.1654-1103.2009.01012.x) [DOI] [Google Scholar]
- 117.Diez J, et al. 2012. Will extreme climatic events facilitate biological invasions? Front. Ecol. Environ. 10, 249–257. ( 10.1890/110137) [DOI] [Google Scholar]
- 118.Jiménez M, Jaksic F, Armesto J, Gaxiola A, Meserve P, Kelt D, Gutiérrez J. 2011. Extreme climatic events change the dynamics and invasibility of semi-arid annual plant communities. Ecol. Lett. 14, 1227–1235. ( 10.1111/j.1461-0248.2011.01693.x) [DOI] [PubMed] [Google Scholar]
- 119.Dreesen F, De Boeck H, Horemans J, Janssens I, Nijs I. 2015. Recovery dynamics and invasibility of herbaceous plant communities after exposure to experimental climate extremes. Basic Appl. Ecol. 16, 583–591. ( 10.1016/j.baae.2015.05.002) [DOI] [Google Scholar]
- 120.Manea A, Sloane D, Leishman M. 2016. Reductions in native grass biomass associated with drought facilitates the invasion of an exotic grass into a model grassland system. Oecologia 181, 175–183. ( 10.1007/s00442-016-3553-1) [DOI] [PubMed] [Google Scholar]
- 121.Davis M, Grime J, Thompson K. 2000. Fluctuating resources in plant communities: a general theory of invasibility. J. Ecol. 88, 528–534. ( 10.1046/j.1365-2745.2000.00473.x) [DOI] [Google Scholar]
- 122.Koerner S, Avolio M, Chang C, Gray J, Hoover D, Smith M. 2015. Invasibility of a mesic grassland depends on the time-scale of fluctuating resources. J. Ecol. 103, 1538–1546. ( 10.1111/1365-2745.12479) [DOI] [Google Scholar]
- 123.Meisner A, De Deyn G, de Boer W, van der Putten W. 2013. Soil biotic legacy effects of extreme weather events influence plant invasiveness. Proc. Natl Acad. Sci. USA 110, 9835–9838. ( 10.1073/pnas.1300922110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Caldeira M, Lecomte X, David T, Pinto J, Bugalho M, Werner C. 2015. Synergy of extreme drought and shrub invasion reduce ecosystem functioning and resilience in water-limited climates. Sci. Rep. 5, 15110 ( 10.1038/srep15110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kreyling J, Beierkuhnlein C, Ellis L, Jentsch A. 2008. Invasibility of grassland and heath communities exposed to extreme weather events—additive effects of diversity resistance and fluctuating physical environment. Oikos 117, 1542–1554. ( 10.1111/j.0030-1299.2008.16653.x) [DOI] [Google Scholar]
- 126.Sheppard C, Alexander J, Billeter R. 2012. The invasion of plant communities following extreme weather events under ambient and elevated temperature. Plant Ecol. 213, 1289–1301. ( 10.1007/s11258-012-0086-5) [DOI] [Google Scholar]
- 127.Hou Q, Chen B, Peng S, Chen L. 2014. Effects of extreme temperature on seedling establishment of nonnative invasive plants. Biol. Invasions 16, 2049–2061. ( 10.1007/s10530-014-0647-8) [DOI] [Google Scholar]
- 128.Knapp A. 2002. Rainfall variability, carbon cycling, and plant species diversity in a mesic grassland. Science 298, 2202–2205. ( 10.1126/science.1076347) [DOI] [PubMed] [Google Scholar]
- 129.De Boeck H, Bassin S, Verlinden M, Zeiter M, Hiltbrunner E. 2016. Simulated heat waves affected alpine grassland only in combination with drought. New Phytol. 209, 531–541. ( 10.1111/nph.13601) [DOI] [PubMed] [Google Scholar]
- 130.Bailey L, van de Pol M. 2016. Tackling extremes: challenges for ecological and evolutionary research on extreme climatic events. J. Anim. Ecol. 85, 85–96. ( 10.1111/1365-2656.12451) [DOI] [PubMed] [Google Scholar]
- 131.Vellend M. 2010. Conceptual synthesis in community ecology. Q. Rev. Biol. 85, 183–206. ( 10.1086/652373) [DOI] [PubMed] [Google Scholar]
- 132.De Laender F, et al. 2016. Reintroducing environmental change drivers in biodiversity–ecosystem functioning research. Trends Ecol. Evol. 31, 905–915. ( 10.1016/j.tree.2016.09.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
One data file containing studies and the study type used to generate figure 1 will be available in the electronic supplementary material. Figure 1 was generated using R statistical software environment (v. 3.2.1) accessible at https://www.rstudio.com/. The R code for this figure will also be provided in the electronic supplementary material.