Abstract
Despite abundant evidence that natural populations are responding to climate change, there are few demonstrations of how extreme climatic events (ECEs) affect fitness. Climate warming increases adverse effects of exposure to high temperatures, but also reduces exposure to cold ECEs. Here, we investigate variation in survival associated with severity of summer and winter conditions, and whether survival is better predicted by ECEs than mean temperatures using data from two coexisting bird species monitored over 37 years in southwestern Australia, red-winged fairy-wrens, Malurus elegans and white-browed scrubwrens, Sericornis frontalis. Changes in survival were associated with temperature extremes more strongly than average temperatures. In scrubwrens, winter ECEs were associated with survival within the same season. In both species, survival was associated with body size, and there was evidence that size-dependent mortality was mediated by carry-over effects of climate in the previous season. For fairy-wrens, mean body size declined over time but this could not be explained by size-dependent mortality as the effects of body size on survival were consistently positive. Our study demonstrates how ECEs can have individual-level effects on survival that are not reflected in long-term morphological change, and the same climatic conditions can affect similar-sized, coexisting species in different ways.
This article is part of the themed issue ‘Behavioural, ecological and evolutionary responses to extreme climatic events’.
Keywords: carry-over costs, climate change, extreme climatic events, passerine birds, size-dependent mortality, Meliphagoidea
1. Introduction
There is now increasing evidence for adverse effects of climate change associated with increased exposure to extreme climatic events (ECEs) in the form of high temperatures (hot ECEs). Exposure to severe heatwaves with temperatures above critical thresholds can have dramatic immediate impacts on wild populations, leading to mass mortalities and major population crashes via fatal hyperthermia [1,2]. In addition, there is growing evidence that pervasive effects of increasing exposure to high temperatures may also have implications for individual phenotypes and hence for fitness. Prolonged exposure (i.e. over successive days) to high temperatures in the mid-30s°C can lead to reductions in adult body mass, as well as affect nestling development, presumably through a combination of dehydration via increased rates of evaporative cooling and energetic constraints that result from reduced foraging opportunities or provisioning effort [3–7].
Body size may play a critical role in mediating the impact of warmer temperatures on wild animal populations. In general, smaller endotherms are expected to cope better with a gradual warming of the climate because their larger surface area to volume ratios allow more efficient dissipation of heat [8–10]. Thus, gradual climate warming may be associated with pervasive reductions in body size [11,12], in line with Bergmann's observation of increases in mean body size with latitude [13]. Despite this prediction, responses to gradual warming might also involve increases in body size. The metabolic costs of maintaining high body temperature for birds might be reduced in a warming climate, leading to increased allocation of resources to growth and body maintenance, with concomitant improvements in body condition [12]. In addition, temperature extremes (hot ECEs), rather than means, may impose selection on small body size. With prolonged exposure to high temperatures above mid-30s°C (hot ECEs), smaller individuals may suffer higher mortality because evaporative water loss used for body cooling increases disproportionately with decreasing body size [14]. Thus, larger individuals can have higher survival during heatwaves [14,15].
In addition to increased exposure to hot ECEs, climate warming may also reduce exposure to cold ECEs [16]. The effects of changing winter conditions have received little attention even though they can cause direct mortality via cold stress, reductions in immune function or energetic constraints associated with resource shortages that can affect body condition with consequences for fitness [17,18]. Indeed, energetic costs of thermoregulation in winter during periods of low food availability can be greater than those during the breeding season [19]. As for hot extremes, how endotherms deal with cold is also expected to be affected by body size. Larger body size may confer thermal benefits in cold conditions both by reducing heat loss (via reduced surface area to volume ratios) and by increasing capacity to carry more fat reserves, thereby increasing resistance to starvation [17,20]. Thus, large body sizes may be beneficial during cold ECEs. Accordingly, reductions in the severity of winter conditions due to climate warming are predicted to increase the survival of smaller individuals, which might lead to a decline in mean body size of a population over time. For example, in Soay sheep Ovis aries, milder winters increased survival of small individuals, which in part contributed to a decrease in mean body size [21].
Although there have been relatively few studies testing for the impacts of warming winters on fitness, there are even fewer that have analysed the effects of summer and winter ECEs simultaneously. Joint consideration is important, because processes in one season may affect performance in another [18]. Thus, for example, the condition of animals emerging from winter will determine their performance during the breeding season, and their breeding performance will in turn determine their condition going into the subsequent winter and hence their winter performance [17]. Comprehensive assessment of such carry-over effects is necessary if we are to quantify the full impact of climate change [18].
Here, we test for links between climatic stressors and survival, and the consequences for morphology, in two similar-sized, coexisting species of insectivorous passerine birds that have been the subject of long-term monitoring over 37 years (1977–2014) at a temperate site in southwestern Australia. These long-term datasets allow us to explore associations between climate and survival and whether changes in survival lead to morphological change over time. Using the long-term recapture data in combination with local temperature and rainfall data, we test whether inter-annual variation in survival is associated with the severity of summer and winter conditions and associated size-dependent mortality over decades, and thus the long-term impact of climate change on avian morphology. Our study populations have experienced increases in mean temperatures in winter and summer. We focus on occurrence of ‘extremes’ (ECEs) defined by the extreme tails of the temperature distribution at our study site, which represent conditions that affect survival in a range of bird species including small passerines [15,22,23]. In particular, we test whether survival is more clearly predicted by occurrence of extreme events than by mean temperatures. We predict that:
(1) Exposure to extreme events, either hot dry in summer or cold wet in winter, reduces survival of both study species.
(2) Survival is also size-dependent, and the effects of body size mediate the effect of extreme events on survival.
We anticipate that the combined implications of these predictions explain any long-term trends in body size in the study species: a priori predictions are difficult, being dependent on the relative impact of hot versus cold ECEs, and their respective interactions with body size.
2. Material and methods
(a). Definition of an extreme climatic event
We have a poor understanding of the ways in which ECEs lead to changes in fitness, selection and microevolution, in part because synthesis is hampered by the lack of a universal definition of what constitutes an ECE. Here, we use a statistical definition of climate extremes, as the tails in either end of the summer and winter temperature range at the study site [24]. Specifically, for each year, we quantified the number of cold wet days on which rainfall more than 0 mm and minima less than 5°C were recorded (NColdWetDays) and the number of hot dry days with no rainfall and maxima more than 30°C (NHotDryDays), because cold wet conditions in winter and hot dry conditions in summer can cause thermal stress and affect fitness [14,22,23].
(b). Study site and study species
Our study populations are two populations of small passerines, the white-browed scrubwren (Sericornis frontalis) and the red-winged fairy-wren (Malurus elegans), that have been the subject of study over a 37 year period (1977–2014) at the Smithbrook Nature Reserve southeast of Manjimup in Western Australia (116°10′ E, 34°20′ S). The 95-ha reserve consists of eucalypt wet forest dominated by Karri (Eucalyptus diversicolor), Jarrah (E. marginata) and Marri (E. calophylla) trees with a dense understorey (for more details, see [25]).
Both study species are small insectivorous passerines that belong to the large and diverse superfamily Meliphagoidea [26]. Mean body weight of adults in this study was 9.7 g (fairy-wren) and 11.2 g (scrubwren). Both species are sedentary, year-round residents, occupying territories that persist year-to-year [27,28]. Both are cooperative breeders, breeding during the austral spring and summer months, and capable of producing multiple clutches each season [27,29]. Both are also relatively long-lived: the maximum ages for red-winged fairy-wrens and white-browed scrubwrens in this study are 15 and 13 years, respectively. The sexes are dichromatic in both species so can be readily distinguished on the basis of plumage [27,30].
(c). Data
(i). Bird data
The morphometric and recapture/survival data used here are from a long-term banding (ringing) programme at Smithbrook Nature Reserve [31]. Additional data for red-winged fairy-wrens were also available from two detailed studies of the social and mating system of this species based on individually marked, colour-banded birds at the same site: Rowley et al. [27] and Russell & Rowley [30] monitored individuals from 30 breeding groups between 1980 and 1995, and Brouwer et al. [28] have monitored individuals from approximately 70 territories since 2008. As a result, more than 99% of adult birds in the fairy-wren population were individually colour-banded.
Birds were captured in mist-nets throughout the year and weighed with a Pesola balance to an accuracy of 0.5 g. Birds were caught and banded with permission from the Australian Bird and Bat Banding Scheme. Primary wing feathers were scored for moult. Wing length was measured as the length of the flattened wing chord from the carpal joint to the tip of the longest primary to the nearest 1.0 mm using a butt-ended ruler. Among passerines, wing length is the best single linear predictor of structural size, and accordingly may be used as an index of body size [32]. Some studies use tarsus length as an index of body size because the trait is less variable over an individual's life. However, appendages such as bills and tarsi are involved in heat dissipation and are predicted to increase in size, relative to body size, with climate warming in accordance with Allen's Rule [33,34]. To avoid these complications, we therefore chose to use wing length an index of structural body size. Birds were either of known age (banded as a nestling) or we estimated minimum age based on recapture data, with unknown adult birds assigned as age one at first capture. We included only adults (more than 12 months old) in our analyses. We assigned an index of feather wear (abrasion score, 1–12) to account for abrasion of the tips of primary feathers, which occurs between successive moults and affects wing length [35].
We analysed summer and winter survival separately. The species are known to be sedentary and strongly site-faithful, so adult disappearances are likely to reflect mortality rather than dispersal [27,29,30]. Thus, we defined ‘winter survival’ as a binary variable, with a bird being defined as having survived winter if it had been captured during autumn/winter (i.e. April–September), and then was resighted/captured again at least one time point subsequently following 1 October. Similarly, ‘summer survival’ was defined as a bird having survived summer if it had been captured during spring/summer (i.e. October–March), and then was resighted/captured again subsequently following 1 April. We also calculated the number of days elapsed between capture and the day on which survival was assessed at the end of autumn/winter (NDaysFromCaptureToOct1) or at the end of spring/summer (NDaysFromCaptureToApr1). For fairy-wrens, data were available on 2258 captures of 1612 individuals over 37 years; for scrubwrens, data were available on 1146 captures of 633 individuals over 36 years.
(ii). Climate data
We calculated environmental variables from climate data, based on standardized daily records from the Pemberton weather station (Station: 009592, 116°00′ E, 34°27′ S; Bureau of Meteorology) located approximately 15 km from the study site. In addition to the effect of summer and winter ECEs on survival (defined above), we also extracted mean temperatures for summer (MeanSummerTemp) for the months December–February, and for winter (MeanWinterTemp) for the months June–August, and total annual rainfall (TotalAnnualRainfall) for each year. To test for temporal changes in climate at our study site we also extracted mean minimum temperature (AnnualMeanMinTemp), mean maximum temperature (AnnualMeanMaxTemp), number of cold days recording temperatures less than 5°C (AnnualNdays less than 5°C) and number of hot days recording temperatures more than 30°C (AnnualNdays more than 30°C) for each calendar year.
(d). Statistical models
Our analyses consisted of three sets of statistical models.
(i). Temporal changes in climate and in body size
We tested for changes in climate at Smithbrook Nature Reserve over the 37 years of the study, fitting each annual climate variable (AnnualMeanMinTemp, AnnualMeanMaxTemp, AnnualNdays less than 5°C and AnnualNdays more than 30°C) as the response and calendar year as a predictor covariate in separate linear regression models.
We tested for long-term changes in adult body size over the 37 years of the study, treating each species separately. We used linear mixed models with body size as the response variable using an identity link function and Gaussian error distribution. We fitted sex (as a two-level fixed factor) and age (because older birds tend to have longer feathers [30]), feather wear and year as continuous fixed-effects covariates. We also fitted age at last capture as an additional variable to distinguish within- versus between-subject effects of age on body size, and hence to model any potential ‘selective disappearance’ [36]. Finally, the random effects fitted were bird identity and year (fitted as a categorical variable) to account for multiple captures of the same individuals, and multiple measures from the same years, respectively.
(ii). Determinants of winter survival
We tested whether winter survival each year was associated with the severity of weather conditions for birds caught in the autumn and winter months between April and September, using generalized linear mixed models (GLMM) with survival as a binary variable and a logit-link function and binomial error. We ran separate models for each species.
We tested for the effects of NColdWetDays recorded in the calendar year in which a bird was captured. Furthermore, NHotDryDays in the preceding summer was also included as a covariate, to test for carry-over effects of high summer temperatures on survival [7]. We predicted that the effects of exposure to these weather conditions would be nonlinear so we also fitted quadratic terms for each variable.
We tested for effects of structural body size on survival by including a measure of wing length corrected for feather wear; this was calculated from a regression of wing length on abrasion score [35]. To test whether associations between survival and climate variables were size-dependent, we fitted interactions between body size and the two climate variables (body size × NColdWetDays; body size × NHotDryDays). Because the probability of survival may also vary with TotalAnnualRainfall, age, sex and NDaysFromCaptureToOct1, we fitted these terms as additional fixed explanatory variables.
To account for multiple observations from each year, we fitted year as a multi-level random effect. In the case of red-winged fairy-wrens, capture effort varied substantially among years, so we also fitted ‘capture effort’ as a three-level factor (low, medium, high) to account for this difference (see the electronic supplementary material). All continuous, explanatory variables were standardized to zero mean and unit variance prior to analysis to facilitate model convergence.
Finally, because responses to climate may be better predicted by mean temperatures rather than extremes, we ran all models with mean temperature variable equivalents replacing those for ECEs (e.g. NColdWetDays with MeanWinterTemp, NHotDryDays with MeanSummerTemp) including in interactions with body size.
(iii). Determinants of summer survival
We used the same model structure and variables to test whether summer survival each year (after 1 April) was associated with the severity of weather conditions for birds caught in the spring and summer months (between October and March). We therefore tested for the effects of NHotDryDays during the current summer, and also for possible carry-over effects of NColdWetDays recorded in the preceding winter. Other variables included were identical to the winter model except that we assessed survival to end of summer (NDaysFromCaptureToApr1) (above). As with the winter analysis, we also fitted models with mean temperature variables including interactions with body size.
(e). Model fitting
To account for model selection uncertainty, we adopted a multi-model inference approach. We used Akaike information criteria [37,38] corrected for the sample size (AICc) to select the most parsimonious model out of the set of models with all possible combinations of variables. Models with lower AICc values are better supported by the data. We used an all-subset approach and reported the top models within two ΔAICc of the best supported model only. We calculated the Akaike weights to assess the relative likelihood of competing models. In total, 95% confidence intervals (CI) of estimates of individual predictor variables were used as indicators of parameter importance [39].
All analyses were conducted in R 3.3.2 [40]. Linear mixed models were fitted with maximum-likelihood (rather than REML) using the package lme4 [41], generalized linear mixed models were fitted using the glmer function from the lme4 package [42] and model selection was conducted using the package MuMIn [43].
Because climate means and extremes were highly correlated (electronic supplementary material, table S1), we did not include mean and extreme temperature variables in the same model simultaneously. Instead, we used AICc to identify whether models with extremes better explained the data, over and above those with mean temperature variables. In all but one case, replacing climate extremes with climate means increased AICc values. Thus, our results are confined to models with extreme temperatures for three of the four analyses; see electronic supplementary material, table S2 for the top 100 models for each analysis.
3. Results
(a). Temporal trends in climate
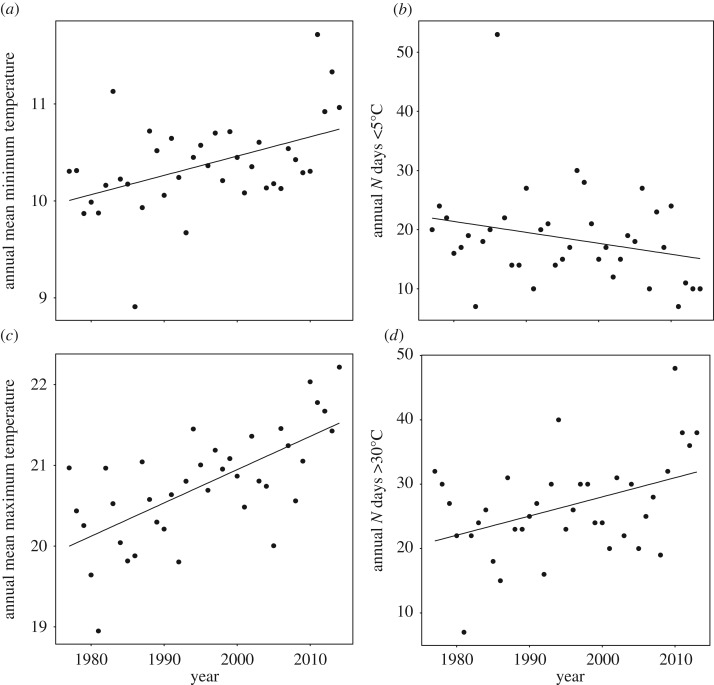
The climate changed at the site over the 37 years of the study (figure 1). Annual mean minimum and mean maximum temperatures rose over time (AnnualMeanMinTemp: 0.020 ± 0.006 s.e. °C yr−1, t = 3.11, P = 0.004; AnnualMeanMaxTemp: 0.041 ± 0.008 s.e. °C yr−1, t = 5.34, P < 0.001). The AnnualNdays less than 5°C showed a marginally non-significant decline (−0.246 ± 0.123 s.e. days yr−1, t = −2.0, P = 0.052), and the AnnualNdays more than 30°C increased (0.297 ± 0.109 s.e. days yr−1, t = 2.737, P = 0.01). TotalAnnualRainfall did not change over time (0.768 ± 2.404 s.e. mm yr−1, t = 0.319, P = 0.751).
Figure 1.
Temporal trends in annual climate between 1977 and 2014 at the Smithbrook Nature Reserve in southwestern Western Australia. Shown are (a) annual mean minimum temperature; (b) annual number of days with minimum temperature below 5°C; (c) annual mean maximum temperature; (d) annual number of days with maximum temperature above 30°C.
(b). Determinants of winter survival
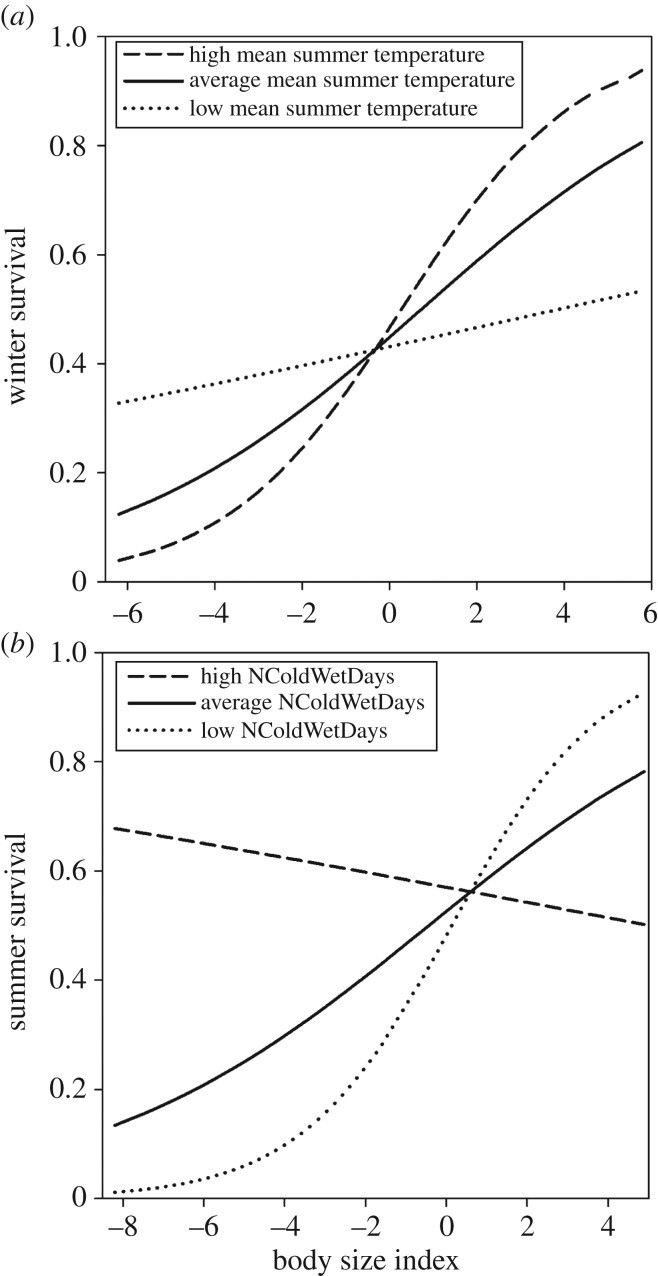
Fairy-wrens. Overall, models with mean temperature variables better explained the data than did those with ECE variable equivalents (electronic supplementary material, table S2). In contrast with our predictions, the winter ECEs (NColdWetDays) were not included in any of the top models and thus there was no evidence that winter survival decreased with an increasing frequency of cold, wet days (table 1a). However, body size was consistently included in the top models (table 1a), thus there was strong evidence that body size was positively associated with survival (figure 2a). Moreover, the association between body size and survival was mediated by a carry-over effect of mean temperature in the preceding summer, with body size having a stronger effect on winter survival if the previous summer was warmer (table 1a and figure 2a). Thus, size-dependent survival in winter was associated with warmer conditions in the previous summers, but there was no evidence that hot ECEs explained this variation in survival better than mean summer temperature (ΔAICc = +2.7, electronic supplementary material, table S2, model 1 versus model 16). There was no evidence that survival was associated with annual rainfall (table 1a).
Table 1.
Summary of model selection results investigating survival to the end of winter, assessed at 1 October (i.e. start of spring) for (a) red-winged fairy-wrens and (b) white-browed scrubwrens captured during the autumn and winter months (April–September) between 1977 and 2014. Shown are standardized coefficients and their standard errors (s.e.) for variables included in the top models with ΔAICc less than 2 (or the top 10 models with ΔAICc less than 2), following model selection based on AICc. In addition, the last model in each table shows the top model from the set of models that do not include any ECEs or mean climate effects. Variables identified as important in the top model have 95% CIs of estimated coefficients that do not overlap zero and are shown in italics. Models were ranked by ΔAICc values. n.a. represents variables that were not included in the model. + indicates that a variable was included in model.
| (a) red-winged fairy-wrens | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| model | intercept | catch effort | mean summer temp (carry-over) | mean summer temp2 (carry-over) | mean winter temp (direct) | mean winter temp2 (direct) | total annual rainfall | NDays from capture to Oct1 | age | sex (male) | body size | body size × mean summer temp (carry-over) | body size × mean winter temp (direct) | d.f. | AICc | ΔAICc |
| 1 | −0.207 ± 0.119 | + | 0.069 ± 0.100 | n.a. | n.a. | n.a. | n.a. | n.a. | 0.117 ± 0.074 | n.a. | 0.282 ± 0.076 | 0.211 ± 0.074 | n.a. | 8 | 1219.028 | 0 |
| 2 | −0.216 | + | 0.058 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.314 | 0.212 | n.a. | 7 | 1219.459 | 0.431 |
| 3 | −0.273 | + | 0.067 | n.a. | n.a. | n.a. | n.a. | n.a. | 0.120 | + | 0.245 | 0.212 | n.a. | 9 | 1220.606 | 1.578 |
| 4 | −0.214 | + | 0.072 | n.a. | n.a. | −0.049 | n.a. | n.a. | 0.118 | n.a. | 0.281 | 0.212 | n.a. | 9 | 1220.834 | 1.806 |
| 5 | −0.214 | + | 0.072 | n.a. | −0.048 | n.a. | n.a. | n.a. | 0.118 | n.a. | 0.281 | 0.212 | n.a. | 9 | 1220.841 | 1.813 |
| 6 | −0.206 | + | 0.069 | n.a. | n.a. | n.a. | n.a. | 0.031 | 0.117 | n.a. | 0.285 | 0.211 | n.a. | 9 | 1220.897 | 1.869 |
| 7 | −0.209 | + | 0.082 | n.a. | n.a. | n.a. | −0.024 | n.a. | 0.117 | n.a. | 0.282 | 0.211 | n.a. | 9 | 1221.027 | 1.999 |
| 8 | −0.259 | + | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.117 | n.a. | 0.282 | n.a. | n.a. | 6 | 1223.577 | 4.549 |
| (b) white-browed scrubwrens | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| model | intercept | NHotDryDays (carry-over) | NHotDryDays2 (carry-over) | NColdWetDays (direct) | NColdWetDays2 (direct) | total annual rainfall | NDays from capture to Oct1 | age | sex (male) | body size | body size × NHotDryDays (carry-over) | body size × NColdWetDays (direct) | d.f. | AICc | ΔAICc | |
| 1 | 0.042 ± 0.128 | −0.176 ± 0.126 | n.a. | −1.188 ± 0.496 | 0.970 ± 0.489 | n.a. | −0.152 ± 0.082 | 0.134 ± 0.083 | n.a. | 0.081 ± 0.079 | 0.138 ± 0.079 | n.a. | 9 | 1080.563 | 0.000 | |
| 2 | 0.037 | −0.181 | n.a. | −1.207 | 0.981 | n.a. | −0.162 | 0.150 | n.a. | n.a. | n.a. | n.a. | 7 | 1080.571 | 0.007 | |
| 3 | 0.009 | n.a. | n.a. | −1.063 | 0.893 | n.a. | −0.151 | 0.156 | n.a. | n.a. | n.a. | n.a. | 6 | 1080.672 | 0.109 | |
| 4 | 0.044 | −0.180 | n.a. | −1.199 | 0.997 | n.a. | −0.165 | n.a. | n.a. | 0.113 | 0.133 | n.a. | 8 | 1081.184 | 0.621 | |
| 5 | 0.027 | n.a. | n.a. | −1.091 | 0.887 | −0.149 | −0.165 | 0.157 | n.a. | n.a. | n.a. | n.a. | 7 | 1081.220 | 0.656 | |
| 6 | 0.170 | −0.176 | n.a. | −1.177 | 0.953 | n.a. | −0.149 | 0.126 | + | 0.153 | 0.139 | n.a. | 10 | 1081.544 | 0.981 | |
| 7 | 0.036 | −0.182 | n.a. | −1.205 | 0.974 | n.a. | −0.162 | 0.129 | n.a. | 0.081 | n.a. | n.a. | 8 | 1081.554 | 0.991 | |
| 8 | 0.049 | −0.186 | n.a. | −1.278 | 1.074 | n.a. | −0.167 | 0.132 | n.a. | 0.077 | n.a. | −0.111 | 9 | 1081.588 | 1.025 | |
| 9 | 0.007 | n.a. | n.a. | −1.060 | 0.886 | n.a. | −0.151 | 0.135 | n.a. | 0.081 | n.a. | n.a. | 7 | 1081.638 | 1.074 | |
| 10 | 0.050 | −0.179 | n.a. | −1.240 | 1.039 | n.a. | −0.156 | 0.135 | n.a. | 0.078 | 0.114 | −0.078 | 10 | 1081.714 | 1.151 | |
| 11 | −0.059 | n.a. | n.a. | n.a. | n.a. | n.a. | −0.126 | 0.161 | n.a. | n.a. | n.a. | n.a. | 4 | 1082.006 | 1.443 | |
Figure 2.
The predictions of the effect of body size on survival for (a) red-winged fairy-wrens in winter given for varying carry-over effects of mean temperature of the preceding summer and (b) white-browed scrubwrens in summer given for varying carry-over effects of NColdWetDays (number of wet days less than 5°C) of the preceding winter. Predictions are based on model 1, table 1a and model 1, table 2b, respectively. Body size index is residual body size, calculated from a regression between wing length and abrasion score to account for changes in wing length due to feather abrasion (zero = mean body size) and is given in standard deviations of the mean. A high mean summer temperature/NColdWetDays depicts the mean +1 s.d. whereas a low mean summer temperature/NColdWetDays depicts the mean −1 s.d.
Scrubwrens. In accordance with our predictions, there was strong evidence that white-browed scrubwrens were less likely to survive winters with higher frequencies of cold ECEs (table 1b). However, this effect was nonlinear (table 1b, NColdWetDays2). There was only weak evidence that survival was associated with carry-over effects of the preceding summer (table 1b, model 2 versus model 3). Furthermore, similar to the red-winged fairy-wrens, there was a body size-mediated carry-over effect of the previous summer, with body size having a stronger effect on winter survival if the previous summer had more hot ECEs (NHotDryDays), although this effect did not receive strong support (table 1b model 1 versus model 2; CIs overlap zero). There was no evidence that survival was associated with annual rainfall (table 1b).
(c). Determinants of summer survival
Fairy-wrens. There was no association between summer survival and any climate variable for red-winged fairy-wrens (table 2a). Neither was survival associated with body size, but males and older individuals had higher survival than did females or younger individuals (table 2a).
Table 2.
Factors affecting the survival to the end of summer, assessed at 1 April (i.e. start of autumn) for (a) red-winged fairy-wrens and (b) white-browed scrubwrens captured during the spring and summer months (October–March) between 1977 and 2014. Shown are coefficients and their standard errors (s.e.) for variables included in the 10 top models with ΔAICc less than 2, following model selection based on AICc. In addition, the last model in each table shows the top model from the set of models that do not include any ECE or mean climate effects. Variables identified as important in the best model have 95% CIs of estimated coefficients that do not overlap zero and are shown in italics. Models were ranked by ΔAICc values. n.a. represents variables that were not included in the model. + indicates that a variable was included in model.
| (a) red-winged fairy-wrens | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| model | intercept | catch effort | NHotDryDays (direct) | NHotDryDays2 (direct) | NColdWetDays (carry-over) | NColdWetDays2 (carry-over) | total annual rainfall | NDays from capture to Oct1 | age | sex (male) | body size | body size × NHotDryDays (direct) | body size × NColdWetDays (carry-over) | d.f. | AICc | ΔAICc |
| 1 | −0.319 ± 0.370 | + | n.a. | n.a. | n.a. | n.a. | n.a. | 0.179 ± 0.070 | 0.185 ± 0.082 | 0.570 ± 0.133 | n.a. | n.a. | n.a. | 7 | 1492.398 | 0.000 |
| 2 | −0.301 | + | −0.023 | n.a. | n.a. | n.a. | n.a. | 0.186 | 0.209 | + | −0.058 | 0.159 | n.a. | 10 | 1493.789 | 1.391 |
| 3 | −0.323 | + | n.a. | n.a. | n.a. | n.a. | n.a. | 0.181 | 0.191 | + | −0.040 | n.a. | n.a. | 8 | 1494.223 | 1.825 |
| 4 | −0.309 | + | n.a. | n.a. | n.a. | −0.064 | n.a. | 0.178 | 0.185 | + | n.a. | n.a. | n.a. | 8 | 1494.291 | 1.893 |
| 5 | −0.545 | + | n.a. | n.a. | n.a. | n.a. | n.a. | 0.161 | 0.173 | n.a. | n.a. | n.a. | n.a. | 5 | 1495.621 | 3.223 |
| (b) white-browed scrubwrens | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| model | intercept | NHotDryDays (direct) | NHotDryDays2 (direct) | NColdWetDays (carry-over) | NColdWetDays2 (carry-over) | total annual rainfall | NDays from capture to Oct1 | age | sex (male) | body size | body size × NHotDryDays (direct) | body size × NColdWetDays (carry-over) | d.f. | AICc | ΔAICc | |
| 1 | 0.104 ± 0.156 | n.a. | n.a. | 0.178 ± 0.157 | n.a. | n.a. | n.a. | n.a. | n.a. | 0.240 ± 0.106 | n.a. | −0.296 ± 0.115 | 5 | 618.633 | 0.000 | |
| 2 | 0.112 | n.a. | n.a. | 0.112 | n.a. | −0.182 | n.a. | n.a. | n.a. | 0.239 | n.a. | −0.295 | 6 | 619.306 | 0.673 | |
| 3 | 0.098 | n.a. | n.a. | 0.168 | n.a. | n.a. | n.a. | 0.112 | n.a. | 0.224 | n.a. | −0.295 | 6 | 619.604 | 0.970 | |
| 4 | −0.045 | n.a. | n.a. | 0.190 | n.a. | n.a. | n.a. | n.a. | + | 0.164 | n.a. | −0.299 | 6 | 619.715 | 1.082 | |
| 5 | −0.094 | n.a. | n.a. | 0.182 | n.a. | n.a. | n.a. | 0.141 | + | 0.122 | n.a. | −0.298 | 7 | 620.149 | 1.516 | |
| 6 | −0.049 | n.a. | n.a. | 0.121 | n.a. | −0.194 | n.a. | n.a. | + | 0.157 | n.a. | −0.297 | 7 | 620.206 | 1.572 | |
| 7 | 0.114 | n.a. | n.a. | −0.168 | 0.367 | n.a. | n.a. | n.a. | n.a. | 0.236 | n.a. | −0.307 | 6 | 620.222 | 1.589 | |
| 8 | 0.109 | n.a. | n.a. | 0.179 | n.a. | n.a. | −0.068 | n.a. | n.a. | 0.239 | n.a. | −0.292 | 6 | 620.272 | 1.638 | |
| 9 | 0.107 | n.a. | n.a. | 0.106 | n.a. | −0.175 | n.a. | 0.104 | n.a. | 0.224 | n.a. | −0.294 | 7 | 620.425 | 1.792 | |
| 10 | 0.105 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.262 | n.a. | n.a. | 3 | 622.787 | 4.154 | |
Scrubwrens. In white-browed scrubwrens, summer survival was not associated with the severity of summer ECEs. However, survival was associated with body size and this effect of body size on survival was mediated by conditions in the preceding winter: larger individuals survived better following mild winters (low number of NColdWetDays), whereas smaller individuals survived better following cold winters (high number of NColdWetDays; table 2b and figure 2b), although the advantage for small size under extreme cold is minimal (figure 2b).
The results from the top models in tables 1 and 2 are summarized in table 3.
Table 3.
Summary of the nature and direction (+ positive,−negative) of direct and carry-over effects and body size on the survival of red-winged fairy-wrens and white-browed scrubwrens in summer (for birds captured during the spring and summer months, October–March) and winter (for birds captured during the autumn and winter months, April–September), and between October and March between 1977 and 2014. n.a. represents variables that were not included in the final top model;+represents significant positive effect.
| species | direct effects | carry-over effects | body size |
|---|---|---|---|
| winter | |||
| red-winged fairy-wrens | n.a. | positive interaction between body size × MeanSummerTemp | + |
| white-browed scrubwrens | negative effect of NColdWetDays | n.a. | n.a. |
| summer | |||
| red-winged fairy-wrens | n.a. | n.a. | n.a. |
| white-browed scrubwrens | n.a. | negative interaction between body size × NColdWetDays | + |
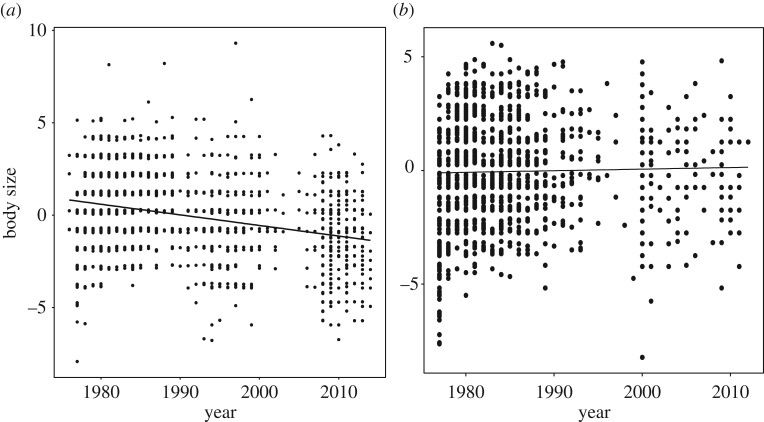
(d). Temporal trends in body size
Structural body size (wing length) decreased by 0.029 mm per year over the 37 years of the study in red-winged fairy-wrens, but there was no change in body size of white-browed scrubwrens (table 4 and figure 3). The analysis of body size in the fairy-wrens also showed that size increased with age, and that there was selective disappearance of smaller individuals (i.e. larger individuals had an older age of last capture; table 4a).
Table 4.
Changes in structural body size (wing length) between 1977 and 2014 for (a) red-winged fairy-wrens and (b) white-browed scrubwrens. Shown are coefficients and their standard errors (s.e.) for variables included in the top models with ΔAICc less than 2, following model selection based on AICc. Variables identified as important in the best model have 95% CIs of estimated coefficients that do not overlap zero and are shown in italics. Models were ranked by ΔAICc values. n.a. represents variables that were not included in the model.
| model | intercept | abrasion score | age at last capture | age | year | sex (male) | years×sex | d.f. | AICc | ΔAICc |
|---|---|---|---|---|---|---|---|---|---|---|
| (a) red-winged fairy-wrens | ||||||||||
| 1 | 49.615 ± 0.065 | n.a. | 0.046 ± 0.020 | 0.179 ± 0.017 | −0.024 ± 0.004 | 2.036 ± 0.085 | 0.010 ± 0.006 | 9 | 10653.865 | 0.000 |
| 2 | 49.630 | n.a. | 0.047 | 0.177 | −0.030 | + | n.a. | 8 | 10654.583 | 0.718 |
| (b) white-browed scrubwrens | ||||||||||
| 1 | 52.019 ± 0.110 | −0.062 ± 0.019 | n.a. | 0.269 ± 0.025 | n.a. | 2.513 ± 0.139 | n.a. | 7 | 3224.929 | 0 |
Figure 3.
Temporal change in body size between 1977 and 2014 of (a) red-winged fairy-wrens and (b) white-browed scrubwrens. Body size is residual body size, calculated from a regression between wing length and abrasion score to account for changes in wing length due to feather abrasion.
4. Discussion
Our aim in this study was to explore the relationship between climate and survival and the long-term impacts on morphology in two coexisting species of small insectivorous passerine, using data from two study populations that have been monitored for 37 years in southwestern Australia. Associations between climate and survival (recapture) were complex and varied between the species, but survival was more strongly associated with temperature extremes than mean temperatures, except in one case. Survival was also associated with body size in both species, and with inter-annual variation in the severity of winter conditions in white-browed scrubwrens. There was also evidence that size-dependent mortality was mediated by carry-over effects of climate conditions in the previous season: in both species, larger individuals survived winter better following hotter summers, and larger scrubwrens had higher summer survival following mild winters (figure 2). In accordance with predictions that warming winters should result in a reduction in body size, we found that body size of red-winged fairy-wrens declined over 37 years. However, this reduction in mean body size cannot be explained by size-dependent mortality, which showed positive effects of body size on winter survival and hence should lead to an increase in mean body size through either within- or between-generation changes. Our study therefore demonstrates how climate can have complex effects on fitness (survival), but no apparent effect on morphology across years.
5. Direct effects of climate on survival
Cold, wet conditions in winter were directly associated with declines in winter survival in white-browed scrubwrens; there was also evidence for size-mediated carry-over effects of winter conditions on their survival in the subsequent summer (see below). Cold exposure can cause mortality of small passerines in winter, even in conditions that are relatively mild [17,22,44]. For example, prolonged exposure to cold wet weather with daily minima less than 5°C was associated with high mortality in a range of similar-sized passerine species in Britain [23]. Although such conditions are relatively mild, they have been shown to cause thermal stress in small passerines, increasing the metabolic costs of keeping warm at a time when food is limited [22,23,29]. In the case of species at our study area, temperatures rarely dropped below zero (2 days in 37 years) so temperatures less than 5°C were the most severe experienced, and can be considered to represent ECEs. Unexpectedly, in white-browed scrubwrens, the effect of winter conditions on survival was nonlinear, with survival increasing in years with the harshest conditions, when cold wet days exceeded 20 days per year (NColdWetDays2; table 1b). However, the positive quadratic term was driven by a single outlier year (1986) and disappeared when data from 1986 were removed from analysis; all other effects remained the same. The year 1986 was the one with the highest number of cold, wet days (a total of 26; the next highest, 1989, was 19), but had 50% average survival rates of scrubwrens (n = 12): the results therefore indicate that across all other years, survival dropped with increasing numbers of cold, wet days, but for reasons unknown, not in 1986.
In contrast with that of scrubwrens, winter survival of red-winged fairy-wrens was not associated with severity of winter conditions. This might conceivably relate to differences between the species in roosting behaviour and use of microclimates that can buffer individuals from extreme conditions [45,46]. Like other fairy-wrens, red-winged fairy-wrens are thought to roost communally, huddling together at night, which presumably conserves heat in cold conditions, while scrubwrens apparently roost individually and presumably have higher thermoregulatory costs in cold conditions [47].
In contrast with our prediction, hot ECEs were not associated with lower survival in either species, suggesting that the climate is not hot enough to drive reductions in survival. In the few studies demonstrating fitness costs of high temperatures, negative effects on body condition and survival are associated with air temperatures more than or equal to 35°C [3–5,7]. Studies of physiology show that for many passerines, the onset of panting, which facilitates evaporative cooling, occurs at slightly lower temperatures, at about 30°C, but only when air temperatures approach body temperature (approx. 40°C) do the costs of evaporative cooling increase dramatically, and body temperatures of approximately 45°C or higher are lethal [14,15,48]. At Smithbrook, although maximum temperatures have risen over the study period, there were few days where temperatures exceeded 35°C: only 190 days over 37 years. This suggests that the increase in the annual frequencies of days more than 30°C (figure 1d) that presumably increase energetic costs associated with heat dissipation behaviours has no measureable effects on survival at these levels. However, as temperatures continue to rise and the costs of heat dissipation behaviours increase, fitness consequences may change.
6. Carry-over effects of climate on survival
Both winter and summer survival were associated with size-mediated carry-over effects of the preceding season. In white-browed scrubwrens, larger individuals survived better in summers that followed mild winters (low NColdWetDays) (figure 2b). Mild winter conditions were directly associated with higher scrubwren survival in the same winter (above). We speculate that perhaps higher survival in mild winters leads to greater competition among individuals for resources in the following summer, conditions that might give larger individuals a competitive advantage. Such density-dependent phenomena are common and associated with climatic variation [49], although as we do not have direct measures of population density in these study populations for all years, we are unable to explicitly test for density-dependence here. More work is required to understand carry-over effects of climate between seasons and the consequences for selection on body size.
In red-winged fairy-wrens, larger individuals survived winter better than smaller ones, especially after summers with higher mean temperatures (table 2a and figure 2a). Performance in winter can be enhanced by pre-winter conditions and organisms in better condition heading into winter can have higher winter survival [17]. For example, in yellow-bellied marmots (Marmota flaviventris), earlier emergence from hibernation in response to warming spring temperatures led to increased growth and improved body condition before the start of the following winter, and consequently increased overwinter survival [50]. Surprisingly, the positive effect of warmer summers was only evident for larger individuals; smaller individuals had higher winter survival following cooler summers (figure 2a). We would expect all fairy-wrens to benefit from warmer (mean) summer temperatures at Smithbrook, given the absence of summer ECEs, not just larger individuals. We have no explanation for this patterns that suggests complex interactions between climate, body size and survival [21,50,51].
7. Temporal trends in body size
We have demonstrated positive viability selection on body size in both species, involving both direct effects within a season, as well as carry-over effects between seasons, and these relate to ECEs and to mean temperatures. The evidence that body size interacts with carry-over effects of climate in the previous season suggests that selection on body size is stronger under recent climatic conditions (figure 3a,b). Despite the observed patterns of survival, we found no evidence for an evolutionary response to this selection in the form of increased body size over time. In fact, the mean body size of fairy-wrens declined over time and there was no change in the mean size of scrubwrens.
The temporal decline in fairy-wren body size is consistent with predictions arising from Bergmann's Rule for pervasive reductions in body size as the climate warms [11]. However, such reductions are not related to a survival advantage for smaller individuals, and indeed we showed that there was selective disappearance of smaller individuals (i.e. larger individuals were older at last capture, and older birds are larger (wing length) (table 1b). Accordingly, our results are inconsistent with the thermoregulatory explanations we outline here. This may be due to a variety of factors. For example, phenotypic plasticity may underlie shifts in body size that are associated with density-dependent phenomena or changes in other ecological conditions, such as predation pressure and habitat degradation [52,53]. In addition, selection may act via reproduction in addition to viability, although it is worth noting that selection on body size via reproduction is typically positive (e.g. [54]).
8. Conclusion
Our study demonstrates that responses to climate are complex and can vary between ecologically similar species coexisting at the same site and experiencing the same weather conditions. We found evidence for changes in climate, and for associations between survival and climate and between survival and body size, but these factors did not combine to generate population-level changes in morphology over multiple years. Changes in survival were associated with temperature extremes more strongly than with mean temperatures. Exposure to these extremes of climate had direct effects on survival, within the same season, in one case, but also had carry-over effects of body size on survival in the following season. The results illustrate the value of considering the effects of changes in climate across the year on wild animal populations, rather than considering just a single season. Finally, the differences in the responses to ECEs we observed between two closely related, similar-sized insectivores experiencing the same weather conditions highlight the potential diversity in effects of climate change on natural populations, and hence the difficulties inherent in drawing general conclusions about avian responses to extreme climatic events.
Supplementary Material
Supplementary Material
Acknowledgements
The Western Australian Department of Parks and Wildlife (DPAW) gave permission for fieldwork. We are grateful to numerous people for assistance in the field, and acknowledge the contribution made by Dick and Molly Brown who set up the study in 1974. We thank Adrian Wayne and other staff of the DPAW Science division in Manjimup, John Angus and Karen and Michael Keely for logistical support and hospitality. Finally, we thank Loeske Kruuk and Martijn van de Pol for comments on the draft manuscript and Loeske Kruuk for advice on analyses.
Data accessibility
Data available from the Dryad Digital Repository: Data from: Effects of extreme weather on two sympatric Australian passerine bird species. Provisional DOI: doi:10.5061/dryad.cv853. Data files: Gardner et al.data.PhilTransB.
Authors' contributions
J.L.G. conceived the study and L.B., E.R., P.d.R. and A.d.R. collected the data. J.L.G. and L.B. designed the analysis, and J.L.G. analysed the data. J.L.G. wrote the manuscript and L.B. assisted with interpretation of results and all authors contributed manuscript feedback and approved the final manuscript.
Competing interests
We have no competing interests.
Funding
The compilation of datasets was partly undertaken at Monash University while J.L.G. was supported by an Australian Research Council grant (DP120102651). L.B. was supported by a Rubicon Fellowship of the Netherlands Organisation for Scientific Research (NWO825.08.003) and a Fellowship from the Australian Research Council (DE130100174), and J.L.G. was supported by Loeske Kruuk and an Australian Research Council Future Fellowship (FT150100139).
References
- 1.Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO. 2000. Climate extremes: observations, modeling, and impacts. Science 289, 2068–2074. ( 10.1126/science.289.5487.2068) [DOI] [PubMed] [Google Scholar]
- 2.McKechnie AE, Hockey PAR, Wolf BO. 2012. Feeling the heat: Australian landbirds and climate change. EMU 112, i–vii. ( 10.1071/MUv112n2_ED) [DOI] [Google Scholar]
- 3.du Plessis KL, Martin RO, Hockey PAR, Cunningham SJ, Ridley AR. 2012. The costs of keeping cool in a warming world: implications of high temperatures for foraging, thermoregulation and body condition of an arid-zone bird. Glob. Change Biol. 18, 3063–3070. ( 10.1111/j.1365-2486.2012.02778.x) [DOI] [PubMed] [Google Scholar]
- 4.Cunningham SJ, Martin RO, Hojem CL, Hockey PAR. 2013. Temperatures in excess of critical thresholds threaten nestling growth and survival in a rapidly-warming arid savanna: a study of common fiscals. PLoS ONE 8, e74613 ( 10.1371/journal.pone.0074613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kruuk LEB, Osmond HL, Cockburn A. 2015. Contrasting effects of climate on juvenile body size in a Southern Hemisphere passerine bird. Glob. Change Biol. 21, 2929–2941. ( 10.1111/gcb.12926) [DOI] [PubMed] [Google Scholar]
- 6.Edwards EK, Mitchell NJ, Ridley AR. 2015. The impact of high temperatures on foraging behaviour and body condition in the Western Australian Magpie Cracticus tibicen dorsalis. Ostrich J. Afr. Ornithol. 86, 137–144. ( 10.2989/00306525.2015.1034219) [DOI] [Google Scholar]
- 7.Gardner JL, Amano T, Sutherland WJ, Clayton M, Peters A. 2016. Individual and demographic consequences of reduced body condition following repeated exposure to high temperatures. Ecology 97, 786–795. ( 10.1890/15-0642.1) [DOI] [PubMed] [Google Scholar]
- 8.Scholander PF, Hock R, Walters V, Johnson F, Irving L. 1950. Heat regulation in some arctic and tropical mammals and birds. Biol. Bull. 99, 237–258. ( 10.2307/1538741) [DOI] [PubMed] [Google Scholar]
- 9.Brown JH, Lee AK. 1969. Bergmann's rule and climatic adaptation in woodrats (Neotoma). Evolution 23, 329–338. ( 10.2307/2406795) [DOI] [PubMed] [Google Scholar]
- 10.James FC. 1970. Geographic size variation in birds and its relationship to climate. Ecology 51, 365–390. ( 10.2307/1935374) [DOI] [Google Scholar]
- 11.Gardner JL, Peters A, Kearney MR, Joseph L, Heinsohn R. 2011. Declining body size: a third universal response to warming? Trends Ecol. Evol. 26, 285–291. ( 10.1016/j.tree.2011.03.005) [DOI] [PubMed] [Google Scholar]
- 12.Teplitsky C, Millien V. 2014. Climate warming and Bergmann's rule through time: is there any evidence? Evol. Appl. 7, 156–168. ( 10.1111/eva.12129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergmann C. 1847. Über die verhältnisse der wärmeökonomie der thiere zu ihrer grösse. Göttinger Stud. 3, 595–708. [Google Scholar]
- 14.McKechnie AE, Wolf BO. 2010. Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol. Lett. 6, 253–256. ( 10.1098/rsbl.2009.0702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyles JG, Seebacher F, Smit B, McKechnie AE. 2011. Adaptive thermoregulation in endotherms may alter responses to climate change. Integr. Comp. Biol. 51, 676–690. ( 10.1093/icb/icr053) [DOI] [PubMed] [Google Scholar]
- 16.Ummenhofer CC, Meehl GA. 2017. Extreme weather and climate events with ecological relevance: a review. Phil. Trans. R. Soc. B 372, 20160135 ( 10.1098/rstb.2016.0135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams CM, Henry HAL, Sinclair BJ. 2015. Cold truths: how winter drives responses of terrestrial organisms to climate change. Biol. Rev. 90, 214–235. ( 10.1111/brv.12105) [DOI] [PubMed] [Google Scholar]
- 18.Harrison XA, Blount JD, Inger R, Norris DR, Bearhop S. 2011. Carry-over effects as drivers of fitness differences in animals. J. Anim. Ecol. 80, 4–18. ( 10.1111/j.1365-2656.2010.01740.x) [DOI] [PubMed] [Google Scholar]
- 19.Sgueo C, Wells ME, Russell DE, Schaeffer PJ. 2012. Acclimatization of seasonal energetics in northern cardinals (Cardinalis cardinalis) through plasticity of metabolic rates and ceilings. J. Exp. Biol. 215, 2418–2424. ( 10.1242/jeb.061168) [DOI] [PubMed] [Google Scholar]
- 20.Cushman JH, Lawton JH, Manly FJ. 1993. Latitudinal patterns in European ant assemblages: variation in species richness and body size. Oecologia 95, 30–37. ( 10.1007/BF00649503) [DOI] [PubMed] [Google Scholar]
- 21.Ozgul A, Tuljapurkar S, Benton TG, Pemberton JM, Clutton-Brock TH, Coulson T. 2009. The dynamics of phenotypic change and the shrinking sheep of St. Kilda. Science 325, 464–467. ( 10.1126/science.1173668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown CR, Brown MB. 1998. Intense natural selection on body size and wing and tail symmetry in cliff swallows during severe weather. Evolution 52, 1461–1475. ( 10.2307/2411315) [DOI] [PubMed] [Google Scholar]
- 23.Robinson RA, Baillie SR, Crick HQP. 2007. Weather-dependent survival: implications of climate change for passerine population processes. Ibis 149, 357–364. ( 10.1111/j.1474-919X.2006.00648.x) [DOI] [Google Scholar]
- 24.Bailey LD, van de Pol M. 2016. Tackling extremes: challenges for ecological and evolutionary research on extreme climatic events. J. Anim. Ecol. 85, 85–96. ( 10.1111/1365-2656.12451) [DOI] [PubMed] [Google Scholar]
- 25.Rowley I, Russell E, Brown R, Brown M. 1988. The ecology and breeding biology of the red-winged fairy-wren Malurus elegans. Emu 88, 161–176. ( 10.1071/MU9880161) [DOI] [Google Scholar]
- 26.Gardner JL, Trueman JWH, Ebert D, Joseph L, Magrath RD. 2010. Phylogeny and evolution of the Meliphagoidea, the largest radiation of Australasian songbirds. Mol. Phylogenet. Evol. 55, 1087–1102. ( 10.1016/j.ympev.2010.02.005) [DOI] [PubMed] [Google Scholar]
- 27.Ambrose SJ, Davies SJJF. 1989. The social organisation of the White-browed Scrubwren Sericonis frontalis Gould. Acanthizidae) in arid, semi-arid and mesic environments in Western Australia. Emu 89, 40–46. ( 10.1071/MU9890040) [DOI] [Google Scholar]
- 28.Brouwer L, van de Pol M, Atema E, Cockburn A. 2011. Strategic promiscuity helps avoid inbreeding at multiple levels in a cooperative breeder where both sexes are philopatric. Mol. Ecol. 20, 4796–4807. ( 10.1111/j.1365-294X.2011.05325.x) [DOI] [PubMed] [Google Scholar]
- 29.Lejeune L, van de Pol M, Cockburn A, Louter M, Brouwer L. 2016. Male and female helper effects on maternal investment and adult survival in red-winged fairy-wrens. Behav. Ecol. 27, 1841–1850. ( 10.1093/beheco/arw121) [DOI] [Google Scholar]
- 30.Russell E, Rowley I. 2000. Demography and social organisation of the red-winged fairy-wren, Malurus elegans. Aust. J. Zool. 48, 161–200. ( 10.1071/ZO99066) [DOI] [Google Scholar]
- 31.Brown RJ, Brown MN, Russell EM. 1990. Survival of four species of passerine in karri forests in southwestern Australia. Corella 14, 69–78. [Google Scholar]
- 32.Gosler AG, Greenwood JJD, Baker JK, Davidson NC. 1998. The field determination of body size and condition in passerines: a report to the British Ringing Committee. Bird Study 45, 92–103. ( 10.1080/00063659809461082) [DOI] [Google Scholar]
- 33.Symonds MRE, Tattersall GJ. 2010. Geographical variation in bill size across bird species provides evidence for Allen's rule. Am. Nat. 176, 188–197. ( 10.1086/653666) [DOI] [PubMed] [Google Scholar]
- 34.Tattersall GJ, Arnaout B, Symonds MRE. 2016. The evolution of the avian bill as a thermoregulatory organ. Biol. Rev. ( 10.1111/brv.12299) [DOI] [PubMed] [Google Scholar]
- 35.Gardner JL, Amano T, Mackey BG, Sutherland WJ, Clayton M, Peters A. 2014. Dynamic size responses to climate change: prevailing effects of rising temperature drive long-term body size increases in a semi-arid passerine. Glob. Change Biol. 20, 2062–2075. ( 10.1111/gcb.12507) [DOI] [PubMed] [Google Scholar]
- 36.van de Pol M, Verhulst S. 2006. Age-dependent traits: a new statistical model to separate within-and between-individual effects. Am. Nat. 167, 766–773. ( 10.1086/503331) [DOI] [PubMed] [Google Scholar]
- 37.Arnold TW. 2010. Uninformative parameters and model selection using Akaike's information criterion. J. Wildl. Manage. 74, 1175–1178. ( 10.1111/j.1937-2817.2010.tb01236.x) [DOI] [Google Scholar]
- 38.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information theoretic approach, 2nd edn Berlin, Germany: Springer. [Google Scholar]
- 39.Galipaud M, Gillingham MAF, David M, Dechaume-Moncharmont FX. 2014. Ecologists overestimate the importance of predictor variables in model averaging: a plea for cautious interpretations. Methods Ecol. Evol. 5, 983–991. ( 10.1111/2041-210X.12251) [DOI] [Google Scholar]
- 40.R Development Core Team. 2012. R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria). See http://www.R-project.org/.
- 41.Bates D, Maechler M, Bolker B. 2012. lme4: Linear mixed-effects models using S4 classes R package. See http://cran.project.org/web/packages/lme4/index.html.
- 42.Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th edn New York, NY: Springer. [Google Scholar]
- 43.Bartoń K. 2016. MuMIn: multi-model inference. R package version 1.
- 44.Deville A, Labaude S, Jean-Patrice Robin J, Béchet A, Gauthier-Clerc M, Porter W, Fitzpatrick M, Paul Mathewson P, Grémillet D. 2014. Impacts of extreme climatic events on the energetics of long-lived vertebrates: the case of the greater flamingo facing cold spells in the Camargue. J. Exp. Biol. 217, 3700–3707. ( 10.1242/jeb.106344) [DOI] [PubMed] [Google Scholar]
- 45.Suggitt AJ, Gillingham PK, Hill JK, Huntley B, Kunin WE, Roy DB, Thomas CD. 2011. Habitat microclimates drive fine-scale variation in extreme temperatures. Oikos 120, 1–8. ( 10.1111/j.1600-0706.2010.18270.x) [DOI] [Google Scholar]
- 46.Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology, and adaptation. Phil. Trans. R. Soc. B 367, 1665–1679. ( 10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higgins PJ, Peter JM. 2002. Handbook of Australian, New Zealand and Antarctic Birds. vol. 6: Pardalotes to Shrike-thrushes. Melbourne, Australia: Oxford University Press. [Google Scholar]
- 48.Smit B, Zietsman G, Martin RO, Cunningham SJ, McKechnie AE, Hockey PAR. 2016. Behavioural responses to heat in desert birds: implications for predicting vulnerability to climate warming. Clim. Change Resp. 3, 9 ( 10.1186/s40665-016-0023-2) [DOI] [Google Scholar]
- 49.Sæther BE, et al. 2016. Demographic routes to variability and regulation in bird populations. Nat. Commun. 7, 12001 ( 10.1038/ncomms12001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson LE, Tuljapurkar S, Coulson T. 2010. Coupled dynamics of body mass and population growth in response to environmental change. Nature 466, 482–485. ( 10.1038/nature09210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teplitsky C, Mills JA, Alho J, Yarrall JW, Merilä J. 2008. Bergmann's Rule and climate change revisited: disentangling environmental and genetic responses in a wild bird population. Proc. Natl Acad. Sci. USA 105, 13 492–13 496. ( 10.1073/pnas.0800999105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charlesworth B. 1971. Selection in density-regulated populations. Ecology 52, 469 ( 10.2307/1937629) [DOI] [Google Scholar]
- 53.Selwood KE, McGeoch MA, Mac Nally R. 2014. The effects of climate change and land-use change on demographic rates and population viability. Biol. Rev. 90, 837–853. ( 10.1111/brv.12136) [DOI] [PubMed] [Google Scholar]
- 54.Bonnet T, Wandeler P, Camenisch G, Postma E. In press Bigger is fitter? Quantitative genetic decomposition of selection reveals an adaptive evolutionary decline of body mass in a wild rodent population. PLoS ONE 15, e1002592 ( 10.1371/journal.pbio.1002592) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: Data from: Effects of extreme weather on two sympatric Australian passerine bird species. Provisional DOI: doi:10.5061/dryad.cv853. Data files: Gardner et al.data.PhilTransB.