Abstract
Global climate warming results in an increase in mean temperatures and in the frequency of extreme climatic events (ECEs), which could both strongly impact ecosystems and populations. Most studies assessing the impact of global warming on ecosystems have focused on warming trends while neglecting ECEs. In particular, the effects of multiple ECEs on fitness, and their consequences for selection, are still missing. Here we explored the effects of daily extreme rainfalls, as well as the occurrence of extremely hot and cold days, on clutch size and laying date in a wild blue tit population (Cyanistes caeruleus) monitored over 25 years. During the nestling phase (8–15 days old), the number of fledglings in a brood was negatively correlated with extremely hot days. The presence of extremely hot days between days 8 and 15 was also associated with an increase in the strength of selection acting on laying date, independently of mean temperature trends during the same period: when 10% of broods in the population experienced this type of ECE, selection for earlier breeding increased by 39%. Our results represent a unique quantification of the impact of multiple ECEs on the fitness landscape and emphasize their role as climatic drivers of selection.
This article is part of the themed issue ‘Behavioural, ecological and evolutionary responses to extreme climatic events’.
Keywords: natural selection, Cyanistes caeruleus, extreme climatic events, laying date, long-term dataset, blue tit
1. Introduction
Global climate change results in an increase in mean temperatures over global land and sea areas and generates new environmental conditions for wild populations [1]. The influence of this warming trend on many taxa, such as plants [2], birds [3], insects [4] and mammals [5], is now a primary research area. Along with a rise in mean temperatures, climate change is also characterized by an increase in the frequency of extreme climatic events (ECEs) such as floods, hurricanes and droughts [6,7]. However, most studies interested in ecosystem responses to changes in climate in an evolutionary ecology context have focused on mean climatic trends, generally ignoring the potential effects of ECEs [8–10]. Recently, studies exploring the impact of ECEs on wild populations have shown striking effects on animals [11] and plants [12,13]. For instance, droughts significantly affect above-ground biomass [14] and phenology [15] of several plant species in Europe. In animals, ECEs were shown to impact the reproductive success and survival of many species [11,16]. In particular, partial or complete reproductive failure was observed in bird populations after ECEs such as the 1982–1983 El Nino Southern Oscillation Event [17], a significant cooling in 1991 in the Arctic [18] or flooding events [19]. Although less explored, ECEs occurring in the non-breeding season, such as harsh winters, have been shown to reduce adult survival in birds [20] and mammals [21].
Beyond such quantitative assessment of the impact of ECEs on organisms’ survival and reproductive output, there is now a necessity to understand how wild populations will adapt to this new threat. Theory predicts that populations could adapt to perturbations such as rapid climate change via phenotypic plasticity and evolutionary change. Evidence for plastic changes in response to climate warming in wild populations has been often reported in taxa such as plants [22], birds [23] and mammals [5]. Evidence for evolutionary responses to rapid warming remains relatively rare [24]; less than 20 studies have shown genetic changes in adaptive traits such as morphology, phenology or dispersal (see [25] for a review). However, evidence for plastic or genetic responses to ECEs is still missing [11,16], largely because of lack of investigation. To our knowledge, the evolutionary response of beak size in Darwin finches (Geospiza sp.) after a severe El Nino event [26] remains the only example of an adaptive response to an ECE.
An evolutionary response to an ECE will occur only if the event causes a selective impact on heritable traits in the population. A selective impact refers to a fitness differential (e.g. reproductive success, survival) caused by a difference in phenotypes. In other words, an ECE should discriminate phenotypes based on their fitness consequences in order to affect the fitness landscape and thereby result in selection. A few studies have shown a selective impact of ECEs in wild populations (see table 1). For example, Brown & Brown [30] showed that cold weather during late spring in 1996 reduced a population of cliff swallows (Petrochelidon pyrrhonota) by about 53%, while resulting in stronger selection for larger body sizes. Previous studies exploring the selective pressure imposed by a single ECE could, however, seldom quantify the selective impact of the number and/or the frequency of ECEs experienced by populations (table 1), nor disentangle them from the effect of mean weather conditions (but see [19]). To clearly identify the selective impact of ECEs, studies exploring multiple ECEs and comparing them with mean climatic variables are currently needed [16].
Table 1.
Studies assessing the impact of ECEs on the selection pressures acting on key adaptive traits. In all these studies, ECEs reinforced natural selection.
| species | traits | extreme event | references |
|---|---|---|---|
| Fulmarus glacialoides | individual qualitya | extreme sea ice conditions | [27] |
| Geospiza fortis | beak size | el Niño event | [26] |
| Haematopus ostralegus | nest elevation | flooding | [19] |
| Hirundo rustica | arrival date | extremely cold spring | [28] |
| Passer domesticus | body size | extreme storm | [29] |
| Petrochelidon pyrrhonota | body size | cold weather | [30] |
| Riparia riparia | body size | severe drought | [31] |
aIndividual quality refers here to the acquisition and allocation of resources.
Here we investigated the impact of ECEs in a wild population of blue tits (Cyanistes caeruleus). More specifically, we assessed the effects of extreme daily temperatures and rainfall during the breeding season, on fitness and selective pressures acting on two fundamental life-history traits: laying date (date of first egg laid) and clutch size (number of eggs laid). The blue tit is an insectivorous passerine living in temperate forests of Europe and Western Asia, breeding from March to June in southern France [32]. Both laying date and clutch size are typically under directional selection in blue tits, where early breeding birds laying large clutches are favoured [33,34]. Parental care for young in the nest lasts about 21 days and is crucial for the fledgling success of the brood, which is strongly determined by nestlings’ body mass. During the rearing period, nestlings are almost exclusively fed with leaf-eating caterpillars, which are available for only two to three weeks [35]. Ideally, nestlings should be 9–11 days old at the peak of caterpillar abundance, with a mistiming between food demand and abundance resulting in high energetic costs and low reproductive success [36]. Since recent climate warming has resulted in a shift towards earlier caterpillar phenology [9,37], tit species, which rely on caterpillars to feed their nestlings, have been extensively studied in their responses to climate change [38,39]. Weather conditions such as temperature and rainfall have been shown to cause complex effects on the growth of blue tit nestlings [40], independently of caterpillar phenology. For example, warm temperatures during spring were shown to both increase [41] and decrease [40] the growth rate of blue tit nestlings. Similarly, heavy rainfalls were positively related to growth rate in blue tit nestlings [40]; yet in great tits (Parus major), parents significantly reduced their feeding rate during rainfalls [42], causing a 10–20% reduction in nestling growth rate [43]. An important limit of these studies is that the impact of ambient weather conditions during the nestling period has only been explored within the average range of climatic values, without considering ECEs.
The objective of this study was twofold. First, we explored the impact of extreme daily rainfalls, extremely cold and extremely warm temperatures across nestling growth stages. Second, we identified the ECEs and the nestling stages influencing fledgling success, and we tested the consequences of ECEs on the directional selection acting on laying date and clutch size. In doing so, we also included daily mean temperatures and rainfalls for each nestling stage within these analyses, in order to compare their impacts to ECEs.
2. Material and methods
(a). Study area and fieldwork
Our analyses were based on data from a long-term study of blue tits in the forest of La Rouvière, near the city of Montpellier (43°40′ N, 03°40′ E), southern France. Since 1991, nest boxes have been routinely monitored from the onset of nest construction until all nestlings have fledged (see [32] for further details), which is when chicks are 19–21 days old [44]. For each brood, laying date (date of the first egg laid, 1 March = 1) and clutch size (number of eggs laid) are recorded. Parents are captured in nest boxes when chicks are 9 days or older, and are uniquely marked with metal rings (provided by C.R.B.P.O, France). Nestlings are ringed when 9–15 days old.
(b). Climatic data
Daily temperatures and rainfalls were obtained from a weather station (43°44′30″ N, 3°35′40″ E) located approximately 9 km from the breeding site. Daily mean temperatures were estimated as (daily minimum temperatures + daily maximum temperatures)/2. Anomalies in temperature were estimated as the difference between daily temperatures and monthly temperatures over the 1991–2015 period.
(c). Extreme climatic event variables
We defined an ECE as a 5% or less probability of event occurrence across the study period (1991–2015), regardless of the calendar month when the event occurred. Commonly used by climatologists [11,16], this threshold facilitates the comparison of ECE responses among study systems [16]. Moreover, this 5% probability was the smallest possible threshold guaranteeing at least one ECE for each climatic variable explored. A 1% probability resulted in at least one ECE variable having no occurrence over the study period, thereby preventing us from using such an extreme threshold. This analysis could have been done differently using a ‘biological’ definition (i.e. when ECEs are defined based on their effect on populations), as, for example, a threshold based on the decrease in the population growth rate following an ECE. However, because our aim was to compare the extreme (i.e. rare) versus the mean climate selective impact, we used a ‘statistical’ definition. Note that the definition of ECEs is still actively debated in the literature, with some authors using a statistical [45,46] or a biological [12,47] threshold.
We explored the presence/absence of three types of ECEs: (i) an extremely cold day (corresponding to a daily anomaly in mean temperature of −5.31°C or less, abbreviated COLD T°C), (ii) an extremely hot day (corresponding to a daily anomaly in mean temperature of 4.98°C or more, abbreviated HOT T°C), and (iii) a daily extreme rainfall (corresponding to a rainfall exceeding 15 mm, abbreviated Ext RAIN). The presence/absence of these three ECEs was explored across three nestling periods: (i) from the hatching date to the point when the chicks are 7 days old (abbreviated HAT for hatchlings), chicks becoming self-thermoregulated at 7 days [44]; (ii) from day 8 to day 15 after hatching (abbreviated NES for nestlings), corresponding to the peak of food requirement for nestlings [48]; and (iii) from day 16 to day 21, corresponding to the pre-fledgling period (abbreviated FLE for fledglings). Broods are not visited after day 15 but nestling survival until fledging is estimated based on observations of nest content at day 22 or later. Overall, we thus explored the impact of the presence/absence of nine different ECE types (three daily ECEs in three chick periods), all coded as binomial variables.
Because we wanted to compare ECE effects with mean-weather effects, we also used two mean climatic variables within each nestling stage. We estimated the mean temperature (abbreviated MEAN T°C) and rainfall (abbreviated MEAN RAIN) during each chick stage, hence a total of six mean climatic variables explored. The statistical analyses (below) accommodate for collinearity between some of the climatic variables.
(d). Statistical analyses
All statistical analyses were carried out using the software R (v. 3.1.1) [49]. Analyses were conducted on female individuals that were not subject to any experimental manipulation, with a dataset of 1389 breeding observations on 762 female blue tits breeding between 1991 and 2015. Out of 1869 first broods over the course of the study (including experimental broods), only 29 (1.6%) were followed by a second brood, which is why we considered first broods only in our analyses.
(i). Brood-centred extreme climatic events and number of fledglings
Our aim here was to assess the impact of our 15 climatic variables (nine ECEs + six mean climatic variables) on the number of fledglings per brood, hence our best annual estimation of reproductive success. We used a linear mixed model relating the number of fledglings to the 15 climatic predictors, including female identity and year as random effects. Because we have no a priori hypothesis regarding which variable should affect the number of fledglings, we used a model-averaging approach [50,51]. This approach allows one to test multiple hypotheses in the same analysis using the AIC (Akaike Information Criterion, [52]). The method is based on three steps, which are (i) the generation of all possible sub-models from the set of predictors of interest (our 15 climatic variables, leading to 32 768 models generated); (ii) the selection of the best models given their AIC (here we selected the 95% confidence set of models, leading to 1912 models selected); and (iii) the averaging of estimates of predictors among all selected models weighted by the Akaike weight of each model (see [50] for further details on this approach). Subsequently, we calculated the relative importance of each predictor by summing the Akaike weights from each model in which the specific variable appeared. This relative importance can be interpreted as the probability that the variable of interest is a component of the best model [51]. We considered that a variable significantly affected the number of fledglings if its relative importance was above 80%. The model-averaging analysis and mixed-model analysis were conducted using the MuMIn [53] and the lme4 [54] packages, respectively, in R.
(ii). Population-centred extreme climatic events and the force of natural selection
After identifying climatic variables affecting the number of fledglings, we explored their impact on the selection gradient estimated for laying date and clutch size (simultaneously). The linear selection gradients for laying date and clutch size were defined as the slope of the regression of relative fitness (i.e. number of fledglings) on the traits [55]. Relative fitness corresponded to individual fitness divided by the annual mean fitness of the population and did not strongly deviate from a Gaussian distribution (electronic supplementary material, figure S1). We did not further explore nonlinear selection acting on laying date and clutch size because quadratic and correlational selection estimates were not significant for both traits (results not shown). Female identity was included as a random effect. Year was not added in the model since relative fitness is estimated within each year. The effect of previously selected ECEs was estimated by including interaction terms between reproductive traits and ECEs in the fixed part of the selection models. The significance of these interaction terms was estimated using an F test using the lmerTest [56] package in R. Note that ECE variables used in selection models were not coded as presence/absence of brood-centred variables, as used in the model-averaging analyses. Indeed, because we were interested in the fluctuations in selective pressures (acting at the population level), we constructed population-centred ECE variables. These variables corresponded to the number of broods experiencing at least one ECE during each chick period within each year. However, since the number of available nest boxes changed across years, our ECE variables strongly depended on the absolute number of occupied nest boxes within each year. Hence, we divided these ECE variables by the number of broods within each year in order to compare the ECE selective effect across years independently of the number of occupied nest boxes. In short, for a given nestling stage, an annual ECE variable referred to the percentage of broods experiencing at least one ECE among all broods during 1 year.
(iii). Mean climate versus extreme climatic event influences on selection
After identifying ECE variables correlated with selection gradients, we aimed to estimate their effects independently of the mean weather. For this purpose, we constructed four models of selection:
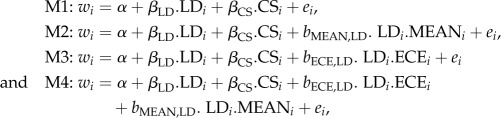 |
where w is the relative fitness; i refers to the individual values; LD and CS are measured traits for laying date and clutch size, respectively; and ei are residuals. β values can be interpreted as directional selection gradients [30] for laying date and clutch size. bECE,LD and bMEAN,LD are the estimated terms for the interaction of values of laying date and the annual values of the ECE (ECEi) and the MEAN (MEANi) climatic variables. MEAN variable refers to the mean daily temperature and/or precipitation experienced by broods during a specific nestling period, averaged across all broods. The four models were compared two by two using a likelihood ratio test (LRT), to test specific assumptions: (i) a comparison of models M1 versus M2 and M1 versus M3 tested whether selection acting on laying date was related to mean weather and ECE, respectively; (ii) a comparison of models M2 versus M4 indicated if selection acting on laying date was related to ECE independently of mean weather.
3. Results
(a). Annual reproductive success and climate fluctuations
Our first aim was to identify any ECE and/or mean climatic variable related to reproductive success (i.e. number of fledglings), depending on the nestling stage at which they occurred. A preliminary model, based on the regression of the number of fledglings on the nestling stages at which our three ECEs occurred, indicated to us that the effect of ECEs on the number of fledglings depended on the nestling stage in which they occur (electronic supplementary material, table S1). Among our 15 potential predictors explored, two were significantly related to the number of fledglings (relative importance more than 80%, table 2): (i) NES HOT T°C was negatively correlated with the number of fledglings (−0.841 ± 0.254); (ii) FLE HOT T°C was positively correlated with the number of fledglings (0.705 ± 0.348). These ECEs were also significantly related to the number of fledglings when tested in a ‘null-hypothesis’ framework (using an F test; all p < 0.01). The number of fledglings was also negatively correlated with the presence of heavy rainfalls (Ext RAIN) during the NES (−0.505 ± 0.387) and the FLE (−0.429 ± 0.320) stages, yet this effect was only marginally significant since their relative importance indices were 75% and 77%, respectively. However, these effects of rainfall during the NES and FLE stages were significant when tested in ‘null-hypothesis’ frameworks (using F test; p < 0.001 and p = 0.041 for extreme rainfalls during the NES and FLE nestling stages, respectively). All climatic variables occurring during the hatchling period (0–7 days) were uncorrelated with the subsequent number of fledglings (relative importance did not exceed 29% for this nestling stage, table 2). Interestingly, the number of fledglings was not correlated with any mean climatic variable (relative importance did not exceed 50%, table 2). Note that our 15 predictors were uncorrelated (electronic supplementary material, table S2).
Table 2.
Summary results of the model-averaging approach exploring the effect of average climatic and ECE variables on the number of fledglings in blue tits. The climatic measures concern three successive nestling stages: 0–7 days (HAT), 8–15 days (NES) and 17–21 days (FLE). The averaged slope corresponds to the averaging of predictor estimates among all selected models weighted by the Akaike weight of each model. These slopes can be interpreted as the effect of the presence of ECEs and the mean climate (temperature and precipitation) during each nestling stage on the number of fledglings. See §2 for abbreviations used here. Relative importance represents the probability that the variable was included in the best model. Variables with a relative importance above 80% are shown in italics.
| ECE and MEAN climate by stage | averaged slope | relative importance (%) |
|---|---|---|
| Intercept | 7.988 ± 0.304 | — |
| HAT MEAN T°C | −0.012 ± 0.038 | 14 |
| HAT MEAN RAIN | −0.001 ± 0.007 | 3 |
| HAT HOT T°C | −0.067 ± 0.182 | 27 |
| HAT COLD T°C | 0.078 ± 0.194 | 29 |
| HAT Ext RAIN | −0.038 ± 0.124 | 21 |
| NES MEAN T°C | 0.007 ± 0.029 | 9 |
| NES MEAN RAIN | −0.038 ± 0.061 | 33 |
| NES HOT T°C | −0.841 ± 0.254 | 99 |
| NES COLD T°C | 0.018 ± 0.171 | 23 |
| NES Ext RAIN | −0.505 ± 0.387 | 75 |
| FLE MEAN T°C | −0.075 ± 0.087 | 50 |
| FLE MEAN RAIN | 0.001 ± 0.014 | 5 |
| FLE HOT T°C | 0.705 ± 0.348 | 93 |
| FLE COLD T°C | 0.414 ± 0.476 | 60 |
| FLE Ext RAIN | −0.429 ± 0.320 | 77 |
(b). Natural selection variation with extreme climatic events
Both laying date and clutch size were under directional selection (βLaying date = −0.036 ± 0.011 and βClutch size = 0.125 ± 0.010). We focused on NES HOT T°C and FLE HOT T°C, the two ECEs significantly correlated with the number of fledglings (table 2), to explore their impact on selection acting on clutch size and laying date. Between these two ECEs, only the proportion of broods experiencing at least one extremely hot day during the nestling stage (NES HOT T°C) was significantly and negatively correlated with the directional selection on laying date (table 3). This means that the proportion of broods in the population experiencing at least one extremely hot day between days 8 and 15 increased the strength of selection for earlier laying date (since laying date is under negative directional selection). Given both the value of the interaction term (−0.130 ± 0.037, table 3) and the directional selection gradient for laying date, it suggests that the magnitude of the directional selection gradient is increasing by approximately 39% for every 10% of broods experiencing an extremely hot day during the nestling stage (day 8–day 15). None of the interactions between ECEs and clutch size (which itself was under positive directional selection) was significant (table 3).
Table 3.
Effect size (±s.d.) of ECE effects on the directional selection gradients for laying date and clutch size. The coefficients are those of interaction terms of ECEs with the life-history trait, and can be interpreted as the absolute change in selection gradients for every 10% of the population experiencing an extremely hot day during the nestling (NES) or the fledgling (FLE) stage. See §2 for meanings of abbreviations. The significant interaction term between laying date and the percentage of broods experiencing at least one extremely hot day during the nestling stage within each year is shown in italics.
| ECE in interaction with the trait | laying date | p-value | clutch size | p-value |
|---|---|---|---|---|
| NES HOT T°C | −0.130 ± 0.037 | <0.001 | 0.060 ± 0.038 | 0.113 |
| FLE HOT T°C | −0.070 ± 0.044 | 0.117 | 0.026 ± 0.044 | 0.556 |
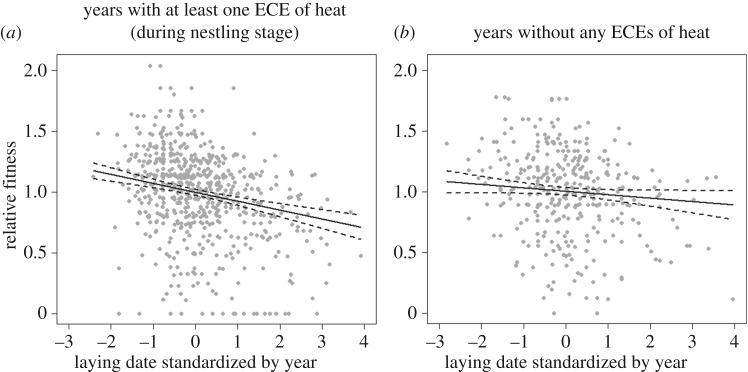
To further understand the effect of NES HOT T°C on the selection acting on laying date, we conducted a selection model on two sub-datasets: a sub-dataset containing only years (n = 14) with at least one extremely hot day experienced by some broods during the nestling stage (NES HOT T°C > 0), and one containing years (n = 11) without any extremely hot day experienced by broods (NES HOT T°C = 0). The directional selection acting on laying date was significant only in the sub-dataset of years with at least one extremely hot day during the nestling stage (table 4). Moreover, selection was 2.5 times stronger in the dataset with NES HOT T°C > 0 (figure 1).
Table 4.
Directional selection gradients (β) for blue tit laying date estimated on two datasets including either at least one ECE or no ECE. The coefficients express the change in relative fitness for one standard deviation of the life-history trait. ECE here represents a brood experiencing an extremely hot day during the nestling stage (8–15 days old). p-Values were obtained from a LRT. n, numbers of broods.
| dataset used | β | p-value | number of years | n |
|---|---|---|---|---|
| at least one ECE detected | −0.073 ± 0.012 | <0.001 | 14 | 843 |
| no ECE detected | −0.028 ± 0.015 | 0.060 | 11 | 543 |
Figure 1.
Directional selection gradient for laying date (solid lines) estimated on a dataset containing only years that did (a) or did not (b) experience at least one extremely hot day during the nestling period. The datasets (a) and (b) included 14 (n = 843) and 11 (n = 543 broods) years, respectively. Dashed curves represent 95% CIs. See detailed estimates in table 4.
(c). Influence of mean versus extreme climate
We focused here only on NES HOT T°C, i.e. the ECE identified as related to selection for earlier laying date. We constructed the four models presented in §2, exploring the effects of MEAN and ECE climatic variables on selection acting on laying date (electronic supplementary material, table S3). Results of LRT conducted between each model pair showed that: (i) the average mean temperature during the nestling period within each year was not significantly related to the strength of selection on laying date (M1 versus M2 LRT, p = 0.45); (ii) the proportion of broods experiencing at least one extremely hot day during the nestling period was significantly related to the selection on laying date (M1 versus M3 LRT, p < 0.001); and (iii) the proportion of broods experiencing this ECE was related to the strength of selection on laying date independently of the mean temperature during the same chick stage (M2 versus M4 LRT, p < 0.001).
4. Discussion
Extreme climatic events explored in this study affect both absolute fitness of individual blue tits and the force of natural selection acting on their timing of reproduction. The presence of an extremely hot day was negatively and positively correlated with the number of fledglings when occurring either during the NES or the FLE stages, respectively. Also, the number of fledglings tended to be negatively affected by the presence of a daily rainfall exceeding 15 mm during both the NES and FLE periods, but this influence was only marginal. Importantly, the presence of an extremely hot day during the NES period strengthened the selection gradient acting on laying date, independently of effects of the mean temperature on selection. We thus show here that punctual events of extreme heat can result in stronger selection for precocious phenology in breeding, in a more important way than can high average spring temperatures.
(a). Effect of extreme climatic events on the number of fledglings
Our model-averaging approach showed that the number of fledglings per brood was negatively affected by the presence of an extremely hot day (HOT T°C) during the nestling (8–15 days old, NES) fast growth stage. This finding is consistent with a recent study on blue tit nestlings, which showed that growth rates in several morphological traits were lower when ambient temperatures were high [40]. Also, negative impacts of temperature on nestling growth rate have been shown in Eastern Kingbirds (Tyrannus tyrannus) [57] and white-crowned sparrows (Zonotrichia leucophrys) [58]. However, our results partly contradict previous studies that showed a positive effect of high ambient temperatures on nestling growth rate in other passerines (e.g. [59,60]). Here, we only detect a positive effect of extremely hot days during the fledging (16–21 days) late growth stage. It should also be noted, however, that all these previous studies investigated temperature effects until chicks reach the adult size (day 15), and within the average range of climatic values, excluding ECEs. Instead, our results suggest that temperature effects on chicks mainly arise from extreme temperature values rather than mean values. The differential effect of temperature across nestling stages (negative and positive for the NES and FLE stages, respectively) could be mediated by parental feeding behaviour. Indeed, previous studies showed a decline in the parental feeding rate after 14–16 days [61,62]. Hence, if extremely hot days have a detrimental effect on caterpillar abundance, or on the ability of parents to forage, then the extreme heat will be particularly harmful during the fast growth stage, that is 8–15 days. However, while we would have predicted less or no effect of adverse conditions during the last, less energetically demanding nestling stage, the positive effect of hot weather after 15 days is still difficult to explain. Any interpretation is at this point speculative, yet it could be interesting to investigate whether heat waves during the last nestling stage have substantial negative effects on nestling predators (or merely reduce predator activity), thereby positively affecting nestling survival.
Although only marginally non-significant, the presence of extreme rainfall after the 7th day (NES and FLE stage) is negatively correlated with the number of fledglings. Previous studies exploring the effect of rainfall on nestlings highlighted complex effects. Indeed, while rainfalls were shown to cause a 10–20% reduction in nestling growth rate in great tits [43], resulting in a reduction of fledgling weight [42], other investigations demonstrated a null correlation [60] or a positive correlation between nestling growth rate and rainfall [40]. As for temperatures, these previous studies only explored the impact of ‘heavy’ rainfalls (i.e. rainfall higher than 1 mm [42,43]), and could not disentangle it from extreme rainfalls. Yet, our results demonstrate that the mean rainfall during each nestling stage does not influence the number of fledglings, thus highlighting the impact of rain through ECEs solely (i.e. rainfall higher than 15 mm).
Our analytical approach allows the decomposition of ECE effects within each nestling growth stage, providing a fine resolution of processes acting at each stage and for each brood. Contrastingly, an analysis conducted on ECEs over the entire breeding season did not identify any ECE affecting the number of fledglings in our population (see electronic supplementary material, table S4). An obvious limitation of our correlative approach, however, is that the causal path of each effect is unknown. For instance, the negative effect of extremely hot days during the NES stage could come either from a decrease in the feeding rate (through an effect on parents; [42]) or from a direct impact on nestling physiology [43], or both.
(b). Selective impact of extreme climatic events
Although ECEs have been previously shown to strongly impact the reproductive success of wild bird populations (e.g. [63–65]), their impact on the selective landscape remains poorly assessed (but see table 1). Our analyses reveal an increase in natural selection acting on laying date due to extremely hot days during the NES stage: selection is more negative (i.e. favouring earlier laying birds) during these events. The underlying cause of this selective effect could be related to an increase in ECE occurrence/probability as the breeding season advances, which could result in partial reproductive failure for later breeders. Indeed, in our population, the probability that an extremely hot day occurs during the NES stage increases by a factor of 1.41 (95% confidence intervals: 1.24–1.63 extracted from a binomial regression) for one standard deviation in laying date (s.d. = 7.6 days). The proportion of broods experiencing a partial reproductive failure increases significantly from 33% to 66% (χ2 = 19.01, p < 0.001) for broods affected by an extremely hot day during the NES stage. These results emphasize the selective impact of ECEs on laying date, which seems maximized during the NES stage. However, our analyses only used the number of fledglings as a fitness proxy, ignoring the selection acting through the number of recruits. Indeed, the number of recruits might be influenced by the extreme weather in winter, potentially shaping the selection patterns driven by the climate we detected. We did not explore the effects of climate on the number of recruits because (i) this fitness component is less affected by parents’ traits than the number of fledglings, and (ii) the number of recruits is potentially affected by the winter climate, increasing drastically the number of potential climatic agents to explore. Moreover, we only explored the selective impact of ECEs identified in the model averaging, which means that we focused only on ECEs acting on mean fitness. This approach would not detect an ECE having no effect on the mean fitness of the population yet with a differential effect on individual fitness depending on the phenotype. For example, an ECE decreasing the fitness of later breeders and increasing the fitness of earlier breeders would not be detected in our model-averaging procedure because its effect on mean fitness would be null. These types of selection patterns could be detected by fitting an interaction term between ECEs and traits in our model-averaging procedure, yet unfortunately increasing drastically the number of models generated. Finally, although a strong directional positive selection acting on clutch size is detected, no ECE is identified as a selective agent, meaning that the negative impact of ECEs on fitness is independent of the number of eggs laid.
(c). Mean versus extreme climate selective impact
Our results suggest that the ECE effects on selection pressures are independent of the mean weather conditions. In fact, our analysis reveals no effect of the average temperature on selective pressure acting on laying date, when including ECEs in the analysis. This result emphasizes the importance of considering ECEs when analysing selective pressures in the context of climate change and suggests a predominant role of ECEs in shaping the fitness landscape and its temporal fluctuations [66]. Several previous studies explored the impact of mean temperature on laying date [67,68] and natural selection acting on laying date [69], without considering ECEs. More studies are thus needed in order to disentangle the effect of ECEs versus mean climate on natural selection acting on adaptive traits. This is particularly important since ECEs are by definition rare and unpredictable, hence potentially preventing adaptive plastic response in populations [70].
This study represents the first investigation of the influence of ECEs on natural selection in comparison to that of the average weather. Increase in mean temperatures has been actively investigated in the literature, yet its impact on natural selection acting on laying is null in our study site. Moreover, because the frequency and the intensity in ECEs are increasing [6], they could potentially become one of the major threats for populations in the future.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Michael Morrissey and an anonymous reviewer for comments that greatly improved our manuscript. We thank the Forecast team at the CEFE for providing climatic data. We thank all the people who helped to maintain the study site and conduct the blue tit monitoring since 1991, in particular Jacques Blondel, Philippe Perret, Marcel Lambrechts, Arnaud Grégoire and Claire Doutrelant.
Ethics
The monitoring protocol was approved by the Animal Care and Use Committee Languedoc-Roussillon (CEEA-LR-12066).
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.452b4.
Authors' contribution
P.M., D.G. and A.C. designed the research. P.M. and A.C. collected field data. P.M. conducted statistical analyses. P.M., D.G. and A.C. wrote the paper.
Competing interests
We have no competing interests.
Funding
This project was funded by the Agence Nationale de la Recherche (BioAdapt grant ANR-12-ADAP-0006-02-PEPS to A.C.), the European Research Council (Starting grant ERC-2013-StG-337365-SHE to A.C.), the OSU-OREME and the Natural Sciences and Engineering Research Council of Canada (NSERC discovery grant to D.G.).
References
- 1.IPCC. 2014. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds CB Field et al.). Cambridge, UK and New York, NY: Cambridge University Press.
- 2.Svenning J-C, Sandel B. 2013. Disequilibrium vegetation dynamics under future climate change. Am. J. Bot. 100, 1266–1286. ( 10.3732/ajb.1200469) [DOI] [PubMed] [Google Scholar]
- 3.Crick HQP. 2004. The impact of climate change on birds. Ibis 146, 48–56. ( 10.1111/j.1474-919X.2004.00327.x) [DOI] [Google Scholar]
- 4.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 5.Boutin S, Lane JE. 2014. Climate change and mammals: evolutionary versus plastic responses. Evol. Appl. 7, 29–41. ( 10.1111/eva.12121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coumou D, Rahmstorf S. 2012. A decade of weather extremes. Nat. Clim. Change 2, 491–496. ( 10.1038/nclimate1452) [DOI] [Google Scholar]
- 7.Beniston M, Stephenson DB. 2004. Extreme climatic events and their evolution under changing climatic conditions. Glob. Planet. Change 44, 1–9. ( 10.1016/j.gloplacha.2004.06.001) [DOI] [Google Scholar]
- 8.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 9.Visser ME. 2008. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659. ( 10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawson CR, Vindenes Y, Bailey L, van de Pol M. 2015. Environmental variation and population responses to global change. Ecol. Lett. 18, 724–736. ( 10.1111/ele.12437) [DOI] [PubMed] [Google Scholar]
- 11.Moreno J, Møller AP. 2011. Extreme climatic events in relation to global change and their impact on life histories. Curr. Zool. 57, 375–389. ( 10.1093/czoolo/57.3.375) [DOI] [Google Scholar]
- 12.Gutschick VP, BassiriRad H. 2003. Extreme events as shaping physiology, ecology, and evolution of plants: toward a unified definition and evaluation of their consequences. New Phytol. 160, 21–42. ( 10.1046/j.1469-8137.2003.00866.x) [DOI] [PubMed] [Google Scholar]
- 13.Jentsch A, Beierkuhnlein C. 2008. Research frontiers in climate change: effects of extreme meteorological events on ecosystems. C. R. Geosci. 340, 621–628. ( 10.1016/j.crte.2008.07.002) [DOI] [Google Scholar]
- 14.Peñuelas J, et al. 2004. Nonintrusive field experiments show different plant responses to warming and drought among sites, seasons, and species in a North–South European gradient. Ecosystems 7, 598–612. ( 10.1007/s10021-004-0179-7) [DOI] [Google Scholar]
- 15.Jentsch A, Kreyling J, Boettcher-Treschkow J, Beierkuhnlein C. 2009. Beyond gradual warming: extreme weather events alter flower phenology of European grassland and heath species. Glob. Change Biol. 15, 837–849. ( 10.1111/j.1365-2486.2008.01690.x) [DOI] [Google Scholar]
- 16.Bailey LD, van de Pol M. 2016. Tackling extremes: challenges for ecological and evolutionary research on extreme climatic events. J. Anim. Ecol. 85, 85–96. ( 10.1111/1365-2656.12451) [DOI] [PubMed] [Google Scholar]
- 17.Schreiner RW, Schreiner EA. 1984. Central Pacific seabirds and the El Niño southern oscillation: 1982 to 1983 perspectives. Science 225, 713–716. ( 10.1126/science.225.4663.713) [DOI] [PubMed] [Google Scholar]
- 18.Ganter B, Boyd H.. 2000. A tropical volcano, high predation pressure, and breeding biology of Arctic waterbirds: a circumpolar review of breeding failure in the summer of 1992. Arctic 53, 289–305. ( 10.14430/arctic859) [DOI] [Google Scholar]
- 19.Van De Pol M, et al. 2010. Do changes in the frequency, magnitude and timing of extreme climatic events threaten the population viability of coastal birds? J. Appl. Ecol. 47, 720–730. ( 10.1111/j.1365-2664.2010.01842.x) [DOI] [Google Scholar]
- 20.Altwegg R, Roulin A, Kestenholz M, Jenni L. 2006. Demographic effects of extreme winter weather in the barn owl. Oecologia 149, 44–51. ( 10.1007/s00442-006-0430-3) [DOI] [PubMed] [Google Scholar]
- 21.Garrott RA, Eberhardt LL, White PJ, Rotella J. 2003. Climate-induced variation in vital rates of an unharvested large-herbivore population. Can. J. Zool. 81, 33–45. ( 10.1139/z02-218) [DOI] [Google Scholar]
- 22.Nicotra AB, et al. 2010. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 15, 684–692. ( 10.1016/j.tplants.2010.09.008) [DOI] [PubMed] [Google Scholar]
- 23.Charmantier A, Gienapp P. 2014. Climate change and timing of avian breeding and migration: evolutionary versus plastic changes. Evol. Appl. 7, 15–28. ( 10.1111/eva.12126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merilä J. 2012. Evolution in response to climate change: in pursuit of the missing evidence. Bioessays 34, 811–818. ( 10.1002/bies.201200054) [DOI] [PubMed] [Google Scholar]
- 25.Merilä J, Hendry AP. 2014. Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evol. Appl. 7, 1–14. ( 10.1111/eva.12137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant RB, Grant PR. 1993. Evolution of Darwin's finches caused by a rare climatic event. Proc. R. Soc. Lond. B 251, 111–117. ( 10.1098/rspb.1993.0016) [DOI] [Google Scholar]
- 27.Jenouvrier S, Péron C, Weimerskirch H. 2015. Extreme climate events and individual heterogeneity shape life-history traits and population dynamics. Ecol. Monogr. 85, 605–624. ( 10.1890/14-1834.1) [DOI] [Google Scholar]
- 28.Møller AP. 1994. Phenotype-dependent arrival time and its consequences in a migratory bird. Behav. Ecol. Sociobiol. 35, 115–122. ( 10.1007/BF00171501) [DOI] [Google Scholar]
- 29.Johnston RF, Niles DM, Rohwer SA. 1972. Hermon Bumpus and natural selection in the house sparrow Passer domesticus. Evolution 26, 20–31. ( 10.2307/2406980) [DOI] [PubMed] [Google Scholar]
- 30.Brown CR, Brown MB. 1998. Intense natural selection on body size and wing and tail asymmetry in cliff swallows during severe weather. Evolution 52, 1461–1475. ( 10.2307/2411315) [DOI] [PubMed] [Google Scholar]
- 31.Jones G. 1986. Selection against large size in the sand martin Riparia riparia during a dramatic population crash. Ibis 129, 274–280. ( 10.1111/j.1474-919X.1987.tb03208.x) [DOI] [Google Scholar]
- 32.Charmantier A, Doutrelant C, Dubuc-Messier G, Fargevieille A, Szulkin M. 2016. Mediterranean blue tits as a case study of local adaptation. Evol. Appl. 9, 135–152. ( 10.1111/eva.12282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porlier M, Garant D, Perret P, Charmantier A. 2012. Habitat-linked population genetic differentiation in the blue tit Cyanistes caeruleus. J. Hered. 103, 781–791. ( 10.1093/jhered/ess064) [DOI] [PubMed] [Google Scholar]
- 34.Marrot P, Garant D, Charmantier A. 2015. Spatial autocorrelation in fitness affects the estimation of natural selection in the wild. Methods Ecol. Evol. 6, 1474–1483. ( 10.1111/2041-210X.12448) [DOI] [Google Scholar]
- 35.Banbura J, Blondel J, de Wilde-Lambrechts H, Galan M-J, Maistre M. 1994. Nestling diet variation in an insular Mediterranean population of blue tits Parus caeruleus: effects of years, territories and individuals. Oecologia 100, 413–420. ( 10.1007/BF00317863) [DOI] [PubMed] [Google Scholar]
- 36.Thomas DW, Blondel J, Perret P, Lambrechts MM, Speakman JR. 2001. Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science 291, 2598–2600. ( 10.1126/science.1057487) [DOI] [PubMed] [Google Scholar]
- 37.Perrins CM. 1991. Tits and their caterpillar food supply. Ibis 133, 49–54. ( 10.1111/j.1474-919X.1991.tb07668.x) [DOI] [Google Scholar]
- 38.Reed TE, Jenouvrier S, Visser ME. 2013. Phenological mismatch strongly affects individual fitness but not population demography in a woodland passerine. J. Anim. Ecol. 82, 131–144. ( 10.1111/j.1365-2656.2012.02020.x) [DOI] [PubMed] [Google Scholar]
- 39.Visser M, Holleman LM, Gienapp P. 2006. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147, 164–172. ( 10.1007/s00442-005-0299-6) [DOI] [PubMed] [Google Scholar]
- 40.Mainwaring MC, Hartley IR. 2016. Local weather conditions have complex effects on the growth of blue tit nestlings. J. Therm. Biol. 60, 12–19. ( 10.1016/j.jtherbio.2016.05.005) [DOI] [PubMed] [Google Scholar]
- 41.Matthysen E, Adriaensen F, Dhondt AA. 2011. Multiple responses to increasing spring temperatures in the breeding cycle of blue and great tits (Cyanistes caeruleus, Parus major). Glob. Change Biol. 17, 1–16. ( 10.1111/j.1365-2486.2010.02213.x) [DOI] [Google Scholar]
- 42.Radford AN, McCleery RH, Woodburn RJW, Morecroft MD. 2001. Activity patterns of parent great tits Parus major feeding their young during rainfall. Bird Study 48, 214–220. ( 10.1080/00063650109461220) [DOI] [Google Scholar]
- 43.Keller LF, van Noordwijk AJ. 1994. Effects of local environmental conditions on nestling growth in the great tit Parus major L. Ardea 82, 349–362. [Google Scholar]
- 44.Perrins CM. 1979. British tits. Glasgow, UK: Collins. [Google Scholar]
- 45.Smith MD. 2011. The ecological role of climate extremes: current understanding and future prospects. J. Ecol. 99, 651–655. ( 10.1111/j.1365-2745.2011.01833.x) [DOI] [Google Scholar]
- 46.Easterling DR, Evans JL, Groisman PY, Karl TR, Kunkel KE, Ambenje P. 2000. Observed variability and trends in extreme climate events: a brief review. Bull. Am. Meteorol. Soc. 81, 417–425. ( 10.1175/1520-0477(2000)081%3C0417:OVATIE%3E2.3.CO;2) [DOI] [Google Scholar]
- 47.Jentsch A, Kreyling J, Beierkuhnlein C. 2007. A new generation of climate-change experiments: events, not trends. Front. Ecol. Environ. 5, 365–374. ( 10.1890/1540-9295(2007)5%5B365:ANGOCE%5D2.0.CO;2) [DOI] [Google Scholar]
- 48.Blondel J, Thomas DW, Charmantier A, Perret P, Bourgault P, Lambrechts MM. 2006. A thirty-year study of phenotypic and genetic variation of blue tits in Mediterranean habitat mosaics. Bioscience 56, 661–673. ( 10.1641/0006-3568(2006)56%5B661:ATSOPA%5D2.0.CO;2) [DOI] [Google Scholar]
- 49.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 50.Grueber CE, Nakagawa S, Laws RJ, Jamieson IG. 2011. Multimodel inference in ecology and evolution: challenges and solutions. J. Evol. Biol. 24, 699–711. ( 10.1111/j.1420-9101.2010.02210.x) [DOI] [PubMed] [Google Scholar]
- 51.Symonds ME, Moussalli A. 2011. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike's information criterion. Behav. Ecol. Sociobiol. 65, 13–21. ( 10.1007/s00265-010-1037-6) [DOI] [Google Scholar]
- 52.Akaike H. 1974. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 19, 716–723. ( 10.1109/TAC.1974.1100705) [DOI] [Google Scholar]
- 53.Bartoń K. 2009. MuMIn: multi-model inference R package. Version 3.1-113.
- 54.Bates D, Mächler M, Bolker B, Walker S.. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 48 ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 55.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 56.Kuznetsova A, Brockhoff PB, Christensen RHB. 2015. Package ‘lmerTest’. R package version 2.0-29. [Google Scholar]
- 57.Murphy MT. 1985. Nestling eastern kingbird growth: effects of initial size and ambient temperature. Ecology 66, 162–170. ( 10.2307/1941316) [DOI] [Google Scholar]
- 58.Pérez JH, et al. 2016. Nestling growth rates in relation to food abundance and weather in the Arctic. Auk 133, 261–272. ( 10.1642/AUK-15-111.1) [DOI] [Google Scholar]
- 59.Dawson RD, Lawrie CC, O'Brien EL. 2005. The importance of microclimate variation in determining size, growth and survival of avian offspring: experimental evidence from a cavity nesting passerine. Oecologia 144, 499–507. ( 10.1007/s00442-005-0075-7) [DOI] [PubMed] [Google Scholar]
- 60.McCarty JP, Winkler DW. 1999. Relative importance off environmental variables in determining the growth off nestling tree swallows Tachycineta bicolor. Ibis 141, 286–296. ( 10.1111/j.1474-919X.1999.tb07551.x) [DOI] [Google Scholar]
- 61.Blondel J, Dervieux A, Maistre M, Perret P. 1991. Feeding ecology and life history variation of the blue tit in Mediterranean deciduous and sclerophyllous habitats. Oecologia 88, 9–14. ( 10.1007/BF00328397) [DOI] [PubMed] [Google Scholar]
- 62.Barba E, Atiénzar F, Marín M, Monrós JS, Gil-Delgado JA. 2009. Patterns of nestling provisioning by a single-prey loader bird, great tit Parus major. Bird Study 56, 187–197. ( 10.1080/00063650902792049) [DOI] [Google Scholar]
- 63.Christman BJ. 2002. Extreme between-year variation in productivity of a bridled titmouse (Baeolophus wollweberi) population. Auk 119, 1149–1154. ( 10.1642/0004-8038(2002)119%5B1149:EBYVIP%5D2.0.CO;2) [DOI] [Google Scholar]
- 64.Bolger DT, Patten MA, Bostock DC. 2005. Avian reproductive failure in response to an extreme climatic event. Oecologia 142, 398–406. ( 10.1007/s00442-004-1734-9) [DOI] [PubMed] [Google Scholar]
- 65.Fletcher JR, Koford RR. 2004. Consequences of rainfall variation for breeding wetland blackbirds. Can. J. Zool. 82, 1316–1325. ( 10.1139/z04-107) [DOI] [Google Scholar]
- 66.Arnold SJ, Pfrender ME, Jones AG. 2001. The adaptive landscape as a conceptual bridge between micro- and macroevolution. Genetica 112, 9–32. ( 10.1023/A:1013373907708) [DOI] [PubMed] [Google Scholar]
- 67.Visser ME, Holleman LJM, Caro SP. 2009. Temperature has a causal effect on avian timing of reproduction. Proc. R. Soc. B 276, 2323–2331. ( 10.1098/rspb.2009.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LEB, Sheldon BC. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803. ( 10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 69.Visser ME, Gienapp P, Husby A, Morrisey M, de la Hera I, Pulido F, Both C. 2015. Effects of spring temperatures on the strength of selection on timing of reproduction in a long-distance migratory bird. PLoS Biol. 13, e1002120 ( 10.1371/journal.pbio.1002120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Jong G. 1995. Phenotypic plasticity as a product of selection in a variable environment. Am. Nat. 145, 493–512. ( 10.1086/285752) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.452b4.