Abstract
Purpose
Recurrent disease following thermal ablation therapy is a frequently reported problem. Preoperative identification of patients with high risk of recurrent disease might enable individualized treatment based on patients’ risk profile. The aim of the present work was to investigate the role of metabolic parameters derived from the pre-ablation 18F-FDG PET/CT as imaging biomarkers for recurrent disease in patients with colorectal liver metastases (CLM).
Methods
Included in this retrospective study were all consecutive patients with CLM treated with percutaneous or open thermal ablation therapy who had a pre-treatment baseline 18F-FDG PET/CT available. Multivariable cox regression for survival analysis was performed using different models for the metabolic parameters (SULpeak, SULmean, SULmax, partial volume corrected SULmean (cSULmean), and total lesion glycolysis (TLG)) corrected for tumour and procedure characteristics. The study endpoints were defined as local tumour progression free survival (LTP-FS), new intrahepatic recurrence free survival (NHR-FS) and extrahepatic recurrence free survival (EHR-FS). Clinical and imaging follow-up data was used as the reference standard.
Results
Fifty-four patients with 90 lesions were selected. Univariable cox regression analysis resulted in eight models. Multivariable analysis revealed that after adjusting for lesion size and the approach of the procedure, none of the metabolic parameters were associated with LTP-FS or EHR-FS. Percutaneous approach was significantly associated with a shorter LTP-FS. It was demonstrated that lower values of SULpeak, SULmax, SULmean , and cSULmean are associated with a significant better NHR-FS, independent of the lesion size and number and prior chemotherapy.
Conclusion
We found no association between the metabolic parameters on pre-ablation 18F-FDG PET/CT and the LTP-FS. However, low values of the metabolic parameters were significantly associated with improved NHR-FS. The clinical implication of these findings might be the identification of high-risk patients who might benefit most from adjuvant or combined treatment strategies.
Electronic supplementary material
The online version of this article (doi:10.1007/s00259-017-3637-0) contains supplementary material, which is available to authorized users.
Keywords: RFA, CLM, PERCIST, Imaging biomarker
Introduction
In recent years, many new treatment modalities have been established in the field of liver-directed oncologic interventions. Local tumour destruction by means of thermal ablation therapy has emerged as a safe and effective treatment modality for patients with colorectal liver metastases (CLM) who are not surgical candidates [1]. Radiofrequency ablation (RFA) and Microwave ablation (MWA) are considered safe ablation techniques with the ability to accomplish local disease control as shown in large cohort studies and meta-analyses [2, 3]. Furthermore, thermal ablation is frequently performed combined with concomitant liver resection [4].
Although ablation therapy has been proven to be effective in many cases, recurrent disease is a frequently reported problem that jeopardizes patients’ prognosis [1, 5]. Recurrent disease can be detected in the periphery of the ablation zone after successful ablation and is referred to as local tumour progression (LTP) [6]. The reported rates of LTP can be as high as 60% of lesions [1]. Recurrent disease also includes intra- and extrahepatic disease, occurring in 56% and 44% of patients, respectively [5, 6]. Identifying robust prognostic biomarkers might help to predict more accurately which eligible patients for thermal ablative therapy are at the highest risk for recurrent disease.
Several studies have investigated the role of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) for selection and staging of patients who are considered for ablative treatment of liver lesions [7]. The role of 18F-FDG PET/CT has only been investigated in terms of detection of hepatic and extrahepatic lesions for staging of patients or for treatment evaluation. However, studies have shown that 18F-FDG PET specific parameters may have a prognostic role in assessing survival of patient with primary or secondary liver malignancies undergoing curative treatment [8, 9]. This association has also been investigated for other malignancies and demonstrated increased tumour aggressiveness with higher 18F-FDG uptake in tumour lesions [10–12].
Up to now, the role of metabolic parameters as imaging biomarkers for recurrent disease following ablative therapy has not been investigated. However, imaging biomarkers can offer a risk stratification tool to identify high-risk patients and allow individualized treatment strategies in order to improve patients’ survival. The aim of this retrospective cohort study was to evaluate the role of metabolic parameters in predicting the prognosis in terms of LTP, intrahepatic, and extrahepatic recurrent disease in patients with CLM treated by means of curative-intent thermal ablation therapy. We hypothesized that an aggressive tumour lesion was associated with a higher metabolic activity, resulting in a higher chance of recurrent disease.
Patients and methods
This retrospective study was reviewed by the institutional review board, and the requirement to obtain informed consent was waived. We retrospectively identified and included all consecutive patients with CLM treated with percutaneous or open RFA or MWA (either alone or combined with liver resection) in Antoni van Leeuwenhoek Hospital Amsterdam, between January 2008 and July 2015. A baseline 18F-FDG PET/CT scan performed within 2 months prior to ablation therapy was an inclusion criterion and all ablation procedures were performed in a curative setting. We excluded tumour lesions that were not assessable on the baseline 18F-FDG PET/CT based on visual inspection. The decision for treatment was made during multidisciplinary tumour-board meeting consisting of at least one experienced liver surgeon, an intervention radiologist, a medical oncologist and a nuclear physician. The main indications for thermal ablation therapy were surgical resection (alone) technically not possible due to multilobar disease or previous liver resection; patient comorbidities could not ensure a safe surgical procedure; and patient preference for minimally invasive treatment. For each procedure, data on clinical and procedural characteristics were collected. Given prior reports of lower 18F-FDG uptake in mucinous type colorectal carcinoma [13], data on histopathology subtypes (mucinous versus non-mucinous type) of the primary colorectal carcinoma was collected as well.
Thermal ablation therapy
Percutaneous thermal ablation procedure was performed under epidural anesthesia using CT-guidance for needle positioning and evaluation of the ablation zone. The open procedure was performed under general anesthesia, using ultrasound guidance for needle positioning. Until 2010, for RFA, the systems used were Covidien Cool-Tip RF Ablation system with switching controller. Thereafter, a Cool-Tip RF Ablation E-series system (Covidien, Mansfield MA, USA) was used. From August 2012, thermal ablation was also performed by means of MWA using an Emprint ablation system (Covidien, Mansfield, MA, USA). In case of RFA, either a single or cluster antenna was used depending on the required ablation volume. Thermal ablation procedure was performed by two experienced interventional radiologists and according to international guidelines [6] ensuring ≥ 5 mm rim of coagulated healthy liver tissue around the tumour lesion.
Follow-up
The standard imaging follow-up schedule consisted of a tri-phasic CT scan within one month in case of percutaneous approach or a 18F-FDG PET/CT within 6–8 weeks in case of open ablation procedure. Subsequently, imaging evaluation by means of MRI, CT, or 18F-FDG PET/CT took place every 3 months until 1-year follow-up and thereafter biannually. All patients were followed at least 12 months after the ablation procedure for detection of recurrent disease. After that, patients were followed until the last follow-up visit or death. The follow-up time was defined as the time between the date of the ablation procedure and the development of recurrent disease or the last date on which follow-up was performed. Overall survival (OS) was defined as interval from ablation to death.
18F-FDG PET/CT and quantitative analysis
Whole-body 18F-FDG PET was performed in one institute on two Gemini TOF scanner (Philips Healthcare, Best, the Netherlands) at 2 min per bed position in 3-dimensional mode. Both scanners were EARL compliant. A graph presenting the recovery curves is included in the supplemental material (Supplemental material, Fig. S1). A low-dose CT was used for attenuation correction and anatomical correlation. Patients were prepared in concordance with the EANM guidelines [14] for tumour imaging. 18F-FDG was injected intravenously using a body mass index (BMI) based dosage scheme. The dose varied between 190 and 240 MBq depending on a BMI higher or lower than 28. In patients with very high or low BMI, the administered dose was in consultation with the physician. Scanning commenced 60 min after administration (mean 66 +/− 14 min).
The quantitative analysis was performed in concordance with the PET Response Criteria in Solid Tumours (PERCIST 1.0) [15] Calculation of the standardized uptake values corrected for lean body mass (SUL) was performed using ROVER evaluation software (ABX GmbH, Radeberg, Germany). The SULpeak, SULmean, partial volume corrected SULmean (cSULmean), SULmax , and the total lesion glycolysis (TLG) were collected for all target lesions. The method for partial volume correction is previously described by Hofheinz et al. [16].
Delineation of liver lesions can be challenging due to the relatively high physiological accumulation in healthy liver tissue. Therefore, the mean SUL of background activity (SULbckgr) was measured by placing a 3-cm diameter spherical volume of interest (VOI) in the healthy liver tissue; the tumour SULpeak was determined in a 1-cm3 spherical VOIpeak. According to the PERCIST 1.0 criteria, a baseline tumour SULpeak has to be ≥1.5 times the SULbckgr of liver tissue plus two times its standard deviation (SD), otherwise quantification can be unreliable. Still, due to the limited number of patients, all measurements were included in the published analysis, also if the tumour SULpeak was below this threshold. A segregated data analysis was performed for measurements that adhered to the strict PERCIST 1.0 criteria (Supplemental material, Table S1).
The threshold for the metabolic tumour volume delineation was set at 70% of the tumour SULpeak (VOI70). However, this approach sometimes resulted in visually inaccurate delineations, especially when VOI70 was much smaller than the VOI1.5bckgr+2SD. In these cases, metabolic tumour volume was redefined as SULbckgr plus two times SD (n = 4 cases). The mean tumour SUL (SULmean) is a direct derivative of this metabolic tumour volume. For the TLG, VOI threshold was defined as SULbckgr plus two times SD (VOIbckgr+2SD) as recommended by the PERCIST 1.0. The latter threshold was not used for the entire dataset since this threshold frequently resulted in inclusion of a large part of the healthy liver tissue in the VOI, especially when SULpeak was much larger than the VOI1.5bckgr+2SD. Visual inspection was performed by comparing the VOI with the lesion margins as detected on a recently performed contrast enhanced CT. Partial volume correction was implemented for each measurement to account for underestimation of the SUL values. For patient-based analysis, the most metabolically active tumour lesion was used as target lesion.
Definitions of outcome
Recurrent disease was stratified in site of recurrence: LTP, intrahepatic, and extrahepatic recurrence. LTP was the primary endpoint of the study and defined as appearance of tumour foci at the edge of the ablation zone (up to 1 cm from the edge), after at least one contrast-enhanced follow-up study has documented adequate ablation and an absence of viable tissue in the target tumour surrounding ablation margin [6]. Accordingly, lesions in which residual unablated tumour was detected on initial follow-up imaging, were excluded from analysis. Secondary endpoints were new hepatic recurrence (NHR) and extrahepatic recurrence (EHR). NHR was defined as new tumour foci outside the ablation zone (>1 cm distance from the ablation zone) in other parts of the liver and EHR was defined as new metastases in other organs than the liver (excluding patients in whom extrahepatic disease was present at baseline).
Reference standard
Follow-up imaging (MRI, CT, or 18F-FDG PET/CT) and clinical data were used as the reference standard. The reports of all post-ablation imaging and the scans were evaluated prospectively by the local investigator in order to evaluate the presence of LTP, NHR, or EHR. In case of ambiguity between the imaging reports and the images, the scans were discussed with an experienced intervention radiologist (W.P.) in order to reach consensus. The evaluation of follow-up imaging was blinded for data of the baseline 18F-FDG PET/CT scan.
Statistics
Descriptive analysis was performed to summarize patient characteristics and treatment characteristics, as well as the metabolic parameters. The median LTP-free survival (LTP-FS), NHR-free survival (NHR-FS), and the EHR-free survival (EHR-FS) were calculated using Kaplan-Meier method. For LTP-FS, analysis was performed based on a per-lesion basis and the NHR-FS and EHR-FS analyses were performed on a per-patient basis where the target lesion was defined as the lesion with the highest SULpeak at baseline.
Univariable cox regression analysis was performed for each metabolic parameter as a risk factor associated with LTP-FS, NHR-FS, or EHR-FS. The metabolic parameter of interest was entered in a multivariable model if the P-value was ≤0.25. Multivariable cox regression analysis was undertaken to evaluate the prognostic potential of the metabolic parameter of interest for prediction of LTP-FS, adjusted for potential effect modifiers such as tumour size, approach of the procedure (open vs. percutaneous), and type of ablation therapy (RFA vs. MWA). Similar analysis was performed for NHR-FS and EHR-FS, adjusted for tumour number and size. The inclusion of covariates in the model was based on clinical relevance. The event per variable rule was used to decide on the appropriate number of variables in the model [17]. In order to investigate the effect of prior chemotherapy on 18F-FDG uptake of tumour lesions, a subset analysis was performed in the chemo-naïve patients. Chemo-naïve was defined as no chemotherapy at least one year prior to ablation therapy.
The proportional hazard assumption for the cox regression model was tested by means of a goodness-of-fit test using chi-square statistics computed for each variable in the model, and adjusted for the other variables in the model. To address the problem of multicollinearity, a correlation coefficient matrix was calculated, with values >0.8 suggesting collinearity between independent variables. In order to obtain clinical relevant results, the median value of the metabolic parameters in the cohort was initially used to retrieve a cut-off value for each parameter. In case of LTP-FS, the median values were based on a per-lesion analysis and in case of NHR-FS and EHR-FS, the median values were based on a per-patient analysis. The multivariable cox regression analysis was performed again including the dichotomized variables. Differences in the survival of the two groups (low and high metabolic value), adjusted for covariates in the model was demonstrated using Kaplan-Meier curves along with the 1-year survival rates. Statistical analyses were performed using RStudio version 3.1.2 open-source software. A P-value <0.05 was considered as statistically significant.
Results
Patient and tumour characteristics
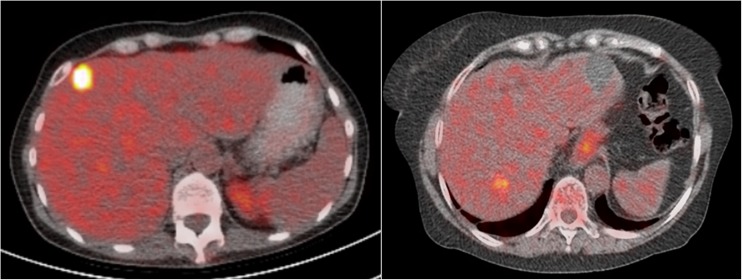
A total of 54 patients underwent 60 thermal ablation procedures and met the inclusion criteria for the study. During the 60 ablation procedures, 90 lesions were ablated. Table 1 summarizes the demographics and the tumour characteristics. The patient characteristics are based on the number of patients, the tumour characteristics are based on the lesion numbers and the procedure characteristics based on the number of procedures. Data on histopathology subtypes of the primary colorectal carcinoma showed only one patient with the mucinous type. RFA was used for ablation of 72 lesion (80%) and MWA for ablation of 18 (20%) lesion. Of the 60 ablation procedures, 31 (52%) were performed open and 29 (48%) percutaneously. The number of lesion on the preoperative imaging ranges between one and four lesions and of these, 17 lesions were resected during a combined procedure (Table 1). The median lesion size was 18 mm (7–55 mm) and 14 (23%) lesions were located ≤1 cm from large vessels. In three patients, incomplete ablation/residual disease was detected on the first follow-up scan in four lesions, so these lesions were excluded from the analysis for LTP-FS. Although all patients had liver dominant disease, extrahepatic disease was present at baseline in 11 patients (20%). These patients were excluded from the analysis for the EHR-FS. According to the PERCIST 1.0 criteria, 68 out of 90 lesions were defined as assessable target lesions (baseline tumour SULpeak ≥1.5 times the SULbckgr of liver tissue plus two times its SD). This resulted in 47 patients for the NHR-FS analysis and 38 patients for the EHR-FS analysis according to PERCIST 1.0 (Supplemental material). Figure 1 demonstrates PET/CT imaging of a patient with high versus low 18F-FDG uptake in the tumour lesion.
Table 1.
Demographics and tumour characteristics
| Characteristic | N (%) |
|---|---|
| Number of patients | 54 |
| Number of procedures | 60 |
| Number of lesions | 90 |
| Age (median), year | 62 (40–84, IQR 14) |
| Gender (Male/female) | 33 (61)/21 (39) |
| RFA/MWA (lesion-based) | 72 (80)/18 (20) |
| Open/Percutaneous approach (procedure-based) | 31 (52)/29 (48) |
| ASA (n = 54) | |
| 1 | 19 (35) |
| 2 | 27 (50) |
| 3 | 2 (4) |
| Missing | 6 (11) |
| Comorbidity (n = 54) | |
| Hearth disease | 5 (9) |
| Pulmonary disease | 4 (7) |
| Renal disease | 1 (2) |
| Other | 9 (17) |
| Extrahepatic disease (n = 54) | 11 (20) |
| Pulmonary | 8 (15) |
| Lymph node | 2 (4) |
| Peritoneal | 1 (2) |
| Prior chemotherapy | 28 (52) |
| Within 1 year from treatment | 8 (15) |
| Adjuvant chemotherapy | |
| Within 6 months after treatment | 11 (20) |
| Directly after treatment | 2 (4) |
| Number of lesions on imaging (n = 60)a | |
| 1 | 33 (55) |
| 2 | 13 (22) |
| 3 | 8 (13) |
| 4 | 6 (10) |
| Number of lesions ablated (n = 60) | |
| 1 | 41 (68) |
| 2 | 10 (17) |
| 3 | 7 (12) |
| 4 | 2 (3) |
| Lesion size in mm (median, n = 90) | 18 mm, range 7–55 mm, IQR 11 mm |
| Distance to large vessel ≤1 cm (n = 90) | 14 (23) |
| Metabolic parameter (based on all lesions) | |
| SULpeak | 4.8 (1.7–10.2, IQR 2) |
| SULmean | 4.0 (1.6–9.1, IQR 1.6) |
| cSULmean | 6.6 (2.3–19.8, IQR 3.7) |
| SULmax | 5.4 (1.9–12.6, IQR 2.3) |
| TLG | 23.7 (2.8–305.2, IQR 31.9) |
| SULmean of normal liver | 2.0 (1.3–2.4, IQR 0.3) |
a Of these, 17 out of 107 lesions were resected during an combined procedure. ASA American Society of Anesthesiologists; IQR interquartile range; MWA microwave ablation; RFA radiofrequency ablation; TLG total lesion glycolysis
Fig. 1.
PET/CT imaging of a patient with high (left panel) versus low (right panel) 18F-FDG uptake in the tumour lesion with the median SULpeak value as cut-off point (median SULpeak of 4.8)
Survival characteristics
The median follow-up time was 29.3 months (range 5.8–91.8 months). Lesion-based analyses resulted in a LTP rate of 46.5% (40 out of 86 lesions). Of these, 36 out of 86 (41.9%) were identified within 1 year and 20 out of 86 (23.3%) were identified within 6 months following the ablation procedure. The 6-month and 1-year LTP-FS were 80.2% (72.2–89.1) and 58.1% (48.3–69.7), respectively. The median number of follow-up moments within 1-year was 4.5 (range 3–8) in patients that did not develop LTP and 4 (range 2–8) in patients with LTP within 1 year. Thirty-two patients developed NHR (59.0%) and 24 out of 43 patients (56.0%), developed EHR during the course of the disease. The 1-year and 3-year NHR-FS and EHR-FS were 47.4% (35.7–63.0), 37.1% (25.4–54.0) and 62.6% (49.7–79.0), 41.9% (29.1–60.4), respectively. Twenty-one patients (39%) died during the entire follow-up. The median OS was 49.3 months with a 1-year and 5-year survival rate of 94.1% (87.9–100.0) and 28.0% (13.6–57.6), respectively.
Cox regression analysis
The univariable analysis resulted in three models for LTP-FS analysis, four models for NHR-FS and one model for EHR-FS (Tables 2 and 3). The type of ablation treatment (RFA vs. MWA) did not meet the proportional hazard assumption and was not found to be an effect-modifier for the relation between the metabolic activity and LTP-FS. Therefore, this variable was not included in the multivariable model. The chi-square test resulted in a P-value >0.05 for the remaining categorical variables in the models. The correlation coefficient between the metabolic parameters of the hepatic metastases was only weakly correlated with lesion size (r <0.40) suggesting no complications caused by multicollinearity in the models.
Table 2.
Univariable cox regression analysis for metabolic parameters as risk factors associated with LTP-FS, NHR-FS and EHR-FS
| LTP-FS HR (95% CI) P-value |
NHR-FS HR (95% CI) P-value |
EHR-FS HR (95% CI) P-value |
||||
|---|---|---|---|---|---|---|
| Metabolic parameter | Not assessable for PERCIST | Assessable for PERCIST | Not assessable for PERCIST | Assessable for PERCIST | Not assessable for PERCIST | Assessable for PERCIST |
| SUL-peak | 0.92 (0.76–1.38) 0.38 |
0.87 (0.68–1.11) 0.25* |
1.31 (1.04–1.66) 0.02* |
1.40 (1.07–1.82) 0.02* |
1.17 (0.89–1.55) 0.26 |
1.22 (0.88–1.69) 0.23* |
| SUL–max | 0.92 (0.78–1.08) 0.30 |
0.86 (0.69–1.08) 0.19* |
1.21 (0.99–1.48) 0.06* |
1.26 (1.00–1.59) 0.05* |
1.13 (0.89–1.43) 0.34 |
1.15 (0.87–1.54) 0.33 |
| SUL–mean | 0.91 (0.72–1.14) 0.41 |
0.85 (0.63–1.14) 0.28 |
1.45 (1.08–1.95) 0.01* |
1.60 (1.15–2.23) 0.01* |
1.14 (0.81–1.61) 0.44 |
1.19 (0.79–1.78) 0.41 |
| cSUL–mean | 0.95 (0.85–1.05) 0.31 |
0.92 (0.81–1.05) 0.23* |
1.18 (1.05–1.33) 0.01* |
1.23 (1.08–1.40) 0.01* |
1.05 (0.90–1.22) 0.53 |
1.06 (0.90–1.26) 0.47 |
| TLG | 1.00 (0.99–1.01) 0.94 |
1.00 (0.99–1.01) 0.94 |
1.00 (1.00–1.01) 0.88 |
1.00 (1.00–1.01) 0.78 |
1.00 (0.99–1.01) 0.57 |
1.00 (0.99–1.01) 0.57 |
EHR-FS extrahepatic recurrence free survival; LTP-FS local tumour progression free survival; NHR-FS new hepatic recurrence free survival. *Relevant metabolic parameters used in the multivariable models are marked
Table 3.
Multivariable cox regression models for LTP-FS (models 1–3), NHR-FS (models 4–7) and EHR-FS (model 8)
| Model | Outcome variable | Covariates | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P |
|---|---|---|---|---|---|---|
| All patients | Chemo-naive patients | |||||
| Model 1 | LTP-FS | Lesion size Percutaneous approach SUL-peak |
1.03 (0.99–1.07) 2.34 (1.25–4.38) 0.88 (0.71–1.08) |
0.104
0.008 0.214 |
NA | |
| Model 2 | LTP-FS | Lesion size Percutaneous approach SUL -max |
1.03 (0.99–1.07) 2.33 (1.24–4.37) 0.89 (0.74–1.06) |
0.108
0.008 0.186 |
NA | |
| Model 3 | LTP-FS | Lesion size Percutaneous approach cSUL-mean |
1.03 (0.99–1.06) 2.32 (1.23–4.35) 0.93 (0.83–1.05) |
0.131
0.009 0.246 |
NA | |
| Model 4 | NHR-FS | Lesion size Lesion number SUL -peak |
0.97 (0.94–1.01) 1.15 (0.84–1.58) 1.38 (1.09–1.74) |
0.168
0.376 0.007 |
0.97 (0.94–1.02) 1.25 (0.92–1.71) 1.33 (1.00–1.78) |
0.273
0.156 0.048 |
| Model 5 | NHR-FS | Lesion size Lesion number SUL -max |
0.98 (0.94–1.02) 1.15 (0.84–1.58) 1.25 (1.02–1.53) |
0.252
0.368 0.029 |
0.98 (0.95–1.02) 1.26 (0.92–1.72) 1.20 (0.95–1.53) |
0.382
0.145 0.123 |
| Model 6 | NHR-FS | Lesion size Lesion number SUL -mean |
0.97 (0.93–1.01) 1.12 (0.82–1.54) 1.60 (1.18–2.17) |
0.106
0.477 0.003 |
0.97 (0.93–1.01) 1.22 (0.89–1.66) 1.59 (1.06–2.40) |
0.177
0.222 0.025 |
| Model 7 | NHR-FS | Lesion size Lesion number cSUL-mean |
0.98 (0.94–1.02) 1.08 (0.79–1.48) 1.20 (1.06–1.35) |
0.214
0.624 0.003 |
0.98 (0.94–1.02) 1.18 (0.86–1.61) 1.20 (1.02–1.40) |
0.315
0.311 0.027 |
| Model 8 | EHR-FS | Lesion size SUL-peak |
0.98 (0.94–1.03) 1.22 (0.92–1.62) |
0.400
0.174 |
NA | |
CI confidence interval; CN Chemo-naïve patients; EHR-FS extrahepatic recurrence free survival; LTP-FS local tumour progression free survival; NA not applicable; NHR-FS new hepatic recurrence free survival
Multivariable analysis revealed that after adjusting for lesion size and the approach of the procedure, none of the metabolic parameters were associated with LTP-FS (Table 3). Percutaneous approach was significantly associated with a shorter LTP-FS (hazard ratio of 2.3, P <0.01), independent of the lesion size. Models 4–7 demonstrated that low values of SULpeak, SULmax, SULmean , and cSULmean are significantly associated with a better NHR-FS, independent of the lesion size and number (Table 3). In other words, new hepatic lesions were earlier observed in patients with more metabolically active tumours. Of these, the SULmean had the highest hazard ratio for a shorter NHR-FS (model 6, hazard ratio of 1.60, P = 0.003). Based on the hazard ratio and the significance level, the cSULmean did not seem to be superior compared to the SULmean (Table 3). The results of the multivariable analysis did not reveal any association between SULpeak , adjusted for lesion size, and the EHR-FS. The multivariable analysis was performed separately in data according to PERCIST 1.0 and results were in line with the results of the analysis in the entire dataset (Supplemental material, Table S1).
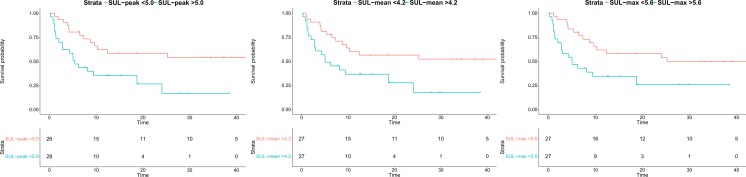
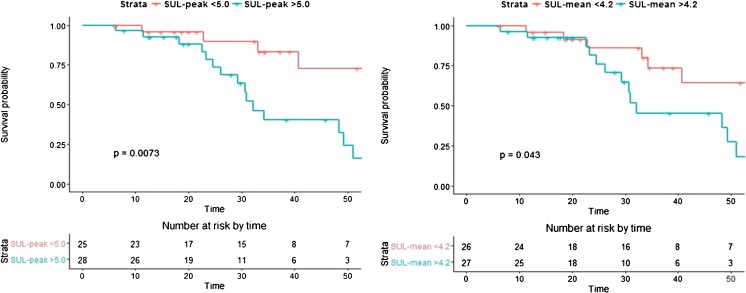
The chosen cut-off values for SULpeak, SULmax, SULmean , and cSULmean were 5.0, 5.6, 4.2, and 6.8, respectively. Multivariable cox regression analysis demonstrated a hazard ratio of respectively 2.7, 2.5, 2.4, and 2.1 for NHR-FS, independent of lesion size and number (Table 4). This can be interpreted as a 2.7 times higher risk of developing intrahepatic disease per unit time in patients with SULpeak >5.0. Only the cut-off value for cSULmean did not remain significant after dichotomizing the metabolic parameter (P = 0.053). Kaplan-Meier survival curves were plotted for NHR-FS stratified for high and low SULpeak, SULmax , and SULmean, adjusted for tumour number and size (Fig. 2). The mean 1-year NHR-free rates for the high versus low values of the metabolic parameters was 35% versus 61%, respectively. Additionally, Kaplan-Meier analysis was performed in order to evaluate differences in OS stratified for high versus low value of the SULpeak and the SULmean. Figure 3 illustrates the significant differences in OS of patients with a preoperative high versus low value of the SULpeak and the SULmean. The calculated 3-year survival rates were 83.3% versus 40.3% in the low versus high SULpeak group. Accordingly, the 3-year survival rate in the low versus high SULmean group was 73.5% versus 45.4% respectively.
Table 4.
Multivariable cox regression models for NHR-FS with dichotomized values of the metabolic parameters
| Model | Covariates | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P |
|---|---|---|---|---|---|
| All patients | Chemo-naive patients | ||||
| Model 4 | Lesion size Lesion number SUL-peak >5.0 |
0.97 (0.93–1.01) 1.17 (0.85–1.61) 2.66 (1.22–5.77) |
0.104
0.330 0.014 |
0.98 (0.93–1.02)
1.29 (0.95–1.77) 1.96 (0.84–4.60) |
0.251
0.107 0.118 |
| Model 5 | Lesion size Lesion number SUL-mean >4.2 |
0.97 (0.93–1.01) 1.17 (0.85–1.60) 2.38 (1.10–5.13) |
0.125
0.336 0.028 |
0.98 (0.94–1.02)
1.28 (0.78–0.93) 1.94 (0.83–4.50) |
0.268
0.129 0.124 |
| Model 6 | Lesion size Lesion number SUL-max >5.6 |
0.97 (0.93–1.01) 1.16 (0.84–1.59) 2.45 (1.15–5.20) |
0.159
0.362 0.020 |
0.98 (0.94–1.02)
1.27 (0.79–0.93) 1.51 (0.68–3.37) |
0.428
0.136 0.316 |
| Model 7 | Lesion size Lesion number cSUL-mean >6.8 |
0.98 (0.94–1.02) 1.03 (0.75–1.43) 2.13 (0.99–4.57) |
0.241
0.841 0.053 |
0.99 (0.95–1.03)
1.22 (0.87–1.71) 1.30 (0.57–2.97) |
0.534
0.240 0.538 |
Fig. 2.
Adjusted Kaplan-Meier curves for NHR-FS with dichotomized values of the metabolic parameters SULpeak, SULmean , and SULmax. The 1-year NHR-free rate was 62.2% (95% CI 46.1–83.9) and 35.2% (95% CI 20.8–59.9) for SULpeak lower and higher than 5.0 respectively, 60.1% (95% CI 44.1–81.9) and 36.6% (95% CI 21.6–61.9) for SULmean lower and higher than 4.2 and 61.2% (95% CI 45.3–82.9) and 61.7% (95% CI 45.7–83.2) and 34.4% (95% CI 20.0–59.2) for SULmax lower and higher than 5.6, respectively
Fig. 3.
Kaplan-Meier curves for OS with dichotomized values of the metabolic parameters SULpeak (left) and SULmean (right)
Analysis in chemo-naïve patients
Eight patients (15%) had received systemic therapy at least 1 year prior to ablation therapy. The univariable and multivariable analysis was repeated in the chemo-naïve patients (Supplemental material, Table S2). The univariable analysis in the chemo-naïve patients resulted in four models corresponding with models 4, 5, 6, and 7 (Table 3). The results of the multivariable analysis in the chemo-naïve patients showed that low values of SULpeak, SULmean , and cSULmean, but not SULmax, were significantly associated with a better NHR-FS. However, the dichotomized values of the SULpeak, SULmean , and cSULmean did not remain significant in the multivariable analysis (Table 4).
Discussion
This study showed that the metabolic parameters calculated based on the pre-ablation 18F-FDG PET/CT were not associated with LTP-FS. In the multivariable model, the percutaneous approach of the procedure remained the most significant predictor of LTP. An important finding was that the metabolic parameters were independent prognostic risk factors for NHR-FS, even in chemo-naïve patients. Remarkably, none of the metabolic parameters were associated with EHR-FS.
Our results suggest that the aggressiveness of the tumour lesions as expressed by means of the metabolic activity is not associated with shorter LTP-FS after thermal ablation therapy. A possible explanation might be that appearance of LTP highly depends on technical aspects of the procedure and tumour size, rather than the metabolic activity. Evidently, the success of ablation therapy greatly depends on the correct positioning of the ablation probe and visibility of the tumour lesion [18]. During an open procedure, localization of the lesion is done by means of intraoperative ultrasound. Use of ultrasound imaging eliminates attenuation by the skin and subcutaneous tissue and ensures a wider window, resulting in an improved visibility and image resolution. Another benefit of the open procedure is the improved controlled positioning of the probe allowing insertion of the probe at different angles with mobilization of the liver if necessary [18].
Despite the differences in the LTP rate, percutaneous ablation is considered the least invasive method and is recommended over the open approach due to the increased mortality and morbidity of the latter [3]. Obviously, this does not count for an ablation procedure that is combined with surgical liver resection [3] Moreover, it would be of interest whether and how the OS or the progression free survival is affected by the approach of the procedure. Unfortunately, our study design did not allow for an analysis for this purpose.
The reported LTP rates after ablation therapy vary widely between studies and comparison between cohorts is hampered due to analysis on a per-lesion or a per-patient basis. Reported lesion-based LTP rate is up to 43% [3] for open ablation and up to 52% for the percutaneous approach [3, 19]. Although higher than expected, the LTP rate in our cohort lies within the range of previous reported rates. Besides the approach of the procedure, the diameter of the lesion (>3 cm) and minimal ablation margin size are important known risk factors [19, 20]. In our cohort, lesion size was not considered as an exclusion criterion. As a result, lesions measuring >3 cm were also included which might explain our LTP rate. Furthermore, current evidence shows that an ablation margin between 5 and 10 mm is a prognostic factor for shorter LTP-FS and ideally, an ablation margin ≥10 mm should be endeavored [19]. Unfortunately, an ablation margin of ≥5 mm was aimed in our clinic.
The prognostic value of the metabolic parameters for time to progression of intrahepatic recurrence is an important finding since patients’ prognosis after ablation therapy seems to be more depending on intrahepatic recurrence rather than LTP. It was shown that LTP alone does not significantly affect the OS of patients treated by means of ablation therapy, but the pattern of disease recurrence does [21]. The OS of patients with LTP combined with intrahepatic recurrence was found to be worse compared to patients with LTP alone. A possible explanation is that a repeated ablation therapy, hence curative treatment, is still feasible in majority of patients with timely detected LTP.
Previous studies reported the prognostic significance of metabolic parameters for the survival of patients with surgically treated CLM [8, 22–24]. However, to our knowledge, this is the first study that investigated this for patients with CLM treated by means of curative-intent thermal ablation with stratified results for site of recurrence. We showed that a higher metabolic value is significantly associated with a worse NHR-FS. In case of a the SULpeak >5.0, multivariable analysis showed a 2.7 times higher risk of developing intrahepatic disease per unit time. Since all PET imaging was performed using an EARL compliant PET scanner, the resulted cut-off values for the metabolic parameters can be useful in other EARL accredited centers [25].
Although we included only patients treated with thermal ablation therapy, our results are consistent with findings by other authors [8, 22–24]. A recent meta-analysis pooled the data on the prognostic significance of metabolic parameters and patients’ survival and found a significant association between a high pretreatment SUV and a poor OS (pooled hazard ratio of 1.24, 95% CI 1.06–1.45) [26]. Lee et al. investigated the prognostic significance of pre-treatment metabolic values and found a significant association between the SUVpeak of hepatic metastases and the recurrence free survival [8]. Other researchers conducted similar studies, but discrepancy in the reported results existed. According to Riedl et al. [22], the SUVmax measured on the pre-operative 18F-FDG PET was associated with a significant shorter OS, while Muralidharan et al. [24] did not find any association between the SUVmax or the SUVmean and OS or recurrence free survival. The differences in the prognostic ability of the metabolic parameters reported by various authors might be the result of variability of technical and biological factors as well as heterogeneity of the population in the studies [27].
Nevertheless, the findings from previous studies, as well as present study show that higher metabolic activity of the tumour lesion is associated with a relatively poor outcome. PET imaging visualizes the increased glucose use by tumour cells using 18F-FDG as tracer. However, the cellular and molecular mechanisms that determine 18F-FDG uptake are poorly understood. Traditionally, histopathological characteristics are considered as reliable markers for biological aggressiveness [28]. The latter is considered a major determinant of clinical outcome of patients [28].
Careful selection of candidates for thermal ablation therapy is important and recommended by the international expert panel [3]. To maintain good survival rates, similar as after surgical resection of liver metastases, adjuvant systemic therapy should be acknowledged after curative thermal ablation [3]. However, it is not clear which patients benefit most from adjuvant systemic treatment. Previous studies showed the beneficial effect of adjuvant chemotherapy on the progression free survival [29–31]. The results of a randomized trial by Ruers et al. showed a significant improvement in progression free survival in patients treated with ablation therapy combined with systemic therapy compared to systemic therapy alone [29]. In the current study, a large difference in the 1-year NHR-free rate of patients with a high versus low metabolic value (35% versus 61%) was demonstrated. These findings might indicate that especially patients with highly metabolic active tumours, as measured based on the pre-procedural 18FDG-PET/CT, might benefit most from adjuvant systemic treatment or combined therapies.
In this study, we found no association between the metabolic activity and the EHD-FS. To our knowledge, no other studies have investigated the correlation of metabolic activity and extrahepatic disease recurrence. Other studies that investigated possible predictors of extrahepatic recurrence after curative-intent surgery, found the following characteristics associated with an increased risk: primary rectal tumour site, primary tumour lymph node metastasis, hepatic tumour size >5 cm, hepatic tumour number >4, as well as receipt of chemotherapy [32–35]. The lack of association between metabolic activity and extrahepatic disease in our cohort is remarkable as our results demonstrated a significant association between metabolic activity and OS. A possible explanation for our findings is the small sample size that was used for this analysis. Hence, the association between the metabolic activity and extrahepatic recurrence after curative-intent surgery needs to be further addressed in future studies with larger cohorts.
There are limitations to this study. The retrospective nature introduces the risk of selection bias. However, we used clearly defined inclusion and exclusion criteria and included all consecutive patients to mitigate this problem. The threshold for the metabolic tumour volume delineation was set at 70% of the tumour SULpeak unless this approach resulted in visually inaccurate delineation. This was the case in four patients for whom a different threshold for delineation (SULbckgr+2SD) was used. The use of different thresholds might have introduced bias in the reported results. However, the segregated analysis for measurements according to the PERCIST 1.0 criteria showed similar results as in the entire dataset. Also, the VOI70 threshold for tumour volume delineation was not according to EANM guidelines [13]. The post injection time varied quite (66 +/− 14 min) between patients which may have affected the tumour to normal-liver uptake ratio and subsequently the resulted metabolic value. Finally, although the SUV corrected for body weight is more often used in the clinical practice, we used the SUL values instead, because the latter is more consistent from patient to patient [36]
There is cumulative evidence on the prognostic significance of pretreatment 18F-FDG PET/CT. The call for more individualized approach to cancer treatment challenges future studies to investigate prospectively whether risk stratification of patients by means of metabolic parameters will lead to change of management and significant improvement in patients’ survival. Moreover, it should be recognized that the benefit of PET imaging can be extended by using different tracers for different biological features such as hypoxia level, which in turn is associated with negative effect on prognosis [37–39]. Perhaps this can lead to promising risk stratification tools that combine different biological imaging markers based on an entirely non-invasive method.
Conclusion
Our findings add to the growing knowledge of the value of 18F-FDG PET/CT in the staging and evaluation setting of patients with CLM. We found no association between the metabolic parameters on pre-ablation 18F-FDG PET/CT and the LTP-FS. However, low values of the metabolic parameters were significantly associated with improved NHR-FS. In the era of tailored approach to patient care, the clinical implication of these findings is the identification of patients who might benefit most from adjuvant or combined treatment strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 36 kb)
(DOC 34 kb)
(DOC 44 kb)
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. MS has received a personal funding for her MD/PhD (Alexandre Suerman Stipendium), provided by the UMC Utrecht.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study had a retrospective nature. For this type of study formal consent was not required.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s00259-017-3637-0) contains supplementary material, which is available to authorized users.
Contributor Information
M. Samim, Phone: 0031-(0)88-7555555, Email: morsalsamim@gmail.com
W. Prevoo, Phone: 0031-(0)20-5129111
B. J. de Wit-van der Veen, Phone: 0031-(0)20-5129111
K. F. Kuhlmann, Phone: 0031-(0)20-5129111
T. Ruers, Phone: 0031-(0)20-5129111
R. van Hillegersberg, Phone: 0031-(0)88-7555555
M. A. A. J. van den Bosch, Phone: 0031-(0)88-7555555
H. M. Verkooijen, Phone: 0031-(0)88-7555555
M. G. E. H. Lam, Phone: 0031-(0)88-7555555
M. P. M. Stokkel, Phone: 0031-(0)20-5129111
References
- 1.Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD, 3rd, Dorfman GS. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28(3):493–508. doi: 10.1200/JCO.2009.23.4450. [DOI] [PubMed] [Google Scholar]
- 2.Groeschl RT, Wong RK, Quebbeman EJ, Tsai S, Turaga KK, Pappas SG, et al. Recurrence after microwave ablation of liver malignancies: a single institution experience. HPB (Oxford) 2013;15(5):365–71. doi: 10.1111/j.1477-2574.2012.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillams A, Goldberg N, Ahmed M, Bale R, Breen D, Callstrom M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontieres meeting 2013. Eur Radiol. 2015;25(12):3438–54. doi: 10.1007/s00330-015-3779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gleisner AL, Choti MA, Assumpcao L, Nathan H, Schulick RD, Pawlik TM. Colorectal liver metastases: recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch Surg. 2008;143(12):1204–12. doi: 10.1001/archsurg.143.12.1204. [DOI] [PubMed] [Google Scholar]
- 5.Govaert KM, van Kessel CS, Steller EJ, Emmink BL, Molenaar IQ, Kranenburg O, et al. Recurrence location after resection of colorectal liver metastases influences prognosis. J Gastrointest Surg. 2014;18(5):952–60. doi: 10.1007/s11605-014-2461-0. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. J Vasc Interv Radiol. 2014;25(11):1691–705.e4. doi: 10.1016/j.jvir.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samim M, El-Haddad GE, Molenaar IQ, Prevoo W, van den Bosch MA, Alavi A, et al. 18F]Fluorodeoxyglucose PET for interventional oncology in liver malignancy. PET Clin. 2014;9(4):469–95. doi: 10.1016/j.cpet.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Lee HS, Kim HO, Hong YS, Kim TW, Kim JC, Yu CS, et al. Prognostic value of metabolic parameters in patients with synchronous colorectal cancer liver metastasis following curative-intent colorectal and hepatic surgery. J Nucl Med. 2014;55(4):582–9. doi: 10.2967/jnumed.113.128629. [DOI] [PubMed] [Google Scholar]
- 9.Jones C, Badger SA, Stevenson M, Diamond T, McKie LD, Taylor MA, et al. PET-CT as a predictor of outcome in resectable colorectal liver metastases. Eur J Gastroenterol Hepatol. 2014;26(4):466–72. doi: 10.1097/MEG.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 10.Lee HY, Lee SW, Lee KS, Jeong JY, Choi JY, Kwon OJ, et al. Role of CT and PET imaging in predicting tumor recurrence and survival in patients with lung adenocarcinoma: a comparison with the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification of Lung Adenocarcinoma. J Thorac Oncol. 2015;10(12):1785–94. doi: 10.1097/JTO.0000000000000689. [DOI] [PubMed] [Google Scholar]
- 11.Lee JW, Kang CM, Choi HJ, Lee WJ, Song SY, Lee JH, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative (1)(8)F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med. 2014;55(6):898–904. doi: 10.2967/jnumed.113.131847. [DOI] [PubMed] [Google Scholar]
- 12.Chang S, Kim SJ. Prediction of recurrence and mortality of locally advanced esophageal cancer patients using pretreatment F-18 FDG PET/CT parameters: intratumoral heterogeneity, SUV, and volumetric parameters. Cancer Biother Radiopharm. 2016;31(1):1–6. doi: 10.1089/cbr.2015.1932. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor OJ, McDermott S, Slattery J, Sahani D, Blake MA. The use of PET-CT in the assessment of patients with colorectal carcinoma. Int J Surg Oncol. 2011;2011:846512. doi: 10.1155/2011/846512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–54. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyun SH, Eo JS, Lee JW, Choi JY, Lee KH, Na SJ, et al. Prognostic value of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with Barcelona Clinic Liver Cancer stages 0 and A hepatocellular carcinomas: a multicenter retrospective cohort study. Eur J Nucl Med Mol Imaging. 2016;43(9):1638–45. doi: 10.1007/s00259-016-3348-y. [DOI] [PubMed] [Google Scholar]
- 16.Hofheinz F, Langner J, Petr J, Beuthien-Baumann B, Oehme L, Steinbach J, et al. A method for model-free partial volume correction in oncological PET. EJNMMI Res. 2012;2(1):16. doi: 10.1186/2191-219X-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–9. doi: 10.1016/S0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 18.Burdio F, Mulier S, Navarro A, Figueras J, Berjano E, Poves I, et al. Influence of approach on outcome in radiofrequency ablation of liver tumors. Surg Oncol. 2008;17(4):295–9. doi: 10.1016/j.suronc.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes--a 10-year experience at a single center. Radiology. 2016;278(2):601–11. doi: 10.1148/radiol.2015142489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sotirchos VS, Petrovic LM, Gonen M, Klimstra DS, Do RK, Petre EN, et al. Colorectal cancer liver metastases: biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016;280(3):949–59. doi: 10.1148/radiol.2016151005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aksoy E, Aliyev S, Taskin HE, Birsen O, Mitchell J, Siperstein A, et al. Clinical scenarios associated with local recurrence after laparoscopic radiofrequency thermal ablation of colorectal liver metastases. Surgery. 2013;154(4):748–52. doi: 10.1016/j.surg.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Riedl CC, Akhurst T, Larson S, Stanziale SF, Tuorto S, Bhargava A, et al. 18F-FDG PET scanning correlates with tissue markers of poor prognosis and predicts mortality for patients after liver resection for colorectal metastases. J Nucl Med. 2007;48(5):771–5. doi: 10.2967/jnumed.106.037291. [DOI] [PubMed] [Google Scholar]
- 23.de Geus-Oei LF, Wiering B, Krabbe PF, Ruers TJ, Punt CJ, Oyen WJ. FDG-PET for prediction of survival of patients with metastatic colorectal carcinoma. Ann Oncol. 2006;17(11):1650–5. doi: 10.1093/annonc/mdl180. [DOI] [PubMed] [Google Scholar]
- 24.Muralidharan V, Kwok M, Lee ST, Lau L, Scott AM, Christophi C. Prognostic ability of 18F-FDG PET/CT in the assessment of colorectal liver metastases. J Nucl Med. 2012;53(9):1345–51. doi: 10.2967/jnumed.112.102749. [DOI] [PubMed] [Google Scholar]
- 25.Quak E, Le Roux PY, Hofman MS, Robin P, Bourhis D, Callahan J, et al. Harmonizing FDG PET quantification while maintaining optimal lesion detection: prospective multicentre validation in 517 oncology patients. Eur J Nucl Med Mol Imaging. 2015;42(13):2072–82. doi: 10.1007/s00259-015-3128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia Q, Liu J, Wu C, Song S, Tong L, Huang G, et al. Prognostic significance of (18)FDG PET/CT in colorectal cancer patients with liver metastases: a meta-analysis. Cancer Imaging. 2015; 15:19-015-0055-z. [DOI] [PMC free article] [PubMed]
- 27.Boellaard R, Oyen WJ, Hoekstra CJ, Hoekstra OS, Visser EP, Willemsen AT, et al. The Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi-centre trials. Eur J Nucl Med Mol Imaging. 2008;35(12):2320–33. doi: 10.1007/s00259-008-0874-2. [DOI] [PubMed] [Google Scholar]
- 28.Neal CP, Garcea G, Doucas H, Manson MM, Sutton CD, Dennison AR, et al. Molecular prognostic markers in resectable colorectal liver metastases: a systematic review. Eur J Cancer. 2006;42(12):1728–43. doi: 10.1016/j.ejca.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 29.Ruers T, Punt C, Van Coevorden F, Pierie JP, Borel-Rinkes I, Ledermann JA, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004) Ann Oncol. 2012;23(10):2619–26. doi: 10.1093/annonc/mds053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–15. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 31.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(3):958–68. doi: 10.1148/radiol.12111851. [DOI] [PubMed] [Google Scholar]
- 32.Angelsen JH, Viste A, Loes IM, Eide GE, Hoem D, Sorbye H, et al. Predictive factors for time to recurrence, treatment and post-recurrence survival in patients with initially resected colorectal liver metastases. World J Surg Oncol. 2015;13:328-015-0738-8. [DOI] [PMC free article] [PubMed]
- 33.Lee H, Choi DW, Cho YB, Yun SH, Kim HC, Lee WY, et al. Recurrence pattern depends on the location of colon cancer in the patients with synchronous colorectal liver metastasis. Ann Surg Oncol. 2014;21(5):1641–6. doi: 10.1245/s10434-013-3477-5. [DOI] [PubMed] [Google Scholar]
- 34.Abbas S, Lam V. In colorectal liver metastases, the presence of extrahepatic disease correlates with the pathology of the primary tumour. ISRN Oncol. 2011;2011:948174. doi: 10.5402/2011/948174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250(3):440–8. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 36.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peeters SG, Zegers CM, Lieuwes NG, van Elmpt W, Eriksson J, van Dongen GA, et al. A comparative study of the hypoxia PET tracers [(1)(8)F]HX4, [(1)(8)F]FAZA, and [(1)(8)F]FMISO in a preclinical tumor model. Int J Radiat Oncol Biol Phys. 2015;91(2):351–9. doi: 10.1016/j.ijrobp.2014.09.045. [DOI] [PubMed] [Google Scholar]
- 38.Lee ST, Scott AM. Hypoxia positron emission tomography imaging with 18f-fluoromisonidazole. Semin Nucl Med. 2007;37(6):451–61. doi: 10.1053/j.semnuclmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 39.van Elmpt W, Zegers CM, Reymen B, Even AJ, Dingemans AM, Oellers M, et al. Multiparametric imaging of patient and tumour heterogeneity in non-small-cell lung cancer: quantification of tumour hypoxia, metabolism and perfusion. Eur J Nucl Med Mol Imaging. 2016;43(2):240–8. doi: 10.1007/s00259-015-3169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 36 kb)
(DOC 34 kb)
(DOC 44 kb)