Abstract
We investigated mitotic delay during replication arrest (the S-M checkpoint) in DT40 B-lymphoma cells deficient in the Chk1 or Chk2 kinase. We show here that cells lacking Chk1, but not those lacking Chk2, enter mitosis with incompletely replicated DNA when DNA synthesis is blocked, but only after an initial delay. This initial delay persists when S-M checkpoint failure is induced in Chk2−/− cells with the Chk1 inhibitor UCN-01, indicating that it does not depend on Chk1 or Chk2 activity. Surprisingly, dephosphorylation of tyrosine 15 did not accompany Cdc2 activation during premature entry to mitosis in Chk1−/− cells, although mitotic phosphorylation of cyclin B2 did occur. Previous studies have shown that Chk1 is required to stabilize stalled replication forks during replication arrest, and strikingly, premature mitosis occurs only in Chk1-deficient cells which have lost the capacity to synthesize DNA as a result of progressive replication fork inactivation. These results suggest that Chk1 maintains the S-M checkpoint indirectly by preserving the viability of replication structures and that it is the continued presence of such structures, rather than the activation of Chk1 per se, which delays mitosis until DNA replication is complete.
Eukaryotic cells respond to DNA damage or blocks to DNA replication by triggering a variety of checkpoint responses which delay cell cycle progression, promote repair, and protect genome integrity (31). Checkpoints are controlled by signal transduction mechanisms which lead to the activation of two checkpoint effector kinases, Chk1 and Chk2 (the Schizosaccharomyces pombe and Saccharomyces cerevisiae [fission and budding yeast] Chk2 homologues are Cds1 and Rad53, respectively), which elicit appropriate checkpoint responses by phosphorylating and modulating the activities of a variety of downstream substrates (31, 40).
Replication arrest triggers at least three mechanistically distinct checkpoints in both yeasts and mammalian cells (31). Two of these are required to protect the inhibited DNA replication machinery and to ensure that replication can resume when conditions permit. In budding and fission yeast, Rad53 and Cds1 are activated when DNA synthesis is inhibited and are required to stabilize slowed or stalled replication structures (12, 26) and to suppress initiation at latent replication origins (20, 43). In the absence of these checkpoint responses, stalled replication forks become inactivated through a poorly defined degenerative process (often referred to as replication fork collapse), while latent replication origins initiate replication even though elongation cannot occur (futile origin firing). Such defects are highly deleterious, and yeast checkpoint mutants which lack these functions exhibit DNA replication abnormalities and diminished cell survival upon release from replication arrest (12, 26).
A third replication checkpoint delays the onset of mitosis while DNA replication is incomplete (the S-M checkpoint). In fission yeast, Cds1 is also considered to be the primary effector of mitotic delay when DNA synthesis is inhibited (25, 30), although paradoxically cds1 null mutants retain the capacity to delay mitosis in response to replication arrest. This residual delay is dependent on Chk1, since cds1 chk1 double mutants lack an effective S-M checkpoint (4, 25, 50). Although Chk1 is activated only by DNA damage in wild-type fission yeast (2, 47), in cds1 null mutant cells Chk1 activation is also observed during replication arrest, either because replication fork collapse gives rise to aberrant DNA structures which are recognized as DNA damage (25) or because in wild-type cells Cds1 suppresses a repair process that leads to Chk1 activation (7).
Regardless of whether Cds1 or Chk1 is responsible, mitotic delay in response to DNA damage and during replication arrest in fission yeast is imposed through the regulation of inhibitory phosphorylation of tyrosine 15 (Y15) of Cdc2 (13, 28). Specifically, the rapid dephosphorylation of Y15-phosphorylated Cdc2, which normally activates Cdc2 catalytic activity and initiates mitosis, is blocked when Cds1 or Chk1 is activated (39, 42). Both Cds1 and Chk1 can phosphorylate and inhibit the Cdc25 phosphatase during replication arrest (50) or DNA damage (15), preventing the dephosphorylation of Cdc2 Y15, and they are required to sustain high levels of Mik1 (10, 41), a Cdc2 Y15 kinase (28). Chk1 also enhances the activity of a second Cdc2 Y15 kinase, Wee1, through direct phosphorylation (32). Although the mechanistic details are not yet fully established, genetic and biochemical evidence suggests that mitotic delay in fission yeast is normally achieved through the coordinated checkpoint modulation of both positive and negative regulators of Cdc2 Y15 phosphorylation (33, 37).
The mechanisms and effectors involved in replication checkpoint responses in higher eukaryotes are less well characterized. In contrast to the case in fission yeast, Chk1 is strongly activated by DNA synthesis inhibitors such as hydroxyurea and aphidicolin as well as by DNA damage in vertebrate cells in culture (14, 49). Furthermore, both biochemical and genetic lines of evidence indicate that the checkpoint functions which preserve replication fork viability and suppress latent origin firing during DNA synthesis inhibition in vertebrate cells are controlled primarily or exclusively by Chk1 rather than the Rad53/Cds1 counterpart Chk2 (14, 49).
These considerations suggest that Chk1 is also involved in S-M checkpoint control in vertebrate cells, but conflicting conclusions regarding the role of Chk1 have been reached with different experimental systems. Chk1 is activated by DNA synthesis inhibition in Xenopus laevis egg extracts, and immunodepletion experiments have revealed that Chk1 activity is required for prolonged mitotic delay (22). Also, Chk1−/− mouse blastocysts were observed to enter mitosis in the presence of DNA replication inhibitors (44). Although these results suggest that Chk1 is required for the S-M checkpoint, at least in embryonic cells, checkpoint failure was not observed after moderate periods of replication arrest in Chk1−/− DT40 B-lymphoma somatic cells (49) or in mouse embryonic fibroblasts (MEFs) deficient in ATR, even though the activation of Chk1 in these cells was largely abolished (9).
To account for these apparent discrepancies, we considered the hypothesis that Chk1 and Chk2 might play partially redundant or complementary roles in S-M checkpoint control in vertebrate cells, much as the case proposed for Cds1 and Chk1 in fission yeast. To evaluate this idea, we investigated the effect of replication arrest on mitotic entry in isogenic mutant DT40 cell lines deficient in either Chk1 or Chk2. Unlike embryonic cells from mice and certain other metazoan species, DT40 cells survive in the absence of Chk1 function (49) and thus provide a uniquely tractable experimental system for such studies. We show here that cells deficient in Chk1, but not those deficient in Chk2, activate Cdc2 kinase activity and enter mitosis with incompletely replicated DNA when DNA synthesis is blocked, but only after an initial delay. Surprisingly, this initial delay is not dependent on compensation by Chk2, nor is the eventual activation of Cdc2 and checkpoint failure due to the removal of the inhibitory Y15 phosphorylation of Cdc2. Instead, our results suggest that the role of Chk1 in the S-M checkpoint is to preserve viable replication structures and that it is the continued presence of such structures in arrested cells which precludes Cdc2 activation and thus the onset of mitosis.
MATERIALS AND METHODS
Generation of Chk2-deficient DT40 cells by gene targeting.
A detailed description of the construction of the Chk2−/− DT40 cell line will be presented elsewhere (M. D. Rainey and D. A. F. Gillespie, unpublished data). Briefly, an avian Chk2 cDNA was isolated from an avian B-cell cDNA library and then sequenced, and oligonucleotides were designed to amplify segments of the genomic chk2 gene which were then used to construct targeting vectors in which a neomycin or puromycin antibiotic selection cassette replaced essential segments of the Chk2 coding region. The vectors were designed to delete the entire Chk2 FHA domain and a majority of the protein kinase domain so that any residual polypeptide encoded by the targeted alleles would be nonfunctional. For the generation of Chk2-deficient DT40 clones, two rounds of targeting were performed to disrupt both chk2 alleles. Hemi- and homozygous gene disruption clones were identified by Southern blotting, and the latter were shown to be devoid of functional Chk2 protein expression by immunoblotting with an anti-Chk2 antiserum.
Antibodies.
Monoclonal antibodies against Chk1 (G-4), Cdc2 (Cl-17), PCNA (PC10), and actin (C-2) and polyclonal antibodies against Cdc2 phosphorylated at Tyr15 or Tyr15/Thr14 were purchased from Santa Cruz Biotechnology. A polyclonal antibody against the phospho-Ser345 form of Chk1 was purchased from New England Biolabs, and a polyclonal antibody against the anti-phospho-Ser10 form of histone H3 was purchased from Upstate Biotechnology. A monoclonal antibody against α-tubulin (DM1A) was purchased from Sigma, and one against lamin B2 was purchased from Cambridge Bioscience. A polyclonal antiserum against cyclin B2 was a kind gift from E. Nigg. A rabbit antiserum specific for avian Chk2 was derived as described previously (49). A mouse monoclonal anti-bromodeoxyuridine (anti-BrdU) antibody was purchased from Dako, and the rat monoclonal antibody clone BU 1/75 against chlorodeoxyuridine (CldU) was purchased from Abcam.
Cell culture and treatments.
DT40 avian B-lymphoma cells were grown in Dulbecco's modified Eagle's medium (GIBCO) containing 10% fetal bovine serum, 1% chicken serum, 10−5 M β-mercaptoethanol, penicillin, and streptomycin at 39.5°C. The cells were treated with 20 μM aphidicolin (Sigma), 1 μg of nocodazole (Sigma)/ml, 100 μM roscovitine (Sigma), and 10 μg of BrdU (Sigma) or CldU (Sigma)/ml, as appropriate. For inhibition, Chk1 cells were treated with 300 nM UCN-01.
Elutriation.
Exponentially growing asynchronous cell cultures were separated in a Beckman JE-6B elutriating rotor system. Cells were elutriated in growth medium at room temperature at a constant flow rate of 40 ml/min, and the G1/S-phase population was eluted by slowing the rotor to a predetermined speed. A more detailed description of the procedure is available upon request.
Detection of phosphorylated histone H3 by flow cytometry.
Cells were fixed in 70% ethanol-phosphate-buffered saline (PBS) at 4°C and labeled with the anti-pS10H3 primary antibody and a fluorescein isothiocyanate-conjugated secondary antibody. The cells were then counterstained with propidium iodide (PI) and analyzed for pS10H3 fluorescence and DNA content by use of a Beckton Dickinson FACScan flow cytometer as described previously (47a).
Cell sorting and fluorescence microscopy.
Cells stained with the anti-pS10H3 primary antibody and a fluorescein isothiocyanate-conjugated secondary antibody were separated according to their fluorescence and then collected by use of a Facsvantage SA cell sorter (Becton Dickinson). After sorting, pS10H3-positive and -negative cell populations were either lysed in sample buffer for Western blot analysis or attached to glass coverslips coated with poly-l-lysine (Sigma) for analysis by fluorescence microscopy. The metabolic incorporation of CldU into DNA was detected after acid denaturation by use of an anti-CldU rat monoclonal antibody and an appropriate secondary antibody conjugated to Texas Red, essentially as described previously (3).
For protein antigens, cells were fixed in 3.7% formaldehyde in PBS for 15 min, permeabilized in 0.1% Triton X-100 in PBS, and sequentially stained with appropriate primary and secondary antibodies at a 1:100 dilution in 10% fetal calf serum-0.5% bovine serum albumin in PBS for 2 h and for 40 min, respectively. All incubations were done at room temperature, and then coverslips were mounted and viewed by fluorescence microscopy.
Kinase assays and immunoprecipitation of Cdc2 with cyclin B2.
Immunoprecipitation-kinase assays for Cdc2 with histone H1 as a substrate were performed by use of a monoclonal anti-Cdc2 antibody (Cl-17; Santa Cruz) essentially as described previously (11). Kinase reactions were transferred to nitrocellulose membranes for autoradiography, after which the precipitated Cdc2 protein was visualized by Western blotting. Cyclin B2/Cdc2 complexes were immunoprecipitated with an anti-cyclin B2 antibody (a kind gift of E. Nigg) under identical conditions, and the cyclin B2-associated Cdc2 protein was visualized by Western blotting.
RESULTS
DT40 cells sustain a prolonged S-M checkpoint delay when DNA synthesis is inhibited.
To investigate the requirement for Chk1 and Chk2 in S-M checkpoint control in vertebrate somatic cells, we devised a protocol by which we could rigorously document the effect of DNA synthesis inhibition on the onset of mitosis in DT40 knockout cell lines that are deficient in each checkpoint kinase. The construction of the Chk1-deficient DT40 cell line has been described previously (49), while the generation of its Chk2-deficient counterpart will be described in detail elsewhere (a brief outline is provided in Materials and Methods). As shown in Fig. 1A, each knockout cell line is devoid of a functional Chk1 or Chk2 protein, respectively.
FIG. 1.
Checkpoint kinase expression in WT, Chk1−/−, and Chk2−/− cells and detection of mitotic cells by flow cytometry. (A) Western blot analysis of Chk1 (left) and Chk2 (right) expression in the cell lines used for this study. (B) Asynchronous cultures of WT, Chk1−/−, or Chk2−/− cells were separated by centrifugal elutriation to generate purified G1/S cell populations. A representative example of a WT cell culture before and after purification is shown. The purified G1/S populations were returned to culture in the presence or absence of 20 μM aphidicolin and the continuous presence of 1 μg of nocodazole/ml. The cells were harvested and analyzed for DNA content and pSer10H3 fluorescence (pH3 fluorescence) by flow cytometry as shown in the bottom right panel (WT cells were cultured with nocodazole alone for 7 h).
Since by definition the S-M checkpoint delays mitosis in cells which have not yet completed DNA replication, we used centrifugal elutriation to remove the G2- and M-phase cells from asynchronous cultures of wild-type (WT), Chk1−/−, and Chk2−/− DT40 cells (Fig. 1B). This allowed us to purify populations of G1- and S-phase cells, which were then returned to culture in the presence or absence of the DNA polymerase inhibitor aphidicolin to inhibit DNA synthesis together with nocodazole to trap cells entering mitosis (Fig. 1B). Subsequent cell cycle progression and mitotic entry were determined by flow cytometry with PI staining in combination with antibodies specific for histone H3 phosphorylated at serine 10 (anti-pS10H3) to identify mitotic cells by immunofluorescence (Fig. 1B) (1). Importantly, this allowed us both to quantify the pS10H3-positive mitotic cells in a given culture and to determine their DNA content (Fig. 1B, bottom right panel).
As shown in Fig. 2A and B, when WT G1/S DT40 cells that had been purified by this procedure were simply returned to culture (control), they progressed rapidly through DNA synthesis, entered G2, and then accumulated in mitosis, with a majority of the cells becoming positive for pS10H3 by 12 h. Mitotic entry was associated with the activation of Cdc2 catalytic activity, as measured by immunoprecipitation kinase assays with histone H1 as a substrate (see Fig. 4A). Importantly, all of the pS10H3-positive WT cells had a 4N DNA content, indicating that mitosis occurred only after the completion of DNA replication (Fig. 2A).
FIG. 2.
Wild-type DT40 cells sustain a prolonged S-M checkpoint delay when DNA synthesis is inhibited. (A) pSer10H3 fluorescence-DNA content flow cytometry analysis of elutriated WT G1/S cells cultured with 1 μg of nocodazole/ml with or without (control [con]) 20 μM aphidicolin for the indicated times. Open boxes indicate total pSer10H3-positive cells (pH3), and the open arrow indicates pH3-positive cells with 4N DNA content. (B) Quantitation of the data shown in panel A. (C) Western blot analysis of total and Ser345-phosphorylated Chk1 in elutriated WT cells cultured for the indicated times as described for panel A.
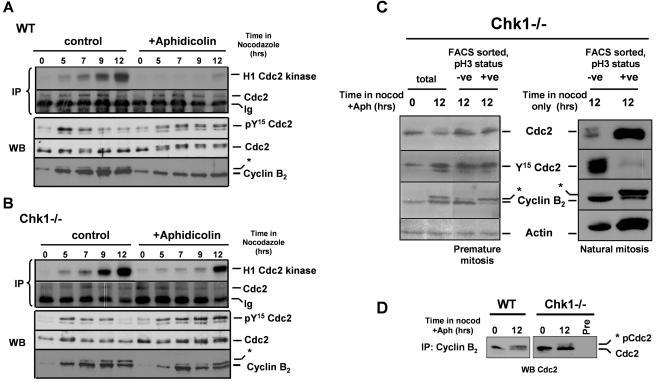
FIG. 4.
Cdc2-associated histone H1 kinase activity, Cdc2 Y15 phosphorylation, and cyclin B2 expression in elutriated WT and Chk1−/− cells during replication arrest. (A) Cdc2-associated H1 kinase activity and Western blot analysis of Tyr15-phosphorylated Cdc2, total Cdc2, and cyclin B2 protein levels in elutriated G1/S WT cultures with 1 μg of nocodazole/ml and with or without (control) 20 μM aphidicolin for the indicated times. The prominent band in the anti-Cdc2 Western blot analysis of the immunoprecipitation-kinase assay corresponds to the light chain of the immunoprecipitating antibody (also shown in panel B). The samples were split in two: one half was analyzed for H1 kinase activity and the second half was used for Western blot analysis. The pY15 Cdc2, total Cdc2, and cyclin B2 results shown in the lower parts of panels A and B were derived from sequential reprobing of the same Western blots. (B) Cdc2-associated H1 kinase activity and Western blot analysis of Tyr15-phosphorylated Cdc2, total Cdc2, and cyclin B2 protein levels in elutriated G1/S Chk1−/− cultures treated as described for panel A. (C) Levels of Tyr15-phosphorylated Cdc2, total Cdc2, cyclin B2, and actin proteins in total (unsorted) and sorted pSer10H3-positive (mitotic) and -negative (nonmitotic) populations of Chk1−/− cells treated with 1 μg of nocodazole/ml and 20 μM aphidicolin for 12 h to induce premature mitosis (left) or with 1 μg of nocodazole/ml alone for 12 h to enrich for natural mitotic cells (right). (D) Cyclin B2 was immunoprecipitated from cultures of WT and Chk1−/− cells treated with 1 μg of nocodazole/ml and 20 μM aphidicolin for 12 h, and the coprecipitated Cdc2 protein was visualized by Western blotting. The positions of phosphorylated (asterisk) and nonphosphorylated Cdc2 protein are indicated.
In contrast, WT cells that were incubated with aphidicolin showed no evidence of cell cycle progression, as judged by an increasing DNA content (Fig. 2A and B), nor was there any significant entry to mitosis or increase in Cdc2 catalytic activity for up to 12 h (see Fig. 4A). Aphidicolin treatment also resulted in a strong and persistent activation of Chk1, as judged by both an electrophoretic mobility shift and phosphorylation on serine 345 (S345) detected by immunoblotting (27, 51), neither of which was observed in untreated cultures (Fig. 2C). Essentially identical results were observed for Chk2−/− cells, which exhibited a similar delay in the appearance of pS10H3-positive cells, the suppression of Cdc2 kinase activity, and the activation of Chk1 when exposed to aphidicolin as that shown by the WT (see Fig. 5B; also data not shown). Taken together, these results demonstrate that WT and Chk2−/− DT40 cells are capable of sustaining a prolonged S-M checkpoint delay when DNA synthesis is blocked and that this delay is associated with a robust activation of Chk1 and an inhibition of Cdc2 kinase activity.
FIG. 5.
Mitotic delay in the absence of Chk1 and Chk2 activity. (A) Elutriated G1/S WT cells were incubated with 1 μg of nocodazole/ml in the presence or absence of 20 μM aphidicolin, with or without 300 nM UCN-01, for the indicated times. At each time point, the percentage of pSer10H3-positive cells was determined by flow cytometry as described in the legends for Fig. 2 and 3. Also included for comparison are data for elutriated Chk1−/− cells treated with 20 μM aphidicolin alone (shaded triangles). (B) Elutriated G1/S Chk2−/− cells treated as described for panel A.
Chk1 is required to maintain but not to initiate mitotic delay during replication arrest.
When elutriated Chk1−/− G1/S cells were cultured under control conditions, they also progressed through S and G2, activated Cdc2 catalytic activity, and accumulated in mitosis (Fig. 3A and B and Fig. 4B). Furthermore, as for WT cells, all of the pS10H3-positive cells that accumulated in the Chk1−/− cultures under these conditions had a 4N DNA content, which is indicative of normal, properly scheduled mitoses (Fig. 3A).
FIG. 3.
Chk1 is required to maintain the S-M checkpoint. (A) pSer10H3 fluorescence-DNA content flow cytometry analysis of elutriated Chk1−/− G1/S cells cultured with 1 μg of nocodazole/ml with or without (control [con]) 20 μM aphidicolin for the indicated times. Open boxes indicate total pSer10H3-positive cells (pH3). The open arrow indicates pSer10H3-positive cells with a 4N DNA content, and the solid arrow indicates pSer10H3-positive cells with a 2N DNA content. (B) Quantitation of the data shown in panel A. (C) More detailed kinetic analysis of the rates at which Chk1−/− cells accumulated in premature or natural mitosis in the presence or absence of aphidicolin, performed as described for panel A. (D) Mitotic spindles in pSer10H3-positive Chk1−/− cells. Elutriated G1/S WT or Chk1−/− cells were treated with aphidicolin for 12 h without nocodazole, fixed, and stained with antibodies against pSer10H3 (red) and α-tubulin (green).
A strikingly different outcome was observed when Chk1−/− cells were cultured in the presence of aphidicolin. Although aphidicolin initially suppressed both the accumulation of pS10H3-positive cells and the increase in Cdc2 kinase activity which occurred in control cultures for up to 9 h, clearly indicating the imposition of an initial checkpoint delay, both then increased abruptly at 12 h (Fig. 3A and B and Fig. 4B). Importantly, a large proportion of the pS10H3-positive Chk1−/− cells which accumulated at 12 h exhibited a 2N DNA content (Fig. 3A), indicating that entry to mitosis had occurred in the absence of DNA replication.
To confirm that pS10H3 staining in this context was a reliable marker of bona fide mitosis, we treated Chk1−/− cells with aphidicolin for 12 h without nocodazole and examined the organization of microtubules in the pS10H3-positive cells by using antibodies against α-tubulin. This revealed that the large majority of the pS10H3-positive Chk1−/− cells possessed bipolar spindles, although unlike natural mitoses these were typically offset from the condensed chromosomes, which often appeared fragmented (Fig. 3D and data not shown). Such structures were not observed in aphidicolin-treated WT cultures (Fig. 3D and data not shown). We also observed that the pS10H3-positive Chk1−/− cells lacked a nuclear envelope, as judged by staining with antibodies specific for lamin B2 (data not shown).
Thus, although Chk1-deficient cells can initially delay mitosis when DNA synthesis is blocked, if the period of arrest exceeds 9 h a premature mitosis characterized by Ccd2 kinase activation, chromosome condensation, nuclear envelope breakdown, and mitotic spindle formation occurs in the absence of genome duplication. To gain more insight into the kinetics of this phenomenon, we performed a more detailed analysis using more time points over a longer time period. As shown in Fig. 3C, this revealed that the mitotic delay induced by aphidicolin treatment persisted at all time points tested up to 14 h and that Chk1−/− cells entered mitosis in the presence and absence of aphidicolin at broadly comparable rates.
S-M checkpoint failure in Chk1-deficient cells is not associated with loss of Cdc2 tyrosine 15 phosphorylation.
In fission yeast, Cds1 or Chk1 delays mitosis during replication arrest by preventing the removal of inhibitory Y15 phosphorylation from Cdc2 (42, 50), but whether this mechanism is solely responsible for S-M checkpoint delay in vertebrate cells is less clear (8). To investigate the relationship between inhibitory phosphorylation of Cdc2 and Chk1-dependent maintenance of the S-M checkpoint, we treated elutriated populations of G1/S WT and Chk1−/− cells with aphidicolin and nocodazole for 0 to 12 h and determined the levels of total and Y15-phosphorylated Cdc2 by immunoblotting analysis.
Consistent with current models of Cdc2 regulation (34), Cdc2 Y15 phosphorylation increased transiently during normal cell cycle progression in both WT and Chk1−/− control cultures but declined again after 12 h, when a majority of cells had arrested in mitosis and Cdc2 kinase activity was maximal (Fig. 4A and B, left panels). We also observed a progressive increase in the level of cyclin B2 (the predominant avian B-type cyclin [16]) and the appearance of an electrophoretically distinct isoform (asterisks in Fig. 4A and B) which was attributable to phosphorylation since it was reversed when extracts were treated with lambda phosphatase (data not shown). This phosphorylated isoform of cyclin B2 has been shown to accumulate during mitosis and may be associated with nuclear translocation (16). In contrast, when WT cells were treated with aphidicolin, Cdc2 Y15 phosphorylation increased and was maintained at maximal levels (Fig. 4A, right panel), indicating that, as expected, mitotic delay and the suppression of Cdc2 kinase activity were associated with the persistence of this inhibitory modification. Aphidicolin treatment also prevented the appearance of the mitotic isoform of cyclin B2 (Fig. 4A, right panel).
Surprisingly, the inhibitory phosphorylation of Cdc2 was also maintained at high levels in Chk1−/− cells during aphidicolin treatment (Fig. 4B, right panel). In particular, it was evident that the abrupt increase in Cdc2 kinase activity seen after 12 h of replication arrest was not associated with any corresponding decrease in total Y15 phosphorylation (Fig. 4B, right panel). Essentially identical results were obtained with an antibody which specifically recognizes Cdc2 phosphorylated at both Y15 and threonine 14 (which also inhibits Cdc2 catalytic activity) (data not shown). In marked contrast to the case for WT cells, however, aphidicolin treatment did not prevent the appearance of the phosphorylated mitotic isoform of cyclin B2 in Chk1−/− cells.
To monitor the modification state of Cdc2 and cyclin B2, specifically in cells which had entered mitosis prematurely compared with the remaining nonmitotic cells, we treated Chk1−/− cells with aphidicolin and nocodazole for 12 h, separated the pS10H3-positive and -negative populations by fluorescence-activated cell sorting (see Fig. 6C for an example), and analyzed cell extracts prepared from the resulting fractions by Western blotting. This analysis (Fig. 4C, left panel) confirmed that there was no significant difference in the level of Cdc2 Y15 phosphorylation between the pS10H3-positive and -negative populations, although the phosphorylated form of cyclin B2 was highly enriched in the mitotic cells. As a control for this experiment, we treated Chk1−/− cells with nocodazole alone for 12 h and purified pS10H3-positive and -negative mitotic and nonmitotic populations. Western blotting of these fractions (Fig. 4C, right panel) revealed that, as expected, Chk1−/− cells which entered mitosis naturally after the completion of DNA synthesis did show a substantial reduction in Cdc2 Y15 phosphorylation compared to nonmitotic cells, together with enrichment for the mitotic isoform of cyclin B2. We also investigated the modification of cyclin B2-associated Cdc2 in WT and Chk1−/− cells that were treated with aphidicolin and nocodazole for 12 h by coimmunoprecipitation with anti-cyclin B2 antibodies. Western blotting of these immunoprecipitates (Fig. 4D) revealed that cyclin B2 was associated with both phosphorylated (asterisk) and nonphosphorylated forms of Cdc2 in aphidicolin-treated WT and Chk1−/− cells, as judged by their electrophoretic mobilities. It was evident, however, that nonphosphorylated Cdc2 predominated in the Chk1−/− immunoprecipitate, whereas both forms were equally abundant in the WT. Thus, although the activation of Cdc2 in Chk1−/− cells during premature entry to mitosis from S phase was not associated with any detectable loss of inhibitory Y15 phosphorylation from the total pool of Cdc2, as occurs during normal mitosis, it was accompanied by the mitotic phosphorylation of cyclin B2, and cyclin B2 was associated with significant amounts of nonphosphorylated and therefore potentially active Cdc2.
FIG. 6.
Loss of viable replication structures precedes checkpoint failure in Chk1-deficient cells. (A and B) Elutriated G1/S WT and Chk1−/− cultures were treated with 20 μM aphidicolin and 1 μg of nocodazole/ml for the indicated times, washed free of drugs, and pulse-labeled with BrdU for 1 h, and the percentages of BrdU-labeled (A) and pSer10H3-positive cells (B) were then determined by flow cytometry. The data shown are means and standard errors for three independent experiments. (C) Elutriated G1/S Chk1−/− cells were treated with 20 μM aphidicolin and 1 μg of nocodazole/ml for 12 h, washed free of drugs, and pulse-labeled with CldU for 1 h. The pSer10H3-positive and -negative populations (top) were then separated by cell sorting and stained for CldU incorporation, and the percentage of cells capable of DNA replication in each population was determined by fluorescence microscopy. Two hundred nuclei were scored in each case. (D) Same data as for panels A and B, except that the cultures were treated with 20 μM aphidicolin and 1 μg of nocodazole/ml for 12 h with or without the continuous presence of 100 μM Roscovitine, washed, and pulse-labeled with BrdU for 1 h, and the percentages of BrdU-labeled and pSer10H3-positive cells were then determined.
Mitotic delay in the absence of Chk1 and Chk2 activity.
In wild-type fission yeast, Cds1 rather than Chk1 is activated by DNA synthesis inhibition and is thought to be responsible for delaying mitosis until replication is complete (30). Fission yeast Cds1 mutants nevertheless remain capable of mitotic delay during replication arrest. This residual delay has been ascribed to the activation of Chk1, which occurs in response to replication arrest specifically in Cds1-deficient strains (4, 25, 50). A remarkably similar, if inverted, phenomenon has been described for DT40 cells, in which DNA synthesis inhibition activates the Cds1 homologue Chk2 only in cells lacking Chk1 (49). These considerations raised the possibility that the initial mitotic delay observed for Chk1-deficient cells that were subjected to replication arrest might be due to the compensatory activation of Chk2 (48). To evaluate this idea, we used UCN-01 (17) to inhibit Chk1 function in Chk2-deficient DT40 cells to mimic a double deficiency of Chk1 and Chk2. Elutriated cultures of WT, Chk1−/−, and Chk2−/− DT40 cells were treated with aphidicolin and UCN-01, and the effect on entry into mitosis was determined as described for Fig. 2 and 3.
Importantly, UCN-01 induced premature mitosis in aphidicolin-treated WT cells at a similar frequency and after a delay comparable to that observed spontaneously in Chk1−/− cells (Fig. 5A, compare filled circles and shaded triangles). We believe that this checkpoint failure was specifically due to the inhibition of Chk1, since UCN-01 had no effect on the incidence or timing of premature mitosis in the Chk1−/− cells themselves (data not shown). In comparison, Chk2-deficient cells that were treated with UCN-01 and aphidicolin also entered mitosis with incompletely replicated DNA (Fig. 5B, filled circles), although crucially, checkpoint failure was again only observed after an initial delay. Thus, the initial mitotic delay which persists when Chk1 is inhibited does not require and therefore cannot be solely attributable to compensation by Chk2.
Chk1-deficient cells enter mitosis with incompletely replicated DNA only after the loss of viable replication structures.
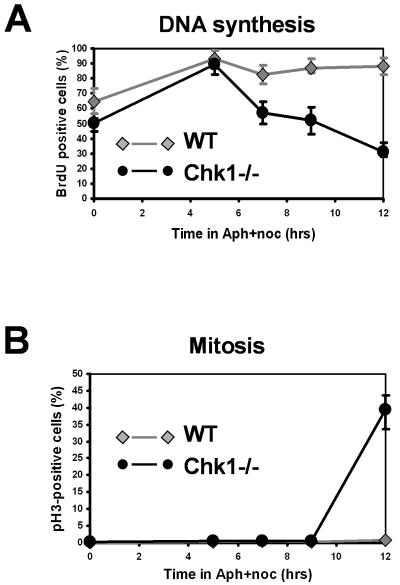
Previous studies have established that Chk1-dependent stabilization of stalled replication forks and suppression of latent origin firing are essential for the resumption of DNA replication after release from a prolonged inhibition of DNA synthesis (14, 49). In the absence of Chk1, replication forks are unstable and become progressively inactivated over a period of hours (14, 49). We therefore explored the possibility that the duration of the initial mitotic delay in Chk1−/− cells during replication arrest might be related to the DNA replication process per se. To this end, we first compared the rates at which cells devoid of detectable replication capacity accumulated during replication arrest in elutriated G1/S populations of WT and Chk1−/− cells. The cells were incubated with aphidicolin and nocodazole for various times, after which they were washed free of drugs and labeled with BrdU for 1 h. Each sample was then split in two; one half was used to determine the proportion of cells that remained capable of resuming DNA synthesis, as judged by the incorporation of BrdU (Fig. 6A), while the other half was used to monitor the entry into mitosis, as judged by pS10H3 staining (Fig. 6B).
As shown in Fig. 6A, this revealed that the number of cells incorporating BrdU increased in the first 5 h of culture after elutriation in both WT and Chk1−/− cultures as G1 cells in the starting population entered and then arrested in early S phase. In WT cultures, this percentage then remained constant for 12 h of aphidicolin treatment, indicating that the large majority of cells in the population remained capable of resuming DNA synthesis upon release from arrest (Fig. 6A). In contrast, the proportion of Chk1−/− cells which incorporated BrdU began to decrease after 5 h, with a particularly marked decline occurring between 9 and 12 h, when mitotic entry was occurring (Fig. 6A and B).
These results demonstrated a temporal correlation between the appearance of cells devoid of viable replication structures and cells undergoing premature entry into mitosis in the Chk1−/− population, but we could not determine from this analysis alone whether these processes occurred concurrently in individual cells. To answer this question, we treated elutriated Chk1−/− cells with aphidicolin and nocodazole for 12 h, after which the drugs were removed and the cells were immediately labeled for 1 h with CldU. The cells were then stained with anti-pS10H3 antibodies to identify mitotic cells, and the pS10H3-negative and -positive populations were isolated by fluorescence-activated cell sorting (Fig. 6C, upper panel). The pS10H3-positive and -negative cells were then denatured and stained for the incorporation of CldU, and the percentage of labeled cells in each population was determined by fluorescence microscopy (Fig. 6C, lower panel).
The results of this analysis were unambiguous; whereas a majority of pS10H3-negative Chk1−/− cells incorporated CldU upon release from aphidicolin arrest, none of those which had become positive for pS10H3 as a consequence of S-M checkpoint failure became labeled (Fig. 6C, lower panel). We do not believe that this was because premature entry to mitosis extinguished DNA replication for several reasons. Firstly, cells devoid of replication capacity were detected before the onset of mitosis, as judged by the appearance of pS10H3-positive cells (Fig. 6, compare panels A and B). Secondly, when similar experiments were performed in the presence of the Cdk inhibitor roscovitine, premature mitosis could be largely or completely blocked in aphidicolin-treated Chk1−/− cells (Fig. 6D, lower panel) without preventing the accumulation of cells devoid of replication capacity (Fig. 6D, upper panel). Finally, the work of others has shown that condensed mitotic chromatin is capable of both the initiation and elongation phases of DNA replication (36).
We also investigated the relationship between premature entry to mitosis and nuclear PCNA accumulation, which concentrates at sites of active DNA replication (5, 6). Elutriated WT and Chk1−/− cells were treated with aphidicolin and nocodazole for 0 to 12 h, after which the cells were washed free of drugs, incubated in fresh medium for 1 h, fixed, and then stained for PCNA and pS10H3. As shown in Fig. 7A, cells with strong nuclear PCNA staining were readily detectable in freshly elutriated G1/S populations of both WT and Chk1−/− cells. In WT cultures, the percentage of cells showing nuclear PCNA staining increased at 5 h and then persisted at this level for at least 12 h of aphidicolin treatment (Fig. 7A). In contrast, in Chk1−/− cultures, cells with weak or undetectable PCNA staining accumulated at later times during replication arrest and constituted approximately 40% of the population after 12 h of aphidicolin treatment, when pS10H3-positive cells were also present (Fig. 7A). Strikingly, strong nuclear PCNA staining was mutually exclusive with pS10H3 staining (Fig. 7B) in individual cells at this time. Since Chk1−/− cells lacking detectable PCNA staining appeared before pS10H3-positive cells (Fig. 7A), we inferred that the loss of PCNA from such sites likely preceded the onset of mitosis and correlated with the loss of replication capacity, as defined by BrdU incorporation. Taken together, these results provide further evidence that premature entry into mitosis from S phase occurs in Chk1−/− cells only when all viable replication structures have been lost and the cells lack the capacity to synthesize DNA, even though genome duplication is incomplete.
FIG. 7.
Loss of nuclear PCNA staining precedes premature mitosis in Chk1−/− cells. (A) Elutriated G1/S WT and Chk1−/− cells were incubated with 20 μM aphidicolin and 1 μg of nocodazole/ml for the indicated times, washed free of drugs, incubated in fresh medium for 1 h, fixed, and stained with antibodies specific for pSer10H3 and PCNA. The percentages of cells exhibiting detectable staining for pSer10H3 and PCNA were determined by scoring 200 cells at each time point by fluorescence microscopy. (B) Representative fields of WT and Chk1−/− cells after 12 h of replication arrest. Essentially all of the WT cells exhibited strong nuclear staining for PCNA but were uniformly negative for pSer10H3, whereas Chk1−/− cells exhibited mutually exclusive staining for PCNA (green) and pSer10H3 (red). Individual examples are indicated by white arrows.
DISCUSSION
To understand the roles of Chk1 and Chk2 in S-M checkpoint control in vertebrate cells, we investigated the effect of DNA synthesis inhibition on mitotic entry in mutant DT40 cell lines deficient in each checkpoint kinase. The results of these studies led to three principal novel conclusions. Firstly, cells deficient in Chk1, but not Chk2, ultimately enter mitosis with incompletely replicated DNA when DNA synthesis is blocked, but only after an initial delay. Secondly, premature entry to mitosis in Chk1−/− cells is associated with an induction of Cdc2 catalytic activity, but this occurs in the absence of any general loss of Cdc2 inhibitory Y15 phosphorylation. Thirdly, mitotic entry from S phase is observed only for Chk1-deficient cells which have lost the capacity to synthesize DNA, even though DNA replication in these cells remains incomplete. We believe that this loss of replication capacity is attributable to the degeneration of viable replication structures, which are normally stabilized by Chk1 during replication arrest (14, 49). Collectively, these findings led us to propose a model (Fig. 8) in which Chk1 maintains the S-M checkpoint indirectly by stabilizing viable replication structures, and it is the continued presence of such structures, rather than Chk1 activation per se, which delays mitosis in vertebrate cells until DNA replication is complete.
FIG. 8.
Proposed model for the role of Chk1 in S-M checkpoint control in vertebrate cells (see the text for details).
This model has several important implications. Firstly, it may help to explain why conflicting findings have been reported concerning the consequences of Chk1 inhibition on S-M checkpoint control, since whether or not checkpoint failure is observed is likely highly dependent on the experimental protocol and system employed. Thus, in an earlier study with Chk1−/− DT40 cells, we did not observe premature entry into mitosis during replication arrest, but in retrospect we know that the maximum arrest period examined (8 h) was insufficient to induce a loss of replication capacity, and thus checkpoint failure, according to this model in a significant proportion of cells (49). A similar argument may also explain why ATR-deficient MEFs retain an apparently normal mitotic delay after aphidicolin treatment, even though Chk1 activation is eliminated (9). For experiments with these MEFs, the cell cultures were synchronized by serum refeeding and the effect of aphidicolin on entry into mitosis was scored at or close to the scheduled time for an unperturbed cell cycle (9). Checkpoint failure might therefore have been observed in ATR-deficient cells if the period of DNA synthesis inhibition were extended sufficiently to allow for a complete loss of replication capacity (assuming that the elimination of Chk1 activation in the absence of ATR also leads to replication fork collapse). Similarly, the acute S-M checkpoint failure reported for Chk1-deficient blastocytes (44) may reflect a more rapid rate of replication structure demise in early embryonic cells. Conversely, the immunodepletion of Chk1 from Xenopus extracts impaired but did not completely abolish mitotic delay during DNA synthesis inhibition (22), indicating the presence of an additional delay mechanism. Further work will be required to resolve these issues, but this model may provide a useful conceptual framework for future studies.
Our model also implies the existence of fundamental differences in the mechanisms of S-M checkpoint control between fission yeast and vertebrate cells. In fission yeast, mitosis can be delayed in response to replication arrest through the activation of either Cds1 or Chk1 (4, 25, 50). Although these kinases are not strictly redundant, since Chk1 is activated by replication inhibitors only in the absence of Cds1 (7, 25), both have the potential to impose a mitotic delay under specific circumstances, and yeast mutants that are doubly deficient in both Cds1 and Chk1 lack an effective S-M checkpoint. Initially, we considered that this might also be true for Chk1 and Chk2 and that a compensatory activation of Chk2 (49) might account for the initial mitotic delay in Chk1-deficient cells. Surprisingly, however, a pharmacological inhibition of Chk1 with UCN-01 (17) in Chk2−/− cells (under conditions which induced checkpoint failure in WT DT40 cells) did not eliminate the mitotic delay in response to aphidicolin treatment. Thus, the activation of Chk2 is neither necessary nor sufficient for the initial mitotic delay in the absence of Chk1, which is consistent with other evidence that vertebrate cells can delay mitosis independent of the ATR/Chk1 and ATM/Chk2 pathways (8, 9).
A second major difference concerns the biochemical mechanism of mitotic delay. For fission yeast, a wealth of evidence indicates that maintenance of the inhibitory Y15 phosphorylation of Cdc2 forms the basis of S-M checkpoint delay (13, 28, 42, 50). In contrast, the abrupt activation of Cdc2 kinase activity which accompanies checkpoint failure in Chk1−/− cells is not associated with any general loss of Cdc2 Y15 phosphorylation. Since it is unlikely that Y15-phosphorylated Cdc2 acquires catalytic activity, we speculate that S-phase cells contain a subpopulation of Cdc2 molecules which are not subject to this inhibitory modification but whose activity is normally restrained through some other mechanism. Although unexpected, this result is also consistent with studies of ATR-deficient MEFs which failed to identify a correlation between mitotic delay and Cdc2 Y15 phosphorylation during replication arrest (8, 9). Interestingly, other biochemical studies have pointed to the existence of Y15 phosphorylation-independent mechanisms of Cdc2 inhibition which can delay mitotic entry in both vertebrate cells (18, 19) and Xenopus extracts (21). Although the nature of these mechanisms has not yet been fully elucidated, Cdc2 and its associated cyclin B regulatory subunit are known to be subject to regulation at several additional levels, including subcellular localization (29). In particular, regulatory phosphorylation within the cytoplasmic retention sequence of B-type cyclins is thought to play an important role in initiating mitosis by promoting nuclear translocation (45). Furthermore, the forced nuclear localization of cyclin B can override mitotic delay under conditions of DNA damage (46). The correlation between cyclin B2 phosphorylation and premature mitosis in Chk1−/− cells suggests that this modification is a functionally significant target of the S-M checkpoint mechanism, but further work will be required to test this idea explicitly.
Other studies have clearly implicated Cdc25 phosphatase in S-M checkpoint control in HeLa cells (35) and Xenopus extracts (23), suggesting that the regulation of Cdc2 inhibitory phosphorylation must also contribute to mitotic delay in response to replication arrest in vertebrate cells under specific circumstances. It therefore seems possible that Cdc2 Y15 phosphorylation, which accumulates during cell cycle progression, reinforces other checkpoint mechanisms which also restrain the activity of Cdc2 during replication arrest. Furthermore, the relative importance of these mechanisms may vary according to cell type and, possibly, between species (24).
Finally, it is important to stress that the role of Chk1 in our model is indirect, i.e., it is the presence of active, or at least potentially active (viable), replication structures that generates the signal which initially delays mitosis in S-phase cells rather than the action of Chk1 itself. Only when all of these structures have degenerated and become nonfunctional, a situation which ultimately eventuates in Chk1-deficient cells through a combination of replication fork collapse and futile origin firing (14, 49), is the mitotic delay signal lost. An analogous role for Cds1 in mediating an intrinsic link between DNA replication and mitosis by sustaining a stable S-phase state has been proposed (10, 25), although Cdc2 Y15 phosphorylation was envisaged as the ultimate target of the checkpoint regardless of the origin of the signal.
Interestingly, a replication-linked mitotic delay mechanism can be more readily reconciled with earlier, pioneering studies that demonstrated the existence of an intrinsic checkpoint which precludes mitosis during DNA synthesis in vertebrate cells (38) than with models which invoke Chk1 as a direct effector of mitotic delay. In heterokaryons formed by the fusion of S- and G2-phase cells, mitosis was found to be delayed in the G2-phase nucleus until DNA synthesis was completed in the companion S-phase nucleus (38). These experiments, however, did not involve replication arrest (38), and it seems unlikely that this intrinsic delay could have been imposed by Chk1, since Chk1 is activated only very weakly, if at all, during an unperturbed S phase (Fig. 2C) (14). These observations can be readily explained, however, in terms of an intrinsic mitotic delay signal emanating either from the replication machinery itself (Fig. 8) or from some as yet unidentified checkpoint mechanism which monitors the functional status of this machinery. The future elucidation of the origin and nature of this intrinsic mitotic delay mechanism in vertebrate cells will be of great interest.
Acknowledgments
We thank Dario Alessi and Carl Smythe for the kind gift of UCN-01, Erich Nigg for anti-cyclin B2 antiserum, Tom Gilbey for cell sorting, Tony Carr for comments on the manuscript, Liz Black and Tom McGuire for help with confocal microscopy and image processing, and Michelle Garrett for moral support.
This work was supported by the Association for International Cancer Research (G.Z.) and Cancer Research UK (D.A.F.G. and M.D.R.).
REFERENCES
- 1.Ajiro, K., K. Yoda, K. Utsumi, and Y. Nishikawa. 1996. Alteration of cell cycle-dependent histone phosphorylations by okadaic acid. Induction of mitosis-specific H3 phosphorylation and chromatin condensation in mammalian interphase cells. J. Biol. Chem. 271:13197-13201. [DOI] [PubMed] [Google Scholar]
- 2.al-Khodairy, F., E. Fotou, K. S. Sheldrick, D. J. Griffiths, A. R. Lehmann, and A. M. Carr. 1994. Identification and characterization of new elements involved in checkpoint and feedback controls in fission yeast. Mol. Biol. Cell 5:147-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aten, J. A., P. J. Bakker, J. Stap, G. A. Boschman, and C. H. Veenhof. 1992. DNA double labelling with IdUrd and CldUrd for spatial and temporal analysis of cell proliferation and DNA replication. Histochem. J. 24:251-259. [DOI] [PubMed] [Google Scholar]
- 4.Boddy, M. N., B. Furnari, O. Mondesert, and P. Russell. 1998. Replication checkpoint enforced by kinases Cds1 and Chk1. Science 280:909-912. [DOI] [PubMed] [Google Scholar]
- 5.Bravo, R., R. Frank, P. A. Blundell, and H. Macdonald-Bravo. 1987. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature 326:515-517. [DOI] [PubMed] [Google Scholar]
- 6.Bravo, R., and H. Macdonald-Bravo. 1987. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J. Cell Biol. 105:1549-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brondello, J. M., M. N. Boddy, B. Furnari, and P. Russell. 1999. Basis for the checkpoint signal specificity that regulates Chk1 and Cds1 protein kinases. Mol. Cell. Biol. 19:4262-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, E. J. 2003. The ATR-independent DNA replication checkpoint. Cell Cycle 2:188-189. [PubMed] [Google Scholar]
- 9.Brown, E. J., and D. Baltimore. 2003. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 17:615-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen, P. U., N. J. Bentley, R. G. Martinho, O. Nielsen, and A. M. Carr. 2000. Mik1 levels accumulate in S phase and may mediate an intrinsic link between S phase and mitosis. Proc. Natl. Acad. Sci. USA 97:2579-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark, W., E. J. Black, A. MacLaren, U. Kruse, N. LaThangue, P. K. Vogt, and D. A. Gillespie. 2000. v-Jun overrides the mitogen dependence of S-phase entry by deregulating retinoblastoma protein phosphorylation and E2F-pocket protein interactions as a consequence of enhanced cyclin E-cdk2 catalytic activity. Mol. Cell. Biol. 20:2529-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desany, B. A., A. A. Alcasabas, J. B. Bachant, and S. J. Elledge. 1998. Recovery from DNA replicational stress is the essential function of the S-phase checkpoint pathway. Genes Dev. 12:2956-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enoch, T., and P. Nurse. 1990. Mutation of fission yeast cell cycle control genes abolishes dependence of mitosis on DNA replication. Cell 60:665-673. [DOI] [PubMed] [Google Scholar]
- 14.Feijoo, C., C. Hall-Jackson, R. Wu, D. Jenkins, J. Leitch, D. M. Gilbert, and C. Smythe. 2001. Activation of mammalian Chk1 during DNA replication arrest: a role for Chk1 in the intra-S phase checkpoint monitoring replication origin firing. J. Cell Biol. 154:913-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furnari, B., A. Blasina, M. N. Boddy, C. H. McGowan, and P. Russell. 1999. Cdc25 inhibited in vivo and in vitro by checkpoint kinases Cds1 and Chk1. Mol. Biol. Cell 10:833-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallant, P., and E. A. Nigg. 1992. Cyclin B2 undergoes cell cycle-dependent nuclear translocation and, when expressed as a non-destructible mutant, causes mitotic arrest in HeLa cells. J. Cell Biol. 117:213-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graves, P. R., L. Yu, J. K. Schwarz, J. Gales, E. A. Sausville, P. M. O'Connor, and H. Piwnica-Worms. 2000. The Chk1 protein kinase and the Cdc25C regulatory pathways are targets of the anticancer agent UCN-01. J. Biol. Chem. 275:5600-5605. [DOI] [PubMed] [Google Scholar]
- 18.Jin, P., Y. Gu, and D. O. Morgan. 1996. Role of inhibitory CDC2 phosphorylation in radiation-induced G2 arrest in human cells. J. Cell Biol. 134:963-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin, P., S. Hardy, and D. O. Morgan. 1998. Nuclear localization of cyclin B1 controls mitotic entry after DNA damage. J. Cell Biol. 141:875-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, S. M., and J. A. Huberman. 2001. Regulation of replication timing in fission yeast. EMBO J. 20:6115-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumagai, A., and W. G. Dunphy. 1995. Control of the Cdc2/cyclin B complex in Xenopus egg extracts arrested at a G2/M checkpoint with DNA synthesis inhibitors. Mol. Biol. Cell 6:199-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumagai, A., Z. Guo, K. H. Emami, S. X. Wang, and W. G. Dunphy. 1998. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J. Cell Biol. 142:1559-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumagai, A., P. S. Yakowec, and W. G. Dunphy. 1998. 14-3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts. Mol. Biol. Cell 9:345-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lew, D. J., and S. Kornbluth. 1996. Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr. Opin. Cell Biol. 8:795-804. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay, H. D., D. J. Griffiths, R. J. Edwards, P. U. Christensen, J. M. Murray, F. Osman, N. Walworth, and A. M. Carr. 1998. S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes Dev. 12:382-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopes, M., C. Cotta-Ramusino, A. Pellicioli, G. Liberi, P. Plevani, M. Muzi-Falconi, C. S. Newlon, and M. Foiani. 2001. The DNA replication checkpoint response stabilizes stalled replication forks. Nature 412:557-561. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Girona, A., K. Tanaka, X. B. Chen, B. A. Baber, C. H. McGowan, and P. Russell. 2001. Serine-345 is required for Rad3-dependent phosphorylation and function of checkpoint kinase Chk1 in fission yeast. Proc. Natl. Acad. Sci. USA 98:11289-11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundgren, K., N. Walworth, R. Booher, M. Dembski, M. Kirschner, and D. Beach. 1991. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell 64:1111-1122. [DOI] [PubMed] [Google Scholar]
- 29.Morgan, D. O. 1995. Principles of CDK regulation. Nature 374:131-134. [DOI] [PubMed] [Google Scholar]
- 30.Murakami, H., and H. Okayama. 1995. A kinase from fission yeast responsible for blocking mitosis in S phase. Nature 374:817-819. [DOI] [PubMed] [Google Scholar]
- 31.Nyberg, K. A., R. J. Michelson, C. W. Putnam, and T. A. Weinert. 2002. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 36:617-656. [DOI] [PubMed] [Google Scholar]
- 32.O'Connell, M. J., J. M. Raleigh, H. M. Verkade, and P. Nurse. 1997. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 16:545-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Connell, M. J., N. C. Walworth, and A. M. Carr. 2000. The G2-phase DNA-damage checkpoint. Trends Cell Biol. 10:296-303. [DOI] [PubMed] [Google Scholar]
- 34.O'Farrell, P. H. 2001. Triggering the all-or-nothing switch into mitosis. Trends Cell Biol. 11:512-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng, C. Y., P. R. Graves, R. S. Thoma, Z. Wu, A. S. Shaw, and H. Piwnica-Worms. 1997. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277:1501-1505. [DOI] [PubMed] [Google Scholar]
- 36.Prokhorova, T. A., K. Mowrer, C. H. Gilbert, and J. C. Walter. 2003. DNA replication of mitotic chromatin in Xenopus egg extracts. Proc. Natl. Acad. Sci. USA 100:13241-13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raleigh, J. M., and M. J. O'Connell. 2000. The G(2) DNA damage checkpoint targets both Wee1 and Cdc25. J. Cell Sci. 113:1727-1736. [DOI] [PubMed] [Google Scholar]
- 38.Rao, P., and R. T. Johnson. 1970. Mammalian cell fusion: studies on the regulation of DNA synthesis and mitosis. Nature 225:159-164. [DOI] [PubMed] [Google Scholar]
- 39.Rhind, N., B. Furnari, and P. Russell. 1997. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 11:504-511. [DOI] [PubMed] [Google Scholar]
- 40.Rhind, N., and P. Russell. 2000. Chk1 and Cds1: linchpins of the DNA damage and replication checkpoint pathways. J. Cell Sci. 113:3889-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhind, N., and P. Russell. 2001. Roles of the mitotic inhibitors Wee1 and Mik1 in the G(2) DNA damage and replication checkpoints. Mol. Cell. Biol. 21:1499-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhind, N., and P. Russell. 1998. Tyrosine phosphorylation of cdc2 is required for the replication checkpoint in Schizosaccharomyces pombe. Mol. Cell. Biol. 18:3782-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santocanale, C., and J. F. Diffley. 1998. A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature 395:615-618. [DOI] [PubMed] [Google Scholar]
- 44.Takai, H., K. Tominaga, N. Motoyama, Y. A. Minamishima, H. Nagahama, T. Tsukiyama, K. Ikeda, K. Nakayama, and M. Nakanishi. 2000. Aberrant cell cycle checkpoint function and early embryonic death in Chk1(−/−) mice. Genes Dev. 14:1439-1447. [PMC free article] [PubMed] [Google Scholar]
- 45.Takizawa, C. G., and D. O. Morgan. 2000. Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr. Opin. Cell Biol. 12:658-665. [DOI] [PubMed] [Google Scholar]
- 46.Toyoshima, F., T. Moriguchi, A. Wada, M. Fukuda, and E. Nishida. 1998. Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. EMBO J. 17:2728-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walworth, N., S. Davey, and D. Beach. 1993. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature 363:368-371. [DOI] [PubMed] [Google Scholar]
- 47a.Xu, B., S. T. Kim, D. S. Lim, and M. B. Kastan. 2002. Two molecularly distinct G2/M checkpoints are induced by ionizing radiation. Mol. Cell. Biol. 22:1049-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zachos, G., M. Rainey, and D. A. Gillespie. 2003. Lethal errors in checkpoint control—life without Chk1. Cell Cycle 2:14-16. [DOI] [PubMed] [Google Scholar]
- 49.Zachos, G., M. D. Rainey, and D. A. Gillespie. 2003. Chk1-deficient tumour cells are viable but exhibit multiple checkpoint and survival defects. EMBO J. 22:713-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng, Y., K. C. Forbes, Z. Wu, S. Moreno, H. Piwnica-Worms, and T. Enoch. 1998. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature 395:507-510. [DOI] [PubMed] [Google Scholar]
- 51.Zhao, H., and H. Piwnica-Worms. 2001. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol. 21:4129-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]