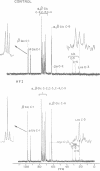
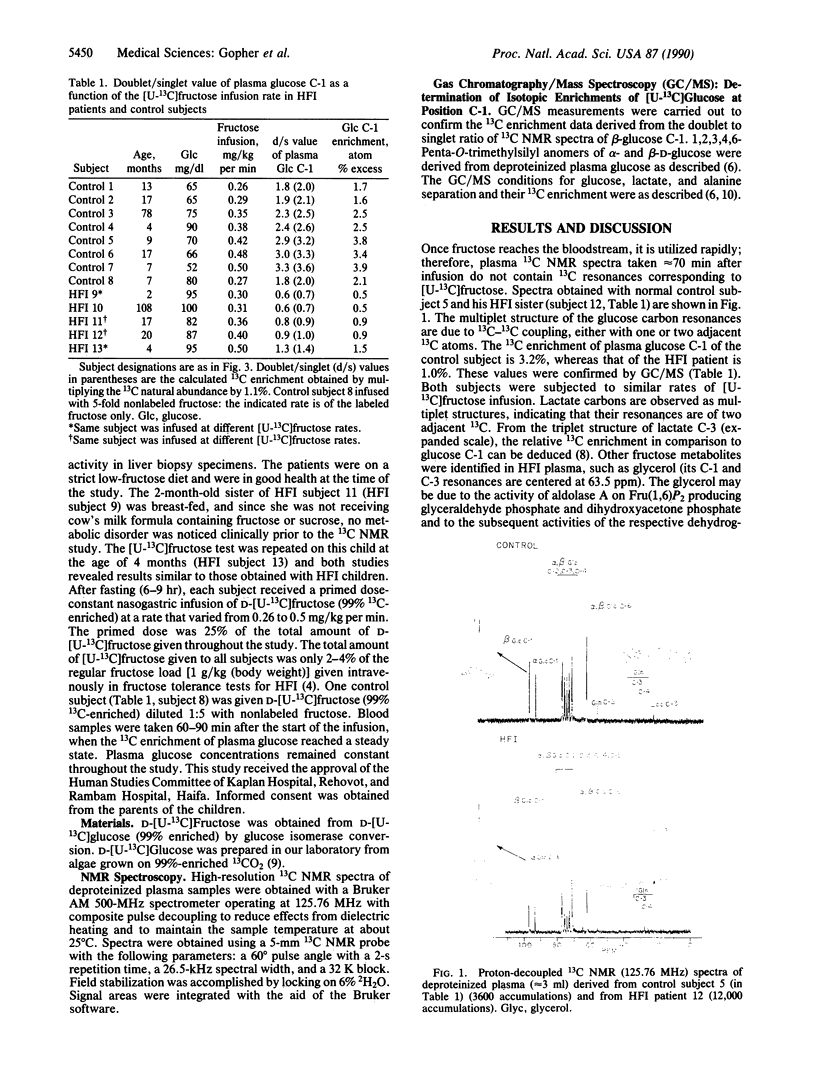
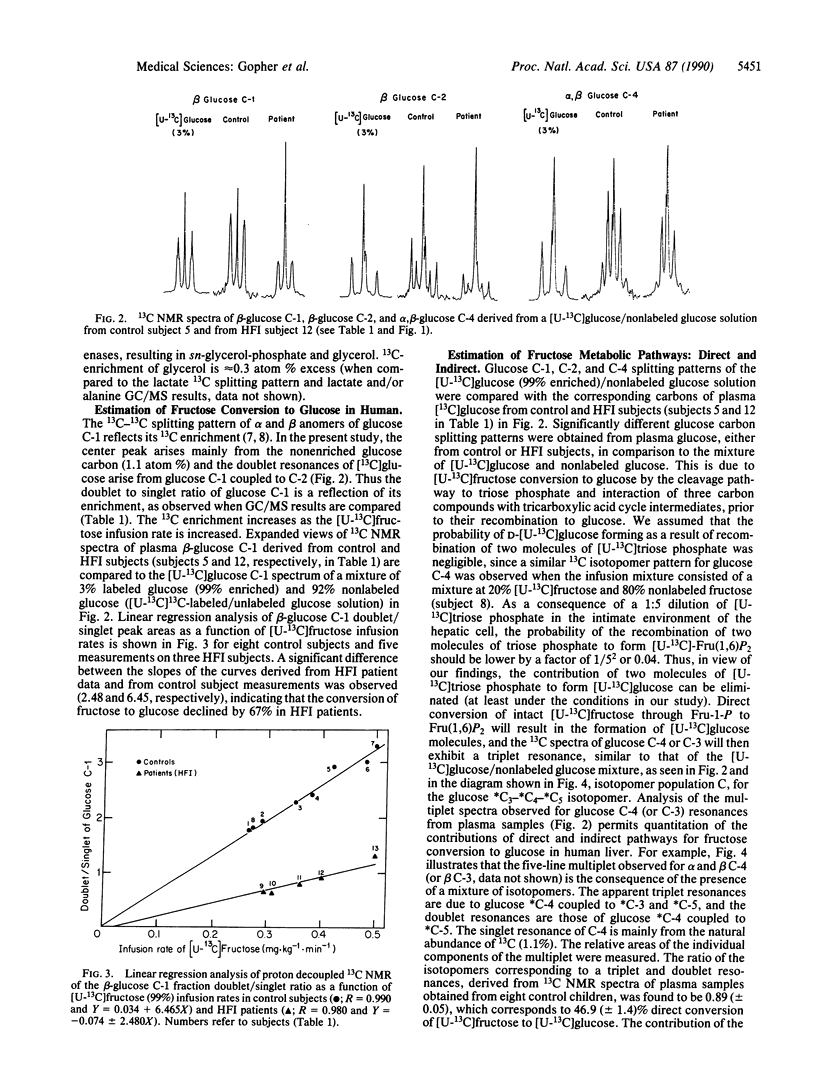
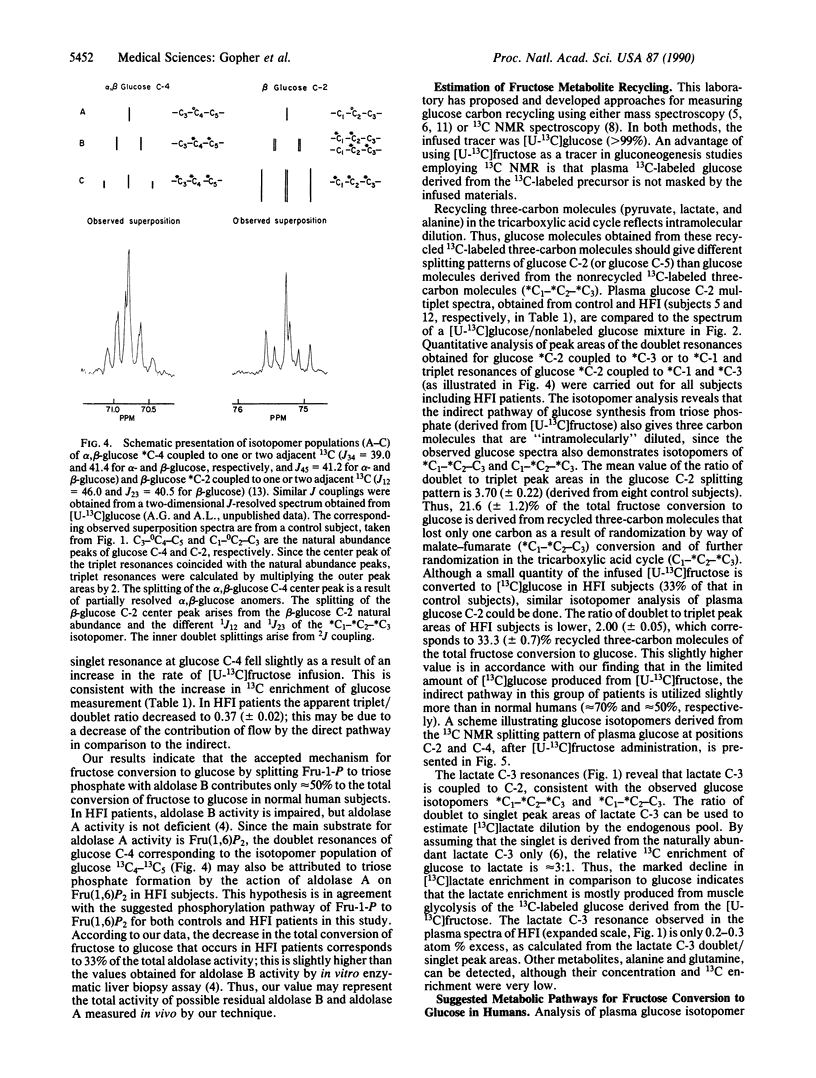
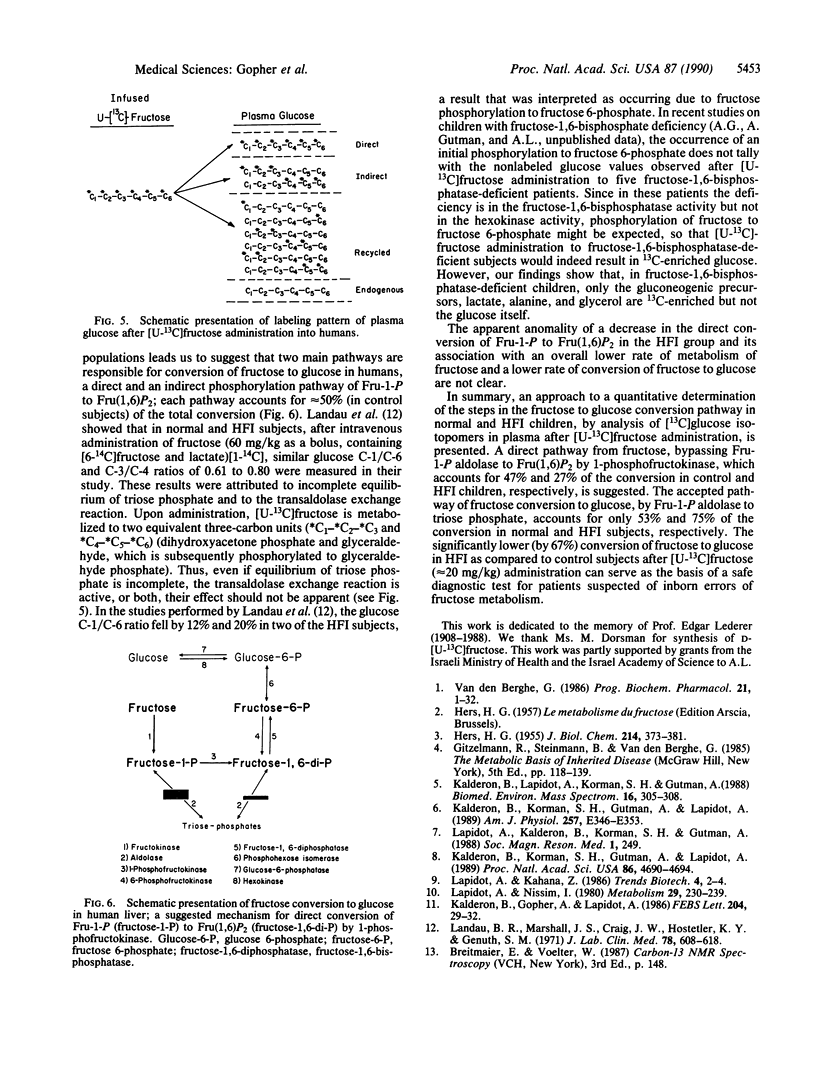
Abstract
An inborn deficiency in the ability of aldolase B to split fructose 1-phosphate is found in humans with hereditary fructose intolerance (HFI). A stable isotope procedure to elucidate the mechanism of conversion of fructose to glucose in normal children and in HFI children has been developed. A constant infusion of D-[U-13C]fructose was given nasogastrically to control and to HFI children. Hepatic fructose conversion to glucose was estimated by examination of 13C NMR spectra of plasma glucose. The conversion parameters in the control and HFI children were estimated on the basis of doublet/singlet values of the plasma beta-glucose C-1 splitting pattern as a function of the rate of fructose infusion (0.26-0.5 mg/kg per min). Significantly lower values (approximately 3-fold) for fructose conversion to glucose were obtained for the HFI patients as compared to the controls. A quantitative determination of the metabolic pathways of fructose conversion to glucose was derived from 13C NMR measurement of plasma [13C]glucose isotopomer populations. The finding of isotopomer populations of three adjacent 13C atoms at glucose C-4 (13C3-13C4-13C5) suggests that there is a direct pathway from fructose, by-passing fructose-1-phosphate aldolase, to fructose 1,6-bisphosphate. The metabolism of fructose by fructose-1-phosphate aldolase activity accounts for only approximately 50% of the total amount of hepatic fructose conversion to glucose. It is suggested that phosphorylation of fructose 1-phosphate to fructose 1,6-bisphosphate by 1-phosphofructokinase occurs in human liver (and intestine) when fructose is administered nasogastrically; 47% and 27% of the total fructose conversion to glucose in controls and in HFI children, respectively, takes place by way of this pathway. In view of the marked decline by 67% in synthesis of glucose from fructose in HFI subjects found in this study, the extent of [13C]glucose formation from a "trace" amount (approximately 20 mg/kg) of [U-13C]fructose infused into the patient can be used as a safe and noninvasive diagnostic test for inherent faulty fructose metabolism.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HERS H. G. The conversion of fructose-1-C14 and sorbitol-1-C14 to liver and muscle glycogen in the rat. J Biol Chem. 1955 May;214(1):373–381. [PubMed] [Google Scholar]
- Kalderon B., Gopher A., Lapidot A. Metabolic pathways leading to liver glycogen repletion in vivo, studied by GC-MS and NMR. FEBS Lett. 1986 Aug 11;204(1):29–32. doi: 10.1016/0014-5793(86)81381-3. [DOI] [PubMed] [Google Scholar]
- Kalderon B., Korman S. H., Gutman A., Lapidot A. Estimation of glucose carbon recycling in children with glycogen storage disease: A 13C NMR study using [U-13C]glucose. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4690–4694. doi: 10.1073/pnas.86.12.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon B., Korman S. H., Gutman A., Lapidot A. Glucose recycling and production in glycogenosis type I and III: stable isotope technique study. Am J Physiol. 1989 Sep;257(3 Pt 1):E346–E353. doi: 10.1152/ajpendo.1989.257.3.E346. [DOI] [PubMed] [Google Scholar]
- Kalderon B., Lapidot A., Korman S. H., Gutman A. Glucose recycling and production in children with glycogen storage disease type I, studied by gas chromatography/mass spectrometry and (U-13C)glucose. Biomed Environ Mass Spectrom. 1988 Oct;16(1-12):305–308. doi: 10.1002/bms.1200160158. [DOI] [PubMed] [Google Scholar]
- Landau B. R., Marshall J. S., Craig J. W., Hostetler K. Y., Genuth S. M. Quantitation of the pathways of fructose metabolism in normal and fructose-intolerant subjects. J Lab Clin Med. 1971 Oct;78(4):608–618. [PubMed] [Google Scholar]
- Lapidot A., Nissim I. Regulation of pool sizes and turnover rates of amino acids in humans: 15N-glycine and 15N-alanine single-dose experiments using gas chromatography-mass spectrometry analysis. Metabolism. 1980 Mar;29(3):230–239. doi: 10.1016/0026-0495(80)90064-5. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G. Fructose: metabolism and short-term effects on carbohydrate and purine metabolic pathways. Prog Biochem Pharmacol. 1986;21:1–32. [PubMed] [Google Scholar]