Abstract
The inhibition effect of persimmon peel extracts (PPE) (0.05(PPE-0.05), 0.1(PPE-0.1), and 0.2 g(PPE-0.2) per meat sample) on lipid and protein oxidation of pork patties during chilled storage for 12 days were investigated and compared to ascorbic acid (As-0.05) and butylhydroxytoluene (BHT) (BHT-0.01). The meat samples treated with PPE had greater (p<0.05) a* values comparing control in raw pork patties meat from day 4 of storage. The addition of PPE at all concentrations on meat samples effectively inhibited the formation of oxidation products as shown by decreasing conjugated dienes (CD), peroxide values (POVs), thiobarbituric acid reaction substances (TBARS), and carbonyl content during chilled storage for 12 d. The PPE-0.2 and BHT-0.01 had the lowest in decrease rate of free thiol content (0.24 and 0.22 times) during chilled storage. Therefore, results of this study suggest that PPE can be considered a potential antioxidant against lipid and protein oxidation of raw meat products.
Keywords: persimmon peel, plant basis antioxidant, lipid oxidation, protein oxidation, pork patties
Introduction
Lipids are extremely susceptible to oxidation upon exposure to the atmosphere, such as grinding, cooking, and storage in the processing of meat products. Lipid oxidation results in undesired flavor (rancidity) and texture, brown color, and the formation of toxic compounds such as malondialdehyde (MDA) and cholesterol oxidation products in meat and meat products (Choe et al., 2014). Antioxidants are therefore needed to prevent the deterioration of meat quality by acting as free radical scavengers. Currently, synthetic antioxidants such as t-butyl-4-hydroxyanisole (BHA) and 2,6-di-t-butyl-p-hydroxytoluene (BHT) are mainly used as antioxidants owing to their chemical stability, strong antioxidant activity, and lower cost (Verhagen et al., 1994). However, there is a significant increase in consumer interest in natural antioxidants, because synthetic antioxidants have potential carcinogenic properties (Ahn et al., 2002). In the past few years, the natural antioxidant effects of natural sources, which contain polyphenol compounds, on various types of meat products have been actively studied (Ganhão et al., 2010; Packer et al., 2015; Selani et al., 2016). Furthermore, the natural antioxidant effects of unutilized or valueless agricultural by-products such as pomegranate rind powder, and almond skin have been studied on muscle foods (Almeida et al., 2015; Naveena et al., 2008; Prasetyo et al., 2008).
Protein oxidation occurs through hydroxyl free radical chain reactions resulting in secondary lipid oxidation products and in the formation of disulfide bonds and dityrosine, fragmentation of the peptide backbone resulting in the modification of amino acid side chains, an increase of carbonyl compounds, and changes in amino acid composition (Stadtman and Levine 2003). Eventually, these changes affect the quality properties of meat and meat products.
Persimmon (Diospyros kaki Thumb), which contains bioactive substances such as phenol compounds, carotenoids, vitamin A, and C, has been used in folk medical treatment for diarrhea, cough, hemostasis, and high blood pressure (Choe et al., 2014; Kim et al., 2003). In particular, it has been studied on antioxidant and antidiabetic activities of persimmon peel in biological system (Gorinstein et al., 1998; Yokozawa et al., 2007). Previous author has been observed that peel contained higher amounts of polyphenol and carotenoids than pulp on persimmon (Gorinstein et al., 1998). However, the persimmon peel is not utilized and this usually disposed of improperly in the environment in spite of potential ability as natural antioxidant. Thus, it is necessary to find the way to utilize the peel as a food source to reduce environmental waste and add value.
No study has investigated the inhibition of lipid and protein oxidation in raw ground meat by persimmon peel, an agricultural by-product and natural antioxidant. Therefore, the aim of this study was to examine the inhibition activity of persimmon peel extract (PPE) at different concentrations (0.05 g, 0.1 g, and 0.2 g per 100 g meat samples) on lipid and protein oxidation of raw ground pork by measuring pH values, color, CD, POVs, TBARS, carbonyl content, and free thiol content during refrigerated storage for 12 d.
Materials and Methods
Preparation of persimmon peel extract (PPE)
Persimmon peel was obtained after drying persimmon from Gwangyang, Korea. The persimmon peel was cut into small pieces, washed 3 times using tap water, dried using a hot-air dryer (Enex-Co-600, Enex, Korea) at 50ºC for 15 h, and powdered using a 35-mesh sieve. The dried persimmon peel was pulverized using a blender (KA-2610, Jworld Tech, Korea) for 30 s and screened through a 35-mesh sieve, and stored at −20°C until use. The dried persimmon peel powder (15 g) was extracted twice with 300 mL ethanol/DW (70/30, v/v) overnight in a shaker (VS-8480, Vison Scientific, Korea) at room temperature. The extract was filtered through Whatman No. 1 filter paper and the solvent was removed using a vacuum evaporator (CCA-1110, Rikakikai, Japan) at 45°C to dryness. After the evaporation of ethanol, the persimmon peel ethanol extracts (5 g) were dissolved in 100 mL of distilled water (DW). The extracted persimmon peel by ethanol/DW (70/30, v/v) had the following color characteristics based on the International Commission for Illumination (CIE) scheme: L*, 9.02; a*, 0.59; and b*, 4.96. Based on the results of previous study (Choe et al., 2014), the extraction solvent (70% ethanol) was selected including the most effective antioxidant ability (total phenols: 12.39 mg gallic acid/g of dried extract, IC50 of DPPH: 243.7 ppm).
Preparation of meat samples
M. biceps femoris, M. semitendinosus, and M. semimembranosus muscle (average pH: 5.72) from fresh pork and back fat were purchased from local market (Hongju Meat Co., Korea), at 48 h postmortem. All subcutaneous and intramuscular fat and visible connective tissues were removed from the fresh ham muscles. For each batch, lean pork meat (7,350 g) and fat (2,000 g) were ground through 3-mm ground plate and samples were assigned to 6 treatments. Each meat sample (1,500 g) was added with cold water (75 g) and salt (22.5 g). The ground meat samples were then treated with no antioxidant (control), with 0.05 g, 0.1 g, or 0.2 g PPE (PPE-0.05, PPE-0.1, or PPE-0.2, respectively), with 0.05 g ascorbic acid (As-0.05), or with 0.01 g buthylhydroxytoluene (BHT-0.01) per 100 g of meat samples. These percentages were based on the formula weight of the minced meat samples without the antioxidant extract. Samples were then hand mixed for 5 min vigorously. Then, 300±5 g (small portion) of mixed meat per treatment from each batch were taken and spread to a thickness of 2.5 cm to obtain in a total of 5 small portion of samples (5 storage time). The samples were packed under vacuum (25%) in polyethylene bags film bags (thickness: 0.07 mm) using a vacuum packaging machine (FJ-500 XL, Fujee Tech, Korea) and stored at 3±1ºC in dark for 1, 4, 7, 10, and 12 d. All treatments were replicated with two times at two different days.
Physicochemical analysis
The instrumental color analyses of raw ground pork were conducted as follows. Color measurements were made using a colorimeter (Chromameter CR-210, Minolta, Japan; Illuminate C), calibrated with a white standard plate (L*=97.83, a*=−0.43, b*=+1.98), consisting of a measuring area of diameter 8 mm, and an illumination area of diameter 50 mm. The color values (L*, a*, and b*) were measured in three different locations and averaged for data analysis.
The pH values of meat samples were determined using a pH meter (Model 340, Mettler-Toledo GmbH, Switzerland). The pH of pork product was measured after blending 5 g of samples with 20 mL of distilled water for 60 s in a homogenizer (Ultra-Turrax SK15, Janke & Kunkel, Germany).
Analysis of conjugated diene (CD) and peroxide value (POV)
Lipids were extracted by the methods Folch et al. (1957), using a chloroform:methanol solvent system (2:1). The lipid extracts were evaporated at 45±2°C and concentrated with a rotary evaporator (Rotary evaporator N-1000, Eyela, Japan), leaving the extracted lipids for CD and POVs analyses immediately.
Fifteen milligrams of extracted sample lipids were placed in a 25 mL volumetric flask and brought to volume with isooctane. The samples were mixed and the absorbance read at 234 nm against an isooctane blank using a UV/VIS spectrophotometer (Libra S22, Biochrom Ltd., England). The CD concentration was calculated using a molar extinction coefficient of 25,200 mol−1 cm−1 and expressed as μmol mg−1 meat lipid sample.
The POVs of all samples were determined according to AOAC method 965.33 (AOAC, 1990). The lipid samples (0.5 g) were treated with 50 mL of solution containing acetic acid: chloroform =3:2 and then shaken vigorously. The mixture was added with 1 mL of saturated potassium iodide solution and then kept in the dark at 25°C for 10 min. The mixture was then added to 30 mL of distilled water and mixed. One milligram of starch solution was added to the mixture, as an indicator. The peroxide values were then determined using titration amount of 0.01 mol L−1 of sodium thiosulfate solutions from iodine and expressed as mEq/kg meat of sample.
TBARS values
Lipid oxidation was measured by the 2-thiobarbituric acid extraction method of Witte, Krause, and Bailey (1970) with modifications. Briefly, 10 g of meat sample was blended with 25 mL of a mixture 2:8 (v/v) of trichloroacetic acid/2 mol L−1 of phosphoric acid. The resultant slurry was filtered through Whatman NO.1 filter paper. Five mL of filtrate was added to vial containing 5 mL of thiobarbituric acid (0.005 mol L−1 in distilled water). The vials were then capped and heated in a boiling water bath for 10 min to develop chromogen and then cooled to room temperature. TBARS values were calculated using absorbance of each sample at 532 nm and a standard curve (8-50 nmol) of malondialdehyde (MDA) was constructed using freshly prepared by acidification of 1,1,3,3-tetraethoxypropane. TBARS values were expressed as MDA mg/kg meat.
Protein oxidation characteristics of pork patties
The measurement of protein carbonyls following their reaction with 2,4-dinitrophenylhydrazine (DNPH) was performed according to the method of Mercier et al. (1998) with some modifications. Meat sample (4 g) was homogenized in 20 mL of KCl (0.15 mol L−1) using a homogenizer (Ultra-Turrax SK15, Janke & Kunkel, Germany) for 30 s. Two equal aliquots of 100 μL were taken from mixture and into 2 mL micro tubes. One mixture was treated with 0.5 mL of HCl (2 mol L−1) (protein content measurement) and the other with an equal volume of DNPH in HCl (2 mol L−1) (carbonyl content measurement). Protein content was calculated from absorption at 280 nm using bovine serum albumin, as a standard. The total carbonyl content, expressed as nmol/mg protein, was quantified by a spectrophotometric assay at 370 nm.
Four gram of meat samples were blended with 20 mL of KCl (0.15 mol L−1) and then were filtered through Whatman NO. 1 filter paper. The filtrate was dissolved in buffer (50 mmol L−1 Tris-HCl, 1.25 mmol L−1 EDTA, SDS) and was centrifuged at 10,000 g for 15 min to remove insoluble protein. The total thiol (SH) content was determined by the method of Ellman (1959) using the precipitate and Ellman’s reagent (10 mmol L−1 5,5′-dithiobis-(2-nitrobenzoic acid); DTNB). The absorbance was measured at 412 nm using UV/VIS spectrophotometer (Libra S22, Biochrom Ltd., England). The SH concentration was calculated using a molar extinction coefficient of 13,600 mol−1 L cm−1. The protein content of the soluble protein fraction was determined using Biuret method (Gornall et al., 1949).
Statistical analysis
The entire data analyzed using two-way analysis of variance (ANOVA) including batch, treatment and storage time as fixed effects in the model. The fixed effect of batches was not significant difference for all data. This was performed on all the variables measured using the General Linear Model (GLM) procedure of the SAS statistical package (SAS version 9.3 for window, SAS Institute Inc., USA). The Duncan’s multiple range test (p<0.05) was used to determine differences between treatment means. Interaction between treatment and storage time served as fixed effect for physico-chemical properties, lipid oxidation, and protein oxidation (pH, CD values, POVs, TBARS, carbonyl and free thiol content).
Results and Discussion
Effect of PPE on physicochemical characteristics of pork patties
The incorporation of plant sources in meat and meat products is prone to change in quality properties such as color and flavor (Šulniūtė et al., 2016). Also, meat color is affected by various factors such as treatment, storage temperature and time, enzyme activity within meat tissue, and pH values. In present study, the addition of persimmon peel significantly affected the instrumental color evaluation (L*, a*, and b* values) of raw ground pork during the refrigerated storage for 12 d (Table 1). The L* values were greater on day 12 of chilled storage than on day 1 in control and As-0.05 (p<0.05). However, there was no significant difference in L* values of ground pork with PPE between at day 1 and day 12 of storage. The meat samples with PPE had higher L* values (p<0.05) until day 7, and then lower L* values (p<0.05) than that of control.
Table 1. The L* and a* values of raw pork patties containing various levels of persimmon peel extract (PPE) during chilled storage for 12 days.
| Treatments1) | Storage (d) | ||||
|---|---|---|---|---|---|
| 1 | 4 | 7 | 10 | 12 | |
| L* | |||||
| Control | 57.9±1.072)Cc | 62.1±0.92Da | 59.8±1.31Cb | 61.8±0.92Aa | 61.8±1.27Aa |
| PPE-0.05 | 61.0±1.62Bc | 66.2±0.66Ba | 62.6±1.20Bb | 59.4±1.35Bd | 61.2±1.19ABbc |
| PPE-0.1 | 60.8±1.33Bb | 64.4±1.25Ca | 62.1±0.97Bb | 58.0±1.60Cc | 61.1±1.06Bb |
| PPE-0.2 | 60.1±0.63Bc | 64.4±1.49Ca | 62.0±1.41Bb | 55.7±1.40Dd | 60.2±1.45Bc |
| As-0.05 | 58.1±1.45Cc | 62.9±1.31Da | 60.7±1.30Cb | 60.0±1.20Bc | 61.4±1.29ABb |
| BHT-0.01 | 63.8±1.69Ac | 68.9±1.42Aab | 65.9±0.37Ab | 60.5±0.64ABd | 61.7±1.10Ad |
| a* | |||||
| Control | 6.4±0.75Ba | 5.8±0.48Ba | 5.5±0.53Bb | 4.9±0.49Dc | 3.8±0.65Dd |
| PPE-0.05 | 6.2±1.29Bb | 8.6±0.77Aa | 8.3±1.18Aa | 6.1 ±0.75Bb | 7.7±0.83Bab |
| PPE-0.1 | 6.3±0.65Bb | 8.3±1.54Aa | 8.3±0.59Aa | 6.5±0.83Bb | 7.7±0.45Aab |
| PPE-0.2 | 6.4±0.38Bb | 8.6±0.44Ab | 8.4±0.88Aa | 6.7±0.39Bb | 7.9±0.87Abc |
| As-0.05 | 8.5±01.22A | 9.3±0.96A | 9.2±0.49A | 9. 8±0.46A | 9.4±0.45A |
| BHT-0.01 | 7.1±1.23Ba | 6.7±1.03Ba | 6.3±0.81Bab | 5.9±0.73Cb | 5.6±0.31Cb |
A-DMeans within columns with different superscript letters are significantly different (p<0.05).
a-dMeans within rows with different superscript letters are significantly different (p<0.05).
1)Control, ground pork without antioxidant; PPE-0.05, ground pork with 0.05% persimmon peel extract; PPE-0.1, ground pork with 0.1% persimmon peel extract; PPE-0.2, ground pork with 0.2% persimmon peel extract; As-0.05, pork with 0.05% ascorbic acid; BHT-0.01, pork with 0.01% butylhydroxytoluene (BHT). 2)All values are mean±standard deviation (n=4).
The a* values decreased with increasing storage time in the control and BHT-0.01 samples (p<0.05). There were no significant differences in a* values of meat samples treated with PPE and As between at initial and end of storage day. On day 12 of chilled storage, As-0.05 and meat samples treated with PPE samples maintained a* values compared to control and BHT-0.01 samples (p<0.05). These result indicates that addition of PPE, which contains phenolic compounds, can minimize the change in a* values compared with control and BHT-0.01 samples during chilled storage relatively. Sebranek et al. (2005) found that the a* values of raw pork sausage treated with BHT/BHA decreased until 84 d of storage, and then remained constant during frozen storage.
The b* values of control samples increased significantly with storage, whereas b* values of samples treated with PPE, As-0.05, and BHT-0.01 increased until day 4 and decreased thereafter (data was not shown). The results of this study presented incorporation of PPE into pork patties can minimize change of redness during 12 d of refrigerated storage.
The pH value can indicate amounts of microbial and chemical reaction influencing on food deterioration. In present study, there was no significant interaction between treatment and storage days on pH values. The pH values increased with the duration of storage in all treatments except As-0.05 (Table 2; p<0.05). The pH values of the As-0.05 samples increased during the first 10 d, and significantly decreased thereafter. Deymer and Vandekerckhove (1979) indicated that pH values may increase because of reactions between protein and ions, resulting in the production of ammonia as storage progresses. The pH values of pork product samples treated with PPE (pH 5.56) tended to be lower (p>0.05) than that of control values. This observation is likely due to low pH (5.56) of persimmon peel.
Table 2. The pH values of raw pork patties containing various levels of persimmon peel extract (PPE) during chilled storage for 12 days.
| Treatments1) | Storage (d) | ||||
|---|---|---|---|---|---|
| 1 | 4 | 7 | 10 | 12 | |
| Control | 5.81±0.052)Ab | 5.79±0.01Ab | 5.82±0.04Ab | 5.91±0.09Aa | 5.86±0.04Aa |
| PPE-0.05 | 5.66±0.05Bb | 5.70±0.06Bb | 5.80±0.06Aa | 5.81±0.06Ba | 5.81±0.06Aa |
| PPE-0.1 | 5.59±0.06Cb | 5.65±0.08Bb | 5.81±0.04Aa | 5.83±0.04ABa | 5.80±0.09Aa |
| PPE-0.2 | 5.62±0.04BCc | 5.62±0.03Bc | 5.79±0.02ABb | 5.90±0.03Aa | 5.83±0.06Aab |
| As-0.05 | 5.62±0.05BCd | 5.69±0.03Bc | 5.74±0.03Bb | 5.87±0.02ABa | 5.69±0.04Bc |
| BHT-0.01 | 5.61±0.04BCc | 5.63±0.04Bc | 5.74±0.04Bb | 5.89±0.04ABa | 5.87±0.03Aa |
A-CMeans within columns with different superscript letters are significantly different (p<0.05).
a-dMeans within rows with different superscript letters are significantly different (p<0.05).
1)Control: ground pork without antioxidant, PPE-0.05: ground pork with 0.05% persimmon peel extract, PPE-0.1: ground pork with 0.1% persimmon peel extract, PPE-0.2: ground pork with 0.2% persimmon peel extract, As-0.05: pork with 0.05% ascorbic acid, BHT-0.01: pork with 0.01% butylhydroxytoluene (BHT). 2)All values are mean±standard deviation (n=4).
Effect of PPE on lipid oxidation characteristics of pork patties
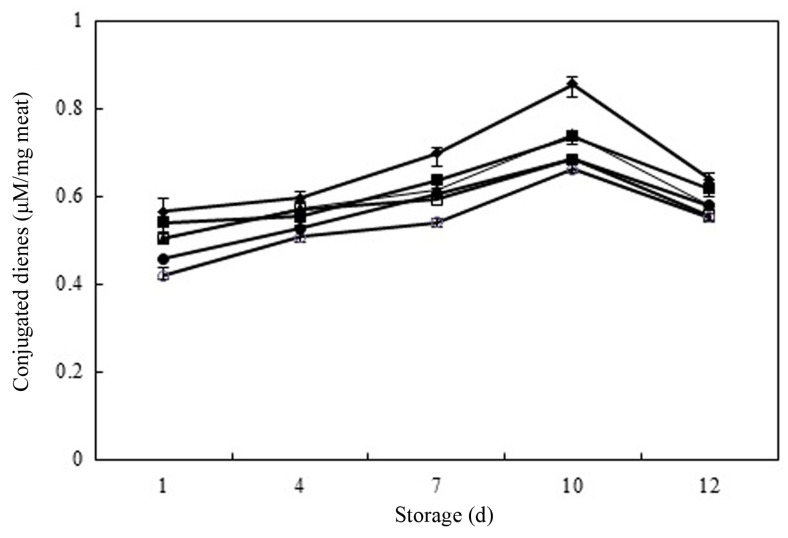
Lipid oxidation produces primary lipid oxidation products, hydroperoxides, which cause oxidation deterioration of lipids, during the initiation and propagation stages of oxidation. The amounts of primary lipid oxidation products can be determined by measuring the amount of CDs and POVs, which are most common parameters used to measure the oxidation process (Damodaran et al., 2008). Fig. 1 shows the effect of different concentrations of PPE on the formation of CD, which ranged from 0.419 to 0.855 μM/mg meat in raw pork patties during chilled storage. On day 1, the CD concentrations in meat samples followed the order: control ≥ As-0.05 ≥ PPE-0.05 ≥ PPE-0.1 > BHT-0.01 > PPE-0.2. Meat samples treated with PPE had significantly lower CD values than did control and As-0.05 samples during entire of storage period (p<0.05). The PPE-0.2 samples had the lowest peak (p<0.05) and reduced CD formation (~23% of the control value) on day 10. The CD concentration peaked on 10 d of chilled storage and decreased thereafter for all treatment (p<0.05). The reduction in CD values after day 10 indicated that the rate of formation of primary lipid oxidation products is slower than their rate of decomposition to secondary lipid oxidation products in the latter stages of oxidation. The PPE-0.2 samples had lower CD values than did the BHT- 0.01 samples on day 12 of chilled storage (p<0.05). This result indicates that PPE-0.1 and 0.2 had most effective inhibition of CD formation among the treatments during chilled storage, possibly due to the amount of phenolic compounds in PPE (Choe et al., 2014).
Fig. 1. Conjugated dienes (CD) (μM/mg meat) in raw pork patties containing various levels of persimmon peel extract (PPE) during chilled storage for 12 d. Values are Mean±S.D. (n=4). Error bars indicate standard deviation.
(◆) Control: ground pork without antioxidant, (▲) PPE-0.05: ground pork with 0.05% persimmon peel extract, (□) PPE-0.1: ground pork with 0.1% persimmon peel extract, (○) PPE-0.2: ground pork with 0.2% persimmon peel extract, (■) As-0.05: ground pork with 0.05% ascorbic acid, (●) BHT-0.01: ground pork with 0.01% butylhydroxytoluene (BHT).
POVs of raw ground pork with PPE are ranged between 3.23 and 16.85 mEq/kg meat during chilled storage for 12 d (Table 3). On day 1, control samples had the highest POVs and there were no differences (p>0.05) in the POVs of the PPE-0.05, PPE-0.1, As-0.05, and BHT-0.01 samples. POVs significantly increased until day 10 and were the highest values at day 10 of refrigerated storage in all treatments. These results were similar to that of CD values. The control sample had the highest rate of increase (29%; p<0.05) between days 4 and 7. In particular, POVs dramatically increased in all treatments except control between days 7 and 10. Increasing concentrations of PPE resulted in inhibition of the POV increase, with PPE-0.05, PPE-0.1, and PPE-0.2 exhibiting 23.3%, 33.8%, and 44.6% reduction compared to the control on storage day 10. Moreover, PPE-0.1 and PPE-0.2 showed inhibition of 11.1% and 25.5% in the POV increase at day 10 when compared with As-0.05. These results exhibited that addition of PPE can inhibit lipid oxidation effectively as polyphenols chelate or scavenge free radical compared with control and As-0.05. After day 10, POV decreased (p<0.05) due to the degradation of hydroperoxides for control, PPE-0.2, As- 0.05, and BHT-0.01. The inhibitory effects of PPE on lipid oxidation were in the following declining order: PPE-0.2 > BHT-0.01 > PPE-0.1 > As-0.05 > PPE-0.05 > control. Thus, incorporation of PPE, BHT, and As retard the production of hydroperoxides in raw pork samples during the initiation and propagation stage of oxidation relatively.
Table 3. Peroxide values (POVs) (mEq of peroxides/kg meat) and free thiol content (nmol/g) in raw pork patties containing various levels of persimmon peel extract (PPE) during chilled storage for 12 d.
| Treatments1) | Storage (d) | |||||
|---|---|---|---|---|---|---|
| 1 | 4 | 7 | 10 | 12 | ||
| POVs | Control | 5.7±0.202)Ae | 9.3±0.57Ad | 13.1±0.71Ac | 16.9±0.07Aa | 15.0±0.35Ab |
| PPE-0.05 | 4.1±0.28Bd | 6.5±0.71Bc | 8.4±0.28Bb | 12.9±1.44Ba | 12.1±0.25Ba | |
| PPE-0.1 | 3.8±1.12BCd | 5.3±0.41Cc | 6.7±0.42Cb | 11.2±0.53Ca | 10.4±0.53Da | |
| PPE-0.2 | 3.2±0.71Ce | 4.2±0.28Dd | 5.9±0.70Dc | 9.3±0.23Da | 8.6±0.23Eb | |
| As-0.05 | 3.8±0.28BCe | 6.1±0.14Bd | 8.7±0.41Bc | 12.6±0.42Ba | 11.6±0.42Cb | |
| BHT-0.01 | 3.4±0.59BCe | 5.2±0.42Cd | 7.1±0.29Cc | 10.7±0.41Ca | 9.9±0.41Db | |
| Free thiol content | Control | 33.0±2.00a | 29.0±1.54Bb | 25.6±1.68Dc | 21.3±2.02Dd | 18.7±0.28Dd |
| PPE-0.05 | 32.0±1.61a | 31.7±0.87ABa | 29.4±1.55BCb | 25.9±1.12Bc | 24.5±1.10Bc | |
| PPE-0.1 | 31.9±1.71a | 32.4±2.96ABa | 30.7±1.10ABab | 27.8±0.74ABbc | 26.0±1.67ABc | |
| PPE-0.2 | 33.9±1.94a | 33.7±0.46Aa | 32.1±0.45Aa | 28.7±0.39Ab | 26.9±0.78Ab | |
| As-0.05 | 30.6±0.61a | 30.1±1.85ABa | 28.0±0.48Cb | 24.0±0.79Cc | 21.0±1.11Cd | |
| BHT-0.01 | 33.8±2.09a | 33.2±0.76ABab | 31.0±0.83ABb | 28.4±0.88Ac | 25.9±0.31ABd | |
A-EMeans within columns with different superscript letters are significantly different (p<0.05).
a-eMeans within rows with different superscript letters are significantly different (p<0.05).
1)Control, ground pork without antioxidant; PPE-0.05, ground pork with 0.05% persimmon peel extract; PPE-0.1, ground pork with 0.1% persimmon peel extract; PPE-0.2, ground pork with 0.2% persimmon peel extract; As-0.05, pork with 0.05% ascorbic acid; BHT-0.01, pork with 0.01% butylhydroxytoluene (BHT). 2)All values are mean±standard deviation (n=4).
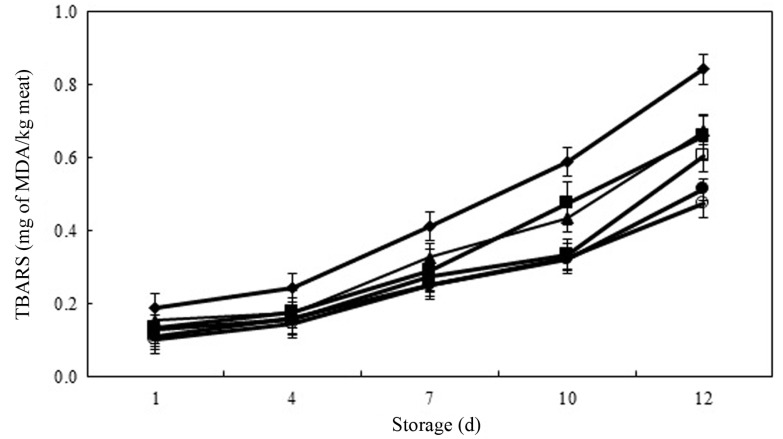
TBARS include secondary lipid oxidation products such as MDA, which forms a red color on reacting with thiobarbituric acid, resulting in a strong red color at high MDA concentrations. Fig. 2 shows the changes in TBARS values (0.10-0.84 mg of MDA/kg meat) in raw ground pork treated with PPE during 12 d of chilled storage. There was no significant difference in TBARS values among the all treatments on day 1 of storage. However, with increasing time of storage, TBARS values continually increased in all treatments. The TBARS values of meat product samples treated with PPE and BHT were significantly lower than the that of control on each day of chilled storage. The PPE-0.05 had similar inhibition ability of lipid oxidation as As-0.05 during the entire storage period. At the end of storage time, PPE-0.2 had similar TBARS value to BHT-0.01 (p>0.05). Furthermore, PPE-0.2 and BHT-0.01 were able to inhibit lipid oxidation to the extent of 42.9% and 39.3% in meat product samples, respectively. According to Han and Rhee (2005), the addition of various natural ingredients resulted in notable reduction (p<0.05) in the TBARS value, an indicator of lipid oxidation, in meat products during chilled storage. Also, Ibrahim et al. (2010) reported that the amounts of various phenolic substances contained in food additives strongly correlated with the suppression of lipid oxidation as inhibiting free radical formation and chelating transition metal ions in meat and meat products. In current study, inhibition activity of lipid oxidation increased in meat product samples with increasing of PPE concentrations. This result might be caused that the polyphenol derived from PPE chelated or scavenged the free radical and, as a result, delayed or inhibited the lipid oxidation of meat samples.
Fig. 2. Thiobarbituric acid reaction substances (TBARS) values (mg of MDA/kg meat) in raw pork patties containing various levels of persimmon peel extract (PPE) during chilled storage for 12 d.
Values are Mean±S.D. (n=4). Error bars indicate standard deviation. (◆) Control: ground pork without antioxidant, (▲) PPE-0.05: ground pork with 0.05% persimmon peel extract, (□) PPE-0.1: ground pork with 0.1% persimmon peel extract, (○) PPE-0.2: ground pork with 0.2% persimmon peel extract, (■) As-0.05: ground pork with 0.05% ascorbic acid, (●) BHT-0.01: ground pork with 0.01% butylhydroxytoluene (BHT).
Effect of PPE on protein oxidation characteristics of pork patties
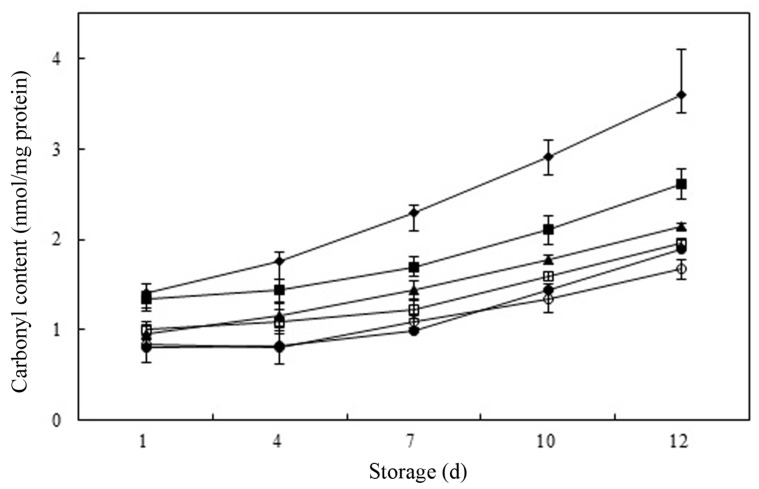
Amino acids such as lysine, histidine, proline, and arginine in meat result in a decrease protein functionality and solubility, and in deterioration of the quality of meat and meat products because they react with radicals, hydroperoxides, and secondary compounds that are products of lipid oxidation (Gardner, 1979). The extent of protein oxidation can be estimated by measuring carbonyl content using the method of derivatization with DNPH (Levine et al., 1994). In general, non-oxidized muscle tissue has a carbonyl content of 1 nmol/mg protein, whereas the carbonyl content in oxidized muscle tissue ranges from 2-14 nmol/mg protein, depending on the extent of oxidation, muscle type, and the actual initiator of oxidation (Mercier et al., 1998; Reznick and Packer, 1994). In this study, the protein carbonyl content ranged from 0.80 to 3.60 nmol/mg protein in raw meat product samples (Fig. 3). With increasing storage time, the amount of protein oxidation increased, as evidenced by the increase of carbonyl content in raw meat samples after day 4. At initial storage day, no significant differences were observed in carbonyl content between control and As-0.05 and treatment added with PPE and BHT. The meat samples with PPE, As-0.05, and BHT-0.01 showed a significantly greater (p<0.05) reduction in carbonyl formation than seen in control raw pork product samples after day 1. The carbonyl content sharply increased between days 7 and 10 in raw meat samples in all treatment. The control had the highest carbonyl content (3.60 nmol/mg protein) indicating the highest level of protein oxidation among the treatments. The PPE-0.05, PPE-0.1, PPE-0.2, As-0.05, and BHT-0.01 samples had percent inhibition values of 40.6%, 45.6%, 53.6%, 27.2%, and 47.5%, respectively, for protein oxidation at the end of the storage period, compared to control raw meat product samples. Ibrahim et al. (2010) found addition of avocado peel could retard protein oxidation (around 50%) compared to control in raw patties at the end of chilled storage (day 15). As expected, increasing concentrations of PPE resulted in increased of antioxidant activity during lipid oxidation of raw meat samples. The PPE-0.2 samples had the lowest carbonyl content (p<0.05) and this observation might be because the phenolic compounds in PPE, retard protein oxidation by either binding to the proteins, forming complexes with them, or inhibiting lipid oxidation (Choe et al., 2014; Siebert et al., 1996). To confirm the mechanism as antioxidant against protein oxidation, activity of phenolic compounds from persimmon peel such as radical scavenging and metal chelating activity in myofibrilar proteins would be studied.
Fig. 3. Carbonyl content (nmol/mg protein) in raw pork patties containing various levels of persimmon peel extract (PPE) during chilled storage for 12 d.
Values are Mean±S.D. (n=4). Error bars indicate standard deviation. (◆) Control: ground pork without antioxidant, (▲) PPE-0.05: ground pork with 0.05% persimmon peel extract, (□) PPE-0.1: ground pork with 0.1% persimmon peel extract, (○) PPE-0.2: ground pork with 0.2% persimmon peel extract, (■) As-0.05: ground pork with 0.05% ascorbic acid, (●) BHT-0.01: ground pork with 0.01% butylhydroxytoluene (BHT).
The free thiol content ranged from 18.69 to 33.90 nmol/mg protein, and no significant differences were observed in the free thiol content among all treatments in raw meat product samples at day 1 (Table 3). The free thiol content significantly declined after day 7 in all treatments except for PPE-0.1. Eymard et al. (2009) have reported similar results, studying protein oxidation in minced fish during refrigerated storage. The loss of thiol content was significantly different (p<0.05) in control, PPE-0.05, PPE-0.1, PPE-0.2, As-0.05, and BHT-0.01 raw meat patties, with decreasing percent values (43.4%, 23.7%, 18.5%, 20.6%, 31.4%, and 23.5%, respectively) during chilled storage for 12 d. In other words, PPE treatment significantly reduced the formation of disulfide bonds and it might be due to chelating or scavenging activity of PPE against free radicals compared to control and As-0.05 raw meat samples.
Conclusion
This study investigated the inhibition effect of PPE with various concentrations (0.05, 0.1, and 0.2 g per 100g meat samples) on lipid and protein oxidation in raw pork patties during chilled storage for 12 d compare to ascorbic acid (As-0.05) and BHT (BHT-0.01). The addition of PPE, which contains phenolic compounds, led to more stable a* values than those of control and BHT-0.01 samples during chilled storage. The PPE-0.2 had lower CDs and TBARS than other treatments during 12 d of chilled storage. Increasing concentrations of PPE resulted in inhibition of the POVs increase, with PPE-0.05, PPE-0.1, and PPE-0.2 exhibiting 23.3% and 16.5%, 33.8% of control POVs in each of raw pork patties on storage day 10 having a peak in primary lipid oxidation. The addition of PPE retarded protein oxidation as shown by inhibiting the formation of carbonyl and disulfide bond in pork meat during chilled storage for 12 d. Given the results of this study, PPE has potential inhibition effects on lipid and protein oxidation and improvement on color stability as economic natural antioxidant in meat products during chilled storage. Further study in inhibition mechanism and effect of PPE on lipid and protein oxidation in purified muscle protein system should be required.
References
- 1.Ahn J., Grün I. U., Fernando L. N. Antioxidant properties of natural plant extracts containing polyphenolic compounds in cooked ground beef. Food Chem. Toxicol. 2002;67:1364–1369. [Google Scholar]
- 2.Almeida de P. L., Lima de S. N., Costa L. L., Oliveira de C. C., Damasceno K. A., Santos dos B. A., Campagnol P. C. B. Effect of jabuticaba peel extract on lipid oxidation, microbial stability and sensory properties of Bologna-type sausages during refrigerated storage. Meat Sci. 2015;110:9–14. doi: 10.1016/j.meatsci.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 3.AOAC. Official methods of analysis. 15th ed. Association of Official Analytical Chemists; Washington, DC, USA: 1990. pp. 777–788. [Google Scholar]
- 4.Choe J. H., Kim H. Y., Kim Y. J., Yeo E. J., Kim C. J. Antioxidant activity and phenolic content of persimmon peel extracted with different level of ethanol. Int. J. Food Prop. 2014;17:1779–1790. doi: 10.1080/10942912.2012.731460. [DOI] [Google Scholar]
- 5.McClements D. J., Decker E. A., editors. Fennema’s food chemistry. CRC Press; NY: 2008. pp. 207–209. [Google Scholar]
- 6.Deymer D. I., Vandekerckhove P. Compounds determining pH in dry sausage. Meat Sci. 1979;3:161–168. doi: 10.1016/0309-1740(79)90033-0. [DOI] [PubMed] [Google Scholar]
- 7.Ellman G. L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 8.Eymard S., Baron C. P., Jaconsen C. Oxidation of lipid and protein in horse mackerel (Trachurus trachurus) mince and washed minces during processing and storage. Food Chem. 2009;114:57–65. doi: 10.1016/j.foodchem.2008.09.030. [DOI] [Google Scholar]
- 9.Folch J., Lees M., Sloane Stanley G. H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 10.Ganhão R., Morcuende D., Estévez M. Protein oxidation in emulsified cooked burger patties with added fruit extracts: Influence on colour and texture deterioration during chill storage. Meat Sci. 2010;85:402–409. doi: 10.1016/j.meatsci.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Gardner H. W. Lipid hydroperoxides reactivity with proteins and amino acids a review. J. Agr. Food Chem. 1979;43:651–656. [Google Scholar]
- 12.Gorinstein S., Kulasek G. W., Bartnikowska E., Leontowicz M., Zemser M., Morawiec M., Trakhtenberg S. The influence of persimmon peel and persimmon pulp on the lipid metabolism and antioxidant activity of rats fed cholesterol. J. Nutr. Biochem. 1998;9:223–227. doi: 10.1016/S0955-2863(98)00003-5. [DOI] [Google Scholar]
- 13.Gornall A. C., Bardawill C. J., David M. M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949;177:751–767. [PubMed] [Google Scholar]
- 14.Han J., Rhee K. S. Antioxidant properties of selected Oriental non-culinary/nutraceutical herb extracts as evaluated in raw and cooked meat. Meat Sci. 2005;70:25–33. doi: 10.1016/j.meatsci.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim H. M., Abou-Arab A. A., Abu S. F. M. Addition of some natural plant extracts and their effects on lamb patties quality. J. Food Technol. 2010;8:134–142. doi: 10.3923/jftech.2010.134.142. [DOI] [Google Scholar]
- 16.Kim S. K., Lee G. D., Chung S. K. Monitoring on fermentation of persimmon vinegar from persimmon peel. Korean J. Food Sci. Technol. 2003;35:642–647. [Google Scholar]
- 17.Levine R. L., Williams J. A., Stadtman E. R., Shacter E. Carbonyl assays for determination oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 18.Mercier Y., Gatellier P., Viau M., Remigonon H., Renerre M. Effect of dietary fat and vitamin E on colour stability and on lipid and protein oxidation in turkey meat during storage. Meat Sci. 1998;48:301–318. doi: 10.1016/S0309-1740(97)00113-7. [DOI] [PubMed] [Google Scholar]
- 19.Naveena B. M., Sen A. R., Vaithiyanathan S., Babji Y., Kondaiah N. Comparative efficacy of pomegranate juice, pomegranate rind powder, extract and BHT as antioxidants in cooked chicken patties. Meat Sci. 2008;80:1304–1308. doi: 10.1016/j.meatsci.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Packer V. G., Melo P. S., Bergamaschi K. B., Selani M. M., Villanueva N. D. M., de Alencar S. M., Contreras-Castillo C. J. Chemical characterization, antioxidant activity and application of beetroot and guava residue extracts on the preservation of cooked chicken meat. J. Food Sci. Technol. 2015;52:7409–7416. doi: 10.1007/s13197-015-1854-8. [DOI] [Google Scholar]
- 21.Prasetyo M., Chia M., Hughey C, Were L. M. Utilization of electron beam irradiated almond skin powder as a natural antioxidant in ground top round beef. J. Food Sci. 2008;73:T1–T6. doi: 10.1111/j.1750-3841.2007.00641.x. [DOI] [PubMed] [Google Scholar]
- 22.Reznick A. Z., Packer L. Oxidative damage to proteins spectrophotometric method for carbonyl assay. Method Enzymol. 1994;233:357–363. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 23.Sebranek J. G., Sewalt V. H. H., Robbins K. L., Houser T. A. Comparison of a natural rosemary extract and BHA/BHT for relative antioxidant effectiveness in pork sausage. Meat Sci. 2005;69:289–296. doi: 10.1016/j.meatsci.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Selani M. M., Contreras-Castillo C. J., Shirahigue L. D., Gallo C. R., Plata-Oviedo M., Montes-Villanueva N. D. Wine industry residues extracts as natural antioxidants in raw and cooked chicken meat during frozen storage. Meat Sci. 2011;88:397–403. doi: 10.1016/j.meatsci.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Selani M. M., Shirado G. A. N., Margiotta G. B., Rasera M. L., Marabesi A. C., Piedade S. M. S., Contreras-Castillo C. J., Canniatti-Brazaca S. G. Pineapple by-product and canola oil as partial fat replacers in low-fat beef burger: Effects on oxidative stability, cholesterol content and fatty acid profile. Meat Sci. 2016;115:9–15. doi: 10.1016/j.meatsci.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Siebert K. J., Troukhanova N. V., Lynn P. Y. Nature of polyphenol-protein interactions. J. Agr. Food Chem. 1996;44:80–85. doi: 10.1021/jf9502459. [DOI] [Google Scholar]
- 27.Stadtman E. R., Levine R. L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 28.Šulniūtė V., Jaime I., Rovira J., Venskutonis P. R. Rye and wheat bran extracts isolated with pressurized solvents increase oxidative stability and antioxidant potential of beef meat hamburgers. J. Food Sci. 2016;81:519–527. doi: 10.1111/1750-3841.13197. [DOI] [PubMed] [Google Scholar]
- 29.Verhagen H., Deerenberg I., Marx A., Hoor F., Henderson P. T., Kleinjans J. C. S. Estimate of the daily dietary intake of butylated hydroxyanisole and butylated hydroxytoluene in the Netherlands. Food Chem. Toxicol. 1994;28:215–220. doi: 10.1016/0278-6915(90)90033-j. [DOI] [PubMed] [Google Scholar]
- 30.Witte V. C., Krause G. F., Bailey M. E. A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage. J. Food Sci. 1970;35:582–585. doi: 10.1111/j.1365-2621.1970.tb04815.x. [DOI] [Google Scholar]
- 31.Yokozawa T., Kim Y. A., Kim H. Y., Lee Y. A., Nonaka G. Protective effect of persimmon peel polyphenol against high glucose-induced oxidative stress in LLC-PK1 cells. Food Chem. Toxicol. 2007;45:1979–1987. doi: 10.1016/j.fct.2007.04.018. [DOI] [PubMed] [Google Scholar]