Abstract
The aim of the present study was to investigate on the effects of a low-frequency pulsed electromagnetic field (LF-PEMF) in an experimental cell model of Alzheimer's disease (AD) to assess new therapies that counteract neurodegeneration. In recent scientific literature, it is documented that the deep brain stimulation via electromagnetic fields (EMFs) modulates the neurophysiological activity of the pathological circuits and produces clinical benefits in AD patients. EMFs are applied for tissue regeneration because of their ability to stimulate cell proliferation and immune functions via the HSP70 protein family. However, the effects of EMFs are still controversial and further investigations are required. Our results demonstrate the ability of our LF-PEMF to modulate gene expression in cell functions that are dysregulated in AD (i.e., BACE1) and that these effects can be modulated with different treatment conditions. Of relevance, we will focus on miRNAs regulating the pathways involved in brain degenerative disorders.
1. Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder with irreversible progression that primarily affects the hippocampal and neocortical regions of the brain. Since the incidence of AD increases in the elderly and with the lengthening of human life, this disease is becoming one of the major health problems associated with aging [1]. There is currently no effective treatment against AD, and its pathogenesis remains unclear [2]. A lot of studies on AD have highlighted the possible involvement of genetic [3], immunological [4], and environmental causes [5]. Oxidative stress, disruption of calcium homeostasis, hormonal factors, inflammation, and vascular and cell cycle dysregulations have been associated with the disease [6]. The major microscopic abnormalities of AD, which form the basis of the histologic diagnosis, are β-amyloid (Aβ) plaques and neurofibrillary degeneration (tangles). Notably, the neuritic plaques are mainly composed of Aβ secreted through an aberrant proteolytic cleavage of the amyloid precursor protein (APP) [7]. There are progressive and eventually severe neuronal loss, synaptic loss, and reactive gliosis in the same regions that bear the burden of the plaques and tangles. The involvement of the hippocampus and amygdale in the early phases of AD causes synaptic dysfunctions, such as the block of long-term potentiation (LTP), with consequent damage of the processes of learning and memory [8].
On the other hand, a new study about the brain and the electromagnetic fields (EMFs) showed, in vivo, that the EMFs could protect from the cognitive impairment or improve the memory in mice [9], whereas other in vitro studies indicated a possible role of EMFs as copromoters of tumor growth [10].
Besides age, family history, and inheritance, which are considered important risk factors, emerging evidence suggests that also environmental factors can influence AD development and progression, especially with regard to the sporadic disease which represents the most widespread form. In general, the physiopathological conditions within cells, tissues, and organs can be influenced by changes in the electromagnetic context to the extent that even their phenotype and functions can be altered by EMF exposure [11, 12]. Some literature data indicate that EMFs seem to play a role in the etiology of neurodegenerative disorders, including AD [13, 14]. Interestingly, although the debate on EMFs is still controversial, a pioneering field of research in AD is the deep brain stimulation via EMFs, which seems to modulate the neurophysiological activity of the pathological circuits and produce clinical benefits in AD patients [15]. Of relevance, in recent years, EMF brain stimulation techniques, such as the transcranial magnetic stimulation (TMS) (which noninvasively interacts with the brain activity), have been developed and applied to treat neurological diseases. TMS-induced cortical changes have resulted in enhanced neural plasticity. Indeed, an enhancement of the brain cortical excitability might induce a specific potentiation-like phenomenon, which would enable synaptic plasticity and promote recovery of a degraded function. Given these premises, there is currently a growing interest in applying EMFs as a therapeutic approach in psichiatric and neurological disorders [16]. Moreover, EMFs could be clinically used to re-establish cognitive performance in stroke patients [17, 18] and in patients suffering from neurodegenerative diseases [19, 20]. Presently, various clinical trials are ongoing to further investigate the possible positive effects of EMFs and TMS on AD (www.clinicaltrials.gov).
Despite the significant use of brain stimulation in clinical treatments, as mentioned, the effects of EMFs on the biological systems are not completely understood. In fact, it has been observed that, depending on the EMFs' “dose” and wavelength, the effects can shift from cytotoxicity to cytoprotection [21–23]. As recently reported [24], the electromagnetic waves are able to modulate the cytoskeleton function and to promote the neuronal differentiation of the bone marrow mesenchymal stem cells; in particular, EMFs promote the neuronal differentiation in vitro and the hippocampal neurogenesis in vivo by upregulating the Cav-1 channel activity [25–28], β-III-tubulin, MAP2 [29], and the brain-derived neurotrophic factor [30].
At a molecular level, it has been postulated that EMFs can affect the redox status within cells, thus evoking a general stress response [31] and increasing the expression of stress-related proteins [32]. Moreover, it has been reported that EMFs can delay cellular senescence [33]. As previously shown on an AD mice model, a high-frequency EMF treatment induced an improvement of cognitive functions, ascribed to an enhanced clearance of the amyloid plaques [9]. Conversely, in an in vitro cellular AD model overexpressing APP, prolonged EMFs caused a significantly increased secretion of Aβ1–42 [34], one of the most prone-to-aggregation APP derived fragments [7].
Of interest, it has been widely demonstrated, in vitro, that both low- and high-frequency EMFs can also modulate gene expression by acting on both transcriptional and posttranscriptional regulatory mechanisms [35–37]. Within this context, in both physiological and pathological conditions, posttranscriptional mechanisms are key determinants of the gene expression modulation, since they allow a rapid adaptation of protein levels to changing environmental conditions and can differently influence the cell fate. These mechanisms include the implication of a class of small noncoding RNA molecules, called miRNAs, able to regulate the gene expression mainly by base pairing to the 3′-UTR of specific target mRNAs [38]. Considering that miRNAs are predicted to regulate up to 90% of human genes [39], their physiological activity is critical for the maintenance of healthy conditions and their aberrant expression is associated with the pathological features of many diseases [38, 40].
In particular, mRNA is ~5% of the total cellular RNA and is poorly correlated with protein levels. It is increasingly clear that mRNA translation is a key focal point of gene expression regulation. Noteworthy for this project, miRNAs regulate the expression of key proteins involved in AD pathogenesis and the expression of certain miRNAs is altered in AD patients [41–45], thus suggesting that a dysfunctional miRNA-based regulatory system may represent a new etiologic factor for AD. Notably, an alteration of several miRNAs has been related to Aβ insult [46]. Many other miRNAs are emerging as regulators of the expression not only of APP but also of proteins involved in fundamental cellular processes such as cellular clearance and quality control systems which are altered in AD [47]. Recently, it has been suggested that miRNAs are also able to modulate cognitive and immune processes through direct or indirect alterations of the neuron-to-glia and/or the brain-to-body signaling [48]. In line with this concept, gene expression studies on AD and control subjects have shown differences in some miRNAs not only in the affected brain areas and in the cerebral spinal fluid but also in the peripheral districts, such as blood [49]. Very recently, the potential contribution of miRNAs to AD pathophysiology in humans and in various cellular and animal models has been remarked [50]. Furthermore, a lot of studies have documented the presence of miRNAs (and other RNAs) in the extracellular space after their release from the cells and in the circulating blood. These miRNAs are contained within a variety of different structures and protein/lipoprotein complexes [51, 52]. The circulating miRNAs appear to escape degradation via endogenous ribonuclease activity by residing in membrane-structured bodies as well as protein and lipid complexes [53]. miRNAs previously move through the bloodstream from one district to the others [54, 55]. Circulating miRNAs have emerged as candidate biomarkers for a long list of diseases and medical conditions [56]. Therefore, miRNAs may represent a fine-tuning of the signaling able to reach different body districts and able to integrate multiple inputs and outputs [57]. In this scenario, a deeper understanding of the relation between AD, EMFs, and miRNAs may help to shed more light on the molecular bases of this pathology, also opening the possibility towards the use of miRNAs as potential clinical biomarkers. For instance, it has been demonstrated that the transcranial electromagnetic stimulation of the brain through pulsed electromagnetic fields (PEMFs) can establish the reactivation of cognitive processes in AD patients and the reduction of Aβ in transgenic mice models for APP [9].
Considering these preconditions, the relationships between low-frequency PEMF (LF-PEMF) exposure and miRNAs regulating the proteins connected with altered functions in AD might explain the molecular basis of neuropathologies and show new therapies. miRNAs could be used as drugs to block the production of harmful proteins in new therapeutic strategies, because of their capacity to downregulate gene expression up to silencing, through the interaction with their target messengers. It is important to identify miRNAs that are modulated by exposure to LF-PEMFs in order to characterize the mechanisms associated with AD. Finally, since there are conflicting data about the effects of the electromagnetic fields and various publications deal toxic actions, further studies on LF-PEMFs' effects are necessary to verify whether the exposure to certain dosages may induce therapeutic advantages or, on the contrary, constitute an additional risk factor.
As a consequence, the aim of our study was to evaluate the modulation of miRNAs induced by LF-PEMF in the peripheral blood mononuclear cells (PBMCs) obtained from AD patients. PBMC exposure was realized using an electromagnetic bioreactor, with a frequency of 75 Hz [58]. Significant miRNAs were selected following a search in miRBase, TarBase, and miRTarBase databases. hsa-miR-107 regulates the enzyme BACE1, which exerts its action determining the amyloidogenic pathway of APP protein. Previous research identified a reduced expression of miRNA 107 in AD patients; since this miRNA negatively regulates BACE1, its lower expression promotes the production of toxic peptides Aβ40 and/or Aβ42. Then, we decided to check whether the treatment with LF-PEMF leads to an increased expression of miRNA 107 and, so, to a lower production of toxic peptides of Aβ, achieving a clinical benefit.
Moreover, we considered other significant miRNAs such as hsa-miR-335-5p that targets the MAPK1 gene, which encodes for one of the extracellular signal-regulated kinase (ERK) proteins, a mitogen-activated protein involved in cell growth and in the long-term potentiation (LTP) and acting in synapses regeneration. The same miRNA targets the GRIA1 (glutamate ionotropic receptor AMPA type subunit 1) gene, encoding for the AMPA receptor 1, which is essential for the first phase of LTP induction. Consequently, after LF-PEMF stimulation, a low expression of miR-335, which determines an increase of ERK and AMPA receptor, may be positive for both the cell regeneration and the neurological processes that regulate memory and learning. hsa-miR-26b-5p regulates the expression of the SLC17A6 gene, which encodes for the transporter vGLUT2. This transporter puts glutamate in presynaptic vesicles, which will be released to reach the postsynaptic terminal, where they can interact with AMPA and NMDA receptors. So, we decided to determine whether the action of the LF-PEMF can modulate the expression of this miRNA, since a possible increased vGLUT2 level may cause a higher intake of glutamate within the presynaptic vesicles. This protects the nervous system from the excitotoxicity of the glutamate itself and triggers LTP processes, improving memory and cognition.
1.1. Electromagnetic Fields and ROS in Alzheimer's Disease
At the molecular level, PEMFs have been hypothesized to affect the redox status of the cells, causing protein stress [32]. Also, antioxidant activity is modulated by PEMFs. A stimulation of the antioxidant activity, demonstrated by a decrease of 58.31% of the average in malondialdehyde value and by the balancing of the redox status, was observed in healthy volunteers [59]. The balance between the free radicals and antioxidants (redox equilibrium) is a critical point for the maintenance of homeostasis in a biological system: reactive oxygen species (ROS) at high doses are deleterious because they cause pathophysiological actions, whereas at low doses, they may be beneficial for normal physiological functions such as signal transduction, gene expression, and regulation of the immune response and for the strengthening of antioxidant defense mechanisms [60].
During the experiments on PBMCs of AD patients, electromagnetic waves have been observed to cause a growth of the total production of ROS; this increase seems to be linked to the timing of exposure [61]. The stimulus applied is able to primarily determine a strong increase of ROS until reaching a plateau and then, a decrease with the time. An initial increase, linked to the timing applied, suggests a ROS-mediated amplification of the inflammatory response [62]. The same trend is observed in cultured neurons treated with Aβ, suggesting the role of EMFs in the further activation of the cells defending the tissue damaged by Aβ. A ROS increase could also be responsible for an increase of autophagy and “phagocytic clearance” by microglia which can eliminate the Aβ. The increase of ROS could acquire the role of a “priming agent” as being responsible for the creation of a preconditioning aimed at the clearance of potentially hazardous substances [63]. So, the cognitive improvement and the reduction of Aβ plaques, after electromagnetic fields stimulation, may depend primarily on the enhancement of ROS-mediated inflammatory response after exposure.
1.2. Electromagnetic Fields and Synaptic Plasticity
Despite the effects of PEMFs as still controversial, it has been shown that deep brain stimulation by PEMFs can modulate the activity of neurophysiological circuits producing clinical benefits in AD patients [15]. Recently, brain stimulations with PEMFs have been developed and applied for the treatment of neurological disorders: for instance, the stimulation known as TMS which interacts in a noninvasive way with the nervous system [17]. Cortical changes induced by electromagnetic waves have shown results in improving the neuronal plasticity [18]. Indeed, an excitability increase of the cerebral cortex may affect the phenomenon of LTP, which in turn would support the synaptic plasticity and promote the recovery of degenerated functions [64]. Under these preconditions, there is a growing interest in the application of PEMFs as a possible therapeutic approach in psychiatric and neurological disorders [16]. PEMFs could be used to restore the cognitive performance, for instance, in clinical trials on AD. Recently, the electromagnetic waves have been demonstrated to modulate the functions of the cytoskeleton and to promote the neuronal differentiation and the neurogenesis in the hippocampus in vivo through the upregulation of the Cav-1 channel, β-III-tubulin, MAP2, and the brain-derived neurotrophic factor (BDNF) [29]. The latter is widely expressed in the brain and contributes to a variety of neuronal processes affecting the neurodevelopment, the survival, and the maintenance of the homeostasis of the nervous system in elderly [27]. In the adult brain, BDNF plays a key role in the modulation of the synaptic plasticity and it is essential for the regulation of memory. For these reasons, obtained data support the hypothesis that the electromagnetic waves could improve the brain neuroplasticity also through the modulation of the expression of neurotrophic factors [64].
2. Materials and Methods
2.1. PBMC Isolation
Peripheral blood mononuclear cells (PBMCs) were obtained from peripheral blood of 13 AD patients by means centrifugation on a 1077-density gradient (Histopaque® 1077, Sigma-Aldrich, Inc.). The mononuclear fraction was recovered and resuspended at the concentration of 2.5 × 106 cells/ml in a RPMI 1640 Medium supplemented with 10% bovine calf serum and 1% penicillin/streptomycin (Euroclone, Logan, UT). Cell vitality was assessed by trypan blue dye exclusion method; then, PBMCs were distributed in a 96-multiwell plate (Corning) with a density of 5 × 105 cells/200 μl medium/well and incubated at 37°C in a humidified atmosphere with 5% CO2. For each patient, 3 PBMC cultures were exposed to LF-PEMF for 3 different durations: 15, 30, and 60 min. Nonexposed (i.e., sham) control cultures were set up in parallel.
2.2. Electromagnetic Bioreactor and PBMC Exposure to LF-PEMF
The experimental setup of our electromagnetic bioreactor was based on two solenoids (i.e., air-cored coils) connected in series and powered by a pulse generator (BIOSTIM SPT Pulse Generator from IGEA, Carpi, Italy) [58]. The solenoids had a quasi-rectangular shape (length, 17 cm; width, 11.5 cm), and their planes were parallel with a distance of 10 cm. According to our mathematical model [65], this distance caused a stimulus characterized by a magnetic induction module of circa 3 mT. In addition, the magnetic induction field was perpendicular to the surface where the cells were seeded and grew; the signal frequency was equal to about 75 Hz.
2.3. RNA Extraction
Total RNA was extracted from untreated and LF-PEMF-treated cells using the RNeasy Mini kit (Qiagen GmbH, Hilden) according to the manufacturer's instructions. Total RNA obtained from the replicate cultures of each treatment was pooled, and the quality of RNA was assessed by determining the RNA integrity number (RIN) (TapeStation, Agilent Technologies). A quantitative RNA analysis was performed using a fluorimetric methods by means of the Qubit® platform (Invitrogen, Grand Island, NY, USA) using the Quant-iT RNA Assay (declared assay range between 5 and 100 ng; sample starting concentration between 250 pg/μl and 100 ng/μl): 2 μl of RNA was added to 198 μl of the working solution obtained by mixing 1 μl of Qubit™ RNA Reagent to 199 μl of Qubit RNA Buffer. The quantitation was performed following the calibration of the instrument with the Quant-iT RNA standards (0 and 10 ng/ml).
2.4. Real-Time Reverse Transcription PCR (qRT-PCR)
Quantitative real-time reverse transcription PCR (qRT-PCR) was performed using cDNA obtained following the reverse transcription reaction with the miRCURY LNA™ Universal RT microRNA PCR kit: 4 μl of total RNA (5 ng/μl) was added to 4 μl of 5x reaction buffer, 2 μl of enzyme mix, 1 μl of synthetic spike-in, and 9 μl of nuclease-free water; and the reaction was performed using a thermocycler (Bio-Rad, MJ Mini) for one reaction cycle at 42°C for 60 min and 95°C for 5 min, and the reaction products were immediately cooled at 4°C.
To evaluate the miRNA expression, qRT-PCR reactions were performed using the Universal cDNA Synthesis and SYBR® Green Master Mix kits. Amplification was performed in a 10 μl reaction mixture containing 4 μl of 1 : 80 diluted cDNA, 5 μl of SYBR Green Master Mix, and 1 μl of specific LNA probe. miR-107 LNA probe (50| AGCAGCAUUGUACAGGGCUAUCA |72), miR-335-5p LNA probe (16| UCAAGAGCAAUAACGAAAAAUGU |38), and miR-26b-5p LNA probe (12| UUCAAGUAAUUCAGGAUAGGU |32) are provided by Exiqon using the following reaction conditions: a first step of 10 min at 95°C followed by 45 amplification cycles of 10 sec at 95°C and a final step at 60°C for 1 min. Small nuclear RNA U6 (snU6) was used to normalize the expression data of miRNAs, and every assay was performed in triplicates using the Eco Real-Time PCR Instrument (Illumina, San Diego, CA).
To evaluate the expression of mRNA of BACE1, a protein that is a target of miRNA 107, specific primers were designed using Primer-BLAST software (http://www.ncbi.nlm.nih.gov/tools/primer-blast): BACE1: f: 5′-GCAGGGCTACTACGTGGAGA-3′; r: 5′-GTATCCACCAGGATGTTGAGC-3′.
GAPDH (glyceraldehyde 3-phosphate dehydrogenase) was considered as endogenous control, and the following specific primers were used: f: 5′-CTGAGAATGGGAAGCTGGTCAT-3′; r: 5′-TGGTGCAGGATGCATTGCT-3′.
qRT-PCR was performed by the Eco Real-Time PCR Instrument (Illumina, San Diego, CA), and the results were analyzed by the comparative ct method (ΔΔct method using the software package of the Eco Real-Time PCR System for the calculus of the 2−ΔΔct value [66].
Statistical analysis: from ct raw data of triplicate analysis, means and standard deviations were calculated and the statistical significance was analyzed by one-way ANOVA with post hoc LSD test (a P value smaller than 0.05 was considered as significant).
3. Results
This paper is intended to investigate the ability of LF-PEMF to modulate the expression of proteins involved in Alzheimer's disease. To this purpose, 3 different miRNAs were selected following a bioinformatics analysis in the specialized database miRTarBase. In addition, a PubMed search was performed for miRNAs and Alzheimer's disease. Two miRNAs (miR-335-5p and miR-26b-5p) were selected because of their involvement in brain signaling, in particular, in the glutamate uptake and in LTP. miR-335-5p is able to downregulate MAPK1 (mitogen-activated protein kinase 1) messenger translation. This gene encodes for a member of the MAP kinase family, also known as extracellular signal-regulated kinase (ERK), which acts as an integration point for multiple biochemical signals and is involved in a wide variety of cellular processes such as proliferation, differentiation, transcription, regulation, and development. ERK activity contributes to the synaptic plasticity; in fact, ERK cascade signals act with a regulatory role on the AMPA glutamate receptor (AMPAR), a non-NMDA type ionotropic transmembrane receptor for glutamate characterized by four types of subunits called GRIA (glutamate receptor ionotropic AMPA, 1–4) [67]. This particular receptor is involved in the fast synaptic transmission of the central nervous system, is activated by the artificial glutamate analog AMPA, and represents the most common receptor in the nervous system. It has been recently demonstrated [68] that AMPAR activation promotes the nonamyloidogenic APP processing and suppresses neuronal Aβ production. In this scenario, miR-335-5p is able to directly downregulate ERK which, in turn, regulates AMPAR which is involved in the first phase of LTP.
hsa-miR-26b-5p regulates the expression of a large number of genes, among which it is noteworthy, the carrier vGLUT2 (SLC17A6) involved in the promotion of the LTP. The same miRNA downregulates the kainate receptors.
In addition, miR-107 was considered because of previously reported studies [39] that observed a reduced expression of this miRNA in AD patients. This miRNA targets the messenger of BACE1 which is involved in the processing of APP toward the Aβ peptide: an increased expression of miR-107 would contrast the APP cleavage which results in a smaller deposition of Aβ plaques in the brain.
The ability of an electromagnetic field to modulate the expression of the selected miRNAs was tested on PBMC freshly isolated from the peripheral blood of 13 AD patients. The cells were exposed to LF-PEMF at 75 Hz for different durations (15, 30, and 60 min); subsequently, total RNA was extracted and cDNA was obtained as described in Section 2. The quantitative expressions of miR-107, miR-335-5p, and miR-26b-5p were determined by qRT-PCR using the small nucleolar RNA U6 as endogenous reference, and the RQ quantitative values were calculated against the untreated control cultures applying the ΔΔct method [66]. The results obtained are shown in Figure 1: mean data of 13 different PBMC cultures exposed for different times to LF-PEMF [3 mT; 75 Hz] are reported.
Figure 1.
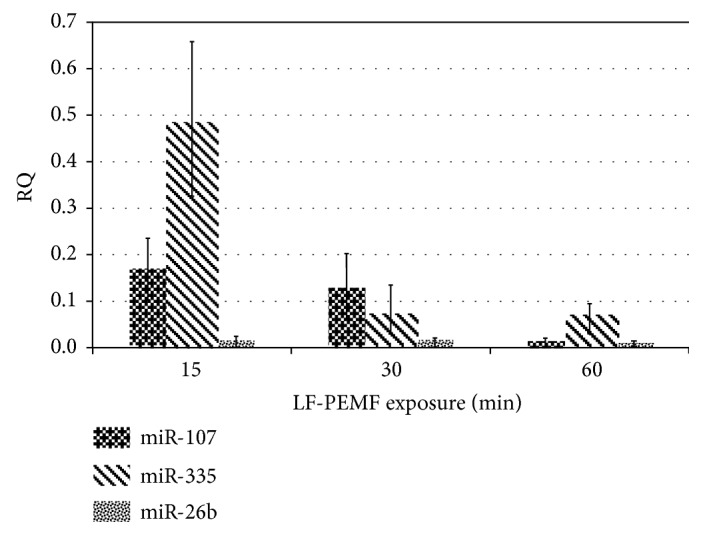
Expression of miR-107, miR-335, and miR-26b in PBMC from AD patients determined by relative quantification RQ (treated versus control sample). The values obtained after different times of exposure (15, 30, and 60 min) are shown (P > 0.05).
We can observe that the exposure to LF-PEMF was able to modulate the expression of all miRNAs considered; in particular, a progressive reduction of all miRNAs with the increasing time of exposure was observed even if the differences between untreated and treated cells were not statistically significant (P > 0.05). Similarly, the expression of BACE1 is affected by LF-PEMF with a progressive reduction of mRNA at the increasing exposure time (Figure 2).
Figure 2.
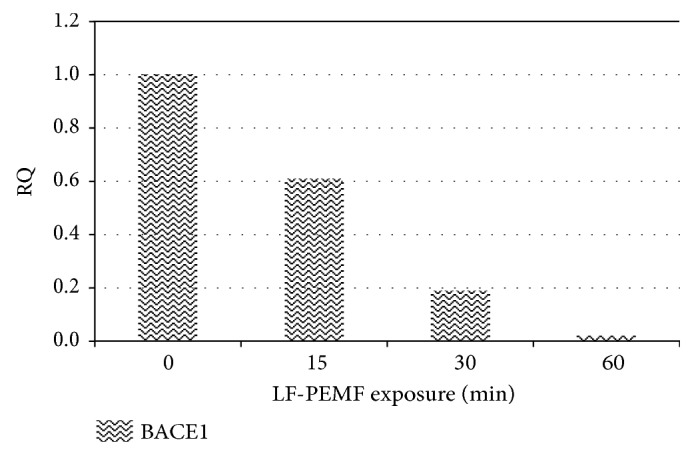
BACE1 expression in PBMC exposed to LF-PEMF for 3 different durations (15, 30, and 60 min). Relative quantification RQ of BACE1 mRNA using GAPDH mRNA as endogenous control (ΔΔct method [66]).
In Figure 3, the RQ values of both miR-107 and BACE1 mRNA obtained in one of the PBMC cultures, before and after LF-PEMF treatment with the different conditions, are compared. It can be observed that LF-PEMF induces a modulation of both miR-107 and of BACE1 mRNA expression. Moreover, a different modulation was observed depending on the duration of exposure.
Figure 3.
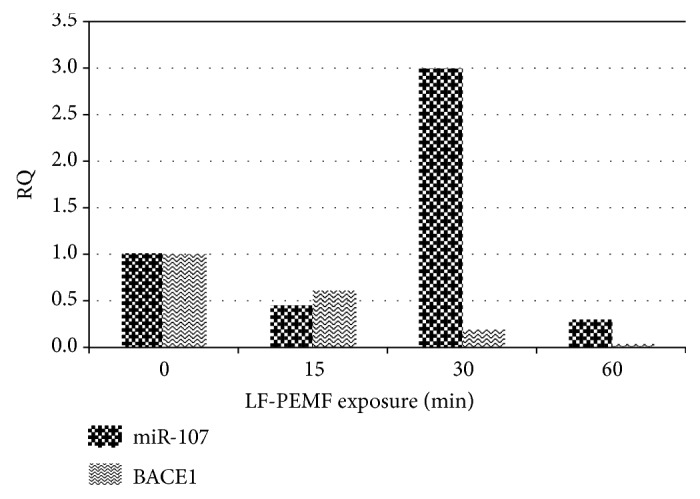
Comparison of miR-107 and BACE1 expression in the same PBMC culture exposed to LF-PEMF (15, 30, and 60 min durations). Results were normalized by U6 values for miR-107 and by GAPDH values for BACE1. Relative quantification RQ values were calculated against the untreated controls and referred to the average of the respective untreated control.
4. Discussion
According to the present data, LF-PEMF (3 mT; 75 Hz) demonstrated to be able to modulate both miRNAs and mRNA involved in AD-related pathways.
miRNAs are molecules acting through direct complementary interaction with sequences of RNA messengers (target mRNA) and are able to interact with a broad range of mRNAs sharing the same sequences; so, each miRNA can be considered the center of a complex network that regulates various protein pathways. miR-107 has been seen to downregulate in addition to BACE1 and other mRNAs that could be involved in brain degenerative disorders, for example, GRN, CYP2C8, DAPK1, and PTEN. From literature data, diseases associated with GRN (granulin) include frontotemporal lobar degeneration with ubiquitin-positive inclusions and progressive nonfluent aphasia [69]. CYPP2C8 gene encodes a member of the cytochrome P450 superfamily of enzymes; these proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids, and other lipids. DAPK1 (death-associated protein kinase 1) is a gene responsible for atherosclerotic plaque development and destabilization. PTEN (phosphatase and tensin homolog) acts as a tumor suppressor downregulating AKT/PKB signaling pathway; moreover, it regulates intracellular levels of phosphatidylinositol-3,4,5-trisphosphate in cells. Another gene, which expression is regulated by miR-107, is SP1 encoding for a zinc finger transcription factor that binds GC-rich motifs of many promoters and is involved in many cellular processes, including cell differentiation, cell growth, apoptosis, immune responses, DNA repair, and chromatin remodeling.
Among the targets of miR-335-5p we consider particularly interesting tenascin C (TNC), an extracellular matrix protein implicated in the guidance of migrating neurons as well as axons during development, synaptic plasticity as well as neuronal regeneration; RASA1 (RAS P21 protein activator) that is an inhibitory regulator of the Ras/cyclic AMP pathway and stimulates the GTPase of normal but not oncogenic Ras p21; and IGFR1 (insulin-like growth factor 1 receptor), a transmembrane receptor that is activated by a hormone called insulin-like growth factor 1 (IGF-1) and by a IGF-1-related hormone called IGF-2. Another interesting target of miR-335-5p is APBB2 (Aβ precursor protein binding family B member 2) that encodes a protein interacting with the cytoplasmic domains of Aβ (A4) precursor protein and of Aβ (A4) precursor-like protein 2. The latter protein contains two phosphotyrosine-binding (PTB) domains, which are thought to function in signal transduction. In Table 1, some of the gene whose expression is regulated by the miRNAs studied are listed (source: miRTarBase, miRDB).
Table 1.
miRNAs studied and some of their targets that are involved in AD-related pathways.
| ID | miRNA | Sequence | Target |
|---|---|---|---|
| MIMAT0000104 | hsa-miR-107 | 50-agcagcauuguacagggcuauca-72 | PLAG1, BACE1, CDK6, GRN, DAPK 1, PTEN, NOTCH 2, NFIA, SERBP1 |
| MIMAT0000765 | hsa-miR-335-5p | 16-ucaagagcaauaacgaaaaaugu-38 | TNC, RASA1, IGFR1, SP1, APBB2 |
| MIMAT0000083 | hsa-miR-26b-5p | 12-uucaaguaauucaggauaggu-32 | SLC17A6, (DNP1/vGLUT2) |
Note. PLAG1: pleiomorphic adenoma gene 1; BACE1: beta-site APP-cleaving enzyme 1; CDK6: cyclin-dependent kinase 6; GRN: granulin; DAPK 1: death-associated protein kinase 1; PTEN: phosphatase and tensin homolog; NOTCH 2: Notch 2; NFIA: nuclear factor I/A; SERBP1: SERPINE1 mRNA-binding protein 1; TNC: tenascin C; RASA1: RAS p21 protein activator (GTPase-activating protein) 1; IGFR1: insulin-like growth factor 1 receptor; SP1: Sp1 transcription factor; APBB2: Aβ precursor protein binding family B member 2; SLC17A6 (DNP1/vGLUT2): solute carrier family 17 member 6 (vesicular glutamate transporter).
In conclusion, the results of the present study, using an ex vivo human PBMC model, demonstrated that LF-PEMF exposure really modulates the expression of miRNAs that regulate the brain signaling, so confirming the capacity of the electromagnetic field to stimulate both tissue regeneration and brain signaling. The analysis of changes in the expression levels of miRNAs, known as the regulatory processes involved in brain signaling and tissue regeneration, after LF-PEMF exposure, has allowed us to verify both the quantitative variations of these miRNAs and to identify other target messengers of the same miRNA. This has been possible through the analysis of protein networks in which the miRNAs are involved. In fact, each miRNA can interact through sequence complementarity with sequences contained in various target mRNAs and also can act in synergy with other miRNAs that regulate the same mRNA. The results of the present study confirmed the capacity of LF-PEMF to influence various networks of physiological functions that are dysregulated in AD. Among the effects observed, a quantitative reduction of β-secretase, following LF-PEMF exposure, could confirm a protective action of the electromagnetic field whose action would counteract the formation of Aβ. Expression values of miR-107 which is a negative regulator of BACE1 decrease with the increasing exposure time, and the same trend was observed for the expression of miR-26b-5p, which is involved in brain signaling and synaptic plasticity.
Differently, the expression of miR-335-5p, which negatively regulates the AMPA receptor, is stimulated by the electromagnetic field, even if this expression decreases with the increasing time of exposure. This result indicates a possible adverse effect depending on the time of exposure.
Overall, the results obtained from the study on our in vitro model demonstrated that LF-PEMF can stimulate an epigenetic regulation mediated by miRNAs, which would lead to a rebalancing of the pathways' deregulation occurring in AD (this deregulation starts in locus coeruleus and then continues in high-order association areas of the neocortex [70]). However, it is necessary to take account of the complex network of epigenetic signals, not yet completely known, and the possibility of some adverse effects. These results suggest that the electromagnetic fields at low frequencies, if properly used, may be useful for the treatment of patients with AD, as suggested by the results of pilot experiments with deep brain stimulation via EMFs, which were reported to produce clinical benefits [15]. However, for the complexity of the epigenetic regulation signals, which are triggered by electromagnetic stimulation [71–74], further in vitro and in vivo studies are needed in order to investigate the effects of LF-PEMF and in order to develop the conditions useful for a therapeutic use (e.g., via a dose-dependent epigenetic regulation mediated by miRNAs [75]), avoiding the possible adverse effects.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Bermejo-Pareja F., Benito-Leon J., Vega S., on behalf of the Neurological Disorders in Central Spain (NEDICES) Study Group Incidence and subtypes of dementia in three elderly populations of central Spain. Journal of the Neurological Sciences. 2008;264(1-2):63–72. doi: 10.1016/j.jns.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 2.Jack C. R., Albert M. S., Knopman D. S., McKhann G. M., Sperling R. A. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheuner D., Eckman C., Jensen M., et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nature Medicine. 1996;2(8):864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 4.Liao P., Yu L., Kuo C., Lin C., Kuo Y. Proteomics analysis of plasma for potential biomarkers in the diagnosis of Alzheimer’s disease. Proteomics. Clinical Applications. 2007;1(5):506–512. doi: 10.1002/prca.200600684. [DOI] [PubMed] [Google Scholar]
- 5.Lambert J. C., Amouyel P. Genetic heterogeneity of Alzheimer’s disease: complexity and advances. Psychoneuroendocrinology. 2007;32(Supplement 1):S62–S70. doi: 10.1016/j.psyneuen.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Mohandas E., Rajmohan V., Raghunath B. Neurobiology of Alzheimer’s disease. Indian Journal of Psychiatry. 2009;51(1):55–61. doi: 10.4103/0019-5545.44908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y. W., Thompson R., Zhang H., Xu H. APP processing in Alzheimer’s disease. Molecular Brain. 2011;4:p. 3. doi: 10.1186/1756-6606-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar V., Abbas A. K., Fausto N., Aster J. C. Robbins & Coltran Pathologic Basis of Disease. 8th. Philadelphia: Saunders/Elsevier; 2010. (Professional Edition). [Google Scholar]
- 9.Arendash G. W., Sanchez-Ramos J., Mori T., et al. Electromagnetic field treatment protects against and reverses cognitive impairment in Alzheimer’s disease mice. Journal of Alzheimer's Disease. 2010;19(1):191–210. doi: 10.3233/JAD-2010-1228. [DOI] [PubMed] [Google Scholar]
- 10.Simkó M. Induction of cell activation processes by low frequency electromagnetic fields. Scientific World Journal. 2004;4(Supplement 2):4–22. doi: 10.1100/tsw.2004.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavalko F. M., Norvell S. M., Burr D. B., Turner C. H., Duncan R. L., Bidwell J. P. A model for mechanotransduction in bone cells: the load-bearing mechanosomes. Journal of Cellular Biochemistry. 2003;88(1):104–112. doi: 10.1002/jcb.10284. [DOI] [PubMed] [Google Scholar]
- 12.Huang S., Ingber D. E. Cell tension, matrix mechanics, and cancer development. Cancer Cell. 2005;8(3):175–176. doi: 10.1016/j.ccr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Davanipour Z., Tseng C. C., Lee P. J., Sobel E. A case-control study of occupational magnetic field exposure and Alzheimer’s disease: results from the California Alzheimer’s Disease Diagnosis and Treatment Centers. BMC Neurology. 2007;7(1):p. 13. doi: 10.1186/1471-2377-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García A. M., Sisternas A., Hoyos S. P. Occupational exposure to extremely low frequency electric and magnetic fields and Alzheimer disease: a meta-analysis. International Journal of Epidemiology. 2008;37(2):329–340. doi: 10.1093/ije/dym295. [DOI] [PubMed] [Google Scholar]
- 15.Laxton A. W., Tang-Wai D. F., McAndrews M. P., et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Annals of Neurology. 2010;68(4):521–534. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- 16.Wassermann E. M., Zimmermann T. Transcranial magnetic brain stimulation: therapeutic promises and scientific gaps. Pharmacology & Therapeutics. 2012;133(1):98–107. doi: 10.1016/j.pharmthera.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miniussi C., Cappa S. F., Cohen L. G., et al. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimulation. 2008;1(4):326–336. doi: 10.1016/j.brs.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Miniussi C., Rossini P. M. Transcranial magnetic stimulation in cognitive rehabilitation. Neuropsychological Rehabilitation. 2011;21(5):579–601. doi: 10.1080/09602011.2011.562689. [DOI] [PubMed] [Google Scholar]
- 19.Cotelli M., Calabria M., Manenti R., et al. Improved language performance in Alzheimer disease following brain stimulation. Journal of Neurology, Neurosurgery, and Psychiatry. 2011;82(7):794–797. doi: 10.1136/jnnp.2009.197848. [DOI] [PubMed] [Google Scholar]
- 20.Finocchiaro C., Maimone M., Brighina F., Piccoli T., Giglia G., Fierro B. A case study of primary progressive aphasia: improvement on verbs after rTMS treatment. Neurocase. 2006;12(6):317–321. doi: 10.1080/13554790601126203. [DOI] [PubMed] [Google Scholar]
- 21.Carmody S., Wu X. L., Lin H., Blank M., Skopicki H., Goodman R. Cytoprotection by electromagnetic field induced HSP70: a model for clinical application. Journal of Cellular Biochemistry. 2000;79(3):453–459. doi: 10.1002/1097-4644(20001201)79:3<453::AID-JCB100>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 22.Coulton L. A., Harris P. A., Barker A. T., Pockley A. G. Effect of 50 Hz electromagnetic fields on the induction of heat-shock protein gene expression in human leukocytes. Radiation Research. 2004;161(4):430–434. doi: 10.1667/RR3145. [DOI] [PubMed] [Google Scholar]
- 23.Pasquini R., Villarini M., Scassellati Sforzolini G., Fatigoni C., Moretti M. Micronucleus induction in cells co-exposed in vitro to 50 Hz magnetic field and benzene, 1,4-benzenediol (hydroquinone) or 1,2,4-benzenetriol. Toxicology in Vitro. 2003;17(5-6):581–586. doi: 10.1016/s0887-2333(03)00137-1. [DOI] [PubMed] [Google Scholar]
- 24.Ross C. L., Siriwardane M., Almeida-Porada G., et al. The effect of low-frequency electromagnetic field on human bone marrow stem/progenitor cell differentiation. Stem Cell Research. 2015;15(1):96–108. doi: 10.1016/j.scr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piacentini R., Ripoli C., Mezzogori D., Azzena G. B., Grassi C. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Ca(v)1-channel activity. Journal of Cellular Physiology. 2008;215(1):129–139. doi: 10.1002/jcp.21293. [DOI] [PubMed] [Google Scholar]
- 26.Cuccurazzu B., Leone L., Podda M. V., et al. Exposure to extremely low-frequency (50 Hz) electromagnetic fields enhances adult hippocampal neurogenesis in C57BL/6 mice. Experimental Neurology. 2010;226(1):173–182. doi: 10.1016/j.expneurol.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 27.Ma J., Zhang Z., Su Y., et al. Magnetic stimulation modulates structural synaptic plasticity and regulates BDNF-TrkB signal pathway in cultured hippocampal neurons. Neurochemistry International. 2013;62(1):84–91. doi: 10.1016/j.neuint.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Lisi A., Ledda M., Rosola E., et al. Extremely low frequency electromagnetic field exposure promotes differentiation of pituitary corticotrope-derived AtT20 D16V cells. Bioelectromagnetics. 2006;27(8):641–651. doi: 10.1002/bem.20255. [DOI] [PubMed] [Google Scholar]
- 29.D’Ascenzo M., Piacentini R., Casalbore P., et al. Role of L-type Ca2+ channels in neural stem/progenitor cell differentiation. The European Journal of Neuroscience. 2006;23(4):935–944. doi: 10.1111/j.1460-9568.2006.04628.x. [DOI] [PubMed] [Google Scholar]
- 30.Di Loreto S., Falone S., Caracciolo V., et al. Fifty hertz extremely low-frequency magnetic field exposure elicits redox and trophic response in rat-cortical neurons. Journal of Cellular Physiology. 2009;219(2):334–343. doi: 10.1002/jcp.21674. [DOI] [PubMed] [Google Scholar]
- 31.Goodman R., Blank M. Insights into electromagnetic interaction mechanisms. Journal of Cellular Physiology. 2002;192(1):16–22. doi: 10.1002/jcp.10098. [DOI] [PubMed] [Google Scholar]
- 32.Osera C., Fassina L., Amadio M., et al. Cytoprotective response induced by electromagnetic stimulation on SH-SY5Y human neuroblastoma cell line. Tissue Engineering. Part a. 2011;17(19-20):2573–2582. doi: 10.1089/ten.TEA.2011.0071. [DOI] [PubMed] [Google Scholar]
- 33.Perez F. P., Zhou X., Morisaki J., Jurivich D. Electromagnetic field therapy delays cellular senescence and death by enhancement of the heat shock response. Experimental Gerontology. 2008;43(4):307–316. doi: 10.1016/j.exger.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Del Giudice E., Facchinetti F., Nofrate V., et al. Fifty Hertz electromagnetic field exposure stimulates secretion of beta-amyloid peptide in cultured human neuroglioma. Neuroscience Letters. 2007;418(1):9–12. doi: 10.1016/j.neulet.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 35.Nikolova T., Czyz J., Rolletschek A., et al. Electromagnetic fields affect transcript levels of apoptosis-related genes in embryonic stem cell-derived neural progenitor cells. The FASEB Journal. 2005;19(12):1686–1688. doi: 10.1096/fj.04-3549fje. [DOI] [PubMed] [Google Scholar]
- 36.Remondini D., Nylund R., Reivinen J., et al. Gene expression changes in human cells after exposure to mobile phone microwaves. Proteomics. 2006;6(17):4745–4754. doi: 10.1002/pmic.200500896. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J., Ming L. G., Ge B. F., et al. Effects of 50 Hz sinusoidal electromagnetic fields of different intensities on proliferation, differentiation and mineralization potentials of rat osteoblasts. Bone. 2011;49(4):753–761. doi: 10.1016/j.bone.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 38.Krützfeldt J., Stoffel M. MicroRNAs: a new class of regulatory genes affecting metabolism. Cell Metabolism. 2006;4(1):9–12. doi: 10.1016/j.cmet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Miranda K. C., Huynh T., Tay Y., et al. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126(6):1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 40.Iorio M. V., Croce C. M. MicroRNA dysregulation in cancer: Diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Molecular Medicine. 2012;4(3):143–159. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson P. T., Wang W. X. MiR-107 is reduced in Alzheimer’s disease brain neocortex: validation study. Journal of Alzheimer's Disease. 2010;21(1):75–79. doi: 10.3233/JAD-2010-091603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith P., Al Hashimi A., Girard J., Delay C., Hébert S. S. In vivo regulation of amyloid precursor protein neuronal splicing by microRNAs. Journal of Neurochemistry. 2011;116(2):240–247. doi: 10.1111/j.1471-4159.2010.07097.x. [DOI] [PubMed] [Google Scholar]
- 43.Junn E., Mouradian M. M. MicroRNAs in neurodegenerative diseases and their therapeutic potential. Pharmacology & Therapeutics. 2012;133(2):142–150. doi: 10.1016/j.pharmthera.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar P., Dezso Z., MacKenzie C., et al. Circulating miRNA biomarkers for Alzheimer’s disease. PloS One. 2013;8(7, article e69807) doi: 10.1371/journal.pone.0069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leidinger P., Backes C., Deutscher S., et al. A blood based 12-miRNA signature of Alzheimer disease patients. Genome Biology. 2013;14(7):p. R78. doi: 10.1186/gb-2013-14-7-r78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schonrock N., Ke Y. D., Humphreys D., et al. Neuronal microRNA deregulation in response to Alzheimer’s disease amyloid-beta. PloS One. 2010;5(6, article e11070) doi: 10.1371/journal.pone.0011070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arduino D. M., Esteves A. R., Silva D. F., et al. Therapeutic intervention at cellular quality control systems in Alzheimer’s and Parkinson’s diseases. Current Pharmaceutical Design. 2011;17(31):3446–3459. doi: 10.2174/138161211798072481. [DOI] [PubMed] [Google Scholar]
- 48.Soreq H., Wolf Y. NeurimmiRs: microRNAs in the neuroimmune interface. Trends in Molecular Medicine. 2011;17(10):548–555. doi: 10.1016/j.molmed.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 49.Schipper H. M., Maes O. C., Chertkow H. M., Wang E. MicroRNA expression in Alzheimer blood mononuclear cells. Gene Regulation and Systems Biology. 2007;1:263–274. doi: 10.4137/grsb.s361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delay C., Mandemakers W., Hébert S. S. MicroRNAs in Alzheimer’s disease. Neurobiology of Disease. 2012;46(2):285–290. doi: 10.1016/j.nbd.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Nana-Sinkam S. P., Croce C. M. Clinical applications for microRNAs in cancer. Clinical Pharmacology and Therapeutics. 2013;93(1):98–104. doi: 10.1038/clpt.2012.192. [DOI] [PubMed] [Google Scholar]
- 52.Chen X., Ba Y., Ma L., et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Research. 2008;18(10):997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 53.Zampetaki A., Willeit P., Drozdov I., Kiechl S., Mayr M. Profiling of circulating microRNAs: from single biomarkers to re-wired networks. Cardiovascular Research. 2012;93(4):555–562. doi: 10.1093/cvr/cvr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weber J. A., Baxter D. H., Zhang S., et al. The microRNA spectrum in 12 body fluids. Clinical Chemistry. 2010;56(11):1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geekiyanage H., Jicha G. A., Nelson P. T., Chan C. Blood serum miRNA: non-invasive biomarkers for Alzheimer’s disease. Experimental Neurology. 2012;235(2):491–496. doi: 10.1016/j.expneurol.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steer C. J., Subramanian S. Circulating microRNAs as biomarkers: a new frontier in diagnostics. Liver Transplantation. 2012;18(3):265–269. doi: 10.1002/lt.23377. [DOI] [PubMed] [Google Scholar]
- 57.Hagen J. W., Lai E. C. MicroRNA control of cell-cell signaling during development and disease. Cell Cycle. 2008;7(15):2327–2332. doi: 10.4161/cc.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fassina L., Visai L., Cusella De Angelis M. G., Benazzo F., Magenes G. Surface modification of a porous polyurethane through a culture of human osteoblasts and an electromagnetic bioreactor. Technology and Health Care. 2007;15(1):33–45. [PubMed] [Google Scholar]
- 59.Vallesi G., Raggi F., Rufini S., Gizzi S., Ercolani E., Rossi R. Effects of cyclotronic ion resonance on human metabolic processes: a clinical trial and one case report. Electromagnetic Biology and Medicine. 2007;26(4):283–288. doi: 10.1080/15368370701768823. [DOI] [PubMed] [Google Scholar]
- 60.Corti A., De Tata V., Pompella A. Agenti e meccanismi di stress ossidativo nella patologia umana. Ligand Assay. 2009;14(1):9–16. [Google Scholar]
- 61.Feychting M., Ahlbom A., Kheifets L. EMF and health. Annual Review of Public Health. 2005;26:165–189. doi: 10.1146/annurev.publhealth.26.021304.144445. [DOI] [PubMed] [Google Scholar]
- 62.Block M. L. NADPH oxidase as a therapeutic target in Alzheimer’s disease. BMC Neuroscience. 2008;9(Supplement 2):p. S8. doi: 10.1186/1471-2202-9-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marchesi N., Osera C., Fassina L., et al. Autophagy is modulated in human neuroblastoma cells through direct exposition to low frequency electromagnetic fields. Journal of Cellular Physiology. 2014;229(11):1776–1786. doi: 10.1002/jcp.24631. [DOI] [PubMed] [Google Scholar]
- 64.Zanardini R., Gazzoli A., Ventriglia M., et al. Effect of repetitive transcranial magnetic stimulation on serum brain derived neurotrophic factor in drug resistant depressed patients. Journal of Affective Disorders. 2006;91(1):83–86. doi: 10.1016/j.jad.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 65.Mognaschi M. E., Di Barba P., Magenes G., Lenzi A., Naro F., Fassina L. Field models and numerical dosimetry inside an extremely-low-frequency electromagnetic bioreactor: the theoretical link between the electromagnetically induced mechanical forces and the biological mechanisms of the cell tensegrity. Spring. 2014;3(1):p. 473. doi: 10.1186/2193-1801-3-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Livak K. J., Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-delta delta C(T) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 67.Peng S., Zhang Y., Zhang J., Wang H., Ren B. ERK in learning and memory: a review of recent research. International Journal of Molecular Sciences. 2010;11(1):222–232. doi: 10.3390/ijms11010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoey S. E., Buonocore F., Cox C. J., Hammond V. J., Perkinton M. S., Williams R. J. AMPA receptor activation promotes non-amyloidogenic amyloid precursor protein processing and suppresses neuronal amyloid-β production. PloS One. 2013;8(10, article e78155) doi: 10.1371/journal.pone.0078155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rohrer J. D., Ridgwaya G. R., Crutcha S. J., et al. Progressive logopenic/phonological aphasia: erosion of the language network. NeuroImage. 2010;49(1):984–993. doi: 10.1016/j.neuroimage.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Braak H., Del Tredici K. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain. 2015;138(10):2814–2833. doi: 10.1093/brain/awv236. [DOI] [PubMed] [Google Scholar]
- 71.Fassina L., Saino E., Sbarra M. S., et al. Ultrasonic and electromagnetic enhancement of a culture of human SAOS-2 osteoblasts seeded onto a titanium plasma-spray surface. Tissue Engineering Part C. 2009;15(2):233–242. doi: 10.1089/ten.tec.2008.0398. [DOI] [PubMed] [Google Scholar]
- 72.Fassina L., Saino E., Visai L., et al. Electromagnetic enhancement of a culture of human SAOS-2 osteoblasts seeded onto titanium fiber-mesh scaffolds. Journal of Biomedical Materials Research. Part a. 2008;87(3):750–759. doi: 10.1002/jbm.a.31827. [DOI] [PubMed] [Google Scholar]
- 73.Fassina L., Di Grazia A., Naro F., Monaco L., Cusella De Angelis M. G., Magenes G. Video evaluation of the kinematics and dynamics of the beating cardiac syncytium: an alternative to the Langendorff method. The International Journal of Artificial Organs. 2011;34(7):546–558. doi: 10.5301/IJAO.2011.8510. [DOI] [PubMed] [Google Scholar]
- 74.Saino E., Fassina L., van Vlierberghe S., et al. Effects of electromagnetic stimulation on osteogenic differentiation of human mesenchymal stromal cells seeded onto gelatin cryogel. International Journal of Immunopathology and Pharmacology. 2011;24(1) Supplement 2:1–6. doi: 10.1177/03946320110241S201. [DOI] [PubMed] [Google Scholar]
- 75.Paolini A., Curti V., Pasi F., Mazzini G., Nano R., Capelli E. Gallic acid exerts a protective or an anti-proliferative effect on glioma T98G cells via dose-dependent epigenetic regulation mediated by miRNAs. International Journal of Oncology. 2015;46(4):1491–1497. doi: 10.3892/ijo.2015.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]


