Summary
Marine group II Euryarchaeota (MG-II) are among the most abundant microbes in oceanic surface waters [1, 2, 3, 4]. So far, however, representatives of MG-II have not been cultivated, and no viruses infecting these organisms have been described. Here, we present complete genomes for three distinct groups of viruses assembled from metagenomic sequence datasets highly enriched for MG-II. These novel viruses, which we denote magroviruses, possess double-stranded DNA genomes of 65 to 100 kilobases in size that encode a structural module characteristic of head-tailed viruses and, unusually for archaeal and bacterial viruses, a nearly complete replication apparatus of apparent archaeal origin. The newly identified magroviruses are widespread and abundant and therefore are likely to be major ecological agents.
Keywords: virus, phage, marine, archaea, replication, DNA polymerase
Highlights
-
•
A novel viral group, magroviruses, likely infects marine group II archaea
-
•
Magroviruses are highly abundant in oceanic surface waters worldwide
-
•
Magroviruses have linear, double-stranded DNA genomes of about 100 kilobases
-
•
Magroviruses encode a near complete replication apparatus of apparent archaeal origin
Philosof et al. report on newly identified viruses (magroviruses), detected using metagenomics, that most likely infect the uncultured marine group II Euryarchaeota. Magroviruses encode a structural module characteristic of tailed viruses and unexpectedly, a nearly complete replication apparatus of apparent archaeal origin.
Results and Discussion
Marine members of the archaeal phylum Euryarchaeota are divided into three groups: marine groups II (MG-II), III (MG-III), and IV (MG-IV) [5]. MG-II archaea dominate the photic zone of oligotrophic oceans [1, 2], show seasonal variation [6], and comprise up to 90% of the total archaea and one-third of all microbial cells during spring blooms in the Atlantic [7]. Metatranscriptomic analyses show that MG-II archaea are among the most transcriptionally active microbial groups in the coastal Pacific Ocean, with transcription levels and patterns similar to those of Pelagibacter ubique and SAR86 [8, 9].
Despite their high abundance and transcription activity, not a single representative of MG-II has been cultured. Nevertheless, MG-II genomes have been assembled for MG-II [10, 11], and the analyses suggest that MG-II are motile, photoheterotrophic, and capable of degrading polymers such as proteins and lipids [5].
To gain further insight into the diel activity of marine Euryarchaeota in the Red Sea, we examined metagenomic bins containing MG-II signatures. The contigs in these bins were retrieved from a cross assembly of microbial, viral, and transcriptomic samples collected at four time points during a single day in the Gulf of Aqaba in the Red Sea (ENA: PRJEB19060). Manual inspection showed that one bin (169) contained a metagenome-assembled genome (MAG) (156409) carrying hallmark viral genes including predicted major capsid protein (MCP), portal protein, and large subunit of the terminase, as well as DNA polymerase of the B family (DNAP). This contig contained overlapping terminal regions, suggesting that it represents a complete, terminally redundant viral genome.
Viruses have been previously isolated from members of several euryarchaeal groups. Euryarchaeal dsDNA viruses show diverse morphologies including spindle-shaped, icosahedral, pleomorphic, and head-tailed viruses. The latter group resembles the bacterial head-tailed phages (order Caudovirales), both in morphology and genome organization, and is currently classified into the families Myoviridae, Podoviridae, and Siphoviridae, each of which includes bacterial and archaeal viruses [12, 13, 14, 15, 16, 17]. No viruses infecting MG-II have been isolated [12], and despite the increasing amount of viral metagenomic data [18, 19], candidate viral contigs from the MG-II group have not been reported either.
The protein sequence of the predicted MCP from MAG 156409 showed significant, albeit moderate (33% identity), similarity solely to the MCPs of haloarchaeal siphoviruses (haloviruses) (Figure 1A). Three additional proteins encoded in MAG 156409, namely, primase (Figure 1C), portal protein, and prohead protease (Figure S2), showed comparable levels of similarity to homologs from haloviruses, indicating a relatively distant evolutionary link. We used these protein sequences as queries in BLAST searches against the complete Red Sea assembly dataset, in an attempt to expand the repertoire of virus-related sequences. This search resulted in additional nine viral MAGs (Table S1), all showing the same pattern of homology to haloviruses.
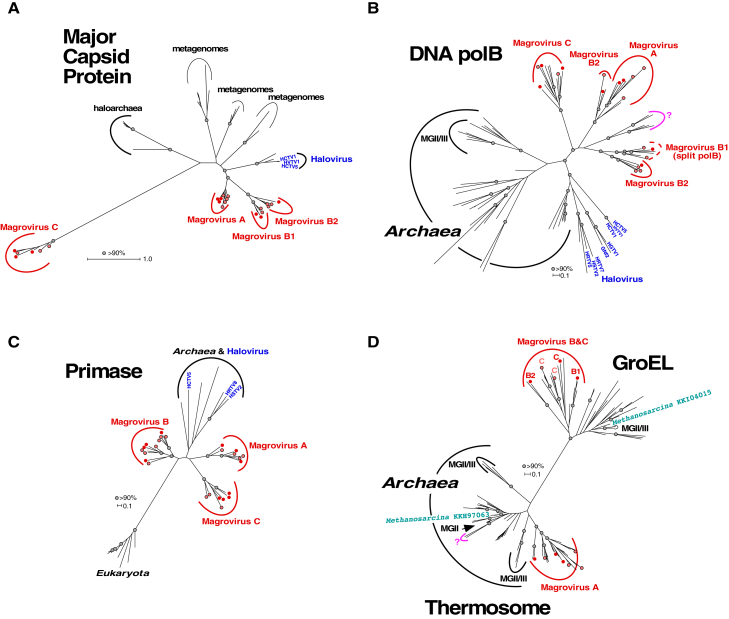
Figure 1.
Unrooted Maximum Likelihood Phylogenetic Trees of Conserved Magrovirus Genes
(A) Major capsid protein (MCP).
(B) DNA polymerase B (DNAP).
(C) Archaeo-eukaryotic primase (AEP).
(D) Chaperonins (thermosome subunit and GroEL).
Metagenome-assembled complete or nearly complete genomes (MAGs) of magrovirus from the Red Sea and Tara Oceans metagenomes are marked with red and light red circles, respectively. Bootstrap support values greater than 90 are marked with gray circles. See also Figures S2 and S3.
To validate and expand our observations on Red Sea metagenomes, we used the same query sequences in a BLAST search against both the original Tara Oceans assembly datasets [20, 21] and a reassembly of the raw sequences from this project (see Supplemental Experimental Procedures). This search yielded 15 additional putative viral genomes related to the Red Sea MAGs (Table S1). All together, we identified 26 putative viral MAGs from two independent metagenomic projects (Red Sea and Tara Oceans).
In the phylogenetic tree of the euryarchaeal virus and provirus MCPs, and their environmental homologs (Figure 1A), the MAG proteins split into three distinct groups, two of which (A, B) join in a clade affiliated with the halovirus MCPs, whereas the third group (C) forms a long branch with an uncertain affiliation. A similar phylogenetic pattern was observed for other hallmark caudoviral genes of the MAGs, namely prohead protease, portal protein, and large subunit of the terminase. Whereas groups A and B cluster together in all these trees, the position of group C changed from tree to tree, suggesting rapid evolution. These findings, along with the fact that 11 MAGs are terminally redundant linear genomes of about 100 kbp in size (Table S1), suggest that these MAGs represent a novel family of head-tailed archaeal viruses. Because, as shown below, these MAGs appear to be strongly associated with MG-II, we provisionally denote them magroviruses (MArine GROup II viruses).
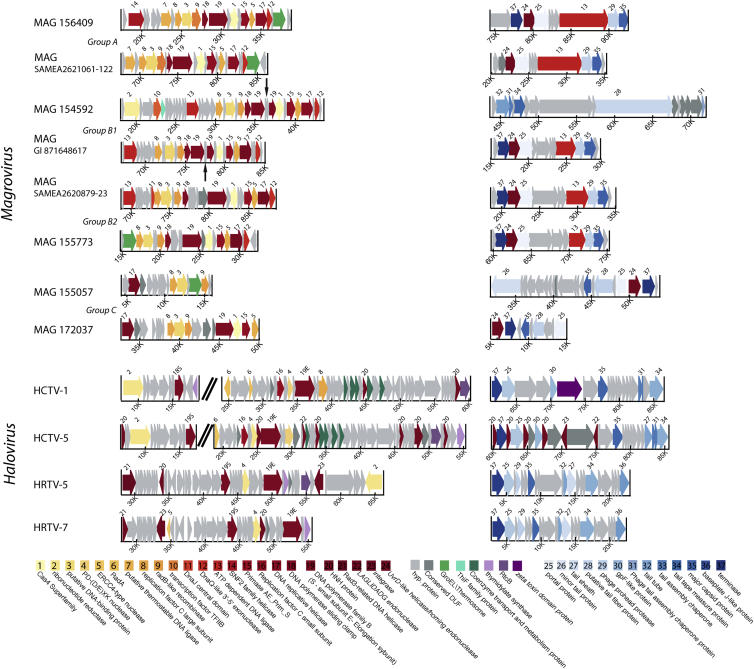
We then compared the gene complements and genome organizations of the three groups of magroviruses in detail. Although, as with many other archaeal viruses [22], most of the magrovirus genes encode proteins without significant similarity to any sequences in current databases, the genomes include two readily identifiable, compact gene blocks, the structural-morphogenetic module and the replicative module (Figure 2). The structural module consists of the genes encoding MCP, portal protein, prohead protease, and terminase and closely resembles the corresponding genomic module of haloviruses (Figure 2). The distinctive feature of magroviruses is the presence of a suit of genes for proteins involved in the genome replication (Figure 2B). All 26 genomes encode DNAP, sliding clamp, clamp loader ATPase (replication factor C), archaeo-eukaryotic primase (AEP), replicative helicase (MCM protein), RadA-like ATPase, single-stranded DNA-binding protein (ssb), and several nucleases; all viruses except for group C also encode one or two ATP-dependent DNA ligases (Figure 2B). Taken together, these proteins could comprise a nearly complete archaeal-type replisome [23], which is unusual among currently known viruses of archaea and bacteria, although some recently discovered bacteriophages also encode expansive suites of replication proteins [24]. Haloviruses that share the morphogenetic gene block with the magroviruses encompass a smaller complement of replicative genes (Figures 2 and S1). The gene order within both the replicative and the structural modules of magroviruses is highly conserved, with limited rearrangements, largely in group C (Figure 2B), possibly, because operonic organization of functionally linked genes is important for virus reproduction. No auxiliary metabolic genes [25] could be observed in magroviruses.
Figure 2.
Genome Organization in Different Groups of Magroviruses and Haloviruses
For each group, detailed genome schemes of the replicative gene block (left, yellow to red) and the structural gene block (right, different shades of blue) are shown. Homologous genes with predicted functions are shown using color code (see key at the bottom). Green arrows indicate thermosome genes, and gray arrows indicate hypothetical proteins. Split DNAP genes from group B1 are marked with a narrow black arrow pointing to the regions between the split genes. See also Figure S1 and Table S1.
With the exception of the DNAP, AEP, and ligases, the replicative proteins of magroviruses show low sequence similarity to homologs from cellular organisms that could be detected only through sensitive profile-profile searches. Nevertheless, most of these proteins show signs of archaeal origin as indicated by the provenance of the closest homologs. Due to the low sequence conservation, informative phylogenetic analysis was feasible only for DNAP and AEP. In the phylogenetic tree of DNAPs, the magrovirus polymerases cluster with the halovirus DNAPs [17, 26], and, together, these viral polymerases are related to archaeal DNApolB III that is involved in lagging strand replication (Figure 1B). This gene is apparently subject to frequent horizontal transfer among archaea that is likely to be at least partially mediated by viruses [27, 28], so that the DNApolB III phylogeny does not follow the archaeal evolutionary tree. Groups A and C that were delineated by analysis of morphogenetic genes retain monophyly in the DNAP tree (Figure 1B), whereas group B splits between two branches, one of which is affiliated with group A (Figure 1B). This discrepancy between the MCP and DNAP phylogenies suggests genetic exchange among diverse magroviruses. In addition to groups A, B, and C, a putative new group (labeled “?” in Figures 1B and 1D) was observed in the DNAP tree. The MAGs encoding this group of DNAPs (tentatively, group D) are not represented in the Red Sea metagenomes but are present among scaffolds originating from the Tara Oceans project. Group D genomes contain genes for DNAP (Figure 1B), thermosome (Figure 1C), resolvase, and DNA-dependent RNA polymerase A, all with the highest similarity to archaeal homologs. So far no closed genomes of this group were assembled, and a morphogenetic gene block was not identified. Therefore, although a connection to magroviruses is apparent, the nature of group D (virus, provirus, mobile element, megaplasmid, or uncultured archaea) remains to be determined.
Notably, seven group B magroviruses possess a split DNAP gene, whereas in all other viral MAGs, the DNAP gene is uninterrupted (Figures 2B and S3). The split is located within the sequence encoding the catalytic domain, similarly to the DNAP genes of the cyanophage P-SSP7 [29] and Methanobacterium thermoautotrophicum. Interrupted DNAP genes are also found in other Euryarchaeota in which the inserts consist of post-transcriptionally excised inteins [30, 31]. The positions of the splits in these archaeal polymerases and the group B magrovirus DNAP are similar, but not identical, suggesting independent, convergent evolutionary events resulting in gene fragmentation. Regardless of the exact evolutionary scenario, the split DNAP is so far unique among archaeal viruses and supports the monophyly of magrovirus group B.
The phylogenetic tree of the AEP supports the monophyly of all three groups of magroviruses as well as the common origin of primases in magroviruses and haloviruses; in this case, however, magrovirus groups A and C cluster with haloviruses, suggesting the possibility of gene exchange between these archaeal viruses (Figure 1C).
Except for group C, all magroviruses encode a DnaQ-like exonuclease that can be implicated in proofreading during viral genome replication. Unlike most of the other magrovirus genes, this nuclease shows significant sequence similarity only to bacterial homologs. Thus, somewhat unexpectedly, magroviruses appear to have acquired genes not only from archaea but also from bacteria.
A notable feature of magroviruses is the presence of two genes encoding distinct ATP-dependent DNA ligases in groups A and B, one within the replicative module and the other one, unexpectedly, embedded in the structural module (Figure 2B). The provenances of these ligases are different as indicated by phylogenetic analysis: the ligase encoded within the structural module represents a distinct family that along with the magrovirus proteins includes ligases from uncharacterized bacteria; the ligase in the replicative block of group B is a typical archaeal variety, whereas the one in group A replicative block belongs to the distinct family known as “thermostable ligases” (Figure S1). Thus, apparently, ligases have been acquired by magroviruses on three independent occasions.
The structural modules of all magroviruses also contain another inserted gene that in different groups encodes distinct nucleases (Figure 2B). In groups A, B, and D, this is a homing endonuclease (LAGLI-DADG family) homologous to nucleases of group I self-splicing introns and inteins, which are also present in many bacteriophages. In contrast, in group C, the inserted gene encodes a homolog of the exonuclease subunit of the archaeal DNA polymerase D [32]. An intriguing possibility is that the two nucleases play analogous roles in magrovirus replication. In addition to the conserved replicative genes, several genes implicated in replication are found in individual groups of magroviruses, e.g., ribonucleotide reductase in group B and SNF2 family helicase in group A.
Apart from the replicative and structural-morphogenetic proteins, 12 of the 26 magroviruses encode either a bacterial-type chaperonin GroEL (groups B, B1, and C) or a thermosome subunit, the archaeal homolog of GroEL (groups A and D) (Figure 2B). Unlike the replicative genes, these magrovirus proteins are highly similar to the archaeal and bacterial homologs. Phylogenetic analysis confirmed the sharp split between GroEL and thermosome subunits (Figure 1D). The thermosome subunits of magroviruses group with homologs from MG-II, which is compatible with relatively recent acquisition of these genes by the viruses. A subset of MG-II archaea encode GroEL instead of the thermosome subunit, conceivably owing to displacement of the ancestral archaeal chaperonin by a bacterial homolog. Although the magrovirus GroEL do not group with those of MG-II in the phylogenetic tree, this could be due to the accelerated evolution in the viruses; acquisition of this gene from MG-II archaea remains likely. Comparison of the topology of the chaperonin tree with those of the MCP, DNAP, and AEP trees suggests that the common ancestor of the magroviruses acquired a GroEL gene that was replaced by the thermosome subunit in group A. Chaperonins are not encoded by any known archaeal viruses but have been detected in several bacteriophages [33, 34]. Notably, these phages do not encode the co-chaperonin GroES, and GroES is not required for the phage chaperonin activity [35]. This is likely to be the case for the magrovirus chaperonins as well. Given that, in magroviruses, the chaperonin genes reside in the replicative gene cluster, the chaperonins might facilitate folding of the replicative proteins and replisome assembly.
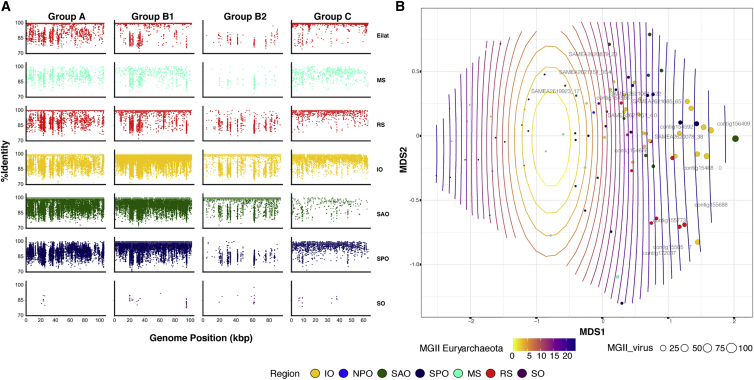
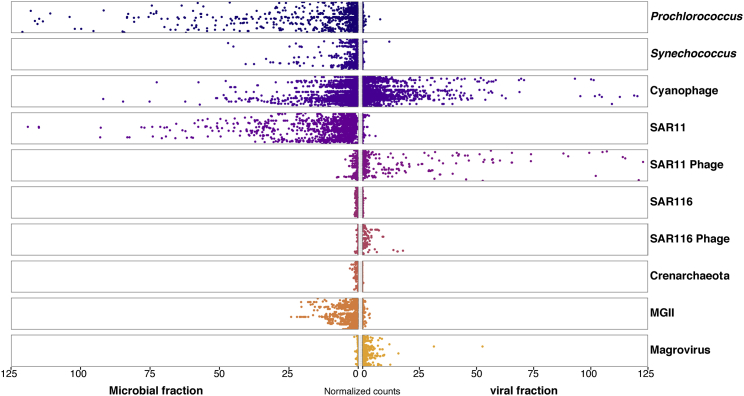
Mapping reads from the Red Sea and from the Tara Oceans on the 26 magrovirus genomes indicate that these viruses are globally widespread, with high abundance in the Indian Ocean, the Red Sea, and the South Pacific and Atlantic Oceans (Figures 3A and 3B), and are composed of different ecotypes (Figure 3A). Magroviruses are highly abundant in the marine environment, third only to SAR11 phages [36] and cyanophages (Figure 4). Surprisingly, the overall abundance of magroviruses was found to be higher than that of SAR116 phages (Figure 4), previously identified as the second most abundant phage group in the oceans [37].
Figure 3.
Global Abundance of Magroviruses and MG-II and/or MG-III
(A) Recruitment plots of representatives from different magrovirus groups (A: MAG 154566, B1: MAG 154680, B2: MAG SAMEA2620879_23, C: MAG 155057), using reads (viral fraction) from surface waters (top 5 m) from the Red Sea and different Tara Oceans stations.
(B) Non-metric multidimensional scaling (NMDS) plot of sampling sites magrovirus abundance. Circle diameter indicates magrovirus abundance at a specific site. MG-II abundance is represented by a gradient of white to red contour lines. A linear response between the magrovirus abundance ordination and the MG-II abundance variable is represented by fitted contours that are equally spaced parallel lines perpendicular to the MG-II abundance vector (R-sq.[adj] = 0.432; deviance explained = 48%; p value = 4.82e−10).
Region abbreviations are as follows: IO, Indian Ocean; RS, Red Sea; SO, Southern Ocean; MS, Mediterranean Sea; NPO, North Pacific Ocean; SAO, South Atlantic Ocean; SPO, South Pacific Ocean.
Figure 4.
Total Abundance of Magroviruses
The abundance of the Magrovirus reads in Red Sea samples and all Tara Oceans microbiomes [21] and viromes [18] is shown along with the abundances of the putative host MG-II, marine group I Crenarchaeota, marine Cyanobacteria (Synechococcus and Prochlorococcus) and their phages, SAR11 bacteria and their phages, and SAR116 and their phages. Plots for halovirus and representatives from cultured Euryarchaeota are not shown as the signals were close to zero. Horizontal axis: the normalized count (genome fragments per kilobase reference sequence per million library reads [GFPM]; see Supplemental Experimental Procedures). Vertical axis: the sampling stations.
So far, based on microscopic measurements [26], head-tailed viruses appeared to be the least abundant morphotype of archaeal viruses. However, our abundance estimates for magroviruses suggest that these previously uncharacterized head-tailed viruses dominate the archaeal virome in surface marine environments. In contrast to the cyanophages, and the SAR11 phages, normalized counts of magroviruses are almost negligible in the microbial fraction (>0.2 μm) but high in the virus-enriched fraction (<0.2 μm) (Figure 4). Thus, the principal source of the magrovirus reads appear to be free virus particles rather than cell-associated viruses, proviruses or megaplasmids. A possible explanation to this observation is that MG-II show seasonal blooming patterns [6]. However, the analysis was done with samples spanning multiple seasons and regions. The low magrovirus signal in the microbial fraction raises interesting questions on how magrovirus virions are maintained in the marine surface waters for long periods of time.
Despite the abundance of MG-II in the oceans and their apparent ecological importance, no viruses infecting these organisms have been identified so far. Here, using metagenomic approaches, we describe three distinct groups of viruses associated with MG-II. Unequivocal demonstration of the virus-host relationship between magroviruses and MG-II is currently unfeasible due to the lack of cultivable MG-II representatives. Nevertheless, several lines of evidence strongly suggest that MG-II archaea are indeed the hosts of magroviruses. First, our abundance estimates show that MG-II is the dominant archaeal group in the samples from which the magrovirus genomes were assembled (Figures 3A and 3B). Second, most of the replicative genes of magroviruses and especially the viral chaperonins show clear signs of archaeal provenance, and in some cases, a specific connection with homologs from MG-II. Furthermore, group B magroviruses encode two tRNAs (tRNALeu and tRNAArg) that are most similar to the respective tRNAs of MG-II archaea. Some of the marine Euryarchaeal fosmids deposited in GenBank lack 16S rDNA genes, therefore their affiliation is not resolved, and they are deposited in GenBank as MG-II/III. In addition, some representatives of MG-III are abundant in surface waters [38], therefore we cannot rule out the possibility that MG-III are the hosts of (some) magroviruses.
The discovery of the magroviruses is part of the growing trend in virology whereby viruses are recognized solely from metagenomics sequence analysis, as recently formalized by the International Committee for Taxonomy of Viruses [39]. Genome analysis of the magroviruses is consistent with modular evolution of viruses whereby the structural and replicative modules have distinct origins. Magroviruses are unusual among viruses of bacteria and archaea in that they encode an elaborate, apparently (almost) self-sufficient replication apparatus. Given the high abundance of both MG-II archaea and magroviruses, the latter are likely to be important ecological agents, similar to cyanophages or archaeal viruses that apparently infect Thaumarchaeota [40].
While this paper was in revision, identification of a group of putative viruses overlapping with the magrovirus set described here has been reported independently [41].
Author Contributions
A.P. and O.B designed the project. A.P., N.Y., J.F.-U., I.S., E.V.K., and O.B. performed bioinformatic analyses. A.P., E.V.K., and O.B. wrote the manuscript with contributions from all authors to data analysis, figure generation, and the final manuscript.
Acknowledgments
The authors would like to thank the captain and crew of the R/V Sam Rothberg of the Inter University Institute in Eilat, Israel for their expert assistance at sea and Hagay Enav and Idan Bodaker for their help with sampling. The authors would also like to thank David Cohen of the Physics Department at the Technion for his help with the HPC ATLAS cluster and Mart Krupovic of the Institut Pasteur (Paris) for critical reading on the manuscript and helpful suggestions. This work was funded by a European Commission ERC Advanced Grant (no. 321647), the Technion’s Lorry I. Lokey Interdisciplinary Center for Life Sciences and Engineering and the Russell Berrie Nanotechnology Institute, and the Louis and Lyra Richmond Memorial Chair in Life Sciences (to O.B.). N.Y. and E.V.K. are supported by intramural funds of the US Department of Health and Human Services (to the National Library of Medicine).
Published: April 27, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, three figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2017.03.052.
Contributor Information
Alon Philosof, Email: aphilosof@gmail.com.
Oded Béjà, Email: beja@tx.technion.ac.il.
Accession Numbers
The raw sequencing data and assembled contigs reported in this paper have been deposited in the European Nucleotide Archive under project ENA: PRJEB19060.
Supplemental Information
References
- 1.Massana R., Murray A.E., Preston C.M., DeLong E.F. Vertical distribution and phylogenetic characterization of marine planktonic Archaea in the Santa Barbara Channel. Appl. Environ. Microbiol. 1997;63:50–56. doi: 10.1128/aem.63.1.50-56.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massana R., DeLong E.F., Pedrós-Alió C. A few cosmopolitan phylotypes dominate planktonic archaeal assemblages in widely different oceanic provinces. Appl. Environ. Microbiol. 2000;66:1777–1787. doi: 10.1128/aem.66.5.1777-1787.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galand P.E., Casamayor E.O., Kirchman D.L., Potvin M., Lovejoy C. Unique archaeal assemblages in the Arctic Ocean unveiled by massively parallel tag sequencing. ISME J. 2009;3:860–869. doi: 10.1038/ismej.2009.23. [DOI] [PubMed] [Google Scholar]
- 4.Lincoln S.A., Wai B., Eppley J.M., Church M.J., Summons R.E., DeLong E.F. Planktonic Euryarchaeota are a significant source of archaeal tetraether lipids in the ocean. Proc. Natl. Acad. Sci. USA. 2014;111:9858–9863. doi: 10.1073/pnas.1409439111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C.L., Xie W., Martin-Cuadrado A.-B., Rodriguez-Valera F. Marine Group II Archaea, potentially important players in the global ocean carbon cycle. Front. Microbiol. 2015;6:1108. doi: 10.3389/fmicb.2015.01108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray A.E., Blakis A., Massana R., Strawzewiski S., Passow U., Alldredge A., DeLong E.F. A timeseries assessment of planktonic archaeal variability in the Santa Barbara Channel. Aquat. Microb. Ecol. 1999;20:129–145. [Google Scholar]
- 7.Pernthaler A., Preston C.M., Pernthaler J., DeLong E.F., Amann R. Comparison of fluorescently labeled oligonucleotide and polynucleotide probes for the detection of pelagic marine bacteria and archaea. Appl. Environ. Microbiol. 2002;68:661–667. doi: 10.1128/AEM.68.2.661-667.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ottesen E.A., Young C.R., Eppley J.M., Ryan J.P., Chavez F.P., Scholin C.A., DeLong E.F. Pattern and synchrony of gene expression among sympatric marine microbial populations. Proc. Natl. Acad. Sci. USA. 2013;110:E488–E497. doi: 10.1073/pnas.1222099110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aylward F.O., Eppley J.M., Smith J.M., Chavez F.P., Scholin C.A., DeLong E.F. Microbial community transcriptional networks are conserved in three domains at ocean basin scales. Proc. Natl. Acad. Sci. USA. 2015;112:5443–5448. doi: 10.1073/pnas.1502883112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iverson V., Morris R.M., Frazar C.D., Berthiaume C.T., Morales R.L., Armbrust E.V. Untangling genomes from metagenomes: revealing an uncultured class of marine Euryarchaeota. Science. 2012;335:587–590. doi: 10.1126/science.1212665. [DOI] [PubMed] [Google Scholar]
- 11.Martin-Cuadrado A.-B., Garcia-Heredia I., Moltó A.G., López-Úbeda R., Kimes N., López-García P., Moreira D., Rodriguez-Valera F. A new class of marine Euryarchaeota group II from the Mediterranean deep chlorophyll maximum. ISME J. 2015;9:1619–1634. doi: 10.1038/ismej.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prangishvili D. The wonderful world of archaeal viruses. Annu. Rev. Microbiol. 2013;67:565–585. doi: 10.1146/annurev-micro-092412-155633. [DOI] [PubMed] [Google Scholar]
- 13.Krupovic M., Prangishvili D., Hendrix R.W., Bamford D.H. Genomics of bacterial and archaeal viruses: dynamics within the prokaryotic virosphere. Microbiol. Mol. Biol. Rev. 2011;75:610–635. doi: 10.1128/MMBR.00011-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter K., Russ B.E., Dyall-Smith M.L. Virus-host interactions in salt lakes. Curr. Opin. Microbiol. 2007;10:418–424. doi: 10.1016/j.mib.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Pina M., Bize A., Forterre P., Prangishvili D. The archeoviruses. FEMS Microbiol. Rev. 2011;35:1035–1054. doi: 10.1111/j.1574-6976.2011.00280.x. [DOI] [PubMed] [Google Scholar]
- 16.Atanasova N.S., Roine E., Oren A., Bamford D.H., Oksanen H.M. Global network of specific virus-host interactions in hypersaline environments. Environ. Microbiol. 2012;14:426–440. doi: 10.1111/j.1462-2920.2011.02603.x. [DOI] [PubMed] [Google Scholar]
- 17.Pietilä M.K., Laurinmäki P., Russell D.A., Ko C.C., Jacobs-Sera D., Butcher S.J., Bamford D.H., Hendrix R.W. Insights into head-tailed viruses infecting extremely halophilic archaea. J. Virol. 2013;87:3248–3260. doi: 10.1128/JVI.03397-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brum J.R., Ignacio-Espinoza J.C., Roux S., Doulcier G., Acinas S.G., Alberti A., Chaffron S., Cruaud C., de Vargas C., Gasol J.M., Tara Oceans Coordinators Ocean plankton. Patterns and ecological drivers of ocean viral communities. Science. 2015;348:1261498. doi: 10.1126/science.1261498. [DOI] [PubMed] [Google Scholar]
- 19.Paez-Espino D., Eloe-Fadrosh E.A., Pavlopoulos G.A., Thomas A.D., Huntemann M., Mikhailova N., Rubin E., Ivanova N.N., Kyrpides N.C. Uncovering Earth’s virome. Nature. 2016;536:425–430. doi: 10.1038/nature19094. [DOI] [PubMed] [Google Scholar]
- 20.Pesant S., Not F., Picheral M., Kandels-Lewis S., Le Bescot N., Gorsky G., Iudicone D., Karsenti E., Speich S., Troublé R., Tara Oceans Consortium Coordinators Open science resources for the discovery and analysis of Tara Oceans data. Sci. Data. 2015;2:150023. doi: 10.1038/sdata.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunagawa S., Coelho L.P., Chaffron S., Kultima J.R., Labadie K., Salazar G., Djahanschiri B., Zeller G., Mende D.R., Alberti A., Tara Oceans coordinators Ocean plankton. Structure and function of the global ocean microbiome. Science. 2015;348:1261359. doi: 10.1126/science.1261359. [DOI] [PubMed] [Google Scholar]
- 22.Iranzo J., Koonin E.V., Prangishvili D., Krupovic M. Bipartite network analysis of the archaeal virosphere: evolutionary connections between viruses and capsidless mobile elements. J. Virol. 2016;90:11043–11055. doi: 10.1128/JVI.01622-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makarova K.S., Koonin E.V. Archaeology of eukaryotic DNA replication. Cold Spring Harb. Perspect. Biol. 2013;5:a012963. doi: 10.1101/cshperspect.a012963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazlauskas D., Krupovic M., Venclovas Č. The logic of DNA replication in double-stranded DNA viruses: insights from global analysis of viral genomes. Nucleic Acids Res. 2016;44:4551–4564. doi: 10.1093/nar/gkw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breitbart M., Thompson L.R., Suttle C.A., Sullivan M.B. Exploring the vast diversity of marine viruses. Oceanography (Wash. D.C.) 2007;20:135–139. [Google Scholar]
- 26.Luk A.W., Williams T.J., Erdmann S., Papke R.T., Cavicchioli R. Viruses of haloarchaea. Life (Basel) 2014;4:681–715. doi: 10.3390/life4040681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makarova K.S., Krupovic M., Koonin E.V. Evolution of replicative DNA polymerases in archaea and their contributions to the eukaryotic replication machinery. Front. Microbiol. 2014;5:354. doi: 10.3389/fmicb.2014.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takemura M., Yokobori S., Ogata H. Evolution of eukaryotic DNA polymerases via interaction between cells and large DNA viruses. J. Mol. Evol. 2015;81:24–33. doi: 10.1007/s00239-015-9690-z. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan M.B., Coleman M.L., Weigele P., Rohwer F., Chisholm S.W. Three Prochlorococcus cyanophage genomes: signature features and ecological interpretations. PLoS Biol. 2005;3:e144. doi: 10.1371/journal.pbio.0030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith D.R., Doucette-Stamm L.A., Deloughery C., Lee H., Dubois J., Aldredge T., Bashirzadeh R., Blakely D., Cook R., Gilbert K. Complete genome sequence of Methanobacterium thermoautotrophicum deltaH: functional analysis and comparative genomics. J. Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klenk H.P., Clayton R.A., Tomb J.F., White O., Nelson K.E., Ketchum K.A., Dodson R.J., Gwinn M., Hickey E.K., Peterson J.D. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature. 1997;390:364–370. doi: 10.1038/37052. [DOI] [PubMed] [Google Scholar]
- 32.Jokela M., Eskelinen A., Pospiech H., Rouvinen J., Syväoja J.E. Characterization of the 3′ exonuclease subunit DP1 of Methanococcus jannaschii replicative DNA polymerase D. Nucleic Acids Res. 2004;32:2430–2440. doi: 10.1093/nar/gkh558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hertveldt K., Lavigne R., Pleteneva E., Sernova N., Kurochkina L., Korchevskii R., Robben J., Mesyanzhinov V., Krylov V.N., Volckaert G. Genome comparison of Pseudomonas aeruginosa large phages. J. Mol. Biol. 2005;354:536–545. doi: 10.1016/j.jmb.2005.08.075. [DOI] [PubMed] [Google Scholar]
- 34.Cornelissen A., Hardies S.C., Shaburova O.V., Krylov V.N., Mattheus W., Kropinski A.M., Lavigne R. Complete genome sequence of the giant virus OBP and comparative genome analysis of the diverse ΦKZ-related phages. J. Virol. 2012;86:1844–1852. doi: 10.1128/JVI.06330-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semenyuk P.I., Orlov V.N., Sokolova O.S., Kurochkina L.P. New GroEL-like chaperonin of bacteriophage OBP Pseudomonas fluorescens suppresses thermal protein aggregation in an ATP-dependent manner. Biochem. J. 2016;473:2383–2393. doi: 10.1042/BCJ20160367. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y., Temperton B., Thrash J.C., Schwalbach M.S., Vergin K.L., Landry Z.C., Ellisman M., Deerinck T., Sullivan M.B., Giovannoni S.J. Abundant SAR11 viruses in the ocean. Nature. 2013;494:357–360. doi: 10.1038/nature11921. [DOI] [PubMed] [Google Scholar]
- 37.Kang I., Oh H.-M., Kang D., Cho J.-C. Genome of a SAR116 bacteriophage shows the prevalence of this phage type in the oceans. Proc. Natl. Acad. Sci. USA. 2013;110:12343–12348. doi: 10.1073/pnas.1219930110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haro-Moreno J.M., Rodriguez-Valera F., López-García P., Moreira D., Martin-Cuadrado A.-B. New insights into marine group III Euryarchaeota, from dark to light. ISME J. 2017 doi: 10.1038/ismej.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simmonds P., Adams M.J., Benkő M., Breitbart M., Brister J.R., Carstens E.B., Davison A.J., Delwart E., Gorbalenya A.E., Harrach B. Consensus statement: virus taxonomy in the age of metagenomics. Nat. Rev. Microbiol. 2017;15:161–168. doi: 10.1038/nrmicro.2016.177. [DOI] [PubMed] [Google Scholar]
- 40.Danovaro R., Dell’Anno A., Corinaldesi C., Rastelli E., Cavicchioli R., Krupovic M., Noble R.T., Nunoura T., Prangishvili D. Virus-mediated archaeal hecatomb in the deep seafloor. Sci. Adv. 2016;2:e1600492. doi: 10.1126/sciadv.1600492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishimura Y., Watai H., Honda T., Mihara T., Omae K., Roux S., Blanc-Mathieu R., Yamamoto K., Hingamp P., Sako Y. Environmental viral genomes shed new light on virus-host interactions in the ocean. mSphere. 2017;2 doi: 10.1128/mSphere.00359-16. e00359–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.