Abstract
Development of intracranial complications from middle ear infections might be difficult to diagnose. We compared radiological and surgical findings of 26 patients affected by otogenic meningitis. Results of our analysis showed that surgery is more reliable than imaging in revealing bone defects. Therefore, suggest that surgery be performed for diagnosis and eventual management of all cases of suspected otogenic meningitis.
Keywords: computed tomography, magnetic resonance imaging, middle ear infection, otogenic meningitis, surgery
Middle ear infections, such as acute otitis media and chronic suppurative otitis media, with or without cholesteatoma, may result in intracranial complications (ECs) with a reported prevalence of 0.4% to 6.4% of cases [1–3].
Among ECs, meningitis is the most common, with a prevalence of 35.0%–46.4% [1, 4]. Identification of meningitis and its otogenic source is easy, but recognizing an underlying bone defects may be more challenging [2, 5].
Pathways of intracranial spread from otogenic focus include anatomical contiguity between meninges and inner ear, hematogenous spread, thrombophlebitis of the blood vessel, and bone dehiscence (such as tegmen tympani erosion) [6].
Imaging (computed tomography [CT] and magnetic resonance imaging [MRI]) is of uttermost importance in documenting bone defects causing otogenic meningitis. Head and temporal bone high-resolution CT scans show middle ear and/or mastoid infection, bone tissue reaction, and its ECs (herniation, hydrocephalus, empyema, otogenic pneumocephalus, venal or arterial infarction, and abscess formation) [7]. Magnetic resonance imaging is superior to CT scan in visualizing otogenic labyrinthitis and retrocochlear or intracranial abnormalities, with contrast-enhanced T1-weighted images having higher specificity and the potential to detect parenchymal-associated abnormalities [4, 8].
PATIENTS AND METHODS
We retrospectively evaluated clinical records of all consecutive patients affected by otogenic meningitis admitted to the Universtity Hospital of Pisa from January 2009 to December 2014. We included in our analysis all patients considered affected by otogenic meningitis because of clinical diagnosis and/or imaging findings, with compatible microbiologic results, who had either CT or MRI or both performed and who underwent surgery. During their stay, patients were first hospitalized in the Intensive Care Unit, then in the Infectious Disease Units for medical treatment, and, after clinical improvement, in the Ear Nose and Throat, Audiology, and Phoniatrics Unit. All patients received intravenous antibiotic therapy for at least 14 days according to cerebrospinal fluid (CSF) direct examination, serological tests, and cultures, performed CT or MRI or both during the initial 2 or 3 weeks, respectively, and underwent surgery within 60 days of the admission. We operated on all the patients on the basis of the clinical diagnosis and/or the imaging. The first outcome was considered 48 hours after the operation. Last follow up was 2.5 years later. After a first comparative analysis, which considered the radiology report drafted by the neuroradiologist on duty, a not-blinded neuroradiologist reviewed the images of the cases with discordant findings between radiology and surgery, to increase the result’s veracity.
RESULTS
Overall, 26 cases were included in our analysis. Twelve (46.2%) patients were male with a median age of 68 years, (range, 32–82). Patients’ characteristics, clinical features, microbiological results, and treatment are summarized in Table 1. The comparison among clinical diagnosis, imaging, and surgical findings, with specific characteristics of CT scan and MRI, surgical indication, and patients outcome, are summarized in Table 2.
Table 1.
Patient Characteristics
| Pt | Age | Sex | Microbiology | Treatment | Clinical Diagnosis | Note |
|---|---|---|---|---|---|---|
| 1 | 65 | F | Neg | Meropenem + Linezolid | Otomastoiditis | First episode of meningitis |
| 2 | 68 | F | Neg | Meropenem + Acyclovir | CSOM | First episode of meningitis |
| 3 | 59 | F | Haemophilus influenzae | Meropenem + Linezolid | AOM | First episode of meningitis |
| 4 | 58 | F | Streptococcus pneumoniae | Ceftriaxone + Vancomycin | Otomastoiditis | First episode of meningitis |
| 5 | 69 | F | S pneumoniae | Ceftriaxone + Ampicillin | AOM | Previous meningioma removal |
| 6 | 35 | M | S pneumoniae | Ceftriaxone | Otomastoiditis | First episode of meningitis |
| 7 | 74 | F | S pneumoniae | Ceftriaxone | Otomastoiditis | First episode of meningitis |
| 8 | 70 | M | S pneumoniae | Ceftriaxone + Levofloxacin | Otomastoiditis + right temporal abscess | First episode of meningitis |
| 9 | 32 | F | S pneumoniae | Ceftriaxone | Otomastoiditis + abscess | First episode of meningitis |
| 10 | 68 | F | S pneumoniae | Ceftriaxone | Unremarkable | First episode of meningitis |
| 11 | 73 | M | S pneumoniae | Ceftriaxone | CSOM | First episode of meningitis |
| 12 | 39 | M | S pneumoniae | Ceftriaxone | AOM | Relapse |
| 13 | 82 | M | S pneumoniae | Ceftriaxone | Unremarkable | First episode of meningitis |
| 14 | 52 | F | S pneumoniae | Ceftriaxone | Unremarkable | Nasal liquorrhea First episode of meningitis |
| 15 | 67 | M | S pneumoniae | Ceftriaxone + Ampicillin | AOM | First episode of meningitis |
| 16 | 46 | M | S pneumoniae | Ceftriaxone | Cholesteatoma | First episode of meningitis |
| 17 | 73 | F | S pneumoniae | Ceftriaxonee | Otomastoiditis | First episode of meningitis |
| 18 | 73 | F | S pneumoniae | Ceftriaxone + Levofloxacin | Otomastoiditis | Relapse |
| 19 | 57 | F | S pneumoniae | Ceftriaxone | AOM | First episode of meningitis |
| 20 | 72 | F | S pneumoniae | Ceftriaxone | CSOM | First episode of meningitis |
| 21 | 65 | F | S pneumoniae | Ceftriaxone | CSOM | First episode of meningitis |
| 22 | 74 | F | S pneumoniae | Ceftriaxone | Unremarkable | First episode of meningitis |
| 23 | 44 | M | S pneumoniae | Ceftriaxone | AOM | First episode of meningitis |
| 24 | 75 | M | S pneumoniae | Ceftriaxone | Cholesteatoma | First episode of meningitis |
| 25 | 71 | M | Pseudomonas aeruginosa | Meropenem | Liquoral fistula | First episode of meningitis |
| 26 | 69 | F | Streptococcus bovis | Ceftriaxone | Unremarkable | First episode of meningitis |
Abbreviations: AOM, acute otitis media; CSOM, chronic suppurative otitis media; Neg, negative; Pt, patient.
Table 2.
Imaging and Surgery Details
| Pt | Clinical Diagnosis | CT Findings | Note | MRI Findings | Note | Surgery Indications | Surgical Findings | Timing (Days) |
Operation Outcome | 30 Weeks Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Otomastoiditis | Tegmen tympani dehiscence | CT brain Without contrast Day 1 |
Unremarkable | MRI brain With contrast Day 8 |
Tegmen tympani dehiscence | Tegmen tympani dehiscence Mastoid erosion |
15 | Survived | Survived |
| 2 | CSOM | Tegmen tympani dehiscence Mastoid erosion |
CT ear Without contrast Day 8 |
Unremarkable | MRI brain With contrast Day 9 |
Tegmen tympani dehiscence | Tegmen tympani dehiscence Mastoid erosion |
17 | Survived | Survived |
| 3 | AOM | Tegmen tympani dehiscence Meningoencephalocele Cholesteatoma |
CT brain Without contrast Day 1 |
Tegmen tympani dehiscence Mastoid erosion |
MRI brain With contrast Day 11 |
Tegmen tympani dehiscence Meningoencephalocele Cholesteatoma |
Tegmen tympani dehiscence Mastoid erosion Meningoencephalocele Cholesteatoma |
30 | Survived | Deceased (no relapse) |
| 4 | Otomastoiditis | Unremarkable | CT ear Without contrast Day 11 |
Not performed | Otomastoiditis | Tegmen tympani dehiscence Mastoid erosion |
17 | Survived | Survived | |
| 5 | AOM | Unremarkable | CT brain Without contrast Day 1 |
Unremarkable | MRI brain With contrast Day 21 |
AOM | Tegmen tympani dehiscence Meningoencephalocele |
45 | Survived | Survived |
| 6 | Otomastoiditis | Unremarkable | CT right ear Without contrast Day 7 |
Unremarkable | MRI brain With contrast Day 21 |
Otomastoiditis | Cholesteatoma | 28 | Survived | Deceased (no relapse) |
| 7 | Otomastoiditis | Unremarkable | CT brain Without contrast Day 1 |
Not performed | Otomastoiditis | Tegmen tympani dehiscence | 14 | Survived | Survived | |
| 8 | Otomastoiditis + right temporal abscess | Tegmen tympani dehiscence | CT brain Without contrast Day 1 |
Right temporal abscess | MRI brain With contrast Day 21 |
Tegmen tympani dehiscence + temporal abscess | Tegmen tympani dehiscence Mastoid erosion Meningoencephalocele |
60 | Survived | Survived |
| 9 | Otomastoiditis + right temporal abscess | Unremarkable | CT brain Without contrast Day 5 |
Right temporal abscess | MRI brain With contrast Day 19 |
Otomastoiditis + temporal abscess | Tegmen tympani dehiscence Cholesteatoma |
28 | Survived | Survived |
| 10 | Unremarkable | Otomastoiditis | CT brain Without contrast Day 1 |
Unremarkable | MRI brain With contrast Day 3 |
Otomastoiditis | Tegmen tympani dehiscence Meningoencephalocele |
10 | Survived | Survived |
| 11 | CSOM | Tegmen tympani dehiscence Cholesteatoma |
CT ear Without contrast Day 7 |
Not performed | Tegmen tympani dehiscence Cholesteatoma |
Cholesteatoma | 60 | Survived | Survived | |
| 12 | AOM | Tegmen tympani dehiscence Cholesteatoma |
CT ear Without contrast Day 4 |
Not performed | Tegmen tympani dehiscence Cholesteatoma |
Tegmen tympani dehiscence Cholesteatoma |
Survived | Unknown | ||
| 13 | Unremarkable | Tegmen tympani dehiscence | CT head and face Without contrast Day 9 |
Unremarkable | MRI brain With contrast Day 10 |
Tegmen tympani dehiscence | Unremarkable | 10 | Deceased | Deceased |
| 14 | Unremarkable | Otomastoiditis | CT head and face Without contrast Day 7 |
Not performed | Otomastoiditis | Unremarkable | 21 | Survived | Survived | |
| 15 | AOM | Tegmen tympani dehiscence | CT ear Without contrast Day 7 |
Not performed | Tegmen tympani dehiscence | Tegmen tympani dehiscence Meningoencephalocele |
6 | Survived | Survived | |
| 16 | Cholesteatoma | Cholesteatoma | CT brain Without contrast Day 1 |
Unremarkable | MRI brain With contrast Day 18 |
Cholesteatoma | Tegmen tympani dehiscence Cholesteatoma |
21 | Survived | Survived |
| 17 | Otomastoiditis | Cholesteatoma | CT brain With contrast Day 5 |
Not performed | Cholesteatoma | Cholesteatoma | 55 | Decead | Deceased (no relapse) | |
| 18 | Otomastoiditis | Tegmen tympani dehiscence | CT brain Without contrast Day 1 |
Mastoid erosion | MRI brain With contrast Day 15 |
Tegmen tympani dehiscence | Tegmen tympani dehiscence Mastoid erosion Meningoencephalocele |
21 | Survived | Survived |
| 19 | AOM | Unremarkable | CT brain and ear With contrast Day 3 |
Unremarkable | MRI brain With contrast Day 21 |
AOM | Unremarkable. | 27 | Survived | Unknown |
| 20 | CSOM | Cholesteatoma | CT brain Without contrast Day 1 |
Unremarkable | MRI brain With contrast Day 11 |
CSOM | Cholesteatoma | 16 | Survived | Deceased (no relapse) |
| 21 | CSOM | Tegmen tympani dehiscence Mastoid erosion Cholesteatoma |
CT ear Without contrast Day 8 |
Tegmen tympani dehiscence Cholesteatoma |
MRI brain With contrast 13 |
Tegmen tympani dehiscence Mastoid erosion Cholesteatoma |
Tegmen tympani dehiscence Mastoid erosion Cholesteatoma |
27 | Survived | Survived |
| 22 | Unremarkable | Tegmen tympani dehiscence Mastoid erosion Cholesteatoma |
CT temporal bone Without contrast Day 1 |
Tegmentympani dehiscence Mastoid erosion Cholesteatoma |
MRI brain and petrous bone With contrast Day 2 |
Tegmen tympani dehiscence Mastoid erosion Cholesteatoma |
Tegmen tympani dehiscence Mastoid erosion Cholesteatoma |
3 | Survived | Survived |
| 23 | AOM | Tegmen tympani dehiscence Cholesteatoma |
CT ear Without contrast Day 5 |
Not performed | Tegmen tympani dehiscence Cholesteatoma |
Tegmen tympani dehiscence Meningoencephalocele Cholesteatoma |
60 | Survived | Survived | |
| 24 | Cholesteatoma | Tegmen tympani dehiscence Cholesteatoma |
CT brain Without contrast Day 1 |
Mastoid erosion Thrombophlebitis |
MRI brain With contrast Day 2 |
Tegmen tympani dehiscence Cholesteatoma |
Tegmen tympani dehiscence Mastoid erosion Cholesteatoma |
3 | Survived | Survived |
| 25 | Liquoral fistula | Cerebrospinal fluid fistula | CT head and face With contrast Day 3 |
Not performed | Liquoral fistula | Tegmen tympani dehiscence Liquoral fistula |
5 | Survived | Survived | |
| 26 | Unremarkable | Tegmen tympani dehiscence | CT brain Without contrast Day 1 |
Not performed | Tegmen tympani dehiscence | Tegmen tympani dehiscence | 21 | Survived | Survived |
Abbreviations: AOM, acute otitis media; CSOM, chronic suppurative otitis media; CT, computed tomography; MRI, magnetic resonance imaging; Pt, patient.
At the first analysis, bone defect was documented by surgery in 19 (73.1%) patients as opposed to radiology, which was positive in 14 (53.8%) cases. Among the latter cases, 2 (14.3%)—patients 11 and 13—turned out to be false-positive diagnoses, because they were considered as tegmen tympani dehiscence, which was described on the CT report but not confirmed by the surgeon.
Only 1 (14.3%) of 7 patients with surgical evidences of meningoencephalocele had concordant imaging findings. Mastoid erosion was diagnosed during surgery in 9 cases, 6 (66.6%) of whom had been previously documented with radiological imaging. All cases of radiologically suspected ECs were confirmed intraoperatively, and no new cases were found at surgical theatre. Among the latter, in 1 patient (n = 25), we identified a CSF fistula.
On the basis of a radiological report drafted at the time of the images execution, sensitivity and specificity of imaging in identifying tegmen tympani dehiscence were 63.2% and 71.4%, respectively. Sensitivity and specificity of imaging in identifying meningoencephalocele were 14.3% and 100%, respectively.
Sensitivity and specificity of imaging in identifying mastoid erosion were 66.7% and 100%, respectively.
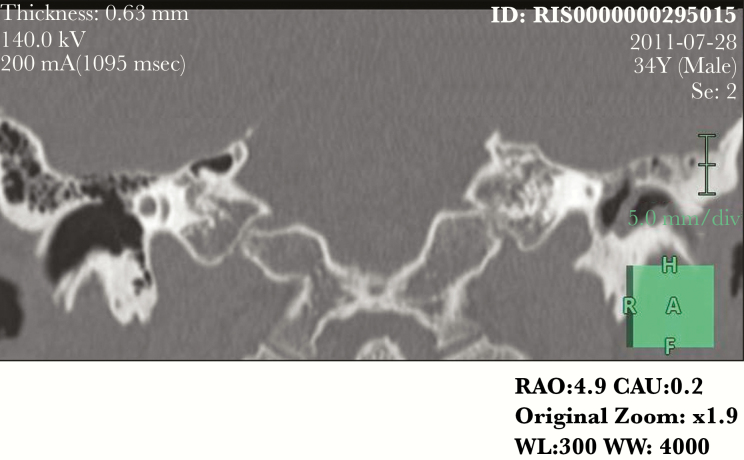
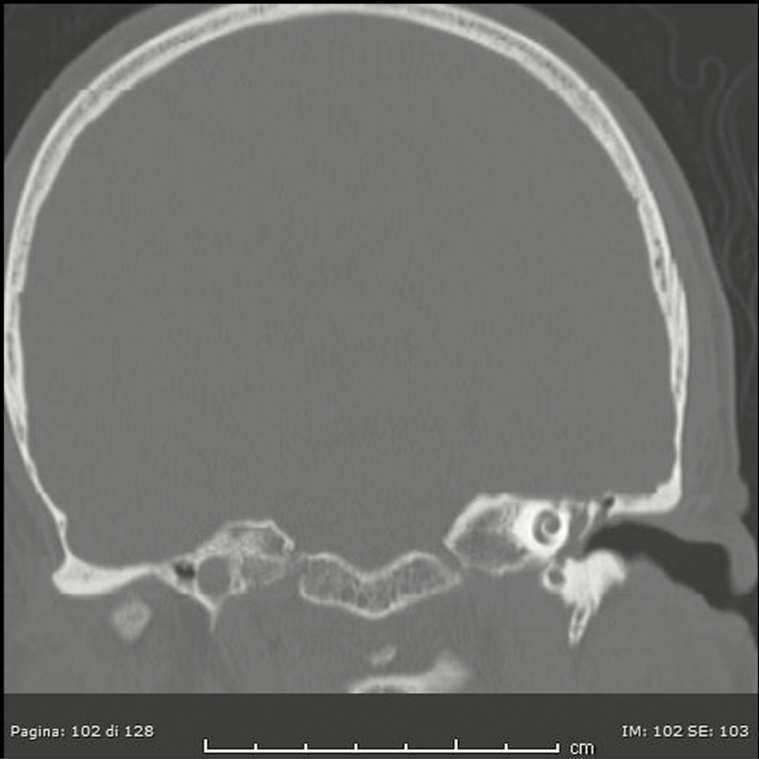
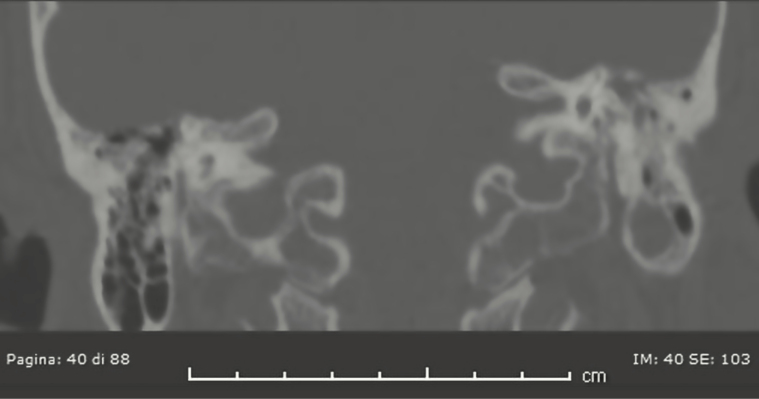
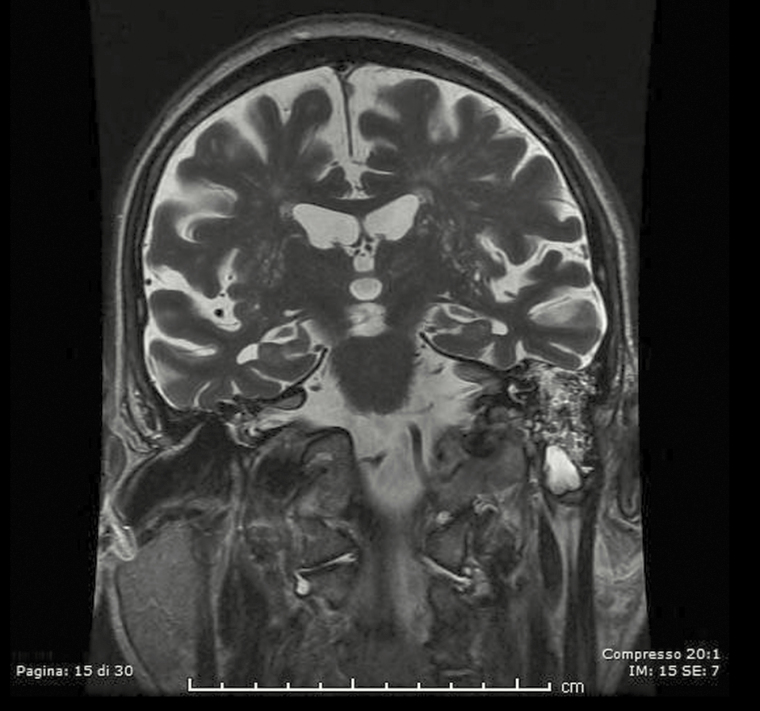
After this first analysis, a neuroradiologist reviewed all available images for these patients with discordant results between imaging and surgical: 4, 5, 6, 7, 9, and 10 because of “false negative” results and patient 13 because “false positive” results. Unfortunately, patients 4 and 5 performed CT scan and MRI elsewhere and the images are not available. During the not-blinded second look, the neuroradiologist confirmed absence of bone defects in patients 7 and 9 at CT scan, but he highlighted a thin tegmen tympani dehiscence on the anterior side and some dehiscences of the anterolateral flogistic petrous bone for patient 6 (Figure 1); he described significant dehiscence both on tegmen tympani and on mastoid for patient 10 (Figure 2). The neuroradiologist confirmed the case of false-positive imaging for patient 13; the CT scan showed probable mastoid erosion (Figure 3) and a meningocele visualized as signal interruption to MRI (Figure 4).
Figure 1.
Computed tomography scan of tegmen thympani dehiscence: false imaging negative.
Figure 2.
Computed tomography scan of tegmen thympani and mastoid erosion: false imaging negative.
Figure 3.
Computed tomography scan of meningocele: false imaging negative.
Figure 4.
Magnetic resonance imagingI of indirect sign of meningocele: false imaging negative.
After the revision of the nonblinded neuroradiologist, the sensitivity in identifying tegmen tympani dehiscence was changed to 73.7% (before 63.2%) and specificity remained at 71.4%. No differences were noted in sensitivity and specificity in identifying mastoid erosion and meningoencephalocele.
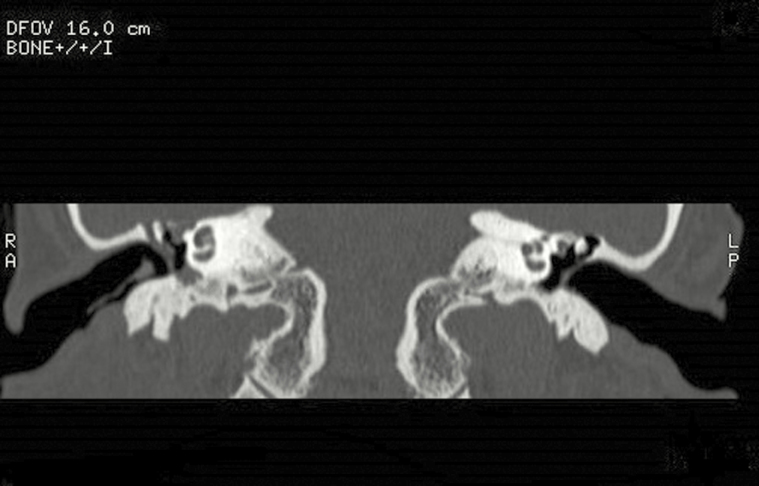
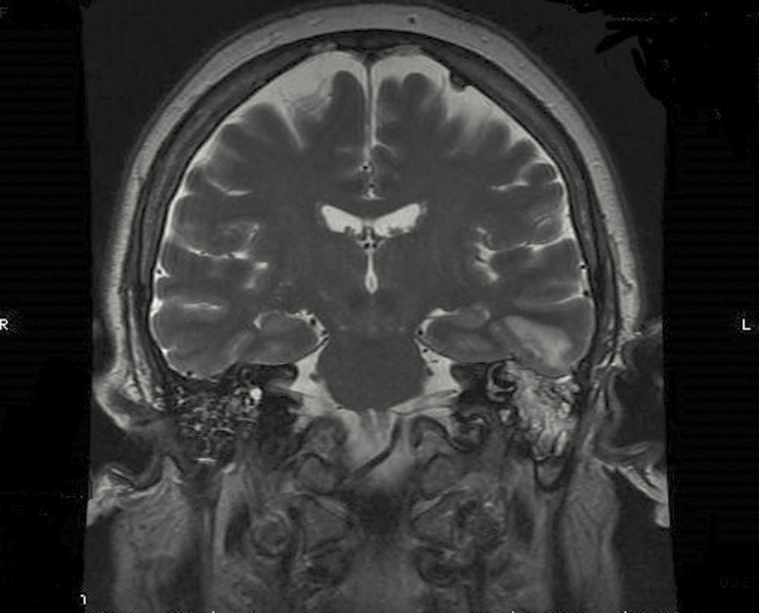
The best resolution to visualize bone defects proved to be the high special resolution bony-algorithm CT scan, with particular relevance for coronal axial plane. No significant performance difference was detected between imaging with or without contrast. Thirteen of 26 patients received the high-resolution bony-algorithm CT scan with coronal axial plane, and 11 of these were with diagnostic correspondence between imaging and surgical findings, in respect of bone erosion. We showed an example of bony-algorithm CT scan in coronal axial plan of tegmen tympani dehiscence (Figure 5) and one of mastoid erosion (Figure 6) and an example of MRI scan of meninogoencephalocele (Figure 7).
Figure 5.
Computed tomography scan, coronal axial plane, of tegmen thympani dehiscence.
Figure 6.
Computed tomography scan, coronal axial plane, of mastoid erosion.
Figure 7.
Magnetic resonance imaging of meningoencephalocele.
Only 1 patient died during the postoperation follow up. Four patients (15.4%) died during the 30-week follow-up period because of coexisting diseases, and no one was readmitted to the hospital. No cases of recurrence of meningitis were observed during follow up.
DISCUSSION
Acute and chronic middle ear diseases are potentially life-threatening because of their ECs [9, 10]. Otogenic meningitis has a high incidence in the general population: some studies report that acute middle otitis might be responsible for 50% of meningitis in adults and 25% in children [11]. The wide use of antibiotics to treat infectious otitis media has decreased the number of complications deriving from all forms of middle ear infections, which can increase the incidence of ECs to 0.13%–1.97% [12].
Tegmen tympani dehiscence, with or without meningoencephalocele, is one possible way the infection spreads from middle ear to brain [13, 14]. Meningitis and brain abscesses are the most common ECs of otitis media, and studies revealed that abscesses are mostly adjacent to the temporal bone and almost exclusively localized at the temporal lobe and cerebellum [15–17]. Computed tomography and MRI scan play an important role in diagnosing middle ear diseases and ECs, but sensibility and specificity of these techniques are not definitively established [18]. Early diagnosis and recognition of pathogenic mechanisms through imaging still represent a challenge for specialists: approximately 80% of skull bone defects in the region of the middle and posterior cranial fossa remain asymptomatic and are usually demonstrated incidentally during otosurgery performed for treating abnormalities that commonly result from chronic inflammatory ear conditions or for the management of ECs [18]. An osseous defect of the petrous bone cannot be recognized before the initial surgery, despite the CT scan and MRI [19]. On the contrary, Migirov maintained that CT has a sensitivity of 97% and a positive predictive value of 94.0% in diagnosis of complicated acute otomastoiditis [4]. Even though our experience is limited to a small number of cases, we have evidenced low sensibility and specificity of imaging in detecting bone defects, particularly for tegmen tympani dehiscence (63.2% and 71.4%, respectively), with 2 cases characterized by positive imaging not confirmed at the operating theatre. Sensibility and specificity in showing mastoid erosion was 66.7% and 100%, respectively. Lower rates were evidenced for meningoencephalocele (14.3% and 100%, respectively).
Of 5 patients evaluated during the not-blinded second look, the neuroradiologist confirmed to be able to visualize in imaging the surgical findings in only 2 patients; in the other 3 patients, he was not able to identify in imaging the defects visualized during the surgical procedures. This procedure improved the imaging sensitivity in identifying tegmen tympani dehiscence to 73.7%.
In our opinion, the resolution that better show bone defects is the high special resolution bony-algorithm CT scan, with particular relevance for coronal axial plane, performed on 13 patients out of 26, with correspondence in imaging and surgical findings in 11 cases out of 13. Moreover, we believe that magnetic resonance imaging could be useful in visualizing indirect signs, such as bone signal interruption or parenchymal modification, or showing direct parenchymal sign, such as meningoencephalocele or parenchymal abscess.
In our series, surgery was performed in all patients regardless of indications derived from CT scan or MRI. Imaging was not fully reliable in showing tegmen tympani dehiscence, mastoid erosion, and meningoencephalocece, and this could highlight the importance of a surgical approach in the diagnostic workout of otogenic meningitis. Moreover, surgery allows for proper definitive treatment along with disease recognition.
CONCLUSIONS
Intracranial complications of middle ear infections, in particular otogenic meningitis, are life-threatening diseases burdened with a high mortality rate. Radiological imaging could be useful for diagnosis, but it has a limited role in identifying tegmen tympani dehiscence, mastoid erosion, and meningencephalocele.
For cases that have a high index of suspicion of otomeningitis and bone defects, to increase the probability to make diagnosis, it is useful to perform a CT scan first, with every possible reconstruction, to exclude all possible focal dehiscences, and, eventually, obtain a second opinion with precise questions. An MRI could clarify intracranial flogistic processes.
Although our analysis has involved a small case series, we could speculate that surgery is more reliable in revealing bone defects compared with imaging. Therefore, we suggest that surgery be performed in all cases of suspected otogenic meningitis.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Austin DF. Complications of acute and chronic otitis media. In: Ballenger JJ. Snow JB (eds). Otolaryngology-Head and Neck Surgery. 15th ed Philadelphia: Williams & Wilkins, 1996: pp 1037–53. [Google Scholar]
- 2. Hafidh MA, Keogh I, Walsh RM, et al. Otogenic intracranial complications. A 7-year retrospective review. Am J Otolaryngol 2006; 27:390–5. [DOI] [PubMed] [Google Scholar]
- 3. Penido Nde O, Borin A, Iha LC, et al. Intracranial complications of otitis media: 15 years of experience in 33 patients. Otolaryngol Head Neck Surg 2005; 132:37–42. [DOI] [PubMed] [Google Scholar]
- 4. Migirov L. Computed tomographic versus surgical findings in complicated acute otomastoiditis. Ann Otol Rhinol Laryngol 2003; 112:675–7. [DOI] [PubMed] [Google Scholar]
- 5. Dubey SP, Larawin V, Molumi CP. Intracranial spread of chronic middle ear suppuration. Am J Otolaryngol 2010; 31:73–7. [DOI] [PubMed] [Google Scholar]
- 6. Patel KM, Almutairi A, Mafee MF. Acute otomastoiditis and its complications: role of imaging. Operative Techniques in Otolaryngology 2014; 25:21–8. [Google Scholar]
- 7. Valvassori GE. Imaging of the temporal bone. Mafee MF, Valvassori GE, Becker M. (eds). Valvassori’s Imaging of the Head and Neck. Stuttgart: Thieme, 2005: pp 1–133. [Google Scholar]
- 8. Osborn AJ, Blaser S, Papsin BC. Decisions regarding intracranial complications from acute mastoiditis in children. Curr Opin Otolaryngol Head Neck Surg 2011; 19:478–85. [DOI] [PubMed] [Google Scholar]
- 9. Geyik MF, Kokoglu OF, Hosoglu S, Ayaz C. Acute bacterial meningitis as a complication of otitis media and related mortality factors. Yonsei Med J 2002; 43:573–8. [DOI] [PubMed] [Google Scholar]
- 10. Syal R, Singh H, Duggal KK. Otogenic brain abscess: management by otologist. J Laryngol Otol 2006; 120:837–41. [DOI] [PubMed] [Google Scholar]
- 11. Moya AJ, Curiel JA, Remiro RG, et al. [Trombosis de seno sigmóide como complicación de otitis media]. An Esp Pediatr 2000; 53:488–91. [PubMed] [Google Scholar]
- 12. Osma U, Cureoglu S, Hosoglu S. The complications of chronic otitis media: report of 93 cases. J Laryngol Otol 2000; 114:97–100. [DOI] [PubMed] [Google Scholar]
- 13. Neely JG. Complications of temporal bone infection. Cummings CW. (ed). Otolarynology-Head and Neck Surgert. 2nd ed St. Louis: Mosby-Year Book, 1993: pp 2840–64. [Google Scholar]
- 14. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 2010; 23:467–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chotmongkol V, Sangsaard S. Intracranial complications of chronic suppurative otitis media. Southeast Asian J Trop Med Public Health 1992; 23:510–3. [PubMed] [Google Scholar]
- 16. Kangsanarak J, Fooanant S, Ruckphaopunt K, et al. Extracranial and intracranial complications of suppurative otitis media. Report of 102 cases. J Laryngol Otol 1993; 107:999–1004. [DOI] [PubMed] [Google Scholar]
- 17. Minks DP, Porte M, Jenkins N. Acute mastoiditis–the role of radiology. Clin Radiol 2013; 68:397–405. [DOI] [PubMed] [Google Scholar]
- 18. Wiatr M, Składzień J, Tomik J, et al. Bony wall damage in the region of the middle and posterior cranial fossa observed during otosurgery. Med Sci Monit 2012; 18:BR215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kou YF, Allen KP, Isaacson B. Recurrent meningitis secondary to a petrous apex meningocele. Am J Otolaryngol 2014; 35:405–7. [DOI] [PubMed] [Google Scholar]