Abstract
ROG, a transcriptional repressor, is a direct target gene of NF-AT and a putative negative regulator of T-cell activation. In addition, overexpression of ROG suppresses the activity of GATA-3, implying a role of ROG in the differentiation and function of Th cells. Despite these observations, the function of ROG has yet to be confirmed by loss-of-function approaches. Here we report that ROG-deficient T cells are hypersensitive to anti-CD3 stimulation and produce more interleukin-2 (IL-2) due to enhanced NF-κB activity. ROG-deficient dendritic cells also produce more IL-12p40, another NF-κB target gene. However, ROG-deficient Th cells are capable of differentiating into Th1 and Th2 cells, and ROG-deficient mice have no defect in mounting appropriate Th immune responses in vivo. Thus, ROG is dispensable for the differentiation and function of Th cells but serves as a mediator of NF-AT-initiated suppression of NF-κB. Its mechanism of action and its expression pattern are distinct from those of other transcription factors negatively regulating the activation of T cells.
Stimulation of T cells through T-cell receptors leads to the induction of many effector genes, including cyclins, cytokines, and adhesion molecules, that mediate the activation and proliferation of T cells. As uncontrolled T-cell activation can result in harmful T-cell-mediated autoimmunity, the activation of T cells must be tightly regulated. NF-AT and NF-κB are two important transactivators of the effector genes during T-cell activation (10, 13, 28). Surprisingly, combined deficiency of two NF-AT members, NF-ATc2 and NF-ATc3, results in T-cell hyperproliferation, overproduction of type 2 Th cytokines, and the development of a lymphoproliferative disease (27). These unexpected observations argue strongly that NF-AT also negatively regulates T-cell activation. We have previously shown that ROG, a transcriptional repressor, is induced rapidly upon T-cell activation by NF-AT, in particular NF-ATc2, and that the induction of ROG is impaired in T cells lacking NF-ATc2 (18). More importantly, restoration of ROG attenuates T-cell hyperproliferation and delays the onset of lymphoproliferative disease in NF-ATc2/NF-ATc3 doubly deficient (NF-AT DKO) mice. Thus, deficiency of ROG may partly explain the paradoxical phenotype of NF-ATc2/NF-ATc3 deficiency. ROG is therefore a part of an NF-AT-initiated negative feedback mechanism regulating the activation of T cells.
In addition to its potential role in regulating T-cell activation, ROG may play a regulatory role in the differentiation and function of Th cells. Th cells can be divided into two functional subsets based on their secreted cytokines (5, 19). Type 1 Th (Th1) cells produce gamma interferon (IFN-γ), and type 1 Th immune responses are responsible for eradicating intracellular microorganisms. Type 2 Th (Th2) cells produce interleukin-4 (IL-4), IL-5, and IL-13 and are important for immunity against parasitic infection. Several transcription factors have been shown to play crucial roles in regulating the differentiation and function of Th cells. The Th1-cell-specific transcription factor, T-bet, is essential for the differentiation of Th1 cells and for mounting effective type 1 adaptive immune responses (31, 32). The counterpart of T-bet in Th2 cells is GATA-3. GATA-3 is preferentially expressed in Th2 cells and is induced by the IL-4/Stat6 signaling pathway (23, 34, 35). Deficiency of GATA-3 results in a profound defect in the differentiation and function of Th2 cells in vivo and in vitro (24). Several groups, including ours, have shown that overexpression of ROG in vitro interferes with the function of GATA-3 and suppresses the expression of Th2 cytokines (6, 17, 22). In addition, the level of ROG is significantly higher in CD8+ Tc than in CD4+ Th cells (22), prompting the investigators to postulate that the higher level of ROG might render CD8+ Tc cells poor IL-4 producers. Despite these reports, the function of ROG has yet to be confirmed by loss-of-function approaches. Furthermore, how ROG suppresses the activation of T cells is still poorly understood.
To address these questions, we have generated ROG-deficient (ROGKO) mice. Our studies indicate that ROG can indeed function as a negative regulator of T-cell activation. The effect of ROG is at least partly mediated by inhibiting the binding of NF-κB to the IL-2 promoter, thereby suppressing anti-CD3-induced proliferation. However, ROGKO Th cells can differentiate into Th1 and Th2 cells normally, and ROGKO mice are capable of mounting appropriate Th immune responses in vivo. Thus, our results depict a novel one-way cross-regulation between NF-AT and NF-κB in T cells and show that ROG is dispensable for the differentiation and function of Th cells.
MATERIALS AND METHODS
Generation of ROGKO mice.
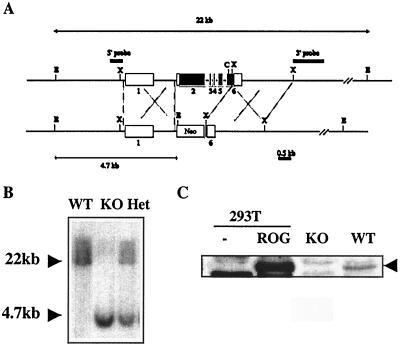
The targeting construct for the generation of ROGKO mice is shown in Fig. 1A. The backbone was derived from pLNTK (32). The 5′ homology region was derived from a 2-kb SalI-NheI fragment, in which a SalI site was added immediately 3′ of the NheI site via PCR amplification. The 5′ homology region was cloned into the SalI site of pLNTK. The 3′ homology region was derived from a 2.6-kb XhoI restriction fragment and was cloned into the XhoI site of the pLNTK. The resulting targeting construct was used to transfect murine embryonic stem cells, which were cultivated with murine embryonic fibroblasts in the presence of neomycin and ganciclovir. Both murine embryonic stem cells and fibroblasts were purchased from Incyte Genomics (Palo Alto, Calif.). Homologous recombination at the ROG locus was initially identified by genomic Southern analysis. A 400-bp 5′ probe immediately upstream of the 5′ homology region detected a 22- or 4.7-kb EcoRI restriction fragment from the wild-type or recombined allele, respectively. A 1.4-kb XhoI-HindIII restriction fragment immediately downstream of the 3′ homology region detecting a 22- or 16-kb EcoRI restriction fragment from the wild-type or recombined allele, respectively, was used to confirm recombination at the 3′ end. Among approximately 200 drug-resistant clones, one has undergone homologous recombination. The embryonic stem cell clone was then injected into 129/sv blastocysts, which were then implanted into pseudopregnant mice. Chimeric mice carrying the recombined ROG allele were identified and backcrossed with C57BL/6 and BALB/c mice, respectively, for at least five generations. Heterozygous mice were then intercrossed to produce ROGKO mice and wild-type control mice. With the exception of that shown in Fig. 7, all the data shown were obtained from mice of BALB/c background. Animals were housed in specific-pathogen-free conditions at the Dana-Farber Cancer Institute under protocols approved by its institutional review board.
FIG. 1.
Generation of ROG-deficient mice. (A) Schematic diagrams of murine wild-type and targeted ROG alleles. The open and filled boxes represent untranslated and translated exons, respectively. C, E, and X stand for ClaI, EcoRI, and XhoI restriction sites, respectively. (B) Genomic Southern analyses of wild-type (WT), ROGKO (KO), and heterozygous (Het) DNA. Genomic DNA was digested with EcoRI and probed with the 5′ genomic probe shown in panel A. (C) Cell extract obtained from untransfected 293T cells, 293T cells transfected with a ROG expression vector, and ROGKO and wild-type Th cells stimulated with anti-CD3/CD28 for 48 h was subjected to Western analyses using rabbit antiserum raised against truncated murine ROG-recombinant proteins containing amino acid residues 171 to 465. The arrowhead indicates the position of ROG protein.
FIG. 7.
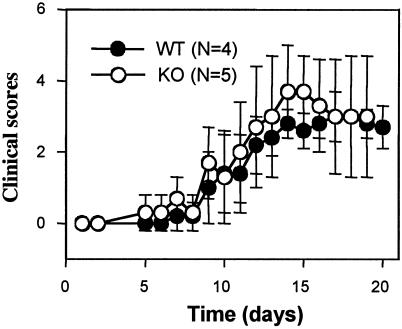
ROGKO (KO) and wild-type (WT) mice are equally susceptible to EAE. ROGKO mice and wild-type littermates of C57BL/6 genetic background were subjected to EAE induction according to the protocol described in Material and Methods and were observed for 20 days. The data shown are the averages of disease scores.
Activation and proliferation assays of Th cells.
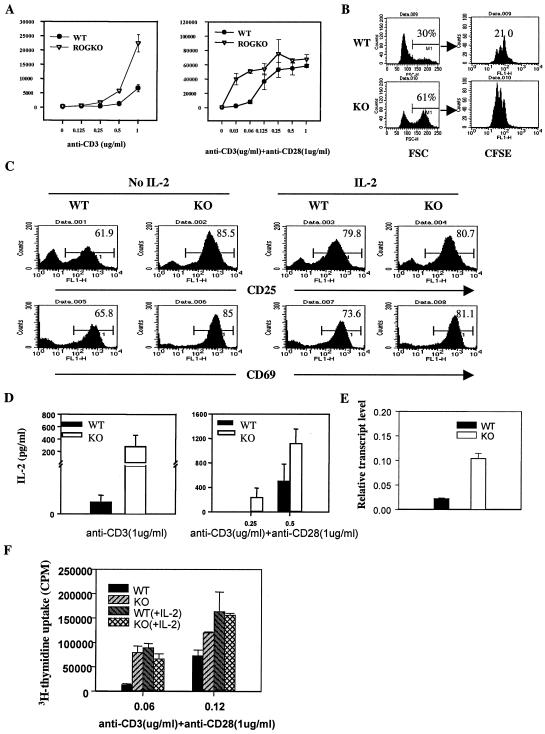
CD4+ and CD8+ T cells were purified from lymph nodes and spleens by using magnetic-activated cell sorting to >90% purity (Miltenyl Biotec, Auburn, Calif.) and stimulated with the indicated dose of anti-CD3 antibody in the presence or absence of anti-CD28 antibody (1 μg/ml). The stimulated cells were then incubated with fluorochrome-tagged antibodies to CD25 and CD69 and were analyzed on a FACSCalibur or FACSort machine. Antibodies against CD25 and CD69 were purchased from Pharmingen (San Diego, Calif.). For [3H]thymidine incorporation assays, the purified T cells were plated in triplicate at 20,000 cells/100 μl/well in a 96-well plate precoated with anti-CD3 in the presence or absence of anti-CD28. Forty-eight hours after stimulation, 1 uCi of [3H]thymidine/50 μl/well was added. The cells were harvested 16 h later, and the incorporation of [3H]thymidine was measured with a liquid scintillation counter (Amersham Pharmacia Biotech, Piscataway, N.J.). For 5 (and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution assay, the purified T cells were labeled with CFSE prior to stimulation. The labeling of T cells with CFSE was previously described (18). At indicated time points, the stimulated cells were collected and analyzed on a FACSCalibur or FACSSort machine.
In vitro differentiation of T cells.
Purified Th or Tc cells were stimulated in vitro with plate-bound anti-CD3 monoclonal antibody (MAb) (2C11; 0.06 μg/ml) and soluble anti-CD28 (PV1; 1 μg/ml) (nonskewing conditions), along with either anti-IFN-γ MAb (R4-682) at 20 μg/ml (Th2-skewing conditions) or anti-IL-4 MAb (11B11; National Cancer Institute [NCI] Preclinical Repository, Bethesda, Md.) at 5 μg/ml (Th1-skewing conditions). Twenty-four hours poststimulation, recombinant human IL-2 (NCI Preclinical Repository) at 50 U/ml was added to all cultures. In addition, IL-4 (PeproTech, Rocky Hill, N.J.) at 10 ng/ml or IL-12 (PeproTech) at 50 U/ml was added to Th2 or Th1 cultures, respectively. Seven days after stimulation, cells were harvested, washed thoroughly, and replated at 106/ml on a 12-well plate precoated with anti-CD3 (0.06 μg/ml). Supernatant was collected, and the concentration of cytokines was measured by enzyme-linked immunosorbent assay (ELISA), which was performed according to a protocol described previously (24). All antibodies except anti-IL-4 were processed by Bioexpress (Kaysville, Utah).
Cytotoxic T-lymphocyte assay.
Cytotoxic T-lymphocyte assay was performed according to the published protocol (32). Briefly, approximately 106 log-phased target cells (p815) or nontarget cells (EL4) in 15 ml of 10% RPMI were labeled with 100 uCi of 51Cr (20 μl at 5 mCi/ml) at 37°C for 1 to 2 h and were plated in triplicate at 104 cells/100 μl of 10% RPMI/well on a 96-well plate. Splenocytes of wild-type and ROGKO mice were harvested and stimulated with concanavalin A (2 μg/ml; Sigma, St. Louis, Mo.) and IL-2 (200 U/ml) for 5 days. CD8+ Tc cells were then isolated from the activated splenocytes by using magnetic-activated cell sorting (Miltenyl Biotec). Various numbers of effector cells in 50 μl of 10% RPMI were added to each well containing p815 or EL4 cells (104 cells in 100 μl of 10% RPMI), and the plate was incubated at 37°C for 4 h. Maximal release of 51Cr was achieved by lysing the labeled cells with 100 μl of 0.2% Triton X-100. Forty microliters of supernatant from each well was spotted on Filtermat (Wallac, Turku, Finland), and the release of 51Cr was measured by a liquid scintillation counter. Normalized maximal release was calculated by subtracting background 51Cr release from the maximal release.
Plasmid, transfection, and luciferase assay.
The murine tumor necrosis factor alpha (TNF-α) and IL-2 promoter (−350 to +45)/luciferase vector were gifts from Laurie Glimcher. The NF-κB, NF-AT, and AP-1 luciferase reporters were purchased from Clontech (Palo Alto, Calif.). The ROG expression vector was previously reported (17). Jurkat cells were maintained in RPMI supplemented with 10% fetal calf serum (FCS). For each transfection, 5 × 106 Jurkat cells (in 400 μl of plain RPMI) at log phase were electroporated with 5 μg of the indicated firefly luciferase reporter, 5 μg of expression plasmid, and 10 ng of a renilla luciferase reporter, pRL-TK (Promega). Electroporation was performed in a Bio-Rad Gene Pulser II (Hercules, Calif.) set at 280 V and 975 uF. Twelve hours after transfection, the cells were either left unstimulated or were stimulated with phorbol myristate acetate (PMA; 50 ng/ml) or ionomycin (iono; 1 uM) for 6 h. Luciferase activity was measured with the Dual Luciferase Reporter System (Promega, Madison, Wis.). The firefly luciferase activity was normalized against renilla luciferase activity of the same sample.
Electrophoretic mobility shift assay (EMSA).
T4 polynucleotide kinase was used to end label 100 ng of the indicated double-stranded oligonucleotides with [32P] dATP (DuPont NEN Research Products, Wilmington, Del.). The labeled double-stranded oligonucleotides were fractionated in 15% polyacrylamide gels, eluted overnight at 37°C in 1× Tris-EDTA (TE), and precipitated in ethanol. Binding assays were performed at room temperature for 20 min using 5 μg of nuclear extract prepared from activated Th cells, 500 ng of poly(dI-dC), and 20,000 counts per million (cpm) of probe in a 15-μl volume of 20 mM HEPES (pH 7.9), 100 mM KCl, 5% glycerol, 1 mM EDTA, 5 mM dithiothreitol, and 0.1% NP-40. The samples were then fractionated in 4% nondenaturing polyacrylamide gels containing 0.5× Tris-borate-EDTA (TBE) at room temperature. For supershift assay, 1 μl of the indicated or control antibody was added to the reaction mixture 10 min after the beginning of the incubation period. The sequences of the double-stranded oligonucleotides used are the following: distal NF-κB site (−213 to −193), 5′-GATCCACCTCATTCAGGTCTCTCTTTCTCTCCG-3′; CD28RE (−166 to −144), 5′-AAAGAAATTCCAGAGAGTCATCA-3′; and NF-AT site (−290 to −260), 5′-CCAAAGAGGAAAATTTGTTTCATACAGAAGGCG . Antibodies against p65, c-Rel, p-50, goat immunoglobulin G (IgG) (control for anti-p65), and rabbit IgG (control for anti-cRel) were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.).
Real-time PCR.
For real-time PCR, 1 μg of total RNA was used in reverse transcription (RT) and amplification by using a Superscript II RT kit according to the manufacturer's protocol (Invitrogen, Carlsbad, Calif.). First-strand cDNA was prepared after DNase I treatment. A master mix of TaqMan or SYBR green reagents was prepared, and 10 ng of each RT product was used in the PCR. The reactions were run in triplicate on a 7700 Sequence Detector (Applied Biosystems, Foster City, Calif.). The sequences of primers and probes are the following: IL-2, 5′-CCTGAGCAGGATGGAGAATTACA-3′, 5′-TCCAGAACATGCCGCAGAG-3′, and FAM-CCCAAGCAGGCCACAGAATTGAAAG-TAMRA; ROG, 5′-TCTGGGCAAGGGTTCACAG-3′, 5′-GAGATGGGCAGTTCAGTGTGC-3′, and FAM-CACACTGGCAACCTGTGTGAGTCAGGAG-TAMRA;β-actin, 5′-GCTCTGGCTCCTAGCACCAT-3′, 5′-GCCACCGATCCACACCGCGT-3′, and FAM-TCAAGATCATTGCTCCTCCTGAGCGC-TAMRA.
Isolation and stimulation of splenic DCs.
Spleens were injected with 3 ml of type IV collagenase (100 U/ml in Dulbecco's modified Eagle medium [DMEM]; Worthington Biochemical, Lakewood, N.J.) and incubated for 15 min at 37°C. The spleens were then disrupted by passing through pipettes several times in 60-mm-diameter dishes containing 5 ml of collagenase (400 U/ml in DMEM) and were incubated at 37°C for 20 min. The process of disruption and incubation was repeated once and was followed by lysis of red blood cells. CD11c-expressing cells were then enriched with magnetic-activated cell sorting (Miltenyl Biotech). The enriched CD11c+ dendritic cells (DCs), at 106 cell/ml, was then stimulated with lipopolysaccharide (LPS) (10 μg/ml; Sigma) for 3 or 24 h prior to harvest.
EAE model.
Mice of 8 to 10 weeks of age were immunized with 100 μg (in 100 μl of phosphate-buffered saline [PBS]) of myelin oligodendrocyte-glycoprotein peptide-complete Freund’s adjuvant antigen (Difco, Detroit, Mich.) subcutaneously and were then injected with 250 ng of pertussis toxin (List Biological Laboratories, Campbell, Calif.) intraperitoneally on days 0 and 2. The mice were observed daily for clinical signs and were scored as follows: score 0, normal mouse with no sign of disease; score 1, limp tail; score 2, weakness or paralysis of one of hind limbs; score 3, complete paralysis of both hind limbs; score 4, forelimb paralysis; and score 5, moribund state.
RESULTS
Generation of ROG-deficient mice.
To study the in vivo function of ROG, we generated ROGKO mice in which the coding regions (exons 2, 3, 4, 5, and a part of exon 6) of ROG were replaced with a neomycin resistance gene (Fig. 1A). When examined by genomic Southern analysis using a probe upstream of the first exon (5′ probe), a 22-kb EcoRI fragment that was detected in the wild-type allele was replaced by a 4.7-kb fragment derived from the mutated allele after homologous recombination (Fig. 1B). CD4+ Th cells obtained from ROGKO mice lacked a 50-kDa protein, comigrating with exogenous ROG, that was detected by anti-ROG polyclonal serum, confirming the deficiency of ROG protein (Fig. 1C). ROGKO mice were born at the expected Mendelian ratio, had normal development and growth, and remained healthy in a specific-pathogen-free environment for up to 1 year.
ROGKO Th cells are hypersensitive to anti-CD3 stimulation.
ROGKO mice also had normal development of the immune system. There was no apparent difference in the total number and maturation status of lymphocytes between wild-type and ROGKO mice. We previously showed that the level of ROG was reduced in NF-AT DKO mice and that partial restoration of ROG delayed the onset of the lymphoproliferative disease in NF-AT DKO mice (18). However, we did not detect any lymphoadenopathy or splenomegaly in ROGKO mice even after 1 year of observation. To further examine whether ROG deficiency would affect activation and/or proliferation of T cells, we isolated CD4+ Th cells from either ROGKO mice or wild-type littermates and stimulated the cells in vitro with anti-CD3 in the presence or absence of anti-CD28. We found that ROGKO Th cells were more sensitive to anti-CD3 stimulation, as judged by enhanced [3H]thymidine uptake (Fig. 2A, left panel). The hyperproliferation was still obvious when ROGKO Th cells were stimulated with suboptimal doses of anti-CD3 in the presence of anti-CD28, but the amount of hyperproliferation became negligible at higher doses of anti-CD3 (Fig. 2A, right panel). The hypersensitivity to anti-CD3 stimulation was observed in Th cells derived from ROGKO mice of either BALB/c or C57BL/6 genetic background and also in ROGKO Tc cells (data not shown).
FIG. 2.
Hypersensitivity of ROGKO Th cells to anti-CD3 stimulation. (A) Wild-type (WT) and ROGKO Th cells were stimulated under the indicated conditions, and the incorporation of [3H]thymidine (in cpm) was determined 3 days later. (B) CFSE dilution assay. ROGKO (KO) or wild-type Th cells were labeled with CFSE and stimulated with 0.06 μg of anti-CD3/ml and 1 μg of anti-CD28/ml for 48 h. Cell size was determined by the FSC reading. The percentage and CFSE content of cycling (high-FSC) cells are shown. The numbers on the right-hand panels represent the numbers of cell division. (C) ROGKO and wild-type Th cells were stimulated as described for panel B in the presence or absence of exogenous IL-2 (100 U/ml) and were stained with fluorescence-conjugated antibodies against CD25 and CD69. The cells were then analyzed on a FACSCalibur or FACSort machine. The numbers represent the percentages of cells stained positive for the indicated antibodies. (D and E) Wild-type and ROGKO Th cells were stimulated under the indicated conditions for panel D or with anti-CD3 (0.06 μg/ml)-anti-CD28 (1 μg/ml) for panel E for 48 h. The concentration of IL-2 in the supernatant was measured with ELISA (D), and the level of IL-2 transcripts was determined by real-time PCR (E). (F) Wild-type or ROGKO Th cells were stimulated as described for panel A in the presence or absence of exogenous IL-2 (100 U/ml), and the incorporation of [3H]thymidine was measured.
The increase in [3H]thymidine uptake was not simply due to enhanced resistance to apoptosis. Examination by CFSE dilution assay showed that more ROGKO Th cells entered the cell cycle in response to anti-CD3 stimulation, as judged by high forward-scatter (FSC) values, and the cycling ROGKO Th cells also had undergone more cell divisions than Th cells obtained from wild-type littermates (Fig. 2B). In addition, more ROGKO Th cells expressed the activation markers CD69 and CD25 (Fig. 2C). ROGKO Th cells also produced more IL-2 (Fig. 2D) and contained more IL-2 transcripts (Fig. 2E), suggesting that deficiency of ROG has a global effect on Th-cell activation. IL-2 is a potent growth factor for T cells, raising the possibility that the enhanced proliferation of ROGKO Th cells might be secondary to overproduction of IL-2. To address this possibility, we stimulated wild-type or ROGKO Th cells with anti-CD3 and anti-CD28 in the presence or absence of IL-2. As shown in Fig. 2F, exogenous IL-2 further enhanced the proliferation of wild-type Th cells but had no effect on ROGKO T cells, thereby eliminating the differences in 3H uptake. Similarly, exogenous IL-2 also augmented the expression of CD69 and CD25 in wild-type Th cells to a level comparable to that of ROGKO Th cells (Fig. 2C). These observations indicate that the hyperproliferation and enhanced expression of activation markers of ROGKO Th cells are at least in part mediated by overproduction of IL-2.
Normal Th- and Tc-cell differentiation and function in the absence of ROG.
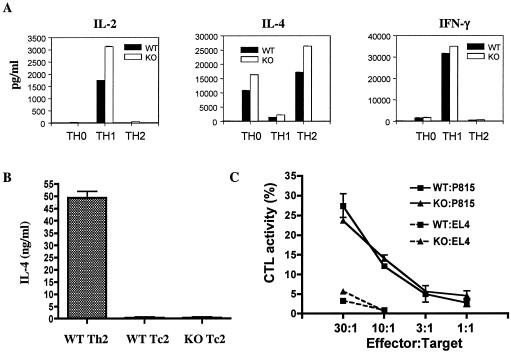
It was previously shown that overexpression of ROG suppressed the activity of GATA-3 and inhibited the production of Th2 cytokines (17, 22). To determine whether deficiency of ROG would alter the differentiation of Th cells, we performed in vitro differentiation of ROGKO Th cells under nonskewing, Th1-, or Th2-polarizing conditions. Exogenous IL-2 was added to equalize the proliferation. The differentiated Th cells were then restimulated with various doses of anti-CD3, and the production of cytokines was examined 24 h later. When the differentiated Th cells were restimulated with a low dose of anti-CD3 (0.06 μg/ml), ROGKO Th1 cells also produced approximately twice more IL-2 than wild-type Th1 cells did (Fig. 3A). The level of IL-2 produced by wild-type and ROGKO Th2 and Th0 cells was below the detectable range, reflecting the fact that IL-2 is preferentially expressed by Th1 cells. There was also a subtle, albeit reproducible, increase in the production of IL-4 by ROGKO Th0 and Th2 cells. The levels of IFN-γ were relatively equal between wild-type and ROGKO Th1 cells. However, when the differentiated Th cells were restimulated with a higher dose of anti-CD3 (1 μg/ml) or PMA/iono, the production of cytokines was comparable between wild-type and ROGKO Th cells (data not shown), indicating that ROGKO Th cells were able to differentiate normally into Th1 and Th2 cells.
FIG. 3.
Normal Th and Tc function in the absence of ROG. (A and B) Wild-type (WT) and ROGKO (KO) Th (A) and Tc (B) cells were subjected to in vitro differentiation under nonskewing (Th0), Th1-, or Th2-skewing conditions. The differentiated T cells were replated at 106 cells/ml and were restimulated with anti-CD3 (0.06 μg/ml in panel A and 1 μg/ml in panel B) for 24 h. The concentration of indicated cytokines in supernatant was measured by ELISA. (C) Tc cells derived from wild-type or ROGKO mice of BALB/c background were subjected to CTL assay as described in Materials and Methods. The data shown is the percentage of normalized maximal release. EL4 cells were used as control nontarget cells.
ROG is expressed in CD8+ Tc cells at a level significantly higher than that of Th cells and was shown to bind to the IL-13 gene at the IL-4/IL-5/IL-13 locus (22). A high level of ROG could at least partly explain why Tc2 cells produce less IL-4 than Th2 cells. We confirmed that Tc2 cells produced a very low level of IL-4 compared to that of Th2 cells. However, ROGKO Tc2 cells did not produce more IL-4 than wild-type Tc2 cells (Fig. 3B). Furthermore, deficiency of ROG did not affect the cytotoxic T-lymphocyte activity of Tc cells in an in vitro killing assay (Fig. 3C), indicating that ROG is dispensable for the in vitro function of Tc cells.
Overexpression of ROG suppresses NF-κB activity.
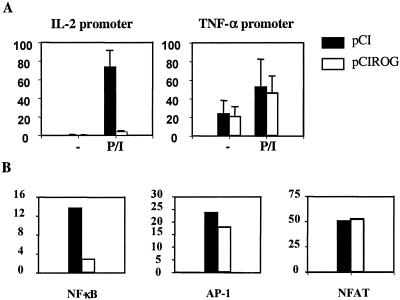
As enhanced production of IL-2 was the key feature of ROG deficiency, we further investigated how ROG suppresses the expression of IL-2 by performing luciferase assay using a luciferase reporter encompassing −350 to +40 of the murine IL-2 promoter. As shown in Fig. 4A, overexpression of ROG dramatically inhibited the activity of the IL-2 promoter, suggesting that a ROG-responsive element is located within the proximal region of the IL-2 promoter. In contrast, overexpression of ROG did not affect the activity of a TNF-α promoter. ROG has been shown to bind to the DNA sequence TGTACAGTGT (33), but no such sequence was found in the IL-2 promoter. We were also unable to demonstrate direct binding of recombinant ROG protein to the IL-2 promoter by EMSA (data not shown). The proximal region of the IL-2 promoter contains binding sites for several transcription factors, including NF-AT, AP-1, and NF-κB (9, 21, 29). ROG might indirectly suppress the expression of IL-2 by inhibiting the activity of those transcription factors. In concordance with this hypothesis, we found that overexpression of ROG significantly suppressed the activity of a luciferase reporter driven by three copies of a consensus NF-κB binding site, whereas overexpression of ROG did not affect the transcriptional activity of AP-1 or NF-AT (Fig. 4B), demonstrating that the negative effect of ROG is restricted to NF-κB proteins.
FIG. 4.
Suppression of NF-κB activity by ROG. Jurkat cells were transfected with the indicated firefly luciferase reporters and an expression vector of ROG (pCIROG) or empty pCI vector along with a renilla luciferase reporter. The transfected cells were left unstimulated or were stimulated with PMA/iono for 6 h, and the luciferase activity in the cell extract was measured. The data shown in panel A is relative luciferase activity after normalization against renilla luciferase activity. The data shown in panel B is the fold induction over unstimulated samples after normalization against renilla luciferase activity. The firefly luciferase reporters used for panel B are driven by three tandem copies of the consensus NF-κB, AP-1, or NF-AT site, cloned immediately upstream of a minimal thymidine kinase promoter.
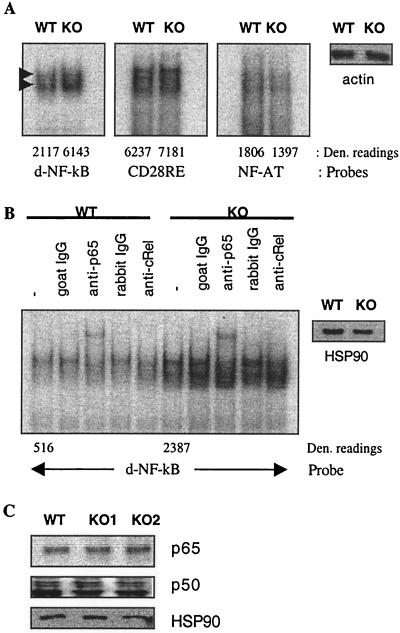
If ROG suppresses the activity of NF-κB, then enhanced NF-κB activity is very likely the cause of IL-2 overproduction by ROGKO Th cells. The proximal IL-2 promoter contains two NF-κB sites (4, 8, 15, 30): CD28RE (−163 to −147), which contains a modified NF-κB site, and a canonical site (−208 to −197) distal to CD28RE. Both sites have been shown to be functionally critical, because mutation or deletion of either site dramatically attenuated the activity of the IL-2 promoter (3, 21, 30). To further determine which NF-κB site is indeed needed for mediating the effect of ROG, we used EMSA to examine the binding of NF-κB complexes to the NF-κB sites. Nuclear extract was prepared from wild-type or ROGKO Th cells, stimulated in vitro with anti-CD3/anti-CD28 for 16 h, and used in EMSA. As shown in Fig. 5A, ROGKO nuclear extract exhibited significantly stronger NF-κB binding activity to the distal NF-κB site. In contrast, no such increase in binding activity was detected on the CD28RE or NF-AT (−287 to −263) site, suggesting that ROG specifically affects the binding of NF-κB to the distal site. The distal NF-κB site bound two distinct NF-κB complexes. Deficiency of ROG did not alter the mobility or relative intensity of the complexes. In addition, the slow-moving complex of both wild-type and ROGKO extract could be further up-shifted by an anti-p65 antibody or partially eliminated by an anti-c-Rel antibody (Fig. 5B). Thus, deficiency of ROG results in a quantitative but not qualitative change in NF-κB binding activity on the distal site.
FIG. 5.
Enhanced NF-κB binding activity to the IL-2 promoter in ROGKO Th cells. (A and B) Electrophoretic mobility shift assay. Nuclear extract was prepared from wild-type (WT) or ROGKO (KO) Th cells stimulated with 0.06 μg of anti-CD3/ml and 1 μg of anti-CD28/ml for 16 h. EMSA was performed as described in Materials and Methods in the presence or absence of anti-p65, anti-cRel, or control antibody. The two NF-κB complexes that bind to the distal NF-κB (d-NF-κB) sites are marked with arrow heads. The arbitrary density (Den.) units of protein/DNA complexes are listed at the bottoms of the lanes. The equal loading of wild-type and ROGKO nuclear extract was confirmed by Western analysis using anti-actin or anti-HSP90 antibody. (C) The levels of nuclear p65 and p50 proteins were examined with Western analysis. Antibody against HSP90 was again used to demonstrate equal loading of proteins.
The enhanced NF-κB binding could be attributed to an increase in nuclear translocation of NF-κB proteins. An alternative, but not mutually exclusive, hypothesis is that ROG may interact with and prevent NF-κB from binding to DNA. Therefore, there is more ROG-free NF-κB available for binding to the distal site in the absence of ROG. To distinguish these two scenarios, we performed Western analysis examining the levels of nuclear NF-κB proteins. As shown in Fig. 5C, nuclear extract obtained from stimulated ROGKO Th cells did not contain more p65 or p50, indicating that deficiency of ROG does not affect the absolute levels or nuclear translocation of NF-κB proteins. This observation also suggests that ROG suppresses the activity of NF-κB by preventing NF-κB from binding to DNA.
Enhanced production of IL-12p40 by ROGKO dendritic cells.
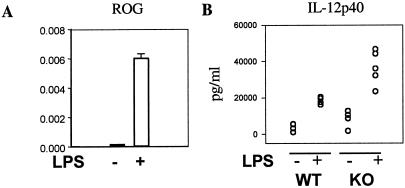
While ROG was expressed at a high level in lymphoid cells, we found that a low level of expression was detected in dendritic cells and that the expression of ROG in DCs could be further induced by LPS stimulation (Fig. 6A). However, the absolute number and subset distribution of splenic DCs were comparable between ROGKO mice and wild-type littermates. To determine whether ROG also play a role in regulating the function of DCs, purified splenic DCs were stimulated with LPS and the expression of IL-12p40 was measured by ELISA. In agreement with the suppressor nature of ROG, ROGKO DCs produced approximately 50% more IL-12p40 (Fig. 6B) than wild-type cells, indicating that the effect of ROG deficiency is not limited to T cells.
FIG. 6.
Overproduction of IL-12p40 by ROG−/− dendritic cells. Splenic CD11c+ dendritic cells were harvested from wild type (WT) or ROGKO (KO) mice and were left unstimulated (−) or were stimulated with LPS (+). The level of ROG transcripts (relative to that of β-actin) of wild-type dendritic cells was measured by real-time PCR 3 h after stimulation (A). The concentrations of IL-12p40 (in picograms/milliliter) in supernatants were determined with ELISA 24 h after stimulation (B).
ROGKO mice have no defect in mounting Th immune responses.
ROGKO Th cells were more sensitive to anti-CD3 stimulation and produced more IL-2. In addition, ROGKO DCs expressed a higher level of IL-12p40, a Th1-polarizing cytokine. However, whether deficiency of ROG would have any effect on in vivo Th immune responses was still unclear. To address this question, we subjected ROGKO mice to the induction of EAE, an animal model of multiple sclerosis. The EAE model was chosen because it is a Th cell-mediated disease. ROGKO mice or wild-type littermates were immunized with myelin oligodendrocyte-glycoprotein peptide and complete Freund's adjuvant. The disease activity was scored daily. As shown in Fig. 7, both ROGKO and wild-type mice were equally susceptible to EAE induction, and there was no difference in the overall disease activity, suggesting that deficiency of ROG does not have any substantial effect on in vivo Th immune responses. In a parallel experiment, we immunized animals with a T-cell-dependent antigen, TNP-KLH, and found that the levels and isotype distribution of antigen-specific immunoglobulin were comparable between wild-type and ROGKO mice (data not shown).
DISCUSSION
We have previously reported that overexpression of ROG is sufficient to repress the activity of GATA-3, thereby inhibiting the production of Th2 cytokines (17). This observation was subsequently confirmed by other groups with different experimental systems. Overexpression of ROG in thymocytes via retroviral transduction impaired the development of CD4 single positive cells, a feature reminiscent of GATA-3 deficiency (6, 25). Endogenous ROG was shown to bind to the IL-13 gene at the IL-4/IL-5/IL-13 locus, and overexpression of ROG counteracted the Th2-promoting effect of GATA-3 (22). However, deficiency of ROG has no effect on thymocyte development, and ROGKO Th cells are capable of differentiating into Th1 and Th2 cells. The discrepancies can be explained by overlapping function between ROG and ROG-related proteins. Of note, the C2H2 zinc finger protein family is by far the largest gene family of mammalian genomes, many of which are capable of functioning as transcriptional repressors.
The observation that ROGKO Th cells are more sensitive to anti-CD3 stimulation is reciprocal to what we observed with ROG transgenic Th cells, confirming the role of ROG in influencing the threshold of Th-cell activation. The negative effect of ROG is at least partly mediated by inhibiting binding of NF-κB to the distal NF-κB site of the IL-2 promoter. As ROG is a direct target gene of NF-AT and is rapidly induced in activated T cells, our results uncover novel NF-AT-initiated and ROG-mediated suppression of NF-κB activity. Upon encountering antigen, signals emanating from T-cell receptors activate NF-AT and NF-κB, which subsequently induce several downstream genes responsible for T-cell activation. Nuclear translocation of NF-AT, in particular NF-ATc2, also induces ROG, which then suppresses the production of IL-2 by attenuating NF-κB activity. The physiological role of the NF-AT-initiated and ROG-mediated inhibition of NF-κB is still unclear, but it is probably to prevent T cells from unwanted overactivation.
How deficiency of ROG results in a quantitative increase in NF-κB binding activity is still unclear. The activity of NF-κB can be regulated at several levels. Our data indicate that ROG does not affect the nuclear translocation of NF-κB. We would like to postulate that ROG may interact with NF-κB, thereby interrupting the formation of NF-κB dimers or sequestering NF-κB from DNA. However, we have not been able to demonstrate the presence of ROG/NF-κB complex in vivo by immunoprecipitation. The physical interaction between ROG and NF-κB may be weak, and more sensitive assays are needed to demonstrate the interaction. One unexpected but interesting finding is that the effect of ROG deficiency is relatively specific to the distal NF-κB site. The neighboring CD28RE also contains a modified NF-κB binding site but only shows a subtle increase in NF-κB binding in the absence of ROG. It has been demonstrated that the distal NF-κB site and CD28RE can bind different NF-κB-containing complexes and that the composition of the NF-κB-containing complexes on CD28RE varies depending on the status of T-cell activation (4, 7, 12, 15, 16, 30). It is possible that ROG may only interfere with DNA binding of certain NF-κB complexes. Alternatively, the inhibitory effect of ROG may also depend on the DNA sequences flanking the NF-κB sites.
The fact that ROG is upregulated in LPS-treated DCs indicates that ROG can be induced by other upstream signals besides NF-AT. LPS binds to TLR4 and activates MyD88-dependent and independent pathways, and it eventually results in the activation of NF-κB and IRF3 (1). We have yet to determine which pathway leads to the induction of ROG by LPS in DCs and how deficiency of ROG causes overproduction of IL-12p40. ROG very likely suppresses the expression of IL-12p40 at the transcriptional level, because overexpression of ROG also strongly attenuated the activity of an IL-12p40 promoter in vitro (data not shown). As IL-12p40 is also a target gene of NF-κB (20, 26), it is temping to speculate that ROG may mediate an autoinhibitory regulation of NF-κB or a novel IRF-3-initiated suppression of NF-κB in DCs. These scenarios remain to be examined.
Thus, ROG is a new member of an expanding group of transcription factors, including LKLF and Foxj1, that negatively regulate the activation of T cells. Deficiency of LKLF causes spontaneous activation of T cells, which then undergo activation-induced cell death, resulting in a dramatic reduction in the number of peripheral T cells (11). Further studies indicate that LKLF suppresses the expression of myc, thereby slowing the progression of the cell cycle (2). Foxj1-deficient T cells are more sensitive to stimulation via T-cell receptors. RAG-deficient mice reconstituted with Foxj1-deficient T cells develop multiorgan systemic inflammation. The effect of Foxj1 deficiency is at least partly mediated by a reduction in the level of I-κBβ, thereby resulting in enhanced nuclear translocation of NF-κB (14). Besides the difference in the mechanism of action, ROG also differs from LKLF and Foxj1 in its expression kinetics. Both LKLF and Foxj1 are highly expressed in naïve T cells and are rapidly downregulated in response to antigen encounter. This expression kinetic suggests that the role of LKLF and Foxj1 is to maintain quiescence of naïve T cells and that LKLF and Foxj1 must be downregulated to allow full-scale T-cell activation to proceed. This expression pattern is in sharp contrast to that of ROG, which is actually induced upon T-cell activation (17). Therefore, we postulate that the function of ROG is not to enforce T-cell quiescence but to finely modulate the extent of T-cell activation.
Despite the in vitro effect on T-cell activation and the production of IL-12p40 by DCs, deficiency of ROG has no obvious effect on in vivo Th immune responses. Functional redundancy as described above is very likely the cause of the discrepancies. Identification of the genes functionally overlapping with ROG may uncover novel negative regulators of innate and adaptive immunity.
Acknowledgments
We thank Sung-Yun Pai for critical review of the manuscript, Elizabeth Leadbetter for assistance in the EAE model, and Kirsten Sigrist for blastocyst injection and implantation.
This work was support by an R01 grant (AI45653) from the National Institutes of Health.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 2.Buckley, A. F., C. T. Kuo, and J. M. Leiden. 2001. Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc-dependent pathway. Nat. Immunol. 2:698-704. [DOI] [PubMed] [Google Scholar]
- 3.Fraser, J. D., B. A. Irving, G. R. Crabtree, and A. Weiss. 1991. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science 251:313-316. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh, P., T. H. Tan, N. R. Rice, A. Sica, and H. A. Young. 1993. The interleukin 2 CD28-responsive complex contains at least three members of the NF kappa B family: c-Rel, p50, and p65. Proc. Natl. Acad. Sci. USA 90:1696-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glimcher, L. H., and K. M. Murphy. 2000. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 14:1693-1711. [PubMed] [Google Scholar]
- 6.Hernandez-Hoyos, G., M. K. Anderson, C. Wang, E. V. Rothenberg, and J. Alberola-Ila. 2003. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity 19:83-94. [DOI] [PubMed] [Google Scholar]
- 7.Himes, S. R., L. S. Coles, R. Reeves, and M. F. Shannon. 1996. High mobility group protein I(Y) is required for function and for c-Rel binding to CD28 response elements within the GM-CSF and IL-2 promoters. Immunity 5:479-489. [DOI] [PubMed] [Google Scholar]
- 8.Hoyos, B., D. W. Ballard, E. Bohnlein, M. Siekevitz, and W. C. Greene. 1989. Kappa B-specific DNA binding proteins: role in the regulation of human interleukin-2 gene expression. Science 244:457-460. [DOI] [PubMed] [Google Scholar]
- 9.Jain, J., C. Loh, and A. Rao. 1995. Transcriptional regulation of the IL-2 gene. Curr. Opin. Immunol. 7:333-342. [DOI] [PubMed] [Google Scholar]
- 10.Karin, M., and A. Lin. 2002. NF-κB at the crossroads of life and death. Nat. Immunol. 3:221-227. [DOI] [PubMed] [Google Scholar]
- 11.Kuo, C. T., M. L. Veselits, and J. M. Leiden. 1997. LKLF: a transcriptional regulator of single-positive T cell quiescence and survival. Science 277:1986-1990. [DOI] [PubMed] [Google Scholar]
- 12.Lai, J. H., G. Horvath, J. Subleski, J. Bruder, P. Ghosh, and T. H. Tan. 1995. RelA is a potent transcriptional activator of the CD28 response element within the interleukin 2 promoter. Mol. Cell. Biol. 15:4260-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, Q., and I. M. Verma. 2002. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2:725-734. [DOI] [PubMed] [Google Scholar]
- 14.Lin, L., M. S. Spoor, A. J. Gerth, S. L. Brody, and S. L. Peng. 2004. Modulation of Th1 activation and inflammation by the NF-κB repressor Foxj1. Science 303:1017-1020. [DOI] [PubMed] [Google Scholar]
- 15.Maggirwar, S. B., E. W. Harhaj, and S. C. Sun. 1997. Regulation of the interleukin-2 CD28-responsive element by NF-ATp and various NF-κB/Rel transcription factors. Mol. Cell. Biol. 17:2605-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGuire, K. L., and M. Iacobelli. 1997. Involvement of Rel, Fos, and Jun proteins in binding activity to the IL-2 promoter CD28 response element/AP-1 sequence in human T cells. J. Immunol. 159:1319-1327. [PubMed] [Google Scholar]
- 17.Miaw, S. C., A. Choi, E. Yu, H. Kishikawa, and I. C. Ho. 2000. ROG, repressor of GATA, regulates the expression of cytokine genes. Immunity 12:323-333. [DOI] [PubMed] [Google Scholar]
- 18.Miaw, S. C., B. Y. Kang, I. A. White, and I. C. Ho. 2004. A repressor of GATA-mediated negative feedback mechanism of T cell activation. J. Immunol. 172:170-177. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann, T. R., and R. L. Coffman. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 20.Murphy, T. L., M. G. Cleveland, P. Kulesza, J. Magram, and K. M. Murphy. 1995. Regulation of interleukin 12 p40 expression through an NF-κB half-site. Mol. Cell. Biol. 15:5258-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novak, T. J., P. M. White, and E. V. Rothenberg. 1990. Regulatory anatomy of the murine interleukin-2 gene. Nucleic Acids Res. 18:4523-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omori, M., M. Yamashita, M. Inami, M. Ukai-Tadenuma, M. Kimura, Y. Nigo, H. Hosokawa, A. Hasegawa, M. Taniguchi, and T. Nakayama. 2003. CD8 T cell-specific downregulation of histone hyperacetylation and gene activation of the IL-4 gene locus by ROG, repressor of GATA. Immunity 19:281-294. [DOI] [PubMed] [Google Scholar]
- 23.Ouyang, W., M. Lohning, Z. Gao, M. Assenmacher, S. Ranganath, A. Radbruch, and K. M. Murphy. 2000. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity 12:27-37. [DOI] [PubMed] [Google Scholar]
- 24.Pai, S. Y., M. L. Truitt, and I. C. Ho. 2004. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc. Natl. Acad. Sci. USA 101:1993-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pai, S. Y., M. L. Truitt, C. N. Ting, J. M. Leiden, L. H. Glimcher, and I. C. Ho. 2003. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity 19:863-875. [DOI] [PubMed] [Google Scholar]
- 26.Plevy, S. E., J. H. Gemberling, S. Hsu, A. J. Dorner, and S. T. Smale. 1997. Multiple control elements mediate activation of the murine and human interleukin-12 p40 promoters: evidence of functional synergy between C/EBP and Rel proteins. Mol. Cell. Biol. 17:4572-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranger, A. M., M. Oukka, J. Rengarajan, and L. H. Glimcher. 1998. Inhibitory function of two NFAT family members in lymphoid homeostasis and Th2 development. Immunity 9:627-635. [DOI] [PubMed] [Google Scholar]
- 28.Rao, A., C. Luo, and P. G. Hogan. 1997. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15:707-747. [DOI] [PubMed] [Google Scholar]
- 29.Rothenberg, E. V., and S. B. Ward. 1996. A dynamic assembly of diverse transcription factors integrates activation and cell-type information for interleukin 2 gene regulation. Proc. Natl. Acad. Sci. USA 93:9358-9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapiro, V. S., K. E. Truitt, J. B. Imboden, and A. Weiss. 1997. CD28 mediates transcriptional upregulation of the interleukin-2 (IL-2) promoter through a composite element containing the CD28RE and NF-IL-2B AP-1 sites. Mol. Cell. Biol. 17:4051-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szabo, S. J., S. T. Kim, G. L. Costa, X. Zhang, C. G. Fathman, and L. H. Glimcher. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100:655-669. [DOI] [PubMed] [Google Scholar]
- 32.Szabo, S. J., B. M. Sullivan, C. Stemmann, A. R. Satoskar, B. P. Sleckman, and L. H. Glimcher. 2002. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science 295:338-342. [DOI] [PubMed] [Google Scholar]
- 33.Tang, C. J., C. K. Chuang, H. M. Hu, and T. K. Tang. 2001. The zinc finger domain of Tzfp binds to the tbs motif located at the upstream flanking region of the Aie1 (aurora-C) kinase gene. J. Biol. Chem. 276:19631-19639. [DOI] [PubMed] [Google Scholar]
- 34.Zhang, D. H., L. Cohn, P. Ray, K. Bottomly, and A. Ray. 1997. Transcription factor gata-3 is differentially expressed murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J. Biol. Chem. 272:21597-21603. [DOI] [PubMed] [Google Scholar]
- 35.Zheng, W. P., and R. A. Flavell. 1997. The transcription factor Gata-3 is necessary and sufficient for Th2 cytokine gene expression in Cd4 T cells. Cell 89:587-596. [DOI] [PubMed] [Google Scholar]