Abstract
Background
The high smoking rate among patients with schizophrenia is an important public health problem, and researchers have been studying how to change the status quo.
Objective
We investigated the effects of high frequency (10Hz) repetitive transcranial magnetic stimulation on the amount of cigarette smoking in patients with schizophrenia.
Method
This study enrolled 37 male patients with schizophrenia who were at a stable stage of treatment. Patients were randomly divided into a treatment group (n=19) and a control group (n=18). The treatment group received repetitive transcranial magnetic stimulation (rTMS) on the left prefrontal dorsolateral cortex (DLPFC), and the control group received placebo treatment. The number of cigarettes smoked were recorded at 7 days before treatment, during the course of treatment (i.e. for 21 days), and 3 weeks after treatment had ended. Assessments using the Positive and Negative Syndrome Scale (PANSS), Wisconsin Card Sorting Test (WCST), and Montgomery Asberg Depression Rating Scale (MADRS) were applied before and after treatment.
Result
Compared to the control group, the number of cigarettes smoked in the treatment group showed a statistically significant reduction in the first week after treatment. However, there was no significant correlation between the scores on PANSS, WCST and MADRS and the number of cigarettes smoked before and after treatment in both groups.
Discussion
High frequency (10Hz) repetitive transcranial magnetic stimulation on the left prefrontal cortex can reduce the number of cigarettes smoked in patients with schizophrenia.
Key words: Repetitive transcranial magnetic stimulation, schizophrenia, cigarette smoking
Abstract
背景
精神分裂症病人的高吸烟率是重要的公共卫生 问题,研究人员一直在探讨如何改变这一现状。
目的
探讨高频(10Hz)重复经颅磁刺激对住院精神 分裂症患者吸烟数量的影响。
方法
本研究纳入37 例处于治疗稳定期的男性精神 分裂症患者。随机分为治疗组和对照组,治疗组(19 例) 在左侧前额叶背外侧皮质进行重复经颅磁刺激(rTMS), 对照组(18 例)采用伪治疗。记录患者治疗前7 天, 整个治疗过程(21 天),治疗结束2 周后连续7 天的 吸烟支数作随访观察,在治疗前后用阳性和阴性症状 评定量表(PANSS)、威斯康星卡片分类测验(WCST) 及蒙格马利抑郁量表(MADRS)进行评定。
结果
治疗组与对照组相比,吸烟数量在治疗第 一周即表现出统计学意义上的降低。治疗前后两组 PANSS、WCST 和MADRS 得分变化与吸烟量无明显相 关性。
结论
左侧前额叶背外侧皮质行高频(10Hz)重复经 颅磁刺激可以减少精神分裂症患者的吸烟数量。
1. Background
Nicotine dependence has always been a large public health concern. According to a report on the harmful health effects of cigarettes in China by the Chinese Ministry of Health and Family Planning Committee in 2012, there were 300 million smokers in our country, and each year the number of death from smoking related diseases was more than 1 million.[1] The rate of cigarette smoking in patients with schizophrenia patients was shown to be 3 to 4 times higher than the general population. In the western countries, it has been estimated that about 76% of male patients with schizophrenia were smokers, while in China the number is about 41%. Currently there is no consensus about the high rate of cigarette smoking in patients with schizophrenia. Some reports pointed out that repetitive transcranial magnetic stimulation (rTMS) on the dorsolateral prefrontal cortex performed on persons without mental disorder could significantly reduce the desire for smoking caused by environmental factors. [5] Other studies showed that rTMS can also reduce the desire for smoking in those patients with schizophrenia.[6,7] This study was conducted to investigate whether rTMS with high frequency on the left prefrontal dorsolateral cortex (DLPFC) can reduce the number of cigarettes smoked by patients with schizophrenia. Another purpose of the study was to understand if there was a correlation between the decrease in the number of cigarettes smoked and the negative symptoms, cognitive symptoms, and depressive symptoms in study participants.
2. Participants and methods
2.1 Participants
There were a total of 440 male patients with schizophrenia who were admitted to our hospital between 1 January 2015 and 15 March 15 2016. Inclusion criteria were the following: 1) meeting diagnostic criteria for schizophrenia according the Diagnositc and Statistical Manual of Mental Disorders 4th edition (DSM IV)[8], and male patients had been in stable condition for at least 6 months. 2) Age 18 to 60 years old. 3) Number of cigarettes smoked is > 10 cigarettes / day and participant has been smoking for more than 2 years. 4) No intention to quit smoking. Exclusion criteria were the following: 1) Comorbidity with other mental disorders, such as affective disorders, anxiety disorders, or personality disorder. 2) Comorbid cardiovascular disease, cerebrovascular disease, endocrine disease, autoimmune disease, or neuronal disease (including epilepsy and EEG abnormalities). 3) Current substance use disorder other than tobacco. 4) Current suicidal ideation. After screening with the inclusion and exclusion criteria, there were a total of 51 potential participants for this study. Upon completion of informed consent process, as well as an explanation regarding the research methods and purpose of this study 10 patients declined to participate. Therefore, a total of 41 participants were eventually included in this research study. The patients were then divided into two groups using a random number table. Upon completion of the study, 2 patients in the treatment group and 2 patients in the control group were unable to adhere with the treatment and dropped out. All the patients in this study had no contraindications related to rTMS and provided written informed consent. This study was approved by the ethics committee of the Tongde Hospital.
2.2 Methods
This study was a randomized, double-blind, controlled study. All the patients were assigned to the treatment group or the control group according to a random number table, and the number of participants in each group was equal. During the treatment and the followup after treatment, the patients decided whether to smoke or not and the number of cigarettes they would smoke. During the study, the patients smoked at a certain place at a certain time, and the most amount of cigarettes was no more than highest level of previous smoking. There were no other restrictions. When the assessors evaluated the patients with scales, they were blinded to the types of treatment the patients were receiving. Only the technicians who operated the rTMS knew the type of treatment for each patient.
2.2.1 Treatment
The YRDCCY-1 transcranial magnetic stimulation instrument was used in the treatment group (Wuhan Yi Rui De company). It has “∞”, and the stimulation parameters are: stimulation site is in the left prefrontal dorsolateral cortex (DLPFC), coil location is according to the positioning method of standard functional area (that is to determine the motor evoked potential (MEP) through the TMS to find the central position of the primary motor cortex (M1), and then forward 5cm along the axis to identify the dorsolateral prefrontal cortical regions), the intensity of stimulation is 110% of motor threshold (MT), the stimulation frequency is 10 Hz, the amount of stimulation is 20 pulses/s, stimulation time is 10s, and the time interval is 30s. 2000 stimuli were given in every stimulus cycle, and it continued for three weeks for a total of 42000 pulses during the treatment period. The patients in the control group were stimulated with the coil of the same shape as the real coil. This special coil can produce the same sound as the real coil but gives no stimulation during the treatment.
2.2.2 Assessments of cigarette smoking and symptoms of schizophrenia
The two groups received rTMS for 3 weeks, with the total number of rTMS sessions totaling 21. Medications that patients were receiving were unchanged during the course of this study. Before and after treatment patients were assessed using the PANSS[9] negative syndrome scale and MADRS[10] depressive syndrome scale by three senior doctors who had been trained in the use of this scale (one of the doctors also was responsible for quality control in this study). All three doctors were blinded to the type of treatment patients were receiving. In addition, we used the computer version of WCST (128 cards) to test the patients before and after the intervention to analyze the total number of errors. Finally, we recorded the number of cigarettes smoked at 7 days before treatment, 21 days into the treatment, and from the 14th day to 17th day after treatment. Any patients who dropped out during the treatment had their data removed from the final analysis.
2.2.3 Safety assessment
Blood and urine testing, liver function, renal function, and electrocardiogram were all examined before and after treatment and any adverse events were recorded.
2.2.4 Statistical methods
SPSS software version 22.0 was used for statistical analysis of data. Mean (standard deviation) was used for continuous data. Independent sample t test was used for the comparisons of demographic data (age, education level, age of onset), baseline assessment, treatment evaluation, and drug use (chlorpromazine dosage, number of cigarettes smoked, smoking age) between the two groups. If there was heterogeneity of variance, t test was applied. Fisher’s exact test was used for comparing first generation antipsychotic drugs and drug combinations between the two groups. Repeated measure of variance analysis was used to compare the number of cigarettes smoked at different points in time over the treatment period. Paired sample t test was used to compare the symptom scores on the scales before and after the treatment in the treatment and control groups. Pearson linear correlation analysis was used to analyze the correlations between the numbers of cigarettes smoked and the symptom score on the scales.
3. Results
3.1 Demographic comparisons between the treatment and control groups
An initial evaluation was done for 51 participants. However, after the informed consent process 10 patients declined to participate in this study, therefore only 41 patients were enrolled in this trial. During the study 2 participants in the treatment group and 2 participants in the control group were unable to complete the treatment. Between the two groups there were no statistically significant differences in age (t=-1.20, df=35, p= 0.239), education (t=-1.49, df=35, p=0.144), and age of onset (t=1.15, df=35, p = 0.259) between the treatment group and control group. At baseline, there was no difference in the numbers of cigarettes smoked between the two groups (t=0.089, df=35, p=0.929). Therefore, the treatment group and the control group were comparable in this study (See table 1).
Table 1.
Comparison of demographic and clinical data between the treatment group and control group (mean [sd])
| Treatment group (n=19) | Control group (n=18) | t /X2 | df | p | |
|---|---|---|---|---|---|
| Demographic data | |||||
| Mean (sd) age | 40.58(3.01) | 39.39(3.03) | -1.20 | 35 | 0.239 |
| Mean (sd) years of education | 10.32(1.29) | 9.33(2.54) | -1.49 | 35 | 0.144 |
| Mean (sd) age of onset | 9.84(2. 22) | 10.72(2.44) | 1.15 | 35 | 0.259 |
| Drug use | |||||
| first generation antipsychotic drugs (yes/no) | 2/17 | 1/17 | --- | --- | 1.000 |
| second generation antipsychotic drugs (yes/no) | 17/2 | 17/1 | --- | --- | 1.000 |
| anti cholinergic drugs (yes/no) | 1/18 | 2/16 | --- | --- | 0.604 |
| mood stabilizer (yes/no) | 2/17 | 2/16 | --- | --- | 1.000 |
| combined medication (yes/no) | 3/16 | 3/15 | --- | --- | 1.000 |
| dosage of chlorpromazine | 184.21(83.42) | 197.22(91.51) | 0.45 | 35 | 0.654 |
| mean (sd) number of cigarettes per day | 22.37(3.93) | 22.50(5.00) | 0.09 | 35 | 0.929 |
| Mean (sd) age started smoking | 13.42(1.51) | 14.34(2.14) | 1.52 | 35 | 0.138 |
| Assessment at baseline (mean [sd]) | |||||
| PANSS positive syndrome score | 9.00(1.63) | 9.72(2.24) | 1.12 | 35 | 0.269 |
| PANSS negative syndrome score | 29.47(3.01) | 30.28(6.29) | 0.49 | 24.08 | 0.628 |
| PANSS general psychiatric syndrome score | 41.58(4.21) | 41.89(6.69) | 0.17 | 28.38 | 0.868 |
| PANSS total score | 81.89(13.48) | 80.05(8.02) | 0.50 | 27.40 | 0.621 |
| MADRS total score | 15.05(5.78) | 12.72(2.14) | -1.65 | 23.08 | 0.113 |
| WCST total errors (n) | 57.37(9.06) | 57.11(4.96) | -0.11 | 28.19 | 0.915 |
| Assessment after treatment (mean [sd]) | |||||
| PANSS positive syndrome score | 9.47(1.68) | 9.67(2.38) | 0.29 | 35 | 0.776 |
| PANSS negative syndrome score | 29.05(2.51) | 30.00(5.77) | 0.64 | 22.92 | 0.527 |
| PANSS general psychiatric syndrome score | 40.68(2.73) | 41.61(6.48) | 0.56 | 22.59 | 0.580 |
| PANSS total score | 79.21(5.34) | 81.28(13.06) | 0.62 | 22.28 | 0.539 |
| MADRS total score | 14.89(5.52) | 12.39(2.57) | -1.79 | 25.76 | 0.086 |
| WCST total errors (n) | 55.47(8.05) | 56.50(6.20) | 0.40 | 35 | 0.689 |
PANSS: Positive and Negative Syndrome Scale;
MADRS: the Montgomery Åsberg Depression Rating Scale;
WCST: Wisconsin Card Sorting Test
3.2 Comparison of the number of cigarettes smoked between the treatment and control groups
The repeated measures analysis of variance showed that there were significant differences in the time effect (Ftime=77.57, p<0.001), between group effect (Fgroup=8.227, p=0.007), and interaction effect (Ftime x group=24.513, p<0.001). Therefore, the changes in the number of cigarettes smoked were significantly different between the two groups along with the treatment period. In addition, we also conducted repeated measures ANOVA with single factor. The number of cigarettes smoked in the treatment group was significantly different over the treatment period (Ftime=73.30, p<0.001), while in the control group there were no differences in the number of cigarettes smoked (F time=1.64, p=0.175). In the treatment group, the mean number of cigarettes smoked 7 days before treatment (mean=22.9) was significantly higher than the first week after treatment (mean=19.9) (F=51.30, p<0.001). There was also a significant difference in the number of cigarettes smoked between the first week and the second week after the treatment (F=71.37, p<0.001). Compared to the second week after treatment, the number of cigarettes smoked in the third week significantly decreased (F=104.25, p<0.001). During the follow up after the treatment, the number of cigarettes smoked continued to decrease and was significantly less than that during the third week after treatment (F=60.87, p<0.001).
Independent sample t test was used to compare the number of cigarettes smoked between the two groups at different time points. The results indicated that there was no statistically significant difference between the two groups in the number of cigarettes smoked during the 7 days before treatment (t=-0.30, df=35, p=0.764). During the first week after treatment, the difference in number of cigarettes smoked between the two groups was statistically significant (t=2.33, df=35, p=0.026). During the second week after treatment, the number of cigarettes smoked in the treatment group was significantly lower than in the control group (t=3.64, df=35, p=0.001). During the third week, the number of cigarettes smoked in the treatment group was also significantly lower than that of the control group (t=5.49, df=35, p<0.001). Finally, the results showed that the number of cigarettes smoked by the treatment group was significantly lower than the control group during the follow-up after the treatment had been completed (t=5.82, df=35, p<0.001).
3.3 Relationship between smoking and change in symptoms in the two groups
We used independent samples t test to compare psychiatric symptoms (PANSS total score, positive symptoms score, negative symptoms score, general psychopathology symptoms score), cognitive function (WCST total errors), and depressive symptoms (MADRS total score) between the two groups at baseline and 3 weeks after treatment. However, we did not find any statistically significant differences in any of these measures (See table 1).
Paired t test was used to compare psychiatric symptoms (PANSS total score, positive symptoms score, negative symptoms score, general psychopathology symptoms score), cognitive function (WCST total errors), and depressive symptoms (MADRS total score) before and after treatment in the treatment group and control group, respectively. However, we did not find any statistically significant differences in any of these measures (See table 3).
Table 3.
Changes in scale scores in the treatment group and control group before and after the rTMS treatment (mean [sd])
| Before treatment (n=19) | 3 weeks after treatment (n=19) | t | p | |
|---|---|---|---|---|
| Treatment group (n=19) | ||||
| PANSS positive syndrome score | 9.00 (1.63) | 9.47 (1.68) | -1.76 | 0.095 |
| PANSS negative syndrome score | 29.47(3.01) | 29.05 (2.51) | 0.60 | 0.559 |
| PANSS general psychiatric syndrome score | 41.58 (4.21) | 40.68 (2.73) | 1.08 | 0.293 |
| PANSS total score | 81.89 (13.48) | 79.21 (5.34) | 0.55 | 0.592 |
| MADRS total score | 15.05 (5.78) | 14.89 (5.52) | 0.43 | 0.674 |
| WCST total errors (n) | 57.37(9.06) | 55.47(8.05) | 1.84 | 0.083 |
| Control group (n=18) | ||||
| PANSS positive syndrome score | 9.72 (2.24) | 9.67(2.38) | 0.19 | 0.854 |
| PANSS negative syndrome score | 30.28 (6.29) | 30.00 (5.77) | 0.39 | 0.702 |
| PANSS general psychiatric syndrome score | 41.89 (6.69) | 41.61(6.48) | 0.33 | 0.745 |
| PANSS total score | 80.05(8.02) | 81.28 (13.06) | 0.36 | 0.722 |
| MADRS total score | 12.72 (2.14) | 12.39 (2.57) | 0.58 | 0.571 |
| WCST total errors (n) | 57.11(4.96) | 56.50 (6.20) | 1.11 | 0.281 |
Using Pearson linear correlation analysis, we did not find any significant differences between the number of cigarettes smoked and psychiatric symptoms (PANSS total score, positive symptoms score, negative symptoms score, general psychopathology symptoms score), cognitive function (WCST total errors), and depressive symptoms (MADRS total score) in either of the groups (See table 4). In conclusion, the change in the number of cigarettes smoked was not related to psychiatric symptoms, cognitive function or depressive symptoms.
Table 4.
Pearson correlation analysis between the number of cigarettes smoked and the different scale scores (Pearson correlation coefficient [p-value])
| treatment group (n) | control group (n) | |||
|---|---|---|---|---|
| Before treatment | 3 weeks after treatment | Before treatment | 3 weeks after treatment | |
| PANSS positive syndrome score | -0.158(0.519) | -0.182(0.457) | 0.126(0.617) | -0.141(0.577) |
| PANSS negative syndrome score | -0.090(0.713) | 0.054(0.827) | 0.130(0.606) | 0.210(0.403) |
| PANSS general psychiatric syndrome score | -0.238(0.326) | -0.008(0.974) | 0.167(0.507) | 0.120(0.634) |
| PANSS total score | -0.191(0.433) | -0.036(0.884) | 0.165(0.513) | 0.127(0.616) |
| MADRS total score | 0.186(0.446) | 0.117(0.634) | 0.126(0.620) | 0.026(0.918) |
| WCST total errors (n) | 0.125(0.609) | 0.423(0.071) | 0.355(0.148) | 0.198(0.430) |
3.4 Comparison of adverse reactions between the two groups
There were no serious adverse events in either of the two groups during the trial. Two patients in the treatment group and one patient in the control group had a mild headache at one time during treatment, but these went away on their own. Other adverse reactions were slight. The results of blood routine, urine routine, blood biochemistry and electrocardiogram (ECG) in the two groups showed no clinical changes before and after treatment.
4. Discussion
4.1 Main findings
The results of this study showed that for patients who receive rTMS treatment there is a tendency to decrease the number of cigarettes smoked when compared with those who do not receive rTMS, with the difference being statistically significant. After the treatment was finished, the follow-up data indicated that the effect of rTMS on reducing the number of cigarettes smoked lasted for at least 3 weeks.
There are several factors which may be associated with the high rates of smoking in patients with schizophrenia such as: change of neurotransmitters in the brain; smoking reduces the negative symptoms of schizophrenia; smoking can improve some cognitive functions (such as attention or short-term memory); and smoking can relieve some of the extrapyramidal symptoms caused by antipsychotic drugs.[11] Some studies found that patients with schizophrenia take in more nicotine than the general population and had faster metabolic rates, which resulted in a tendency towards nicotine dependence.[12] In addition, for a variety of reasons, the rate of successfully quitting smoking is lower among individuals with schizophrenia than the rate seen in the general population. Some studies reported that the rate of successfully quitting smoking is about 42% in the general population, while in individuals with schizophrenia the rate was far lower. [13] In China, some studies reported that the rate of successfully quitting smoking was only 4% among those with schizophrenia.[14]
Generally speaking, those seeking to quit smoking have the following medications available to aid in the process: nicotine replacement therapy (nicotine chewing gum, nicotine capitation tablets, nicotine patch), varenicline, bupropion hydrochloride sustained release tablets. All of the above drugs can further aggravate certain symptoms of schizophrenia [15], therefore the methods for quitting smoking are far more limited for these individuals. Repetitive transcranial magnetic stimulation (rTMS) as a non-invasive brain stimulation technique when given a high frequency of rTMS in the left dorsolateral prefrontal cortex has been shown to reduce nicotine craving in those with nicotine dependence. The exact mechanism of this rTMS technique involves several theories. First of all, in the study of psychoactive substance addiction, the reward system has received a lot of attention as the biological foundation. Dopamine is the most important neurotransmitter in the reward system, and nicotine can activate the dopamine system in the brain’s edge.[16] When people stop smoking, the desire for nicotine is associated with a decreased level of the activity in the brain’s reward system. High frequency rTMS in the frontal lobe can increase the release of dopamine in the brain circuitry of the limbic system. Even if there is only a small amount of dopamine released, the symptoms of substance withdrawal can be improved. Therefore, rTMS treatment simulates the model of dopamine release when people intake nicotine to reduce withdrawal symptoms. There is also a hypothesis that rTMS improves the withdrawal symptoms by stimulating the excitability of the cortex to change the synaptic plasticity thus leading to neuronal adaptation in the brain reward system.[17,18] Finally, some other studies combined the methods of rTMS and functional magnetic resonance imaging. The results suggested that rTMS could change the brain functions of the subcortical brain regions, including the reward system. It is generally believed that the prefrontal cortex is associated with nicotine dependence and addictive behavior. These brain regions are involved in decision making, behavior inhibition and so forth. The treatment of rTMS in the prefrontal cortex can improve the craving phenomenon by intervening in the brain decision making system and inhibiting the central nervous system.
Many studies showed that high frequency rTMS in the left prefrontal cortex had effects on depression to varying degrees,[20] and also could relieve negative symptoms seen in schizophrenia. Those effects however appear to be independent of each other.[21] However, the mechanism of reduction of nicotine craving was seldom considered. The results of this study suggest that, the effects of high frequency rTMS on reducing cigarette smoking may have no significant correlation to the improvement of depressive symptoms or negative symptoms, because high-frequency rTMS used for controlling nicotine dependence in healthy people was also shown to be effective.[5] Therefore, the role of high frequency rTMS in reducing the symptoms of craving is relatively specific, and it is not a secondary result of controlling the symptoms of schizophrenia. In addition, previous studies concluded that rTMS with a of frequency 10Hzr improved the negative symptoms better than rTMS with a frequency 20Hz.[22] Studying the effects of rTMS using different frequencies on the number of cigarettes smoked may provide further understanding of the biological effects of rTMS on the brains neural pathways.
4.2 Limitations
There were several major limitations in our study. First of all, only males were enrolled to participate in this study. Huber et al reported that the effects of rTMS were different for different genders.[23] Secondly, this study only used a self-report questionnaire to record the number of cigarettes smoked. We did not use an objective scale or an instrument measuring CO content at end of the study to assess the level of nicotine dependence. If we had used the above mentioned objective measurements on the assessment, we may be able to find more meaningful results. Thirdly, a decrease in the number of cigarettes smoked due to rTMS treatment does not necessarily indicate successful cessation of smoking as the decrease in amount smoked could be merely temporary. Further studies can try to extend the course of treatment with rTMS in order to assess the long-term effects of rTMS on smoking.
4.3 Implications
This study aimed to evaluate the effects of rTMS on the number of cigarettes smoked in patients with schizophrenia, as well as its association with negative symptoms, cognitive function, and depressive symptoms. We found that high frequency rTMS in the left prefrontal cortex can reduce the number of cigarettes smoked in patients with schizophrenia, and the effects could be sustained for at least three weeks. However, this effect was not correlated with patients’ original negative symptoms, cognitive function or depressive symptoms. There were no cases of obvious adverse reactions on the patients, except for headache, suggesting that rTMS was safe.
Figure 1.
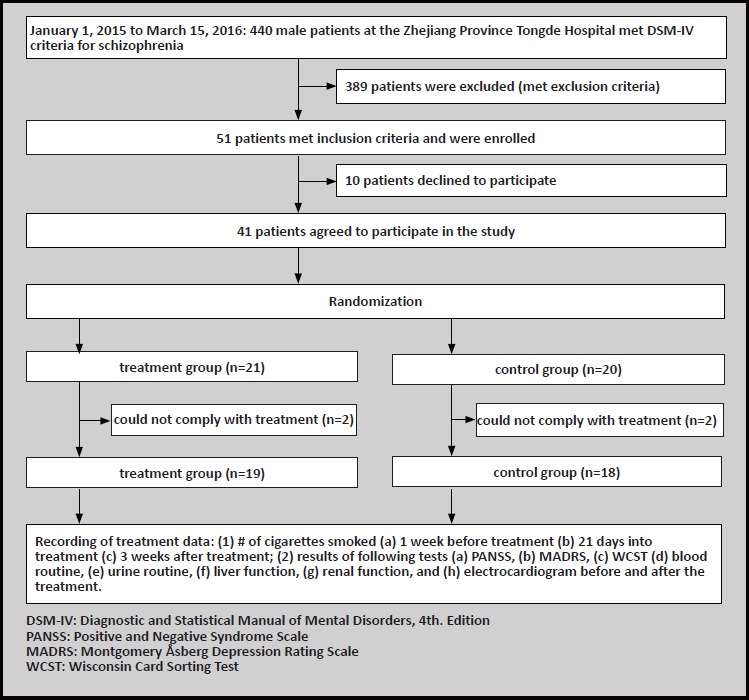
Enrollment of obsessive-compulsive disorder and healthy controls
Table 2.
The comparisons in cigarette smoking between the treatment group and control group before and after rTMS treatment (mean [SD])
| treatment group (n) | control group (n) | |
|---|---|---|
| Before rTMS (7 days before treatment to 1st day of treatment) | 22.90 (4.09) | 22.50 (3.84) |
| After rTMS (1st day to 7th day) | 19.90 (3.45*) | 22.33 (2.89*) |
| After rTMS (8th day to 14th day) | 17.95 (3.15*) | 21.83 (3.35*) |
| After rTMS (15th day to 21st day) | 15.63 (3.04*) | 21.44 (3.40*) |
| Follow-up (14th day to 21st day after treatment) | 15.11 (3.03*) | 21.67 (3.80*) |
*There were statistical significant differences in the smoking of cigarette between the treatment group and the control group (p<0.05)
Biography

Wanli Huang graduated from Central South University, Xiangya Medical College in 2010 and completed a master’s degree in psychopathology and mental health from Zhejiang University in 2013. Since 2013, she has worked in the department of psychiatry at Zhejiang Tongde Hospital as a resident doctor. Her research interests are the pathogenesis and treatment of schizophrenia.
Footnotes
Funding
This study received financial support from the Zhejiang Province Department of Health (ID number is 2015KYB073).
Conflict of interest statement
The authors report no conflict of interest related to this manuscript.
Informed consent
Written informed consent was obtained from all participants.
Ethical approval
The study protocol was approved by the Ethics Committee of Zhejiang Province Tongde Hospital.
Authors’ contribution
HWL and SF were in charge of cases collection and data input.
XBP, SF, and ZJT were in charge of the scale assessments.
ZJT was also in charge of the analysis of data
References
- 1.Wen XZ, Chen WQ, Lu CY, Zhang CX, Luo JY, Deng XQ, et al. [Knowledge and belief related to smoking and health and their association with smoking behaviors among secondary school students]. Zhongguo Gong Gong Wei Sheng. 2005; 21(6): 756-758. Chinese. doi: http://dx.chinadoi.cn/10.3321/j.issn:1001-0580.2005.06.064 [Google Scholar]
- 2.Schneider CE, White T, Hass J, Geisler Daniel, Wallace Stuart R, Roessner Veit, et al. Smoking status as a potential confounder in the study of brain structure in schizophrenia. J Psychiatr Res. 2014; 50: 84-91. doi: http://dx.doi.org/10.1016/j.jpsychires.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005; 76(2-3): 135-157. doi: http://dx.doi.org/10.1016/j.schres.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 4.Xu YM, Chen HH, Li F, Deng F, Liu XB, Yang HC, et al. Prevalence and correlates of cigarette smoking among Chinese schizophrenia inpatients receiving antipsychotic mono-therapy. PloS One. 2014; 9(2): e88478 doi: http://dx.doi.org/10.1371/journal.pone.0088478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Hartwell KJ, Owens M, Lematty T, Borckardt JJ, Hanlon CA, et al. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry. 2013; 73(8): 714-720. doi: http://dx.doi.org/10.1016/j.biopsych.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wing VC, Bacher I, Wu BS, Daskalakis Zafiris J, George Tony P, et al. High frequency repetitive transcranial magnetic stimulation reduces tobacco craving in schizophrenia. Schizophr Res. 2012; 139(1-3): 264-266. doi: http://dx.doi.org/10.1016/j.schres.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 7.Wing VC, Barr MS, Wass CE, Lipsman Nir, Lozano Andres M, Daskalakis Zafiris J, et al. Brain stimulation methods to treat tobacco addiction. Brain Stimul. 2013; 6(3): 221-230. doi: http://dx.doi.org/10.1016/j.brs.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 8.American Psychological Association. Diagnostic and Statistical Manual of Mental Disorder- IV. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 9.Si TM, Yang JZ, Shu L, Wang XL, Kong QM, Zhou M, et al. [The reliability, validity of PANSS and its implication]. Zhongguo Xin Li Wei Sheng Za Zhi. 2004; 18(1): 45-47. Chinese. doi: http://dx.chinadoi.cn/10.3321/j.issn:1000-6729.2004.01.016 [Google Scholar]
- 10.Liu H, Zhang HY, Xiao WD, Liu Q, Wang G, Chen JX, et al. [Comparison of 5 assessment tools for evaluating depressive symptom in patients with schizophrenia]. Zhongguo Xin Li Wei Sheng Za Zhi. 2015; 8: 570-575. Chinese. doi: http://dx.chinadoi.cn/10.3969/j.issn.1000-6729.2015.08.003 [Google Scholar]
- 11.Featherstone RE, Siegel SJ. The role of nicotine in schizophrenia. Int Rev Neurobiol. 2015; 124: 23-78. doi: http://dx.doi.org/10.1016/bs.irn.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 12.Olincy A, Young DA, Freedman R. Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biological Psychiatry, 1997; 42(1): 1-5. doi: http://dx.doi.org/10.1016/S0006-3223(96)00302-2 [DOI] [PubMed] [Google Scholar]
- 13.Kelly C, McCreadie R. Cigarette smoking and schizophrenia. Adv Psychiatr Treat. 2000; 6(5): 327-331. doi: http://dx.doi.org/10.1192/apt.6.5.327 [Google Scholar]
- 14.Zhang XY, Liang J, Xiu MH, Hong X, He J, Cheng W, et al. Cigarette smoking in male patients with chronic schizophrenia in a Chinese population: prevalence and relationship to clinical phenotypes. PloS One. 2012; 7(2): e30937 doi: http://dx.doi.org/10.1371/journal.pone.0030937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo S, Heishman SJ, Raley H, Wright K, Wehring HJ, Moolchan ET, et al. Tobacco craving in smokers with and without schizophrenia. Schizophr Res. 2011; 127(1-3): 241-245. doi: http://dx.doi.org/10.1016/j.schres.2010.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000; 393(1-3): 295-314. doi: http://dx.doi.org/10.1016/S0014-2999(00)00122-9 [DOI] [PubMed] [Google Scholar]
- 17.Berardelli A, Inghilleri M, Rothwell JC, Romeo S, Currà A, Gilio F, et al. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res. 1998; 122(1): 79-84 [DOI] [PubMed] [Google Scholar]
- 18.Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006; 129(7): 1659-1673. doi: http://dx.doi.org/10.1093/brain/awl082 [DOI] [PubMed] [Google Scholar]
- 19.Krain AL, Wilson AM, Arbuckle R, Castellanos F Xavier, Milham Michael P, et al. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. NeuroImage. 2006; 32(1): 477-484. doi: http://dx.doi.org/10.1016/j.neuroimage.2006.02.047 [DOI] [PubMed] [Google Scholar]
- 20.Rachid F, Bertschy G. Safety and efficacy of repetitive transcranial magnetic stimulation in the treatment of depression: a critical appraisal of the last 10 years. Neurophysiol Clin. 2006; 36(3): 157-183. doi: http://dx.doi.org/10.1016/j.neucli.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 21.Goyal N, Nizamie SH, Desarkar P. Efficacy of adjuvant high frequency repetitive transcranial magnetic stimulation on negative and positive symptoms of schizophrenia: preliminary results of a double-blind sham-controlled study. J Neuropsychiatry Clin Neurosci. 2007; 19(4): 464-467. doi: http://dx.doi.org/10.1176/jnp.2007.19.4.464 [DOI] [PubMed] [Google Scholar]
- 22.Shi C, Yu X, Cheung EF, Shum David H K, Chan Raymond C K, et al. Revisiting the therapeutic effect of rTMS on negative symptoms in schizophrenia: a meta-analysis. Psychiatry Res. 2014; 215(3): 505-513. doi: http://dx.doi.org/10.1016/j.psychres.2013.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber TJ, Schneider U, Rollnik J. Gender differences in the effect of repetitive transcranial magnetic stimulation in schizophrenia. Psychiatry Res. 2003; 120(1): 103-105. doi: http://dx.doi.org/10.1016/S0165-1781(03)00170-7 [DOI] [PubMed] [Google Scholar]


