Summary
Bacteriophages kill bacteria very rapidly. Bacteriophage-induced endotoxin release could be an issue during phage therapy. We show that bacteriophages stop cell growth more rapidly than do β-lactams, resulting in a lower endotoxin release.
Keywords: phage therapy, endotoxin, safety, cell lysis, β-lactams.
Abstract
Background.
Other than numerous experimental data assessing phage therapy efficacy, questions regarding safety of this approach are not sufficiently addressed. In particular, as phages can kill bacterial cells within <10 minutes, the associated endotoxin release (ER) in severe infections caused by gram-negative bacteria could be a matter of concern.
Methods.
Two therapeutic virulent phages and 4 reference antibiotics were studied in vitro for their ability to kill 2 pathogenic strains of Escherichia coli and generate an ER. The early interaction (first 3 hours) between these actors was assessed over time by studying the instantaneous cell viability, the colony-forming unit count, the concentration of free endotoxin released, and the cell morphology under light microscope.
Results.
While β-lactams have a relatively slow effect, both tested phages, as well as amikacin, were able to rapidly abolish the bacterial growth. Even when considering the fastest phage (cell lysis in 9 minutes), the concentrations of phage-induced ER never reached the highest values, which were recorded with antibiotic treatments. Cumulative concentrations of endotoxin over time in phage-treated conditions were lower than those observed with β-lactams and close to those observed with amikacin. Whereas β-lactams were responsible for strong cell morphology changes (spheroplast with imipenem, filamentous cells with cefoxitin and ceftriaxone), amikacin and phages did not modify cell shape but produced intracellular inclusion bodies.
Conclusions.
This work provides important and comforting data regarding the safety of phage therapy. Therapeutically relevant phages, with their low endotoxin release profile and fast bactericidal effect, are not inferior to β-lactams.
(See the Editorial Commentary by Reindel and Fiore on pages 1589–90.)
As bacteria, particularly gram-negative, are more resistant than ever to multiple antibiotics, the search for alternative treatments is now strongly promoted by international organizations and governments (United Nations, World Health Organization, and G20 [1]). Besides their ability to control bacterial growth, these alternatives must also be evaluated regarding their safety. In particular, the strategies leading to the physical destruction of bacterial cells have to be studied with respect to their ability to release endotoxin (as many antibiotics do), especially in clinical situations where patients suffer from high-inoculum infections and/or experience severe sepsis or septic shock.
Bacteriophages (phages) are viruses infecting bacteria, and their use to treat bacterial infections (phage therapy) has been the focus of many experimental studies as well as a few clinical trials [2–4]. However, while initiated almost 100 years ago, many aspects of phage therapy safety have not yet been fully investigated. Given their capability of killing their hosts very rapidly, in minutes, the important safety issue related to the release of endotoxin has been poorly explored [5, 6].
Bacterial endotoxin (the hydrophobic domain of the lipopolysaccharide present in the outer membrane of gram-negative bacteria) is one of the most potent inducers of the proinflammatory cytokine response in patients infected by gram-negative pathogens, and it plays an important role in the pathophysiology of septic shock [7]. Accordingly, blood endotoxin level in infected patients is correlated to the sepsis severity and to survival [8–10]. Of note, bactericidal antibiotics provoke an endotoxin release (ER) over time and the amplitude of this release depends on the pharmacological class of the molecule [11]. As assessed by in vitro and in vivo data in animals [11], the use of potent endotoxin-releasing antibiotics can be detrimental in terms of inflammation and survival. In humans, even if the use of such antibiotics could be associated with a higher inflammatory response, this has not translated—to date—in differences in mortality [12, 13].
To our knowledge, only 2 studies have addressed the question of phage-induced ER using a lysis-deficient phage [14] or a nonlytic (filamentous) phage expressing a toxic gene in the host [15]. They showed that such phages released less endotoxin than wild-type ones [14] or than a lytic phage used as control [15]. However, none of these studies compared phages to different antibiotics in their respective ability to release endotoxin within a short time scale corresponding to the rapid lysis induced by phages.
In this study, we aimed to compare the early-stage killing efficiency of 2 virulent phages, 536_P1 and LM33_P1, both infecting clinical isolates of Escherichia coli, to 2 classes of antibiotics, as well as to determine the level of endotoxin released by these antibacterial agents. We found that both phages killed bacteria more rapidly and released less endotoxin than did β-lactams. These results argue that phages are not more harmful than some antibiotics routinely used in the clinical setting.
MATERIAL AND METHODS
Bacteria and Phages
Escherichia coli strain 536 (susceptible to all clinically relevant antibiotics) and strain LM33 (ST131-O25b:H4 clonal complex) are 2 clinical isolates, responsible for a pyelonephritis [16] and a ventilator-associated pneumonia [17], respectively. The infectious parameters of phage 536_P1 (a Myoviridae, 149.4 kbp, infecting strain 536 [18]) were determined as described in [17] and compared with those previously obtained with LM33_P1 (a Podoviridae, 38.9 kbp, infecting strain LM33 [17]). Data are presented in Table 1 and in Supplementary Figure 1. These phages were purified from a bacterial lysate using our standard protocol [19], including a final endotoxin removal step (EndoTrap Blue, Hyglos, Germany) carried out 3 times.
Table 1.
Parameters of the Viral Cycle of Phage LM33_P1 and 536_P1
| Parameter | LM33_P1 | 536_P1 |
|---|---|---|
| Adsorption constanta, mL/min (95% CI) |
K1 = 1.2 × 10-8 (1.1 to 1.3 × 10-8) | K1 = 6.7 × 10-8 (5.0 to 8.4 × 10-8) |
| … | K2 = 5.1 × 10-9 (4.4 to 5.8 × 10-9) | |
| Latent periodb, min | 9 | 19 |
| Eclipse periodc, min | 7 | 17 |
| Burst size, PFU (95% CI) | 317 (289–345) | 176 (161–193) |
Abbreviations: CI, confidence interval; PFU, plaque forming unit.
aPhage LM33_P1 has a 1-step adsorption kinetic whereas phage 536_P1 has a 2-step kinetic (a fast one followed by a slower one; see Supplementary Data), leading to 2 different adsorption constants.
bThe latent period corresponds to the time elapsed between cell infection and cell lysis.
cThe eclipse period corresponds to time elapsed between cell infection and the detection of the first functional intracellular virions, before cell lysis.
Antibiotics: Choice, Susceptibility Testing, and Concentration
The disk diffusion method and Etests (bioMérieux, France) were used to determine susceptibility to antibiotics and minimum inhibitory concentration (MIC), according to EUCAST guidelines. For strain 536, the following antibiotics were tested (the MIC is indicated in brackets): amikacin (2 mg/L), ceftriaxone (0.016 mg/L), and imipenem (0.125 mg/L). For strain LM33, which expresses an extended-spectrum β-lactamase, the antibiotics tested were amikacin (8 mg/L), cefoxitin (2 mg/L), and imipenem (0.125 mg/L). The antibiotic solutions were prepared extemporaneously from intravenous formulations used for humans (Mylan, Canonsburg, Pennsylvania) and were reconstituted with sterile endotoxin-free water (aqua ad iniectabilia, B-Braun, Germany) to be used at a final concentration of 8-fold the MIC. This antibiotic concentration was chosen according to in vitro and in vivo data related to pharmacodynamics and pharmacokinetics of aminoglycosides and β-lactams [20–23]. Such concentrations are also a recommended objective in the clinical situations where severe infections are presents, to ensure an optimal bactericidal activity [24]. For the same purpose, the phages were used at a multiplicity of infection (MOI) >1 (MOI: 5), based on our previous in vivo data [17–19, 25].
Time-Killing Assays
Classical time-killing assays were performed according to the American Society for Microbiology guidelines [26]. In brief, bacteria exponentially grown at 37°C, 150 rpm, in Mueller-Hinton II broth (Sigma-Aldrich, Germany) were diluted with fresh medium to obtain an OD600nm of 0.015 (approximately 5.107 cells/mL). Thirty milliliters were then evenly aliquoted in 5 Erlenmeyer flasks corresponding to the 5 different conditions: a culture without antibacterial agents; cultures with antibiotics 1, 2, and 3; and a culture with phages. At time 0 (T0), antibacterial agents were added and each flask was sampled at 15, 30, 45, 60, and 180 minutes. The control (without antibacterial agents) was also sampled at T0. The bacterial titer (in triplicate), the instantaneous bacterial viability (in triplicate), and the free endotoxin level (in duplicate) were determined. The detection threshold for the count of colony-forming units (CFUs) is 1 × 102 CFU/mL. Killing assays (and subsequent analysis) were independently repeated twice for each strain.
Microscope observations were performed on samples taken at 180 minutes with the remaining volume of culture. After centrifugation (7000g, 5 minutes), the pellet was resuspended in 100 µL of phosphate-buffered saline and 5 µL was dropped on a glass slide to be observed with a phase contrast microscope.
Endotoxin Measurements
Free endotoxin concentrations were quantitatively assayed on filtered cultures (Poly-Ethylen-Sulfone 0.22 µm filter, Sartorius, France) using a Limulus Amebocyte Lysate assay (Endotox R-fact C, Hyglos, Germany), after a 1:1000 dilution and according to the manufacturer instructions. The validity of the assay was checked; no interferences caused by the medium or the phages were noticed.
Instantaneous Cell Viability Assay
Cell viability was assessed by measuring the intracellular adenosine triphosphate content using the BactTiter-Glo assay and a Glomax-MultiDetection System plate reader (Promega, Madison, Wisconsin).
Statistical Analysis and Modeling
Nonlinear regressions from experimental points regarding the evolution of the free endotoxin concentration according to time were performed by using GraphPad Prism version 5.00 (GraphPad Software, La Jolla, California) with the following equations (where y = endotoxin concentration, x = time): the data obtained with Ceftriaxone treatment were ruled by an exponential curve {y = y0.ekx}, amikacin and phages were ruled by a reverse exponential curve {y = y0+(plateau-yo).(1-e-kx)}, whereas imipenem and cefoxitin were ruled by a sigmoid function {y = (Vmax.xh)/(K+xh)} (see Supplementary Tables 1 and 2). The areas under the concentration of free endotoxin vs time curve (AUC) were calculated using the trapeze method with MatLab 8.3 (MathWorks, France). The input for these calculations was the nonlinear regression curves obtained from each replicate (integration from 0 to 180 minutes, sampling frequency: every 20 seconds).
RESULTS
Phages Kill Bacteria Faster Than β-Lactams
Bacteria grown in liquid broth were exposed to antibiotics (8-fold the MIC) or phages (MOI of 5) and multiple samplings were performed during 180 minutes for the evaluation of killing and ER.
Bacterial abundance was assessed using 2 complementary methods that analyze 2 distinct physiological signals: the classical CFU count on agar medium (following an overnight incubation of serial dilutions) and an instantaneous cell viability assay that measures the amount of metabolically active cells. Indeed, the latter parameter is not taken into account with the agar plate method: The cells that are alive but doomed to death a few hours later will not be counted on agar plates, whereas they will be quantified by using the cell viability assay. Conversely, when bacteria are titrated on an agar plate, the inhibitory pressure is removed, especially as a high dilution is performed to count individualized CFUs: in such a setting, weakly metabolically active bacteria are no longer surrounded by the antibacterial agent and could regrow at the time of plating (as persister cells do [27]).
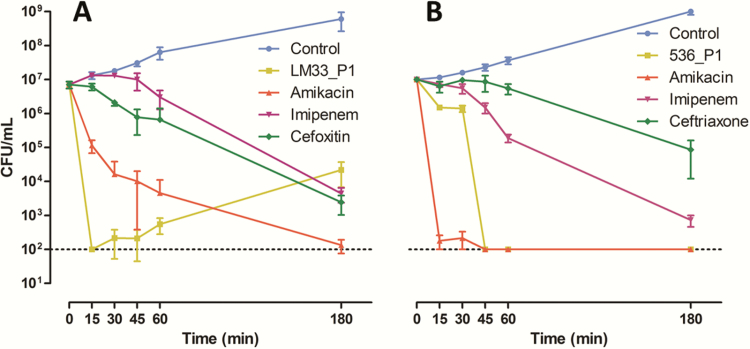
We observed that the decrease of cells able to grow on an agar plate (CFU counts, Figure 1) and the decrease of metabolically active cells (instantaneous cell viability assay, Figure 2) were faster with phages than with β-lactams (whatever the molecule considered). Aminoglycosides and phages were the only antibacterial agents to rapidly decrease the bacterial biomass indirectly assessed by the cell viability assay, while at the earliest stages, β-lactams did not abolish cell growth. After 1 hour, the reduction in CFU counts in phage-treated conditions reached 3.9 and 4.7 log10 for strains LM33 and 536, respectively, while amikacin provided a decrease of 3.0 and 4.7 log10, respectively, and imipenem a decrease of 0.2 and 1.7 log10.
Figure 1.
Colony-forming unit (CFU) counts of strains LM33 and 536 according to time. Strain LM33 (A) and strain 536 (B) were cultured in absence (control) or presence of antibacterial agents: phages (LM33_P1 or 536_P1, multiplicity of infection of 5) or antibiotics (8-fold the minimum inhibitory concentration). Data are means with standard deviation, and the dotted line represents the detection threshold.
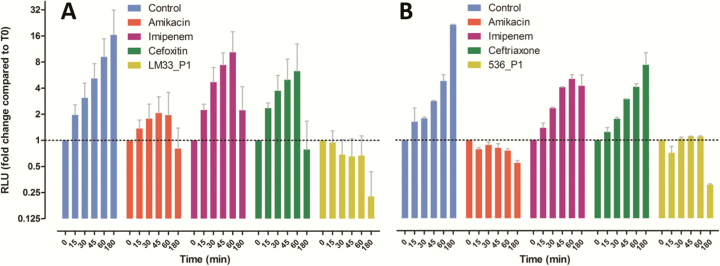
Figure 2.
Relative amount of metabolically active cells according to time in presence of different antibacterial agents. Strain LM33 (A) and strain 536 (B) were cultured in absence (control) or presence of antibacterial agents: phages (LM33_P1 or 536_P1, multiplicity of infection of 5) or antibiotics (8-fold the minimum inhibitory concentration). The viable cells were quantified using an ATP-driven luciferase assay (see Methods). Data are means with standard deviation and the y-axis is in log2 scale. Abbreviation: RLU, relative luminescence unit.
In agreement with its kinetic characteristics (Table 1), phage LM33_P1 was the fastest antibacterial agent on strain LM33 as CFUs were below the threshold of detection (100 cells) at 15 minutes (Figure 1A). Among antibiotics, only amikacin led to a 2 log10 reduction at this time point. When looking at metabolically active cells, we also confirmed that phage LM33_P1 was the fastest killing agent, followed by amikacin, cefoxitin, and imipenem (Figure 2A). It should also be noted that at 180 minutes (Figure 1A), the number of CFUs in the phage-treated sample increased compared to previous time points, while in contrast the number of metabolically active cells remained very low. This suggests that a residual population with low metabolic activity was able to survive in the presence of phages and later to grow on agar plates once the phage pressure is reduced.
As suggested by its intrinsic viral properties (Table 1), phage 536_P1 displayed a slower kinetic than phage LM33_P1 in reducing CFU count and the amount of metabolically active cells. Phage 536_P1 was also slower than amikacin but remained faster than imipenem and ceftriaxone. The fact that the action of amikacin was faster on strain 536 than on strain LM33 is most likely explained by the underlying resistance mechanisms to aminoglycosides of each strain: the MIC of amikacin for strain 536 is 2 mg/L and this strain displays a resistance to streptomycin only whereas strain LM33 has several additional resistances to aminoglycosides (streptomycin, kanamycin, gentamicin, and netilmicin) with an amikacin MIC of 8 mg/L, which is the upper limit to classify E. coli strains as still susceptible [28].
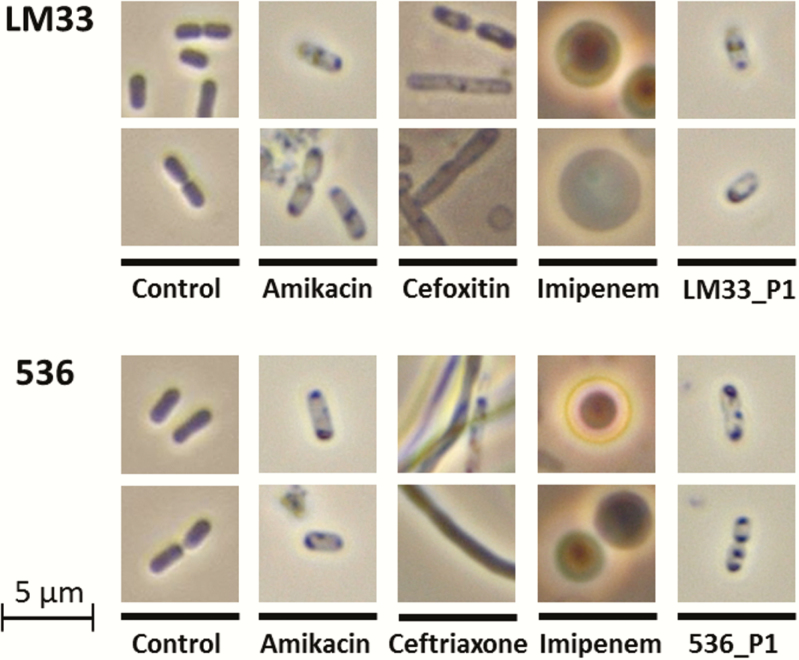
The Morphology of Bacterial Cells Exposed to Amikacin or Phages Is Weakly Affected
Direct microscopic observations, performed after 3 hours of exposition to each of the antibacterial agents, confirmed that β-lactams induced intense cell morphological changes (Figure 3): Ceftriaxone and, to a lesser extent, cefoxitin were responsible for a massive filamentation whereas imipenem was associated with the generation of spherical cells, also known as spheroplasts [29]. Conversely, the cells obtained from cultures treated with amikacin or phages showed a preserved shape. Interestingly, these cells contained typical intracellular inclusion bodies, without any identifiable difference between amikacin and phage-treated cells (Figure 3).
Figure 3.
Morphology of Escherichia coli cells after a 3-hour exposition to different antibacterial agents. The liquid cultures of strains LM33 and 536 in absence (control) or presence of antibacterial agents (antibiotics with a concentration of 8-fold the minimum inhibitory concentration, phages LM33_P1 or 536_P1 with a multiplicity of infection of 5) were centrifuged to concentrate the residual biomass. Wet mount preparations were then observed under phase contrast (×100 lens).
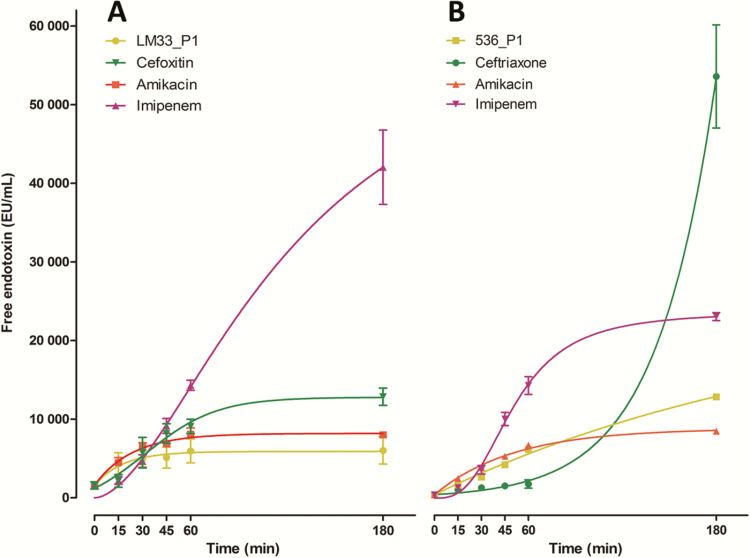
Phages Release as Much Endotoxin as Amikacin but Much Less Than β-Lactams
The levels of released endotoxin were measured over time for each antibacterial agent tested (Figure 4). Initially (T0), the concentrations of free endotoxin in the medium were 1578 ± 652 and 430 ± 20 EU/mL for strains LM33 and 536, respectively. The ER according to time was not identical among the antibacterial agents tested (Figure 4). Whereas the ceftriaxone-associated ER was ruled by an exponential curve, amikacin and phages were ruled by a reverse exponential curve (a rapid rise followed by a plateau) and imipenem with cefoxitin by a sigmoid function. Particularly, the kinetic of ER was initially slow for ceftriaxone but increased suddenly at later time points, a phenomenon also present but to a weaker extent with imipenem.
Figure 4.
Concentration of free endotoxin released in the culture medium over time by cells exposed to different antibacterial agent. Strain LM33 (A) and strain 536 (B) were cultured in presence of phages (LM33_P1 or 536_P1, multiplicity of infection of 5) or antibiotics (8-fold the minimum inhibitory concentration). The symbols are the means of 2 independent experiments with standard deviation. The curves are nonlinear regressions from these points (see Methods). Abbreviation: EU, endotoxin unit.
Compared to T0, the fold change in the amount of endotoxin released by the lysis of strain LM33 after 180 minutes was 3.8 ± 0.03 with phage LM33_P1, 5.5 ± 2.0 with amikacin, 8.7 ± 2.6 with cefoxitin, and 30.0 ± 16.7 with imipenem. With strain 536, this fold change was 19.8 ± 0.1 with amikacin, 29.9 ± 1.3 with phage 536_P1, 53.7 ± 4.2 with imipenem, and 125.1 ± 27.5 with ceftriaxone.
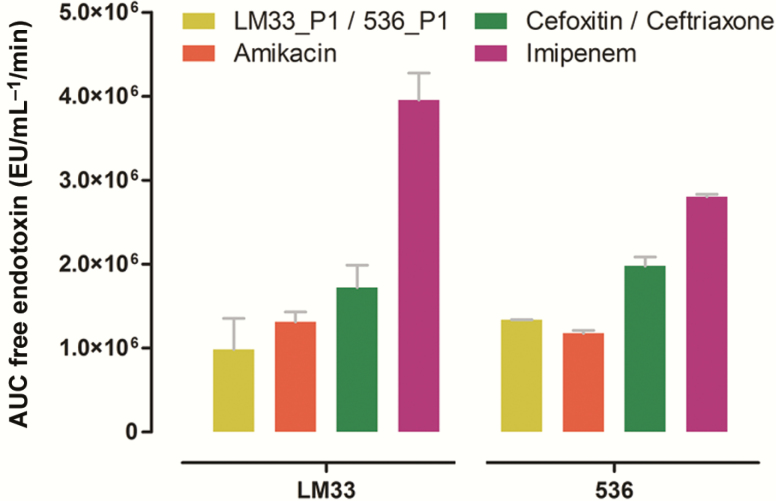
To obtain a more complete overview of the ER, we calculated the AUC that represent the cumulated amount of released endotoxin over time (Figure 5). The lowest recorded values were those obtained with the 2 phages and amikacin whereas the highest values were obtained with imipenem and ceftriaxone. In the experiments with strains LM33 and 536, respectively, the ER was 4- and 2-fold higher with imipenem than with the corresponding phage.
Figure 5.
Areas under the concentration of free endotoxin vs time curve (0 to 180 minutes). Strain LM33 (left side) and strain 536 (right side) were cultured in presence of phages (respectively, LM33_P1 and 536_P1, multiplicity of infection of 5) or antibiotics (8-fold the minimum inhibitory concentration). Data are means with standard deviation. Abbreviations: AUC, area under the curve; EU, endotoxin unit.
DISCUSSION
The safety of antimicrobial agents developed to resolve the progression of antibiotic resistance must be studied and compared to reference treatments. In this respect, the direct immunogenicity of phage particles involving innate immune response (ie, their own ability to trigger an acute inflammatory response) seems to be negligible [30–33] as long as phage preparations are correctly purified [34]. Conversely, the indirect immunogenicity (ie, triggered by cell lysis) is clearly understudied, emphasizing the significance of this work as the first comprehensive study assessing ER and cell killing by phages in comparison with antibiotics. We observed that the antibacterial effect of 2 different phages having a proven therapeutic potential [17, 18] was very close to the one obtained with amikacin, in terms of biomass reduction, speed of action, changes in cell morphology, and ER. In addition, when compared to β-lactams, these 2 phages showed a more rapid bacterial lysis, which was associated with a lower ER.
The mechanisms of action of each of the antibacterial agents tested are different (molecular targets and duration). Phages rely on holins and lysins (peptidoglycan hydrolases) to trigger cell lysis at the end of their infectious cycle [35], while amikacin is a protein synthesis inhibitor binding to the 16S ribosomal RNA of the 30S ribosome subunit. Ceftriaxone (a third-generation cephalosporin), imipenem (a carbapenem), and cefoxitin (a cephamycin) are all β-lactams inhibiting peptidoglycan synthesis by interacting with penicillin-binding proteins (PBPs). More precisely, ceftriaxone (Ro 13–9904) chiefly binds to PBP-3 [36] and induces a high rate of filamentation with delayed lysis and is considered to be a “potent” endotoxin releaser [11, 37]. Imipenem essentially binds to PBP-2, induces spheroplast formation [29], and is considered an “intermediate” endotoxin releaser [11]. Cefoxitin binding is more PBP-1 specific [38] and induces limited filamentation, and could therefore be considered a “weak” endotoxin releaser, like aminoglycosides [11].
Our microscope observations are in agreement with these specific mechanisms of action and the existing data regarding antibiotic-induced changes in cell morphology. Interestingly, phage- and amikacin-treated cells displayed similar inclusion bodies, previously reported when E. coli is exposed to aminoglycosides [39, 40]. We did not find in the literature similar intracellular changes in cells submitted to phage predation. However, as these inclusion bodies are related to protein aggregates induced by translation errors and given that phages are known to interfere with bacterial protein synthesis during the viral cycle, those may have the same origin [41]. More interesting is the putative link between these observations and the lowest levels of endotoxin released by either phage- or amikacin- treated cells. By rapidly turning off the cell growth, in contrast to β-lactams, both phages and amikacin avoid an increase in abnormal cell biomass (eg, with filamentous cells) that will lyse sooner or later, and will release an endotoxin amount proportional to its biomass.
Another key observation is the prompt action of phages. Despite a much more complex and a highly temporally regulated mode of action aiming at hijacking multiple intracellular pathways [42], strictly lytic phages paradoxically appear to be faster than β-lactams in vitro. Among the highly diverse population of phages, this rapidity is a common trait even if important differences exist in terms of cell lysis speed (ranging from <15 minutes to >1 hour, at 37°C [43]). We believe that the 2 phages tested here are particularly good candidates regarding this point.
Several limits have to be discussed in our study. First, our results are related to a limited number of phages and antibiotics. Here, both phages included in this study have favorable kinetic parameters. As the length of the viral cycle is one of the key parameters in the phage pharmacokinetic modeling [44, 45], we cannot rule out the possibility that slower phages could have led to different results. Moreover, we deliberately chose to focus on β-lactams and aminoglycosides because these 2 classes of antibiotics belong to the first-line treatment of many infections in their severe forms (such as pyelonephritis [46]). As “solid value” molecules, they are recommended for the treatment of life-threatening gram-negative infections, especially in neutropenic patients [47, 48]. Other than these valid comparators, what would have been observed if others drugs had been tested (such as quinolones) is open to speculation. Finally, the translation of our results to the in vivo setting could be limited: (1) A possible overestimation of aminoglycosides efficacy may be present in time-kill curve experiments with strain 536 due to antibiotic carryover, as the low number of viable cells required us to plate undiluted samples; (2) in vivo, an additional key player represented by the innate immune system (mainly with polymorphonuclears) is involved in bacterial cell lysis [49]. Additionally, the antibiotic tissue concentrations could be highly variable in the clinical setting among people and fluctuate over time [50]. Thus, periods of time with tissue concentration below the MIC could exist, changing the way cells are lysed.
To conclude, this work provides comforting data regarding the endotoxin-related safety of therapeutically relevant phages, characterized by their low endotoxin release profile and their fast bactericidal effect. An in vivo assessment of the endotoxin release has now to be carried out to confirm and strengthen our findings, which will support further phage therapy development.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Dwayne R. Roach for critical discussions. We warmly thank Yvonne Cloarec for English language revisions and Olivier Sauvage for his helpful contributions using MatLab software.
Financial support. This work was supported by a joint research grant from both Institut Pasteur and Assistance Publique–Hôpitaux de Paris (Poste d’Accueil pour Praticien Hospitalier).
Potential conflicts of interest. L. D. has received grants and personal fees from Ferring SA. J.-D. R. has received coverage of travel expenses to attend scientific meetings from Fisher & Paykel. All other authors report no potential conflicts.All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Implementation of the global action plan on antimicrobial resistance. WHO GAP AMR Newsletter. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2. Kingwell K. Bacteriophage therapies re-enter clinical trials. Nat Rev Drug Discov 2015; 14:515–6. [DOI] [PubMed] [Google Scholar]
- 3. Wright A, Hawkins CH, Anggård EE, Harper DR. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin Otolaryngol 2009; 34:349–57. [DOI] [PubMed] [Google Scholar]
- 4. Sarker SA, Sultana S, Reuteler G, et al. Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: a randomized trial in children from Bangladesh. EBioMedicine 2016; 4:124–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Viertel TM, Ritter K, Horz HP. Viruses versus bacteria-novel approaches to phage therapy as a tool against multidrug-resistant pathogens. J Antimicrob Chemother 2014; 69:2326–36. [DOI] [PubMed] [Google Scholar]
- 6. Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy. Bacteriophage 2011; 1:111–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Remick DG. Pathophysiology of sepsis. Am J Pathol 2007; 170:1435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med 1993; 119:771–8. [DOI] [PubMed] [Google Scholar]
- 9. Marshall JC, Foster D, Vincent JL, et al. ; MEDIC study Diagnostic and prognostic implications of endotoxemia in critical illness: results of the MEDIC study. J Infect Dis 2004; 190:527–34. [DOI] [PubMed] [Google Scholar]
- 10. Opal SM, Scannon PJ, Vincent JL, et al. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J Infect Dis 1999; 180:1584–9. [DOI] [PubMed] [Google Scholar]
- 11. Lepper PM, Held TK, Schneider EM, Bölke E, Gerlach H, Trautmann M. Clinical implications of antibiotic-induced endotoxin release in septic shock. Intensive Care Med 2002; 28:824–33. [DOI] [PubMed] [Google Scholar]
- 12. Simpson AJ, Opal SM, Angus BJ, et al. Differential antibiotic-induced endotoxin release in severe melioidosis. J Infect Dis 2000; 181:1014–9. [DOI] [PubMed] [Google Scholar]
- 13. Prins JM, van Agtmael MA, Kuijper EJ, van Deventer SJ, Speelman P. Antibiotic-induced endotoxin release in patients with gram-negative urosepsis: a double-blind study comparing imipenem and ceftazidime. J Infect Dis 1995; 172:886–91. [DOI] [PubMed] [Google Scholar]
- 14. Matsuda T, Freeman TA, Hilbert DW, et al. Lysis-deficient bacteriophage therapy decreases endotoxin and inflammatory mediator release and improves survival in a murine peritonitis model. Surgery 2005; 137:639–46. [DOI] [PubMed] [Google Scholar]
- 15. Hagens S, Habel A, von Ahsen U, von Gabain A, Bläsi U. Therapy of experimental Pseudomonas infections with a nonreplicating genetically modified phage. Antimicrob Agents Chemother 2004; 48:3817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brzuszkiewicz E, Bruggemann H, Liesegang H, et al. How to become a uropathogen: comparative genomic analysis of extraintestinal pathogenic Escherichia coli strains. Proc Natl Acad Sci U S A 2006; 103:12879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dufour N, Clermont O, La Combe B, et al. ; ColoColi Group Bacteriophage LM33_P1, a fast-acting weapon against the pandemic ST131-O25b:H4 Escherichia coli clonal complex. J Antimicrob Chemother 2016; 71:3072–80. [DOI] [PubMed] [Google Scholar]
- 18. Dufour N, Debarbieux L, Fromentin M, Ricard JD. Treatment of highly virulent extraintestinal pathogenic Escherichia coli pneumonia with bacteriophages. Crit Care Med 2015; 43:e190–8. [DOI] [PubMed] [Google Scholar]
- 19. Henry M, Lavigne R, Debarbieux L. Predicting in vivo efficacy of therapeutic bacteriophages used to treat pulmonary infections. Antimicrob Agents Chemother 2013; 57:5961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Craig WA, Ebert SC. Killing and regrowth of bacteria in vitro: a review. Scand J Infect Dis Suppl 1990; 74:63–70. [PubMed] [Google Scholar]
- 21. Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 1987; 155:93–9. [DOI] [PubMed] [Google Scholar]
- 22. Turnidge JD. The pharmacodynamics of beta-lactams. Clin Infect Dis 1998; 27:10–22. [DOI] [PubMed] [Google Scholar]
- 23. Zelenitsky SA, Harding GK, Sun S, Ubhi K, Ariano RE. Treatment and outcome of Pseudomonas aeruginosa bacteraemia: an antibiotic pharmacodynamic analysis. J Antimicrob Chemother 2003; 52:668–74. [DOI] [PubMed] [Google Scholar]
- 24. Taccone FS, Hites M, Beumier M, Scolletta S, Jacobs F. Appropriate antibiotic dosage levels in the treatment of severe sepsis and septic shock. Curr Infect Dis Rep 2011; 13:406–15. [DOI] [PubMed] [Google Scholar]
- 25. Debarbieux L, Leduc D, Maura D, et al. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis 2010; 201:1096–104. [DOI] [PubMed] [Google Scholar]
- 26. Leber A. 5.14 tests to assess bactericidal activity. In: Leber AL. Clinical Microbiology Procedures Handbook, 4th ed. Washington, DC: ASM Press, 2016. [Google Scholar]
- 27. Lewis K. Persister cells. Annu Rev Microbiol 2010; 64:357–72. [DOI] [PubMed] [Google Scholar]
- 28. European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 6.0, 2016. Available at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf. Accessed 15 June 2016. [Google Scholar]
- 29. Hanberger H, Nilsson LE, Nilsson M, Maller R. Post-antibiotic effect of beta-lactam antibiotics on gram-negative bacteria in relation to morphology, initial killing and MIC. Eur J Clin Microbiol Infect Dis 1991; 10:927–34. [DOI] [PubMed] [Google Scholar]
- 30. Bruttin A, Brüssow H. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob Agents Chemother 2005; 49:2874–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCallin S, Alam Sarker S, Barretto C, et al. Safety analysis of a Russian phage cocktail: from metagenomic analysis to oral application in healthy human subjects. Virology 2013; 443:187–96. [DOI] [PubMed] [Google Scholar]
- 32. Międzybrodzki R, Borysowski J, Weber-Dąbrowska B, et al. Clinical aspects of phage therapy. Adv Virus Res 2012; 83:73–121. [DOI] [PubMed] [Google Scholar]
- 33. Miernikiewicz P, Dąbrowska K, Piotrowicz A, et al. T4 phage and its head surface proteins do not stimulate inflammatory mediator production. PLoS One 2013; 8:e71036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dufour N, Henry M, Ricard JD, Debarbieux L. Commentary: morphologically distinct Escherichia coli bacteriophages differ in their efficacy and ability to stimulate cytokine release in vitro. Front Microbiol 2016; 7:1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Young R. Phage lysis: three steps, three choices, one outcome. J Microbiol 2014; 52:243–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wright RB, Makover SD, Telep E. Ro 13-9904: affinity for penicillin binding proteins and effect on cell wall synthesis. J Antibiot (Tokyo) 1981; 34:590–5. [DOI] [PubMed] [Google Scholar]
- 37. Gould IM, MacKenzie FM. The response of Enterobacteriaceae to beta-lactam antibiotics—“round forms, filaments and the root of all evil.” J Antimicrob Chemother 1997; 40:495–9. [DOI] [PubMed] [Google Scholar]
- 38. Fontana R, Cornaglia G, Ligozzi M, Mazzariol A. The final goal: penicillin-binding proteins and the target of cephalosporins. Clin Microbiol Infect 2000; 6(suppl 3):34–40. [DOI] [PubMed] [Google Scholar]
- 39. Lindner AB, Madden R, Demarez A, Stewart EJ, Taddei F. Asymmetric segregation of protein aggregates is associated with cellular aging and rejuvenation. Proc Natl Acad Sci U S A 2008; 105:3076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bednarska NG, Schymkowitz J, Rousseau F, Van Eldere J. Protein aggregation in bacteria: the thin boundary between functionality and toxicity. Microbiology 2013; 159:1795–806. [DOI] [PubMed] [Google Scholar]
- 41. Drulis-Kawa Z Majkowska-Skrobek G Maciejewska B Delattre AS, Lavigne R. Learning from bacteriophages—advantages and limitations of phage and phage-encoded protein applications. Curr Protein Pept Sci 2012; 13:699–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chevallereau A, Blasdel BG, De Smet J, et al. Next-generation “-omics” approaches reveal a massive alteration of host RNA metabolism during bacteriophage infection of Pseudomonas aeruginosa. PLoS Genet 2016; 12:e1006134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Paepe M, Taddei F. Viruses’ life history: towards a mechanistic basis of a trade-off between survival and reproduction among phages. PLoS Biol 2006; 4:e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cairns BJ, Timms AR, Jansen VA, Connerton IF, Payne RJ. Quantitative models of in vitro bacteriophage-host dynamics and their application to phage therapy. PLoS Pathog 2009; 5:e1000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Paepe M, Tournier L, Moncaut E, Son O, Langella P, Petit MA. Carriage of λ latent virus is costly for its bacterial host due to frequent reactivation in monoxenic mouse intestine. PLoS Genet 2016; 12:e1005861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52:e103–20. [DOI] [PubMed] [Google Scholar]
- 47. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637. [DOI] [PubMed] [Google Scholar]
- 48. Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev 2012; 25:450–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun 2009; 77:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient—concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 2014; 77:3–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.