Summary
This is the largest collection of enteric fever treatment data. The results, from trials conducted in Nepal since 2005, confirm that fluoroquinolones are failing for enteric fever treatment. The World Health Organization enteric fever treatment guidelines should be modified.
Keywords: antimicrobial resistance, typhoid, enteric fever, Nepal, fluoroquinolone
Abstract
Background.
Enteric fever, caused by Salmonella Typhi and Salmonella Paratyphi A, is the leading cause of bacterial febrile disease in South Asia.
Methods.
Individual data from 2092 patients with enteric fever randomized into 4 trials in Kathmandu, Nepal, were pooled. All trials compared gatifloxacin with 1 of the following comparator drugs: cefixime, chloramphenicol, ofloxacin, or ceftriaxone. Treatment outcomes were evaluated according to antimicrobial if S. Typhi/Paratyphi were isolated from blood. We additionally investigated the impact of changing bacterial antimicrobial susceptibility on outcome.
Results.
Overall, 855 (41%) patients had either S. Typhi (n = 581, 28%) or S. Paratyphi A (n = 274, 13%) cultured from blood. There were 139 (6.6%) treatment failures with 1 death. Except for the last trial with ceftriaxone, the fluoroquinolone gatifloxacin was associated with equivalent or better fever clearance times and lower treatment failure rates in comparison to all other antimicrobials. However, we additionally found that the minimum inhibitory concentrations (MICs) against fluoroquinolones have risen significantly since 2005 and were associated with increasing fever clearance times. Notably, all organisms were susceptible to ceftriaxone throughout the study period (2005–2014), and the MICs against azithromycin declined, confirming the utility of these alternative drugs for enteric fever treatment.
Conclusion.
The World Health Organization and local government health ministries in South Asia still recommend fluoroquinolones for enteric fever. This policy should change based on the evidence provided here. Rapid diagnostics are urgently required given the large numbers of suspected enteric fever patients with a negative culture.
Enteric (typhoid) fever is a systemic infection caused by the Salmonella enterica serovars Typhi and Paratyphi A, B, and C. Enteric fever is a significant cause of morbidity and mortality in low-income regions [1] and was responsible for an estimated 12.2 million disability-adjusted life-years and >190 000 deaths globally in 2010 [2]. The fatality rate of enteric fever is low (<1%) but is higher when antimicrobial therapy is delayed or unavailable [3]. Therefore, antimicrobials are essential for the clinical management of enteric fever. Chloramphenicol, ampicillin, and cotrimoxazole were first-line treatments for enteric fever until the early 1990s when the increasing incidence of multidrug-resistant (MDR; defined as resistance to these 3 antimicrobial drugs) S. Typhi organisms led to the use of fluoroquinolones [4, 5]. Yet, organisms with reduced susceptibility against fluoroquinolones became a problem in Asia soon after their introduction [6, 7]. Recent phylogeographic analyses that document an ongoing epidemic of a global antimicrobial resistance (AMR) S. Typhi lineage suggest that the potential for regional or global dispersal of a lineage exhibits resistance to fluoroquinolones is now a real threat [8–10]. In the absence of effective and accessible vaccines and lack of sanitation improvements, development of tailored antimicrobial therapy recommendations is critical to reduce morbidity and prevent disease transmission.
In Kathmandu, Nepal, S. Typhi and S. Paratyphi A are the most commonly isolated organisms from the blood of febrile adults and children [11, 12]. Over the last decade we conducted 4 randomized, controlled trials (RCTs) to evaluate enteric fever treatment in this endemic region [13–16]. Our aim in this study was to use the largest collection of individual patient data assembled to date from enteric fever treatment trials to evaluate the effect of treatment drug on differences in clinical outcome between S. Typhi and S. Paratyphi A infections and those with blood culture-negative enteric fever. We further sought to compare the antimicrobial susceptibility profiles over time between S. Typhi and S. Paratyphi A isolates and to investigate their impact on outcome. An in-depth understanding of trends and clinical implications of AMR enteric fever should guide policymakers and clinicians in decisions regarding treatment in an era of rapidly diminishing therapeutic options.
MethodS
Ethical Approval
Written informed consent, which was required for participation in all trials, was provided by a parent or adult guardian if a patient was aged <18 years. The Nepal Health Research Council Ethics Committee and the Oxford Tropical Research Ethics Committee of the United Kingdom provided ethical approval for all 4 studies.
Patient Populations and Study Procedures
Individual patient data for this study were derived from 4 RCTs conducted at Patan Hospital in Kathmandu, Nepal, between 2005 and 2014, the methods and results of which have been described previously [13–16]. Patients who presented to the outpatient or emergency department with fever lasting longer than 3 days with a clinical diagnosis of enteric fever (undifferentiated fever >38°C with no focus of infection) were eligible. Patients were excluded if they were pregnant or lactating, were aged <2 years or weighed <10 kg, showed any signs of complications (jaundice, shock, gastrointestinal bleeding), showed hypersensitivity to the relevant trial drugs, or had been treated with a study drug in the week prior to going to the hospital. The study procedures between the 4 trials were comparable; however, there were several minor protocol differences between studies (outlined in Supplementary Table 1).
Patients were randomly assigned to 1 of 2 arms in each trial. Each trial was composed of a gatifloxacin arm (10 mg/kg/day, single dose orally for 7 days) and a comparator arm, which was cefixime (20 mg/kg/day, 2 doses orally for 7 days) [13], chloramphenicol (75 mg/kg/day, 4 divided oral doses for 14 days) [14], ofloxacin (20 mg/kg/day, 2 divided oral doses for 7 days) [15], or ceftriaxone (intravenous; 60 mg/kg for patients aged 2–13 years or 2 g for patients aged ≥14 years) [16]. Gatifloxacin was the constant comparator because it is inexpensive and given once daily.
Fever clearance time (FCT) was defined as the time from the first dose of a study drug until the temperature dropped to ≤37.5°C and remained below this temperature for at least 2 days. The composite endpoint treatment failure summarized unfavorable outcomes and was defined as the occurrence of at least 1 of the following: persistent fever (FCT of more than 7 days [trials 1 and 4] or more than 10 days [trials 2 and 3] after treatment initiation), the need for rescue treatment, microbiological failure (blood culture positive for Salmonella) on day 8, relapse or disease-related complications within 31 days of treatment initiation, or death. Blood was taken from all patients for microbiological culture on enrollment and on day 8 for culture-positive individuals or those with a potential relapse.
Microbiological investigations have been described previously [13–16]. Blood samples from adult patients were inoculated into media containing tryptone soya broth and sodium polyanethol sulfonate. For children, BacTEC Ped Plus/F bottles were used. Positive bottles were cultured onto MacConkey agar and presumptive Salmonella colonies were identified using biochemical tests and serotype-specific antisera. During all 4 trials, minimum inhibitory concentrations (MICs) were determined against the following antimicrobials unless otherwise noted: Augmentin, ampicillin, amoxicillin, azithromycin (2006–2011), cefixime (2005), chloramphenicol, ciprofloxacin (2006–2014), ceftriaxone, gatifloxacin, nalidixic acid, ofloxacin (2006–2014), and cotrimoxazole (2006–2009, 2011–2014), and against tetracycline by E-test (AB Biodisk, Sweden).
Statistical Analyses
Data from the trials were combined and analyzed using Stata (v 13.1; College Station, Texas). Plots were drawn in R v3.1.1 (R Foundation, Vienna, Austria) using the ggplot2 package. Demographics and clinical variables were tabulated and compared between serovars. Comparisons of clinical parameters between patient populations were structured as logistic regressions with the patient population (either culture positive/negative or S. Typhi/S. Paratyphi A) as the main covariate and adjustment for age stratum (binary: <16 years/≥16 years). Multivariable models with random effects were fitted to adjust for study heterogeneity as follows: FCT was evaluated using Kaplan-Meier estimates and Cox proportional hazard models with treatment group and age as covariates; logistic regression was used to determine the odds of treatment failure between treatment arms, controlling for age; and linear regression was used to evaluate the relationship between FCT and log2 MIC, also controlling for age. Generalized additive models (GAMs; identity link, cubic spline) were used to examine potential nonlinear trends of MIC over time.
RESULTS
Baseline Characteristics
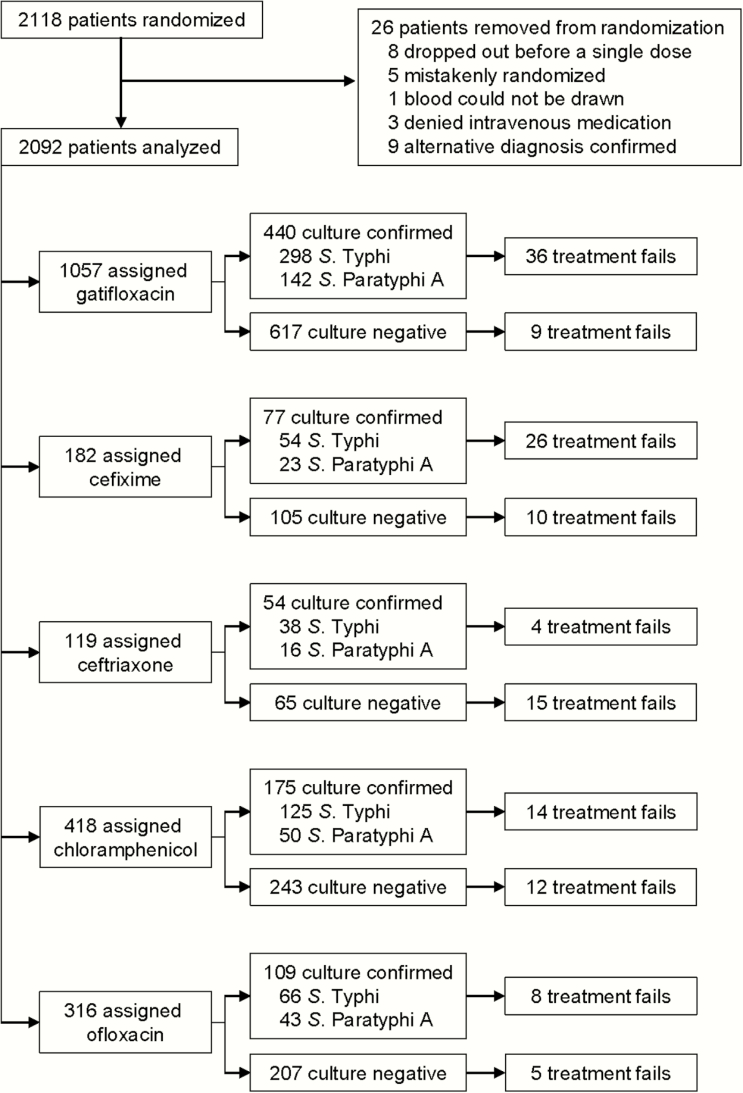
Between 2005 and 2014 there were 2118 patients with clinically suspected enteric fever randomized into 4 trials; data from 2092 (99%) patients were evaluated (Figure 1). Of these, 855 (41%) were culture positive for either S. Typhi (n = 581, 28%) or S. Paratyphi A (n = 274, 13%). Throughout the study period there were 139 (6.6%) treatment failures including 1 death. The median patient age was 17 years (interquartile range [IQR], 10–23); 66% were male (Table 1). There was no significant difference in age between the culture-negative and culture-positive patients; however, S. Typhi patients were significantly younger (median, 16 years; IQR, 9–21) than S. Paratyphi A patients (median, 19.5 years; IQR, 13–24) (P < .001) (Table 2). There was no difference in the sex distribution between culture-positive/culture-negative and S. Typhi/S. Paratyphi A populations (Table 2).
Figure 1.
Enrollment of patients into enteric fever treatment trials in Nepal. Flow chart showing enrollment of patients into the 4 individual, randomized, controlled trials according to antimicrobial treatment and blood culture result.
Table 1.
Baseline Characteristics of Patients Enrolled in 4 Enteric Fever Treatment Trials
| Characteristic | Trial 1 | Trial 2 | Trial 3 | Trial 4 | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | n (%) or Median (IQR) | N | n (%) or Median (IQR) | N | n (%) or Median (IQR) | N | n (%) or Median (IQR) | N | n (%) or Median (IQR) | |
| Age (y) | 382 | 17 (9–23) | 844 | 16 (9–22) | 623 | 17 (9–23) | 239 | 19 (15–23) | 2088 | 17 (10–23) |
| Male sex | 382 | 247 (64.7) | 844 | 540 (64.0) | 627 | 406 (64.8) | 239 | 180 (75.3) | 2092 | 1373 (65.6) |
| Weight (kg) | 382 | 45 (24–53) | 842 | 42 (21–52) | 627 | 45 (25–54) | 237 | 50 (40–56) | 2088 | 45 (24–53) |
| Duration of illness before admission (days) | 382 | 5 (3–6) | 844 | 5 (4–7) | 625 | 5 (4–7) | 180 | 5 (4–7) | 2031 | 5 (4–7) |
| Treatment with antimicrobials in the past 2 weeks | 379 | 238 (62.8) | 724 | 694 (95.9) | 623 | 428 (68.7) | 210 | 109 (51.9) | 1936 | 1469 (75.9) |
| Previous history of typhoid | 382 | 61 (16.0) | 844 | 138 (16.4) | 626 | 103 (16.5) | 238 | 37 (15.5) | 2090 | 339 (16.2) |
| Family history of typhoid | 382 | 62 (16.2) | 844 | 140 (16.6) | 625 | 164 (26.2) | 239 | 35 (14.6) | 2090 | 401 (19.2) |
| Typhoid vaccination | 382 | 2 (0.5) | 844 | 0 (0) | 625 | 0 (0) | 238 | 11 (4.6) | 2089 | 13 (0.6) |
| Temperature at admission (°C) | 379 | 38.9 (38.3–39.5) | 844 | 38.9 (38.2–39.4) | 626 | 38.6 (38.2–39.0) | 235 | 38.8 (38.3–39.4) | 2084 | 38.8 (38.2–39.4) |
| Headache | 382 | 370 (96.9) | 844 | 749 (88.7) | 627 | 541 (86.3) | 239 | 211 (88.3) | 2092 | 1871 (89.4) |
| Anorexia | 382 | 289 (75.7) | 844 | 632 (74.9) | 627 | 455 (72.6) | 239 | 173 (72.4) | 2092 | 1549 (74.0) |
| Abdominal pain | 382 | 32 (8.4) | 844 | 33 (3.9) | 626 | 25 (4.0) | 235 | 62 (26.4) | 2087 | 152 (7.3) |
| Cough | 382 | 142 (37.2) | 844 | 277 (32.8) | 627 | 246 (39.2) | 239 | 91 (38.1) | 2092 | 756 (36.1) |
| Nausea | 382 | 132 (34.6) | 844 | 258 (30.6) | 627 | 174 (27.8) | 239 | 124 (51.9) | 2092 | 688 (32.9) |
| Vomiting | 382 | 57 (14.9) | 844 | 172 (20.4) | 627 | 118 (18.8) | 239 | 69 (28.9) | 2092 | 416 (19.9) |
| Diarrhea | 382 | 86 (22.5) | 844 | 161 (19.1) | 627 | 105 (16.7) | 239 | 59 (24.7) | 2092 | 411 (19.6) |
| Constipation | 382 | 41 (10.7) | 844 | 105 (12.4) | 627 | 79 (12.6) | 239 | 31 (13.0) | 2092 | 256 (12.2) |
| Hepatomegaly | 382 | 19 (5.0) | 844 | 113 (13.4) | 626 | 7 (1.1) | 231 | 0 (0) | 2083 | 139 (6.7) |
| Splenomegaly | 382 | 35 (9.2) | 844 | 119 (14.1) | 626 | 6 (1.0) | 231 | 2 (0.9) | 2083 | 162 (7.8) |
| Haematocrit (%) | 370 | 40 (37–44) | 831 | 39 (36–43) | 624 | 38 (36–42) | 235 | 39 (36–43) | 2060 | 39 (36–43) |
| Leucocyte count (×109/L) | 370 | 7.0 (5.5–9.0) | 831 | 6.3 (5.0–8.1) | 624 | 6.0 (4.8–7.7) | 239 | 5.9 (4.7–7.3) | 2064 | 6.3 (5.0–8.0) |
| Platelet count (×109/L) | 356 | 190 (160–235) | 800 | 190 (164–226) | 615 | 174 (145–216) | 239 | 168 (150–209) | 2010 | 184 (153–220) |
| AST (U/L) | 373 | 47 (36–62) | 835 | 45 (34–61) | 624 | 47 (34–67) | 233 | 49 (36–70) | 2065 | 46 (35–65) |
| ALT (U/L) | 373 | 33 (24–48) | 836 | 29 (20–43) | 624 | 37 (28–53) | 234 | 45 (31–63) | 2067 | 34 (24–50) |
| Salmonella Typhi isolated | 382 | 119 (31.2) | 844 | 249 (29.5) | 627 | 132 (21.1) | 239 | 81 (33.9) | 2092 | 581 (27.8) |
| S. Paratyphi A isolated | 382 | 50 (13.1) | 844 | 103 (12.2) | 627 | 86 (13.7) | 239 | 35 (14.6) | 2092 | 274 (13.1) |
| No growth or culture negative | 382 | 213 (55.8) | 844 | 492 (58.3) | 627 | 409 (65.2) | 239 | 123 (51.5) | 2092 | 1237 (59.1) |
Table 2.
Demographic and Clinical Characteristics of Culture-Negative, Culture-Positive, Salmonella Typhi, and S Paratyphi A Patients
| Characteristic | Culture Negative | Culture Positive | P Valuea | S. Typhi | S. Paratyphi A | P Valuea | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | n (%) or Median (IQR) | N | n (%) or Median (IQR) | N | n (%) or Median (IQR) | N | n (%) or Median (IQR) | |||
| Age (y)b | 1236 | 17 (9–24) | 852 | 17 (10–22) | .692 | 578 | 16 (9–21) | 274 | 19.5 (13–24) | <.001 |
| Male sexb | 1237 | 818 (66.1) | 855 | 555 (64.9) | .565 | 581 | 373 (64.2) | 274 | 182 (66.4) | .525 |
| Weight (kg) | 1234 | 44 (23–54) | 854 | 46 (25–53) | .854 | 580 | 43.5 (22–52) | 274 | 49 (38–55) | <.001 |
| Duration of illness before admission (days) | 1203 | 5 (4–7) | 828 | 5 (4–7) | .500 | 565 | 5 (4–7) | 263 | 5 (4–6) | .102 |
| Treatment with antimicrobials in the past 2 weeks | 1146 | 861 (75.1) | 790 | 608 (77.0) | .330 | 532 | 414 (77.8) | 258 | 194 (75.2) | .440 |
| Previous history of typhoid | 1236 | 208 (16.8) | 854 | 131 (15.3) | .276 | 581 | 68 (11.7) | 273 | 63 (23.1) | <.001 |
| Family history of typhoid | 1236 | 242 (19.6) | 854 | 159 (18.6) | .657 | 580 | 107 (18.4) | 274 | 52 (19.0) | .400 |
| Typhoid vaccination | 1234 | 9 (0.7) | 855 | 4 (0.5) | .511 | 581 | 1 (0.2) | 274 | 3 (1.1) | .073 |
| Temperature at admission (°C) | 1233 | 38.7 (38.1–39.2) | 851 | 39 (38.4–39.5) | <.001 | 577 | 39 (38.5–39.5) | 274 | 38.8 (38.2–39.2) | <.001 |
| Headache | 1237 | 1098 (88.8) | 855 | 773 (90.4) | .348 | 581 | 518 (89.2) | 274 | 255 (93.1) | .237 |
| Anorexia | 1237 | 903 (73.0) | 855 | 646 (75.6) | .190 | 581 | 451 (77.6) | 274 | 195 (71.2) | .036 |
| Abdominal pain | 1237 | 479 (38.7) | 855 | 258 (30.2) | .067 | 581 | 261 (44.9) | 274 | 97 (35.4) | .061 |
| Cough | 1237 | 495 (40.0) | 855 | 261 (30.5) | <.001 | 581 | 193 (33.2) | 274 | 68 (24.8) | .011 |
| Nausea | 1237 | 394 (31.9) | 855 | 294 (34.4) | .310 | 581 | 198 (34.1) | 274 | 96 (35.0) | .853 |
| Vomiting | 1237 | 271 (21.9) | 855 | 145 (17.0) | .010 | 581 | 106 (18.2) | 274 | 39 (14.2) | .324 |
| Diarrhea | 1237 | 210 (17.0) | 855 | 201 (23.5) | <.001 | 581 | 161 (27.7) | 274 | 40 (14.6) | <.001 |
| Constipation | 1237 | 154 (12.4) | 855 | 102 (11.9) | .775 | 581 | 63 (10.8) | 274 | 39 (14.2) | .114 |
| Hepatomegaly | 1234 | 84 (6.8) | 849 | 55 (6.5) | .847 | 578 | 40 (6.9) | 271 | 15 (5.5) | .804 |
| Splenomegaly | 1234 | 85 (6.9) | 849 | 77 (9.1) | .069 | 578 | 48 (8.3) | 271 | 29 (10.7) | .224 |
| Hematocrit (%) | 1219 | 39 (36–43) | 841 | 39 (36–43) | .573 | 569 | 39 (35–43) | 272 | 40 (37–44) | .006 |
| Leucocyte count (×109/L) | 1220 | 6.4 (5.0–8.6) | 844 | 6.1 (4.9–7.5) | <.001 | 572 | 6.2 (4.9–7.5) | 272 | 5.8 (4.8–7.2) | .528 |
| Platelet count (×109/L) | 1187 | 187 (157–229) | 823 | 180 (150–210) | .002 | 555 | 180 (151–214) | 268 | 180 (150–210) | .469 |
| AST (U/L) | 1220 | 42 (32–59) | 845 | 51 (40–69) | <.001 | 573 | 54 (42–71) | 272 | 47 (37.5–66) | .023 |
| ALT (U/L) | 1220 | 31 (21–46.5) | 847 | 38 (28–53) | <.001 | 575 | 39 (28–53) | 272 | 36 (28–49.5) | .564 |
Abbreviations: IQR, interquartile range; AST, asparate aminotransaminase; ALT, alanine aminotransaminase.
a P values derived from logistic regression (categorical variables) or linear regression (continuous variables) with outcome characteristic of interest and a covariate of culture positivity or serovar, controlling for age (<15 years/≥16 years).
b P values derived using Fisher exact test for categorical data and the Kruskal-Wallis test for continuous data (not controlled for age).
There were several significant differences in clinical history between patient populations after controlling for age (Table 2). Culture-negative patients were significantly more likely to report coughing (40%) and vomiting (22%) than culture-positive patients (31% and 17%, respectively). Culture-positive patients, however, reported diarrhea (24%) more often than culture-negative patients (17%) in addition to a higher temperature (median, 39.0°C and 38.7°C, respectively). Among the culture-positive patients, those with an S. Typhi infection were significantly more likely to report a history of anorexia (78%), coughing (33%), and diarrhea (28%) compared to the S. Paratyphi A patients (71%, 25%, and 15%, respectively) and presented with higher temperatures (median, 39.0°C vs 38.8°C). Salmonella Paratyphi A patients were significantly more likely to report a history of previous typhoid illness (23%) compared to S. Typhi patients (12%). Additionally, there were several significant differences in hematology parameters between the culture-negative/culture-positive patients and the S. Typhi/S. Paratyphi A patients (Table 1), despite the majority of the values falling within normal ranges. Asparate aminotransaminase and alanine aminotransaminase were significantly elevated in the culture-positive patients (median, 51 U/L and median, 38 U/L, respectively) compared to culture-negative patients (median, 42 U/L and median, 31 U/L, respectively).
Treatment Failure
The number of patients who failed treatment in each treatment arm is shown in Table 3. Failure rates between antimicrobial treatment arms were largely similar when stratified by microbiological culture result, with a few notable exceptions. Compared to gatifloxacin, culture-positive patients were significantly more likely to fail treatment when administered cefixime (odds ratio [OR], 10.7; 95% confidence interval [CI], 3.72–30.61; P < .001). Culture-negative patients were more likely to fail with cefixime (OR, 7.13; 95% CI, 2.82–18.0; P < .001), ceftriaxone (OR, 19.3; 95% CI, 8.02–46.5; P < .001), and chloramphenicol (OR, 3.67; 95% CI, 1.52–8.86; P = .004) compared to gatifloxacin.
Table 3.
Proportion of Enteric Fever Patients With Treatment Failure by Culture Result and Treatment
| Treatment Arm | Culture Negative | Culture Positive | Salmonella Typhi | Salmonella Paratyphi A | ||||
|---|---|---|---|---|---|---|---|---|
| Total | n (%) | Total | n (%) | Total | n (%) | Total | n (%) | |
| Gatifloxacin | 617 | 9 (1.5) | 440 | 36 (8.2) | 298 | 26 (8.7) | 142 | 10 (7.0) |
| Cefixime | 105 | 10 (9.5) | 77 | 26 (33.8) | 54 | 19 (35.2) | 23 | 7 (30.4) |
| Ceftriaxone | 65 | 15 (23.1) | 54 | 4 (7.4) | 38 | 3 (7.9) | 16 | 1 (6.3) |
| Chloramphenicol | 243 | 12 (4.9) | 175 | 14 (8.0) | 125 | 11 (8.8) | 50 | 3 (6.0) |
| Ofloxacin | 207 | 5 (2.4) | 109 | 8 (7.3) | 66 | 7 (10.6) | 43 | 1 (2.3) |
Fever Clearance Times
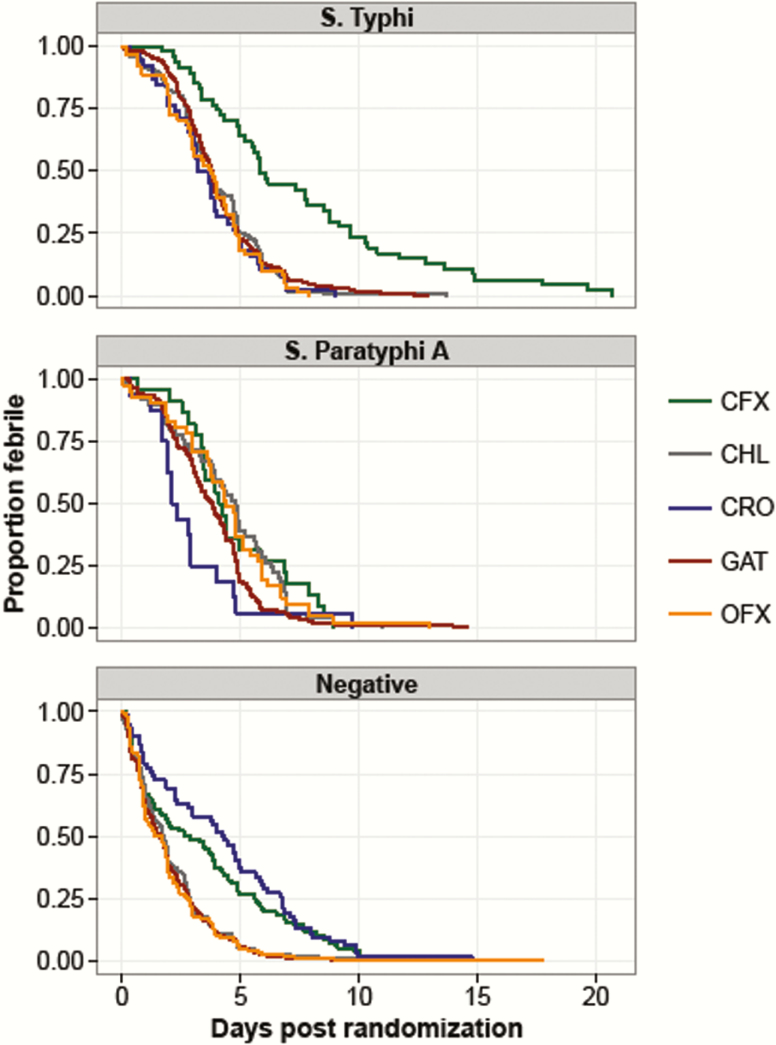
The FCTs of the various patient populations are shown in Figure 2 and Table 4. Among the culture-positive patient population, S. Typhi patients treated with cefixime (hazard ratio [HR], 0.36; 95% CI, 0.25–0.54; P < .001) and ceftriaxone (HR, 1.53; 95% CI,1.01–2.31; P = .043) had significantly longer FCTs than S. Typhi patients treated with gatifloxacin. In the culture-positive patients, those infected with S. Typhi also had significantly longer FCTs than S. Paratyphi A patients when treated with cefixime (HR, 2.18; 95% CI,1.25–3.80; P = .006; Table 4). However, S. Paratyphi A–infected patients had longer FCTs when treated with chloramphenicol compared to S. Typhi–infected patients (HR, 0.069; 95% CI, 0.49–0.97; P = .031). Compared to gatifloxacin, culture-negative patients fared significantly worse when treated with cefixime (HR, 0.56; 95% CI, 0.43–0.71; P < .001) and ceftriaxone (HR, 0.42, 95% CI, 0.31–0.57; P < .001).
Figure 2.
Fever clearance time (FCT) by treatment arm and culture result. FCT (in days) is shown for Salmonella Typhi, S. Paratyphi A, and culture-negative patients. Colors indicate the different treatment arms. Abbreviations: CFX, cefixime; CHL, chloramphenicol; CRO, ceftriaxone; GAT, gatifloxacin; OFX, ofloxacin.
Table 4.
Fever Clearance Time (in hours) for 4 Enteric Fever Patient Populations by Treatment
| Population | Culture Negative | Culture Positive | Salmonella Typhi | Salmonella Paratyphi A | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median FCT (IQR) | Range | N | Median FCT (IQR) | Range | N | Median FCT (IQR) | Range | N | Median FCT (IQR) | Range | |
| Overall | 1178 | 41.3 (18.2–71.3) | 1.0–425.5 | 810 | 92.7 (65.3–124.7) | 1.0–496.0 | 549 | 92.0 (66.4–125) | 1.0–496.0 | 261 | 94.4 (56.1–122.8) | 1.0–349.0 |
| Treatment arm | ||||||||||||
| GAT | 585 | 39.1 (17.0–68.0) | 1.0–285.9 | 416 | 90.9 (64.3–116.9) | 1.0–349.0 | 283 | 90.8 (67.4–117.3) | 1.0–309.6 | 133 | 91.9 (55.8–116.0) | 6.8–349.0 |
| CFX | 96 | 66.5 (18.5–134.5) | 4.0–324.0 | 69 | 134.0 (82.0–205.0) | 16.0–496.0 | 47 | 140.0 (96.0–232.0) | 40.0–496.0 | 22 | 100.0 (81.0–164.0) | 16.0–214.0 |
| CRO | 62 | 102.3 (31.5–161.5) | 1.0–354.3 | 54 | 73.5 (46.0–112.8) | 7.8–232.8 | 38 | 82.6 (54.0–117.5) | 7.8–215.4 | 16 | 53.1 (43.3–83.0) | 7.8–232.8 |
| CHL | 239 | 41.5 (20.2–68.7) | 1.0–304.5 | 169 | 94.2 (65.2–136.3) | 2.8–327.4 | 120 | 89.8 (65.2–121.7) | 2.8–327.4 | 49 | 114.7 (63.4–151.6) | 4.4–262.8 |
| OFX | 196 | 36.8 (17.9–66.4) | 1.0–425.5 | 102 | 94.8 (56.0–122.3) | 1.0–311.8 | 61 | 89.8 (48.0–115.4) | 3.6–189.8 | 41 | 104.4 (71.5–141.6) | 1.0–311.8 |
Abbreviations: CFX, cefixime; CHL, chloramphenicol; CRO, ceftriaxone; FCT, fever clearance time; GAT, gatifloxacin; IQR, interquartile range; OFX, ofloxacin.
Antimicrobial Susceptibility Trends
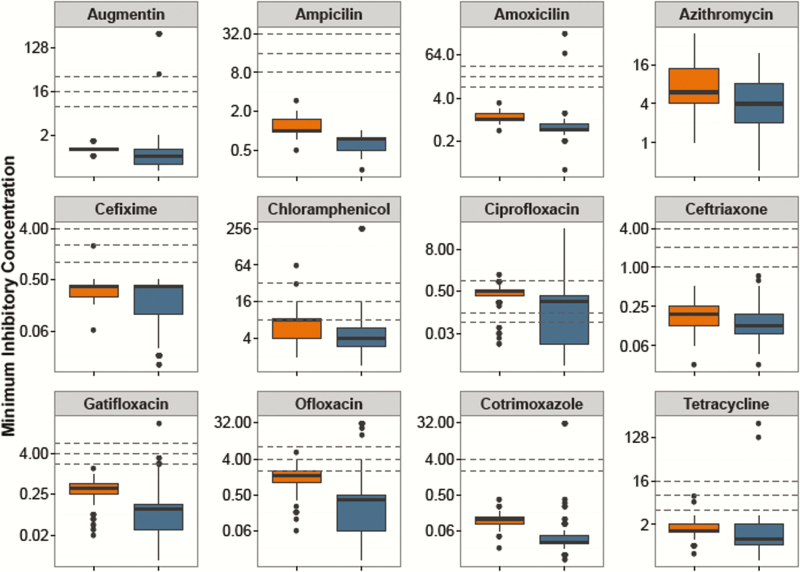
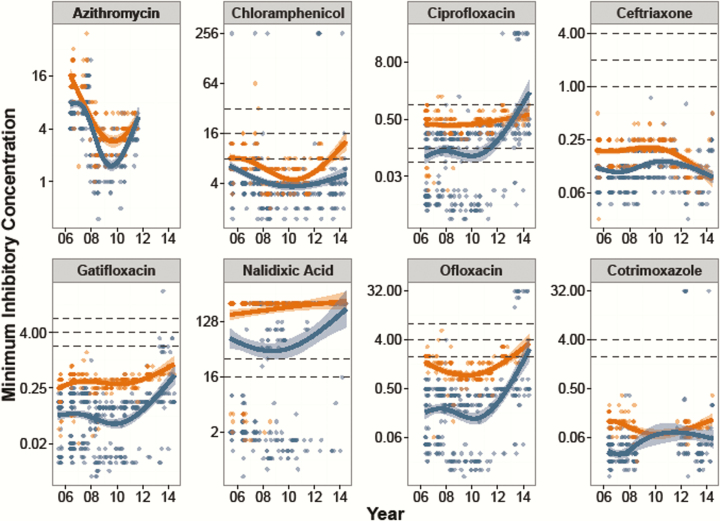
As shown in Figure 3, the MICs for S. Paratyphi A were significantly higher than those for S. Typhi with all antimicrobials (P < .001, Kruskal-Wallis), with the exception of cefixime (P = .375). Figure 4 shows the MIC time trends by serovar, which were significantly nonlinear over time for all antimicrobials in both serovars (GAM, P < .001 with the exception of S. Paratyphi A/ciprofloxacin [P = .052] and S. Paratyphi A/nalidixic acid [P = .003]). Most notably, the MICs against the fluoroquinolones rose significantly over time, and the MICs against azithromycin declined between 2007 and 2010. Last, all isolates were susceptible to ceftriaxone throughout the study period.
Figure 3.
Distribution of minimum inhibitory concentrations (MICs) against antimicrobials for Salmonella Typhi and S. Paratyphi A. MICs shown on a log2 scale against 12 antimicrobials for S. Typhi (blue) and S. Paratyphi A (orange). Lower, middle, and upper horizontal dashed lines represent the current Clinical and Laboratory Standards Institute cutoffs for susceptible/intermediate and intermediate/resistant, respectively.
Figure 4.
Minimum inhibitory concentrations (MICs) over time for Salmonella Typhi and S. Paratyphi A. MICs shown on a log2 scale for 8 antimicrobials over the period 2005–2014. Salmonella Typhi are shown in blue and S. Paratyphi A are shown in orange. The smoothed line is derived from the generalized additive model showing a nonlinear increase in MICs over time, with the shaded region showing the 95% confidence interval. Lower, middle, and upper horizontal dashed lines represent the current Clinical and Laboratory Standards Institute cutoffs for susceptible/intermediate and intermediate/resistant, respectively.
Impact of Antimicrobial Resistance on Clinical Outcomes
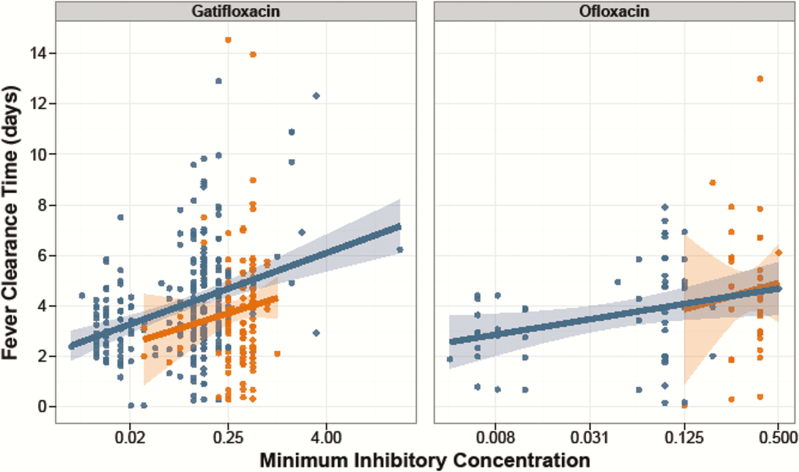
Increasing MICs against fluoroquinolones led to longer FCTs in S. Typhi patients. As shown in Figure 5, an increasing (log2) MIC was associated with longer FCTs in patients treated with gatifloxacin (number of hours increase in FCT for each 2-fold increase in MIC (β = 8.1; 95% CI, 5.3–10.8; P < .001) and ofloxacin (β = 8.4; 95% CI, 2.2–14.5; P = .008). Longer FCTs were also observed with increasing MICs against ciprofloxacin in S. Typhi patients treated with ofloxacin or gatifloxacin (β = 6.88; 95% CI, 4.9–8.9; P < .001). However, we found no significant association between FCT and (log2) MIC against the fluoroquinolones in the S. Paratyphi A patients (all P > .05). Additionally, there was no significant association between FCT and MIC for the other antimicrobials tested. Last, patients infected with an S. Typhi isolate that was nonsusceptible to ciprofloxacin (MIC ≥0.12 μg/mL) were more likely to experience treatment failure (29/211, 13.7%) when treated with ofloxacin or gatifloxacin compared to patients infected with S. Typhi organisms susceptible to ciprofloxacin (MIC < 0.12 μg/mL; 2/79, 2.5%; OR, 5.16; 95% CI, 1.1–23.2; P = .033). Conversely, we did not identify a similar relationship in those infected with S. Paratyphi A (8/149 [5.4%] vs 1/6 [16.7%]; OR, 0.32; 95% CI, 0.03–3.15; P = .329), the majority of which exhibited reduced susceptibility against ciprofloxacin (MIC ≥0.12 μg/mL; 211/221, 96%).
Figure 5.
Fever clearance time and minimum inhibitory concentrations (MICs) against fluoroquinolones for Salmonella Typhi and S. Paratyphi A. Fever clearance time (in days) is shown plotted against log2 MIC for gatifloxacin (left) and ofloxacin (right). Salmonella Typhi isolates are shown in blue and S. Paratyphi A isolates are shown in orange. The lines represent the best-fit linear model, with 95% confidence interval shown by the shaded region.
DISCUSSION
Enteric fever remains the leading cause of febrile bacterial illness in Kathmandu [12]. With alarming AMR rates, a lack of immunization as a public health tool, and slow sanitation improvements, tailored antimicrobial therapies for the prevailing AMR profiles are required. Using systematic, longitudinal, individual patient data, we identified dynamic antimicrobial susceptibility profiles among S. Typhi and S. Paratyphi A isolates and a trend of increasing fluoroquinolone MICs correlating with poor outcome. This phenomenon was particularly apparent among S. Typhi patients. Although ceftriaxone was effective in treating culture-confirmed enteric fever patients, we documented poor clinical response in culture-negative patients. These data suggest that careful consideration is required for antimicrobial therapy of patients with enteric fever. In addition, fluoroquinolones should not be recommended for empirical treatment of this infection in South Asia [17].
By combining the largest number of enteric fever patients from a single location, we were able to identify several notable differences in both clinical presentation and clinical response between S. Typhi and S. Paratyphi A patients. Previous work conducted at the same center showed the 2 serovars to be clinically indistinguishable [18]. We found that, after controlling for age, S. Typhi patients were more likely to report anorexia, diarrhea, and coughing and presented with a higher temperature.
The precise mechanism driving the variability in MICs over time for both S. Typhi and S. Paratyphi A against several antimicrobials throughout 2005–2014 is unknown but may be determined by local prescribing practices. This hypothesis is consistent with notable declines in MDR organisms in both Nepal and India after fluoroquinolones became the first choice of treatment [12, 19, 20]. However, we predict a rapid rebound of MDR organisms with reversion to the prescribing of first-line antimicrobials due to the circulation of MDR plasmids in S. Typhi and other organisms [8, 21].
Our study period captured dynamic changes in MICs against fluoroquinolones, particularly among S. Typhi isolates in more recent years. Through whole genome sequencing, we have determined that this rise in MIC is associated with the emergence of an H58 variant with mutations in the DNA gyrase gene (gyrA) and the DNA topoisomerase IV gene (parC) [10, 16]. Supporting these findings, we can conclusively show that FCTs and the rate of treatment failure increases with elevated MICs in S. Typhi patients treated with a fluoroquinolone, confirming results from small studies conducted elsewhere [7, 22]. However, although S. Paratyphi A isolates had significantly higher MICs against all tested fluoroquinolones in comparison to S. Typhi, poor outcome was not significantly associated with increasing MIC. We suggest continued surveillance of S. Paratyphi A in the region to monitor for the emergence of high-level fluoroquinolone-resistant organisms similar to trends in the S. Typhi population.
As highlighted in our most recent RCT, patients with suspected enteric fever who were blood culture negative were treated effectively with gatifloxacin, yet fared less well when treated with ceftriaxone [16]. The present analysis shows that ofloxacin also performs well in treating those with culture-negative enteric fever. However, due to the low sensitivity of blood culture for the detection of S. Typhi and S. Paratyphi A[23], it is likely ofloxacin may have been effective against undetected enteric fever cases. We have documented that a reasonable proportion (22%, 21/96) of patients enrolled in the third trial included in the present analysis [14] who were blood culture negative were serologically positive for murine typhus [24]. Doxycycline is considered the drug of choice for rickettsial infections, although it seems that fluoroquinolones may also have clinical activity [24].
In 2003 the World Health Organization published guidelines that recommended azithromycin, ceftriaxone, or cefixime for quinolone-resistant S. Typhi and S. Paratyphi A infections [23]. Azithromycin is safe and efficacious for the treatment of uncomplicated typhoid [25, 26]. Although there are no current clinical MIC breakpoints, the majority of isolates (88%) here were susceptible, using the previously suggested cutoff of <16 µg/mL [27]. The low MICs against ceftriaxone and rapid FCTs throughout the study period indicate that this drug is likely to be effective for culture-confirmed enteric fever in Nepal. The cost and parenteral route of administration, however, make ceftriaxone less suitable for patient treatment in low- and middle-income countries, particularly as 60%–90% of enteric fever patients are treated as outpatients [3]. An alternative would be the oral, third-generation cephalosporin cefixime. However, our first trial, in which we compared gatifloxacin with cefixime, had to be stopped early by the Data Safety Monitoring Board because of the high failure rate in the cefixime arm (26/77) compared to the gatifloxacin arm (5/92; OR, approximately 9), despite all strains being cefixime susceptible [13]. Our analysis supports a recommendation for azithromycin or ceftriaxone for culture-confirmed enteric fever, and in the absence of rapid diagnostics for rickettsial infections [28], a combination of ceftriaxone and doxycycline in culture-negative febrile patients in this setting [16]. However, identification of extended-spectrum β-lactamase–producing S. Paratyphi A in India again suggests vigilance is required.
Our study has limitations. First, the poor diagnostic sensitivity of blood culture may lead to misclassification of a significant number of patients. Although a proportion of culture negatives are likely to be positive for Rickettsia spp., this was not directly assessed [24]. Furthermore, by combining patients from individual RCTs with some differing definitions, the data became nonrandomized; however, we included a random effect of study to account for heterogeneity between studies and controlled for age. Therefore, strong associations, such as odds of treatment failure between cefixime and gatifloxacin in culture-positive patients, may be reduced with the larger, nonrandomized data. Additionally, we were unable to access pharmacy records to evaluate the relationship of prescribing patterns for febrile patients and MICs against common antimicrobials. Notwithstanding these limitations, the results from this largest collection of trials with patient recruitment spanning a decade in an endemic location with a high burden of disease will help to inform therapy recommendations.
In conclusion, poor sanitation, low vaccine uptake, and the emergence of extensive ciprofloxacin-resistant S. Typhi in Kathmandu suggest that appropriate antimicrobial usage policies are required in order to limit morbidity, mortality, and transmission. In this large evaluation, we document shifting antimicrobial susceptibility profiles, an association between poor treatment outcome, and S. Typhi MICs in patients treated with a fluoroquinolone and again highlight the need for better diagnostics for febrile diseases in this setting. We reiterate that fluoroquinolones should not be recommended for the empirical treatment of enteric fever in South Asia [8, 29] and advocate the use of azithromycin or ceftriaxone, in addition to surveillance for changes in AMR profiles.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We acknowledge Mangal Rawal, Sumi Munankarmi, Bibek Karki, Radheshyam KC, Sudeep Dhoj Thapa, Rabin Gautam, Priyanka Tiwari, Manisha Risal, Surendra Shrestha, Balmukunda Neupane, Nabraj Regmi, Krishna Prajapati, and Bimal Thapa and the trial monitors Nguyen Thi Phuong Dung and Nguyen Thi Thanh Thuy for their assistance in conducting these trials. We thank Abhusani Bhuju for her painstaking clerical work for this manuscript. We also thank the community medical auxiliaries and all of the patients and their families who participated in the trials.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This project was funded by the Wellcome Trust of Great Britain (106158/Z/14/Z). S. B. is a Sir Henry Dale Fellow, jointly funded by the Wellcome Trust and the Royal Society (100087/Z/12/Z). C. D. was funded by the Li Ka Shing Foundation Global Health Programme at the University of Oxford. A. K. is funded as a leadership fellow through the Oak Foundation.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Crump JA, Mintz ED. Global trends in typhoid and paratyphoid fever. Clin Infect Dis 2010; 50:241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med 2002; 347:1770–82. [DOI] [PubMed] [Google Scholar]
- 4. Bhan MK, Bahl R, Bhatnagar S. Typhoid and paratyphoid fever. Lancet 2005; 366:749–62. [DOI] [PubMed] [Google Scholar]
- 5. Bhutta ZA. Current concepts in the diagnosis and treatment of typhoid fever. BMJ 2006; 333:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown JC, Shanahan PM, Jesudason MV, Thomson CJ, Amyes SG. Mutations responsible for reduced susceptibility to 4-quinolones in clinical isolates of multi-resistant Salmonella typhi in India. J Antimicrob Chemother 1996; 37:891–900. [DOI] [PubMed] [Google Scholar]
- 7. Wain J, Hoa NT, Chinh NT, et al. Quinolone-resistant Salmonella Typhi in Viet Nam: molecular basis of resistance and clinical response to treatment. Clin Infect Dis 1997; 25:1404–10. [DOI] [PubMed] [Google Scholar]
- 8. Wong VK, Baker S, Pickard DJ, et al. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat Genet 2015; 47:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baker S, Duy PT, Nga TV, et al. Fitness benefits in fluoroquinolone-resistant Salmonella Typhi in the absence of antimicrobial pressure. Elife 2013; 2:e01229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pham Thanh D, Karkey A, Dongol S, et al. A novel ciprofloxacin-resistant subclade of H58 Salmonella Typhi is associated with fluoroquinolone treatment failure. Elife 2016; 5:e14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murdoch DR, Woods CW, Zimmerman MD, et al. The etiology of febrile illness in adults presenting to Patan hospital in Kathmandu, Nepal. Am J Trop Med Hyg 2004; 70:670–5. [PubMed] [Google Scholar]
- 12. Maskey AP, Basnyat B, Thwaites GE, Campbell JI, Farrar JJ, Zimmerman MD. Emerging trends in enteric fever in Nepal: 9124 cases confirmed by blood culture 1993–2003. Trans R Soc Trop Med Hyg 2008; 102:91–5. [DOI] [PubMed] [Google Scholar]
- 13. Pandit A, Arjyal A, Day JN, et al. An open randomized comparison of gatifloxacin versus cefixime for the treatment of uncomplicated enteric fever. PLoS One 2007; 2:e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arjyal A, Basnyat B, Koirala S, et al. Gatifloxacin versus chloramphenicol for uncomplicated enteric fever: an open-label, randomised, controlled trial. Lancet Infect Dis 2011; 11:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koirala S, Basnyat B, Arjyal A, et al. Gatifloxacin versus ofloxacin for the treatment of uncomplicated enteric fever in Nepal: an open-label, randomized, controlled trial. PLoS Negl Trop Dis 2013; 7:e2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arjyal A, Basnyat B, Nhan HT, et al. A randomised controlled trial of gatifloxacin versus ceftriaxone for the treatment of uncomplicated enteric fever in Nepal. Lancet Infect Dis 2016; 16:535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryan ET. Troubling news from Asia about treating enteric fever: a coming storm. Lancet Infect Dis 2016; 15:7–8. [DOI] [PubMed] [Google Scholar]
- 18. Maskey AP, Day JN, Phung QT, et al. Salmonella enterica serovar Paratyphi A and S. enterica serovar Typhi cause indistinguishable clinical syndromes in Kathmandu, Nepal. Clin Infect Dis 2006; 42:1247–53. [DOI] [PubMed] [Google Scholar]
- 19. Sood S, Kapil A, Das B, Jain Y, Kabra SK. Re-emergence of chloramphenicol-sensitive Salmonella Typhi. Lancet 1999; 353:1241–2. [DOI] [PubMed] [Google Scholar]
- 20. Menezes GA, Harish BN, Khan MA, Goessens WH, Hays JP. Antimicrobial resistance trends in blood culture positive Salmonella Typhi isolates from Pondicherry, India, 2005–2009. Clin Microbiol Infect 2012; 18:239–45. [DOI] [PubMed] [Google Scholar]
- 21. Holt KE, Phan MD, Baker S, et al. Emergence of a globally dominant IncHI1 plasmid type associated with multiple drug resistant typhoid. PLoS Negl Trop Dis 2011; 5:e1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crump JA, Kretsinger K, Gay K, et al. ; Emerging Infections Program FoodNet and NARMS Working Groups Clinical response and outcome of infection with Salmonella enterica serotype Typhi with decreased susceptibility to fluoroquinolones: a United States FoodNet multicenter retrospective cohort study. Antimicrob Agents Chemother 2008; 52:1278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization. Background document: the diagnosis, treatment and prevention of typhoid fever. Geneva, 2003http://www.who.int/rpc/TFGuideWHO.pdf. [Google Scholar]
- 24. Thompson CN, Blacksell SD, Paris DH, et al. Undifferentiated febrile illness in Kathmandu, Nepal. Am J Trop Med Hyg 2015; 92:875–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Effa EE, Bukirwa H. Azithromycin for treating uncomplicated typhoid and paratyphoid fever (enteric fever). Cochrane Database Syst Rev 2008: CD006083. [DOI] [PubMed] [Google Scholar]
- 26. Effa EE, Lassi ZS, Critchley JA, et al. Fluoroquinolones for treating typhoid and paratyphoid fever (enteric fever). Cochrane database Syst Rev 2011: CD004530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parry CM, Thieu NT, Dolecek C, et al. Clinically and microbiologically derived azithromycin susceptibility breakpoints for Salmonella enterica serovars Typhi and Paratyphi A. Antimicrob Agents Chemother 2015; 59:2756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parry CM, Wijedoru L, Arjyal A, Baker S. The utility of diagnostic tests for enteric fever in endemic locations. Expert Rev Anti Infect Ther 2011; 9:711–25. [DOI] [PubMed] [Google Scholar]
- 29. Basnyat B. Typhoid versus typhus fever in post-earthquake Nepal. Lancet Glob Health 2016; 4:e516–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.