Summary
Coronavirus pneumonia is associated with high rates of oxygen use and mortality in hematopoietic cell transplant (HCT) recipients and patients with hematologic malignancies; mortality in HCT recipients is similar to that seen with respiratory syncytial virus, influenza, and parainfluenza virus.
Keywords: human coronavirus, bronchoalveolar lavage, lower respiratory tract disease, hematopoietic cell transplant, hematologic malignancy.
Abstract
Background.
The possible role of human coronavirus (HCoV) in lower respiratory tract disease (LRTD) in hematopoietic cell transplant (HCT) recipients and patients with hematologic malignancies (HM) has not been well studied.
Methods.
We conducted a retrospective review of HCT/HM patients with HCoV detected in bronchoalveolar lavage (BAL). HCoV strains were identified in BAL samples using strain-specific polymerase chain reaction. Mortality rates were compared among HCT recipients with LRTD caused by HCoV, respiratory syncytial virus (RSV), influenza virus, or parainfluenza virus (PIV) by multivariable Cox regression analysis.
Results.
We identified 35 patients (37 episodes) with HCoV LRTD. Among 23 available BAL samples, 48% were strain OC43, 22% were NL63, 17% were 229E, and 13% were HKU1. Overall, 21 patients (60%) required oxygen therapy at diagnosis and 19 (54%) died within 90 days of diagnosis. Respiratory copathogens were detected in 21 episodes (57%), including viruses (n = 12), fungi (n = 10), and bacteria (n = 8). Mortality rates were not different between patients with and without copathogens (P = .65). In multivariable models, mortality associated with HCoV LRTD was similar to that seen with RSV, influenza, and PIV LRTD in HCT recipients (adjusted hazard ratio, 1.34 [95% confidence interval, .66–2.71], P = .41 vs RSV, adjusted for cell source, cytopenia, copathogens, oxygen use, and steroid use).
Conclusions.
HCoV LRTD in patients with HCT or HM is associated with high rates of oxygen use and mortality. Mortality associated with HCoV LRTD in HCT recipients appears to be similar to that seen with RSV, influenza virus, and PIV.
Respiratory viruses can cause lower respiratory tract disease (LRTD) in immunocompromised hosts, which is associated with significant morbidity and mortality [1–4]. With the development and widespread use of new molecular diagnostic techniques, the clinical impact of previously underdiagnosed respiratory viruses in this population remains uncertain [5]. This is particularly true of human coronavirus (HCoV). In addition to severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronaviruses, 4 other strains of HCoV (229E, OC43, NL63, and HKU1) are now acknowledged to be human pathogens [6, 7]. Previous studies have demonstrated that HCoV is now the second most common virus identified from the upper respiratory tract in hematopoietic cell transplant (HCT) recipients [8]. Cases of fatal pneumonia related to HCoV without copathogens have also been reported mainly in HCT populations [9–12]. Two previous studies describe the possible role of HCoV in LRTD; however, these studies included only limited numbers of HCT recipients and patients with hematologic malignancy (HM), and outcome analyses could not be done [13, 14]. The purpose of this study was to describe the clinical characteristics and outcomes of HCT recipients and patients with HM with HCoV detected in the lower respiratory tract based on testing of bronchoalveolar lavage (BAL) fluid. Mortality rates were compared among HCT recipients with LRTD caused by HCoV, respiratory syncytial virus (RSV), influenza virus, or parainfluenza virus (PIV).
METHODS
Study Design
We identified all HCT recipients and patients with HM with HCoV detected in clinical BAL samples from patients at the Fred Hutchinson Cancer Research Center, University of Washington, or Seattle Children’s Hospital from May 2006 through February 2016. We identified 3 additional HCT recipients with HCoV detected in BAL samples from a previously reported cohort [15]. Demographic and clinical data were collected from the above-mentioned institutions’ databases and medical chart review. We also compared HCT recipients with HCoV LRTD to previously reported cohorts of HCT recipients with LRTD caused by RSV, influenza virus, and PIV [16–18]. The study was approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center.
Laboratory Testing and Definitions
Reverse-transcription polymerase chain reaction (RT-PCR) was performed for HCoV on BAL samples, serum specimens, lung biopsy, and autopsy samples according to a previously published protocol [19]. Viral load of HCoV was determined by quantitative RT-PCR using BAL samples. HCoV was identified from BAL specimens using the consensus HCoV assay, which is part of a multiplex PCR used to detect 12 respiratory viruses. Strain-specific PCR was performed using saved BAL samples as described previously [19]. We performed RT-PCR to detect HCoV RNA in frozen serum samples that were drawn between 23 days before and 23 days after the BAL. When adequate lung tissue was available, curls were cut from fresh frozen tissue or formalin-fixed, paraffin-embedded (FFPE) tissue blocks for RT-PCR. FFPE samples and frozen samples were extracted using RNAeasy FFPE kit and RNAeasy mini kit, respectively (Qiagen, Hilden, Germany). All samples underwent fragment size analysis for quality with RT-PCR targeting amplicons from housekeeping genes with sizes ranging from 100 to 600 base pairs (bp).
HCoV LRTD was defined as HCoV detection in a BAL sample from a patient with signs of LRTD (eg cough, dyspnea) or new pulmonary infiltrates. All BAL specimens underwent broad diagnostic tests including conventional cultures for bacteria, fungi, mycobacteria, and viruses, shell vial culture for cytomegalovirus, immunofluorescent antibody staining for Pneumocystis jirovecii and Legionella, fungal PCR, Aspergillus galactomannan enzyme-linked immunosorbent assay, and cytopathologic examination. HCoV was considered the sole respiratory pathogen if all above-mentioned microbiological test results on BAL specimens were negative. Pulmonary bacterial coinfection was defined as bacterial load of >103 colony-forming units per milliliter of BAL specimen with compatible radiological findings and clinical course. Any virus or fungus detected in BAL samples was considered a respiratory copathogen. Highest steroid doses in the 2 weeks prior to HCoV LRTD and cell counts most immediately prior to HCoV LRTD were recorded. Oxygen-free days and ventilator-free days are defined as days alive and free from oxygen support and mechanical ventilation, respectively [16]. Respiratory death was defined as any death occurring as a consequence of respiratory failure.
Morphologic re-review of available BAL samples, lung biopsies, and autopsy lung tissues was performed on hematoxylin and eosin–stained sections by a board-certified pathologist with expertise in transplant pathology.
Statistical Analysis
Patients’ outcomes were compared using χ2 or Fisher exact test for categorical variables and Wilcoxon rank-sum test for continuous variables, as appropriate. Summary of the various patient cohorts according to analysis type is shown in Supplementary Figure 1. Only the first episode of HCoV LRTD per subject was used for outcome analyses. We also excluded 2 HCT recipients with a history of lung transplantation for outcome analyses except for evaluation of risk factors for mortality following HCoV LRTD. Univariable Cox proportional hazards models were used to evaluate risk factors for overall mortality by day 90 after the diagnosis of HCoV LRTD. Variables with a P value ≤.2 in the univariable models were candidates for multivariable models. A multivariable Cox regression model adjusted for respiratory viruses (HCoV, RSV, influenza, and PIV), cell source, neutrophil counts, lymphocyte counts, monocyte counts, presence of copathogens, steroid dosage, and oxygen use at diagnosis was performed. Patients with any respiratory viral copathogens were excluded for this analysis. The probability of overall mortality in HCT recipients by day 90 following the diagnosis of LRTD was estimated using the Kaplan-Meier method. The log-rank test was used to compare mortality curves among subgroups. Two-sided P values <.05 were considered statistically significant. All statistical analyses were performed using SAS software version 9.4 for Windows (SAS Institute, Inc, Cary, North Carolina).
RESULTS
Patient and Viral Characteristics
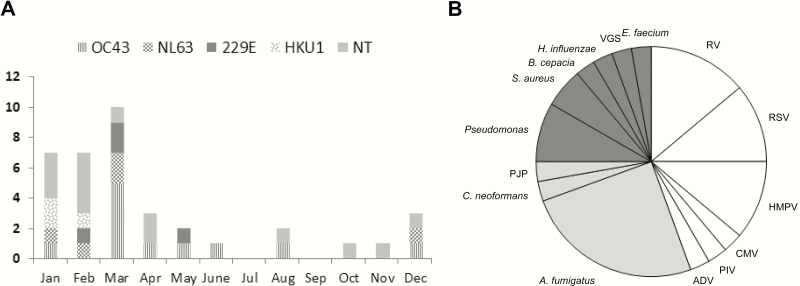
We identified a total of 35 patients (37 episodes) with HCoV detected by RT-PCR from BAL samples. Table 1 shows characteristics of HCT recipients and patients with HM. Two patients developed HCoV LRTD twice. Two HCT recipients had a history of lung transplantation: 1 underwent lung transplantation for bronchiolitis obliterans related to previous HCT and the other received lung transplantation for cystic fibrosis before HCT. Only 1 pediatric patient (8-year-old male) was identified in this cohort. The median time to HCoV LRTD after HCT in 28 recipients was 302 days (range, 8–7045 days): 20 (71%) and 12 (43%) patients developed HCoV LRTD >100 days and >365 days following transplant, respectively. All but 1 of the 20 patients with HCoV LRTD >100 days following transplant received either immunosuppressive therapy or chemotherapy to control their underlying disorders (eg, relapse of hematologic malignancy, graft-vs-host disease) prior to diagnosis of LRTD. Twenty-three recipients were transplanted after 1 May 2006 when respiratory viral PCR panel testing became routine. The median time to HCoV LRTD after HCT in these 23 patients was 340 days (range, 8–3618 days), which was similar to that of entire cohort. At the time of BAL, acute respiratory symptoms and new pulmonary infiltrates were present in the majority of episodes (Table 2). Among 23 available frozen BAL samples, 11 (48%) were identified as OC43, 5 (22%) as NL63, 4 (17%) as 229E, and 3 (13%) as HKU1. The majority of episodes occurred in the winter and spring regardless of strain type (Figure 1A).
Table 1.
Characteristics of All Patients With Human Coronavirus Lower Respiratory Tract Disease
| Characteristic | Total (N = 35) | Hematopoietic Cell Transplant Recipients (n = 28) | Patients With Hematologic Malignancy (n = 7) |
|---|---|---|---|
| Female sex | 10 (29) | 10 (36) | 0 |
| Age, y, median (range) | 53 (8–68) | 53.5 (24–67) | 52 (8–68) |
| Underlying pulmonary disordera | 10 (29) | 10 (36) | 0 |
| Immunosuppressive therapy or chemotherapy | 34 (97) | 27 (96) | 7 (100) |
| Transplant year | |||
| 1996–2006 |
6 (21) | ||
| 2007–2015 |
22 (79) | ||
| Transplant number ≥2 | 10 (36) | ||
| Cell source | |||
| Cord | 1 (11) | ||
| Bone marrow | 6 (21) | ||
| PBSC | 21 (75) | ||
| Donor type | |||
| Autologous | 4 (14) | ||
| Related | 10 (36) | ||
| Unrelated | 14 (50) | ||
| Days between transplant and HCoV LRTD, median (range) | 302 (8–7045) | ||
Data are presented as No. (11) unless otherwise indicated.
Abbreviations: HCoV, human coronavirus; LRTD, lower respiratory tract disease; PBSC, peripheral blood stem cell.
a Bronchiolitis obliterans (n = 4), lung transplantation for bronchiolitis obliterans (n = 1), lung transplantation for cystic fibrosis (n = 1), radiation pneumonia (n = 2), asthma (n = 1), prolonged acute respiratory distress syndrome (n = 1), diffuse alveolar hemorrhage (n = 1), cystic fibrosis (n = 1).
Table 2.
Presentation of Human Coronavirus Lower Respiratory Tract Disease Episodes
| Characteristic | Totala (n = 37) | Hematopoietic Cell Transplant Recipients (n = 30) | Patients With Hematologic Malignancy (n = 7) |
|---|---|---|---|
| Respiratory symptomsb | 34 (92) | 27 (90) | 7 (100) |
| Abnormal lung examinationc | 25 (68) | 21 (70) | 4 (57) |
| Abnormal findings on chest imagingd | 34 (92) | 27 (90) | 7 (100) |
| HCoV strain | |||
| OC43 | 11 (30) | 10 (33) | 1 (14) |
| NL63 | 5 (14) | 4 (13) | 1 (14) |
| 229E | 4 (11) | 4 (13) | 0 |
| HKU1 | 3 (11) | 3 (10) | 0 |
| Unknown | 14 (38) | 9 (30) | 5 (71) |
| Respiratory copathogen | 21 (57) | 18 (60) | 3 (43) |
| None | 16 (43) | 12 (40) | 4 (57) |
| Viruses | 5 (13) | 3 (10) | 2 (29) |
| Bacteria | 4 (11) | 4 (13) | |
| Fungi | 4 (11) | 4 (13) | |
| Multiple | 8 (22) | 7 (23) | 1 (14) |
| Quantitative viral load, log10 copies/mL, median (range) | 5.4 (2.4–9.0) | 5.3 (2.4–7.8) | 6.1 (3.4–7.4) |
| WBC count ≤1000 × 106 cells/L | 11 (30) | 7 (23) | 4 (57) |
| Lymphocyte count ≤300 × 106 cells/L | 19 (51) | 15 (50) | 4 (57) |
| Neutrophil count ≤500 × 106 cells/L | 14 (38) | 9 (30) | 5 (71) |
| Monocyte count ≤300 × 106 cells/L | 24 (65) | 19 (63) | 5 (71) |
| Steroid dosee | |||
| None | 14 (38) | 7 (23) | 7 (100) |
| ≤1 mg/kg | 13 (35) | 13 (43) | 0 |
| >1 mg/kg | 10 (27) | 10 (33) | 0 |
| Oxygen requirement at diagnosis | 23 (62) | 20 (67) | 3 (43) |
Data are presented as No. (11) unless otherwise indicated.
Abbreviations: HCoV, human coronavirus; WBC, white blood cell.
aTwo patients had separated HCoV lower respiratory tract disease (LRTD) episodes. The first patient developed LRTD 361 days and 415 days following hematopoietic cell transplant (HCT), respectively. The second patient developed LRTD 425 days before and 11 days after HCT, respectively.
bCough or dyspnea.
cCrackles, wheeze, rhonchi, or decreased breath sound.
dAny new abnormal lung findings except for single nodule.
eMaximum daily dose within 2 weeks prior to diagnosis.
Figure 1.
A, Human coronavirus strain. Seasonal distribution of human coronavirus lower respiratory tract disease (LRTD). B, Respiratory copathogens in human coronavirus LRTD. Each color indicates category of copathogen as follows: white (viruses), gray (fungi), and dark gray (bacteria). Respiratory viral copathogens were detected in 12 patients, fungal copathogens were detected in 10 patients, and bacterial copathogens were detected in 8 patients. The number of patients (n = 30) with other respiratory copathogens does not equal the sum of detections for each respiratory copathogen (n = 36) owing to codetections of multiple copathogens in some subjects.Abbreviations: ADV, adenovirus; A. fumigutus, Aspergillus fumigatus; B. cepacia, Burkholderia cepacia; CMV, cytomegalovirus; C. neoformans, Cryptococcus neoformans; E. faecium, Enterococcus faecium; H. influenzae, Haemophilus influenzae; HMPV, human metapnuemovirus; NT, nontypeable strain due to unavailable sample; P. aeruginosa, Pseudomonas aeruginosa; PIV, parainfluenza virus; PJP, Pneumocystis jirovecii; RSV, respiratory syncytial virus; RV, rhinovirus; S. aureus, Staphylococcus aureus; VGS, Viridans group streptococci.
Other respiratory pathogens were detected in BAL samples in 21 episodes (57%), including viruses (12 episodes), fungi (10 episodes), and bacteria (8 episodes) (Figure 1B). Two or more other respiratory copathogens were detected in approximately half of these episodes (10/21). Two patients had respiratory copathogen as well as concomitant bacteremia/fungemia; 1 patient was found to have Staphylococcus aureus in blood and BAL, and the other had both Clostridium non-perfringens and Candida glabrata in the blood only.
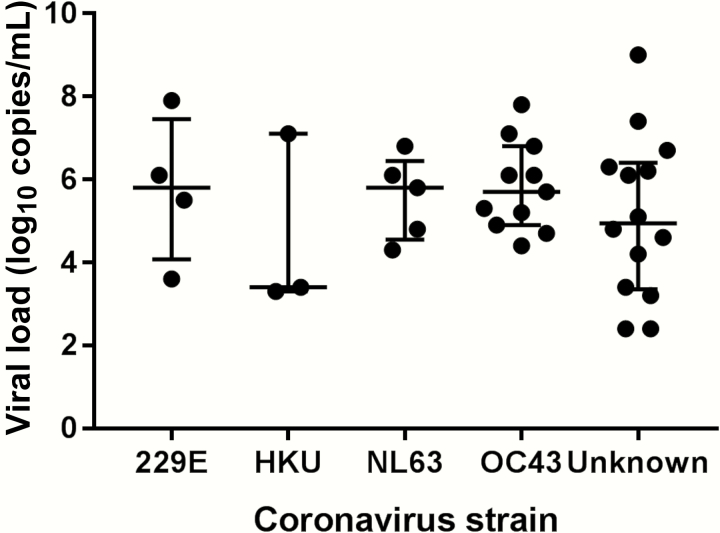
Respiratory copathogens were found in 82% (9/11) of episodes with OC43 and only 42% (5/12) of episodes with other strains (P = .089). The median viral loads of HCoV in BAL samples did not differ among strains (Figure 2). No HCoV RNA was detected in serum samples prior to and following HCoV LRTD available from 21 episodes. Five lung biopsy samples and 4 lung autopsy samples were tested for RT-PCR among 6 patients (3 FFPE samples and 6 fresh frozen samples), all obtained within 67 days after LRTD diagnosis. Quality control fragment size analysis by RT-PCR of the RNA from these samples shows that all FFPE specimens could be reliably amplified to 100 bp while all frozen specimens were reliably amplified to 600 bp. Only 1 sample (lung tissue) was positive for HCoV by RT-PCR, which had been obtained on the same day as BAL.
Figure 2.
Viral load of human coronavirus in bronchoalveolar lavage samples. The bars indicate median values and first and third quartiles.
Outcomes
Patients’ outcomes were summarized after excluding patients with second episodes of HCoV LRTD or a history of lung transplantation (Tables 3 and 4). Outcomes by day 28 and 90 after LRTD diagnosis were compared between HCT recipients and patients with HM. HCT recipients were more likely to have fewer oxygen and ventilator-free days than patients with HM. Outcomes by day 28 and 90 after HCoV diagnosis were also compared between patients with and without respiratory copathogens, with no statistical differences found.
Table 3.
Outcome of Patients With Human Coronavirus Lower Respiratory Tract Disease
| Outcome | Total (N = 33) | Hematopoietic Cell Transplant Recipients (n = 26) | Patients With Hematologic Malignancy (n = 7) | P Value |
|---|---|---|---|---|
| Outcome by day 28 after diagnosis | ||||
| Mechanical ventilation requirement, No. (11) | 7 (21) | 7 (27) | 0 | .30 |
| Oxygen-free days | 17.0 (11.8) | 15.0 (12.2) | 24.7 (5.6) | .04 |
| Ventilator-free days | 22.1 (9.7) | 20.5 (10.4) | 28.0 (0.0) | .03 |
| Days alive without hospitalization | 11.7 (10.9) | 10.5 (10.7) | 16.1 (11.5) | .22 |
| Outcome by day 90 after diagnosis | ||||
| Any death, No. (11) | 18 (55) | 16 (62) | 2 (29) | .20 |
| Respiratory death, No. (11) | 10 (30) | 9 (35) | 1 (14) | .40 |
Data are presented as mean (standard deviation) unless otherwise indicated.
Table 4.
Outcome of Human Coronavirus Lower Respiratory Tract Disease With and Without Respiratory Copathogens
| Outcome | Total (N = 33) | HCoV as Sole Respiratory Pathogen (n = 14) | HCoV Coinfected With Other Respiratory Pathogens (n = 19) | P Value |
|---|---|---|---|---|
| Outcome by day 28 after diagnosis | ||||
| Mechanical ventilation requirement, No. (11) | 7 (21) | 2 (14) | 5 (26) | .67 |
| Oxygen-free days | 17.0 (11.8) | 19.0 (11.3) | 15.6 (12.2) | .36 |
| Ventilator-free days | 22.1 (9.7) | 24.4 (8.1) | 20.4 (10.6) | .16 |
| Days alive without hospitalization | 11.7 (10.9) | 13.4 (11.0) | 10.4 (11.0) | .43 |
| Outcome by day 90 after diagnosis | ||||
| Any death, No. (11) | 18 (55) | 7 (50) | 11 (58) | .65 |
| Respiratory death, No. (11) | 10 (30) | 2 (14) | 8 (42) | .13 |
Data are presented as mean (standard deviation) unless otherwise indicated.
Abbreviation: HCoV, human coronavirus.
Pathology Results
Twenty-eight patients had samples available for pathologic review, including 25 BAL samples, 5 lung biopsy samples, and 4 autopsy lung specimens; 6 of 7 patients without any other respiratory copathogens had either nonspecific findings of multinucleated giant cells or nuclear enlargement (Supplementary Figure 2A and 2B). Lung tissue from 1 patient was positive for HCoV by RT-PCR; the morphologic features noted in the lung biopsy were inflamed tissue with lymphocytes, neutrophils, and cytologic atypia (Supplementary Figure 3A and 3B).
Comparison of Mortality With Other Respiratory Viruses and Risk Factors for Mortality
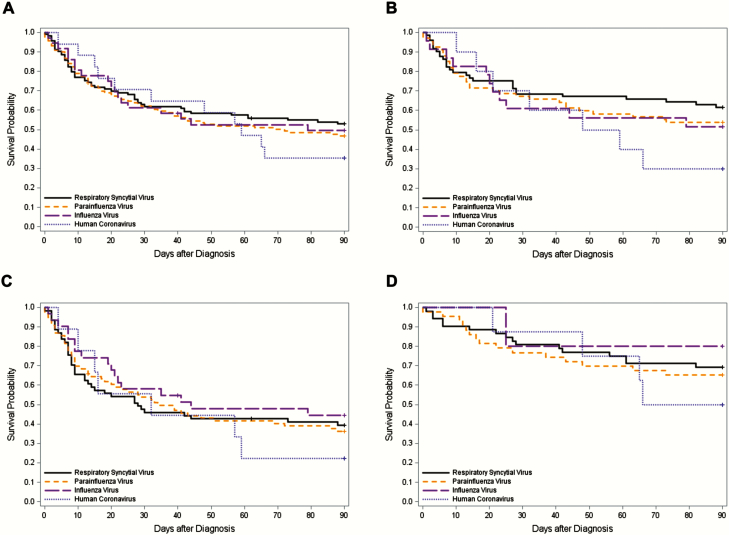
There was a total of 286 HCT recipients with a single respiratory virus identified in BAL samples for whom comparable clinical data were available (HCoV [n = 18], RSV [n = 113], influenza virus [n = 36], and PIV [n = 119]); demographics of these are shown in Supplementary Table 1. Overall mortality rates by day 90 following viral LRTD caused by HCoV, RSV, influenza virus, and PIV among HCT recipients without respiratory viral copathogens and without any copathogens were not different (P = .78 and P = .47, respectively) (Figure 3A and 3B). Furthermore, no difference was seen when the cohort was stratified by those with and without oxygen requirement at the time of LRTD diagnosis (P = . 78 for both) (Figure 3C and 3D). Univariable Cox regression models were used to evaluate risk factors for overall mortality in HCT recipients with LRTD caused by HCoV, RSV, influenza virus, or PIV without respiratory viral copathogens (Table 5). In multivariable models, cell source (bone marrow), respiratory bacterial or fungal copathogens, low neutrophil counts, low monocyte counts, steroid use, and oxygen requirement at diagnosis were associated with overall mortality (Table 6). Mortality due to HCoV LRTD was not significantly different from RSV LRTD (adjusted hazard ratio, 1.34 [95% confidence interval, .66–2.71], P = .41). Similarly, risk factors for overall mortality by day 90 in HCoV LRTD patients alone were evaluated using univariable and multivariable Cox regression models in HCT recipients; no risk factors significantly associated with mortality were found (Supplementary Tables 2 and 3).
Figure 3.
Kaplan-Meier overall survival curve by day 90 after diagnosis of lower respiratory tract disease without respiratory viral copathogens according to respiratory virus classification in hematopoietic cell transplant recipients. A, Kaplan-Meier overall survival curve in overall cohort (n = 286) (log-rank test, P = .78). B, Kaplan-Meier overall survival curve in patients without other copathogens (n = 173) (log-rank test, P = .47). C, Kaplan-Meier overall survival curve in patients with oxygen requirement at diagnosis (n = 178) (log-rank test, P = .78). D, Kaplan-Meier overall survival curve in patients without oxygen requirement at diagnosis (n = 108) (log-rank test, P = .78).
Table 5.
Univariable Cox Regression Analysis for Overall Mortality by Day 90 After Diagnosis of Lower Respiratory Tract Disease (n = 286)
| Covariates | Category | Hazard Ratio (95% CI) |
P Value |
|---|---|---|---|
| Cell source | Peripheral blood stem cell | 1 | |
| Bone marrow | 1.69 (1.22–2.36) | <.01 | |
| Cord | 0.51 (.16–1.62) | .25 | |
| Transplant year | 1993–2006 | 1 | |
| 2007–2015 | 0.87 (.60–1.25) | .45 | |
| Respiratory copathogen | None | 1 | |
| Nonrespiratory virusa ± bacteria/fungi | 1.61 (.93–2.80) | .09 | |
| Bacteria/fungi | 1.54 (1.09–2.19) | .02 | |
| Days between transplant and diagnosis | ≤30 | 1 | |
| 31–365 | 0.94 (.65–1.34) | .71 | |
| >365 | 0.55 (.32–.94) | .03 | |
| White blood cell count, 106 cells/L | ≤1.0 | 1.69 (1.21–2.37) | <.01 |
| >1.0 | 1 | ||
| Neutrophil count, 106 cells/L | <0.5 | 1.76 (1.26–2.47) | <.01 |
| ≥0.5 | 1 | ||
| Lymphocyte count, 106 cells/L | <0.3 | 1.63 (1.17–2.29) | <.01 |
| ≥0.3 | 1 | ||
| Monocyte count, 106 cells/L | <0.3 | 2.38 (1.53–3.70) | <.01 |
| ≥0.3 | 1 | ||
| Steroid use within 2 weeks before diagnosis | No | 1 | |
| <1 mg/kg | 0.96 (.62–1.47) | .85 | |
| 1–2 mg/kg | 1.48 (1.00–2.20) | .05 | |
| >2 mg/kg | 2.23 (1.16–4.27) | .02 | |
| Oxygen use at diagnosis | No | 1 | |
| Any | 2.51 (1.72–3.66) | <.01 | |
| Respiratory virus | Respiratory syncytial virus | 1 | |
| Parainfluenza virus | 1.16 (.81–1.68) | .42 | |
| Influenza virus | 1.08 (.63–1.84) | .78 | |
| Human coronavirus | 1.32 (.69–2.53) | .40 |
Abbreviation: CI, confidence interval.
a Cytomegalovirus, herpes simplex virus, human herpesvirus 6, and Epstein-Barr virus.
Table 6.
Multivariable Cox Regression Analysis for Overall Mortality by Day 90 After Diagnosis of Lower Respiratory Tract Disease (n = 286)
| Covariates | Categories | Adjusted HR (95% CI) | P Value |
|---|---|---|---|
| Cell source | Peripheral blood stem cell | 1 | |
| Bone marrow | 1.64 (1.13–2.40) | .01 | |
| Cord | 0.74 (.23–2.41) | .62 | |
| Respiratory copathogen | None | 1 | |
| Nonrespiratory virusa ± bacteria/fungi | 1.80 (1.00–3.26) | .05 | |
| Bacteria/fungi | 1.66 (1.12–2.45) | .01 | |
| Neutrophil count, 106 cells/L | <0.5 | 1.61 (1.00–2.58) | .05 |
| ≥0.5 | 1 | ||
| Lymphocytes count, 106 cells/L | <0.3 | 0.95 (.62–1.45) | .81 |
| ≥0.3 | 1 | ||
| Monocyte count, 106 cells/L | <0.3 | 1.87 (1.12–3.13) | .02 |
| ≥0.3 | 1 | ||
| Steroid use within 2 wk before diagnosis | No | 1 | |
| <1 mg/kg | 1.27 (.77–2.08) | .35 | |
| 1–2 mg/kg | 1.38 (.87–2.20) | .18 | |
| >2 mg/kg | 2.40 (1.15–5.03) | .02 | |
| Oxygen use at diagnosis | No | 1 | |
| Any | 3.00 (1.98–4.53) | <.01 | |
| Respiratory virus | Respiratory syncytial virus | 1 | |
| Parainfluenza virus | 1.13 (.77–1.67) | .52 | |
| Influenza virus | 0.88 (.47–1.66) | .70 | |
| Human coronavirus | 1.34 (.66–2.71) | .41 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Cytomegalovirus, herpes simplex virus, human herpesvirus 6, and Epstein-Barr virus.
DISCUSSION
In this study, we demonstrated that the presence of HCoV in BAL samples in immunocompromised hosts was significantly associated with high rates of respiratory support and mortality. HCT recipients appeared to be more affected than patients with HM. Although respiratory copathogens were frequently detected, the clinical outcomes of these patients were similar to those without copathogens. The mortality rate of HCT recipients by day 90 after developing HCoV LRTD was similar to rates seen with established respiratory pathogens including RSV, influenza virus, and PIV (Figure 3 and Table 6) [16–18]. All 4 HCoV strains were identified in BAL samples regardless of the presence of copathogens, and at least 2 HCoV strains were present nearly half of the year.
SARS and MERS are recognized as highly human-pathogenic coronaviruses, causing acute, severe, frequently fatal LRTD [20–22]. Although 4 other strains of HCoV (229E, OC43, NL63, and HKU1) are also human pathogens, the clinical impact of HCoV LRTD remains unclear, especially in immunocompromised patients [4, 14, 23]. A previous prospective study with weekly nasal surveillance sampling during the first 100 days after HCT demonstrated prolonged HCoV shedding in the upper respiratory tract (>3 weeks) in half of subjects including asymptomatic patients [8]. In addition, respiratory copathogens were identified in more than half the episodes in this study (57%). Given the prolonged asymptomatic shedding and frequent detection of respiratory copathogens, attributing poor clinical outcomes to HCoV in the lower respiratory tract may be difficult. In the current study, follow-up BAL procedures were not performed to assess prolonged shedding in the lower respiratory tract; however, a prior study that included only 4 patients with cancer demonstrated that only 1 of 10 cases had HCoV detected in follow-up BAL specimens, arguing against asymptomatic prolonged shedding in the lower respiratory tract [14]. More data are needed to define shedding duration in the immunocompromised population. In our study, clinical outcomes including intensity of respiratory support, days alive without hospitalization, and mortality were not significantly different between patients with and without other copathogens, suggesting that HCoV in the lower respiratory tract can contribute to severity of LRTD regardless of copathogens. Lung tissue from 1 patient was positive for HCoV by RT-PCR. This patient subsequently developed prolonged oxygen requirement, which also supports the potential pathogenicity of HCoV.
To further demonstrate the clinical significance of HCoV LRTD in HCT recipients, we compared mortality rates in HCT recipients with HCoV LRTD to other respiratory viruses using multivariable Cox regression analysis and found mortality rates in HCoV LRTD were comparable to those seen with RSV, influenza virus, and PIV. Given the common perception of HCoV as a relatively benign pathogen based on limited data [5, 8], our data are somewhat surprising. The adverse impact of oxygen requirement at the time of diagnosis on subsequent clinical outcome has been suggested [16, 24]. Once substantial acute lung injury occurs, clinical outcome can potentially be affected by inflammation rather than virus itself. Therefore, mortality rates by day 90 following LRTD caused by HCoV, RSV, influenza virus, and PIV were also compared according to oxygen requirement at the time of LRTD diagnosis; no statistically significant difference was found. These data combined suggest that HCoV LRTD is significantly associated with poor clinical outcome in this immunocompromised population.
Previous studies, mainly in immunocompetent hosts, have not demonstrated a distinct association between particular HCoV strains in upper respiratory tract samples and disease severity in LRTD [25–29]. Although there are case reports with each strain identified in lower respiratory tract as a sole pathogen in HCT recipients, systematic data are limited [9–12]. This is the first study to describe that all 4 HCoV strains can be detected in BAL samples with and without any other respiratory copathogens in a large immunocompromised population. However, the few instances of each strain limited our ability to examine if specific HCoV strains are associated with increased disease severity in LRTD. Further studies with larger sample sizes will help to characterize the role of particular HCoV strains.
Our study showed relatively late presentation of HCoV LRTD following transplantation. The median time to HCoV LRTD following HCT was 302 days, which was longer than median days to LRTD caused by RSV (52.5 days), PIV (78 days), and influenza (95 days) [16, 17, 30]. Because this may in part be due to the fact our cohort included some patients who were transplanted prior to the introduction of routine HCoV PCR testing, we separately analyzed patients who underwent transplantation after introduction of a respiratory viral PCR panel in 2006 and determined the median time of diagnosis remained similar (340 days). The majority of patients who developed HCoV LRTD >100 days following transplant had received either chemotherapy or immunosuppressive therapy as predisposing factors. This does not explain why there is a relatively lower incidence of HCoV LRTD early after transplant. Differences in infection control practices early after transplant and factors that affect progression to LRTD early vs late after transplant may play a role. Further studies are needed to determine why HCoV often causes LRTD late after transplant.
This study evaluated the largest cohort of HCoV LRTD confirmed with BAL in patients with HCT and HM by HCoV strain-specific and quantitative PCR in BAL samples. In addition, RT-PCR was performed on serum specimens, lung biopsy samples, and autopsy samples. The main limitation of this study was the relatively small sample size, which prevented us from detecting small differences and performing multivariable analyses to evaluate risk factors for mortality in patients with HCoV LRTD. Another limitation is the fact that BAL samples were available in only two-thirds of the patients for strain identification, which limited our ability to compare clinical and virological differences among each HCoV strain. Among a total of 9 lung biopsy and autopsy samples, only 1 lung biopsy sample, which was taken on the same day as the BAL, was positive for HCoV by RT-PCR. The lower rate of detection may be due to the fact that the negative samples were obtained from 19 days to 67 days after the diagnosis of LRTD, suggesting that the timing of collecting samples may have been too late to identify HCoV in lung specimens. Furthermore, not all samples were optimally preserved for RT-PCR, and thus the sensitivity for HCoV may have been suboptimal.
We demonstrated high rates of respiratory support including oxygen use and mechanical ventilation requirement as well as a high mortality in immunocompromised patients with HCoV identified in the lower respiratory tract. Mortality rates associated with HCoV LRTD in transplant recipients were similar to those seen with other respiratory viral pathogens including RSV, influenza virus, and PIV. Thus, we conclude that HCoV appears to be a significant respiratory pathogen in the populations studied. This is an important observation because HCoVs are highly prevalent in immunocompromised hosts. The appreciation of HCoV as an important lower respiratory tract pathogen could impact clinical management including risk stratification in future studies and provide a rationale to develop antiviral therapies. Further studies are needed to clarify if particular HCoV strains and viral load are correlated with clinical outcome and to identify risk factors for progression to LRTD.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Zachary Stednick for database services; Terry Stevens-Ayers, Reigran Sampoleo, Isabel Palileo, Kristen Shimp, Petrina Mulhauser, and Catherine Spurgeon for laboratory assistance.
Financial support. This work was supported by the National Institutes of Health (grant numbers K24HL093294 to M. B., K23 AI114844 to A. W., CA18029 to W. L., clinical database, CA15704 to H. X.); the Fred Hutchinson Cancer Research Center Vaccine and Infectious Disease Division (biorepository); T32HD00723332and Pediatric Infectious Diseases Society Fellowship Award funded by Horizon Pharma to C. O.
Potential conflicts of interest. M. B. has received research support from and served as a consultant to Gilead Sciences, Merck, Ansun Bioscience, and Aviragen Therapeutics, and has served as a consultant for Humabs Biomed. J. A. E. has received research support from GlaxoSmithKline, Gilead, Pfizer, and Chimerix, and has served as a consultant for Pfizer and GlaxoSmith Kline (data safety monitoring board). C. Y. has received a research grant from Gilead Sciences for unrelated research. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Renaud C, Xie H, Seo S, et al. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol Blood Marrow Transplant 2013; 19:1220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chemaly RF, Shah DP, Boeckh MJ. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis 2014; 59suppl 5:S344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lo MS, Lee GM, Gunawardane N, Burchett SK, Lachenauer CS, Lehmann LE. The impact of RSV, adenovirus, influenza, and parainfluenza infection in pediatric patients receiving stem cell transplant, solid organ transplant, or cancer chemotherapy. Pediatr Transplant 2013; 17:133–43. [DOI] [PubMed] [Google Scholar]
- 4. Weigt SS, Gregson AL, Deng JC, Lynch JP, 3rd, Belperio JA. Respiratory viral infections in hematopoietic stem cell and solid organ transplant recipients. Semin Respir Crit Care Med 2011; 32:471–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Renaud C, Campbell AP. Changing epidemiology of respiratory viral infections in hematopoietic cell transplant recipients and solid organ transplant recipients. Curr Opin Infect Dis 2011; 24:333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cherry JD, Harrison GJ, Kaplan SL, Hotez PJ, Steinbach WJ. Feigin and Cherry’s textbook of pediatric infectious diseases. 7th ed Philadelphia, PA: Elsevier/Saunders, 2014. [Google Scholar]
- 7. Self WH, Williams DJ, Zhu Y, et al. Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis 2016; 213:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Milano F, Campbell AP, Guthrie KA, et al. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood 2010; 115:2088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uhlenhaut C, Cohen JI, Pavletic S, et al. Use of a novel virus detection assay to identify coronavirus HKU1 in the lungs of a hematopoietic stem cell transplant recipient with fatal pneumonia. Transpl Infect Dis 2012; 14:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Folz RJ, Elkordy MA. Coronavirus pneumonia following autologous bone marrow transplantation for breast cancer. Chest 1999; 115:901–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pene F, Merlat A, Vabret A, et al. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin Infect Dis 2003; 37:929–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oosterhof L, Christensen CB, Sengeløv H. Fatal lower respiratory tract disease with human coronavirus NL63 in an adult haematopoietic cell transplant recipient. Bone Marrow Transplant 2010; 45:1115–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hakki M, Rattray RM, Press RD. The clinical impact of coronavirus infection in patients with hematologic malignancies and hematopoietic stem cell transplant recipients. J Clin Virol 2015; 68:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garbino J, Crespo S, Aubert JD, et al. A prospective hospital-based study of the clinical impact of non-severe acute respiratory syndrome (non-SARS)-related human coronavirus infection. Clin Infect Dis 2006; 43:1009–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Englund JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med 2006; 144:344–9. [DOI] [PubMed] [Google Scholar]
- 16. Seo S, Xie H, Campbell AP, et al. Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: viral detection in the lung predicts outcome. Clin Infect Dis 2014; 58:1357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waghmare A, Campbell AP, Xie H, et al. Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin Infect Dis 2013; 57:1731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi SM, Boudreault AA, Xie H, Englund JA, Corey L, Boeckh M. Differences in clinical outcomes after 2009 influenza A/H1N1 and seasonal influenza among hematopoietic cell transplant recipients. Blood 2011; 117:5050–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuypers J, Martin ET, Heugel J, Wright N, Morrow R, Englund JA. Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics 2007; 119:e70–6. [DOI] [PubMed] [Google Scholar]
- 20. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med 2014; 160:389–97. [DOI] [PubMed] [Google Scholar]
- 21. Leung GM, Hedley AJ, Ho LM, et al. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Ann Intern Med 2004; 141:662–73. [DOI] [PubMed] [Google Scholar]
- 22. Chan JW, Ng CK, Chan YH, et al. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS). Thorax 2003; 58:686–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis 2013; 56:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Fontbrune FS, Robin M, Porcher R, et al. Palivizumab treatment of respiratory syncytial virus infection after allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2007; 45:1019–24. [DOI] [PubMed] [Google Scholar]
- 25. Lee J, Storch GA. Characterization of human coronavirus OC43 and human coronavirus NL63 infections among hospitalized children <5 years of age. Pediatr Infect Dis J 2014; 33:814–20. [DOI] [PubMed] [Google Scholar]
- 26. Gerna G, Campanini G, Rovida F, et al. Genetic variability of human coronavirus OC43-, 229E-, and NL63-like strains and their association with lower respiratory tract infections of hospitalized infants and immunocompromised patients. J Med Virol 2006; 78:938–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaunt ER, Hardie A, Claas EC, Simmonds P, Templeton KE. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol 2010; 48:2940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dominguez SR, Robinson CC, Holmes KV. Detection of four human coronaviruses in respiratory infections in children: a one-year study in Colorado. J Med Virol 2009; 81:1597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kristoffersen AW, Nordbø SA, Rognlien AG, Christensen A, Døllner H. Coronavirus causes lower respiratory tract infections less frequently than RSV in hospitalized Norwegian children. Pediatr Infect Dis J 2011; 30:279–83. [DOI] [PubMed] [Google Scholar]
- 30. Choi SM, Xie H, Campbell AP, et al. Influenza viral RNA detection in blood as a marker to predict disease severity in hematopoietic cell transplant recipients. J Infect Dis 2012; 206:1872–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.