Summary
An analysis of serial galactomannan index (GMI) in patients with invasive aspergillosis from a phase 3 clinical trial demonstrated that increases in GMI in the first week of therapy significantly increase the likelihood of death and unsuccessful response.
Keywords: aspergillosis, galactomannan, biomarker, isavuconazole, isavuconazonium sulfate.
Abstract
Background.
The ability to make early therapeutic decisions when treating invasive aspergillosis using changes in biomarkers as a surrogate for therapeutic response could significantly improve patient outcome.
Methods.
Cox proportional hazards model and logistic regression were used to correlate early changes in galactomannan index (GMI) to mortality and overall response, respectively, from patients with positive baseline GMI (≥0.5) and serial GMI during treatment from a phase 3 clinical trial for the treatment of invasive mold disease. Pharmacokinetic/pharmacodynamic (PK/PD) analysis in patients with isavuconazole plasma concentrations was conducted to establish the exposure necessary for GMI negativity at the end of therapy.
Results.
The study included 158 patients overall and 78 isavuconazole patients in the PK/PD modeling. By day 7, GMI increases of >0.25 units from baseline (3/130 survivors; 9/28 who died) significantly increased the risk of death compared to those with no increase or increases <0.25 (hazard ratio, 9.766; 95% confidence interval [CI], 4.356–21.9; P < .0001). For each unit decrease by day 7 from baseline, the odds of successful therapy doubled (odds ratio, 2.154; 95% CI, 1.173–3.955). An area under the concentration-versus-time curve over half-maximal effective concentration (AUC:EC50) of 108.6 is estimated to result in a negative GMI at the end of isavuconazole therapy.
Conclusions.
Early trends in GMI are highly predictive of patient outcome. GMI increases by day 7 could be considered in context of clinical signs to trigger changes in treatment, once validated. Our data suggest that this improves survival by 10-fold and positive outcome by 3-fold. These data have important implications for individualized therapy for patients and clinical trials.
Clinical Trials Registration.
Invasive fungal diseases caused by Aspergillus species are a persistent public health problem [1–3]. Therapeutic options are few and limited by toxicity, an incomplete spectrum of antifungal activity, and acquired drug resistance [4–8]. Treatment is initiated when tissue damage is established, usually evidenced by radiographic abnormalities. Assessing the response to therapy at the bedside is difficult. Clinical signs and symptoms are nonspecific and radiological abnormalities may paradoxically deteriorate despite otherwise successful therapy [3]. Thus, ways to objectively monitor the response to antifungal therapy and make rational therapeutic choices would significantly improve antifungal stewardship and potentially improve clinical outcomes.
The widespread use of galactomannan (GM) detection and quantification has improved the speed and accuracy of a diagnosis of invasive aspergillosis (IA). Serum GM is often detectable prior to the onset of clinical signs and symptoms of infection [2, 3] and has been used as a surrogate for therapeutic response [9–11]. A decline in GM or GM negativity has been linked to clinical outcomes [12–15]. However, interpretive criteria used to direct therapeutic decision making are lacking.
To identify an early pattern of GM index (GMI) change that could guide therapeutic decision-making for patients increasing the likelihood of survival and successful outcome, we analyzed serial GMI from patients treated in a randomized, double-blind trial (SECURE) in the treatment of IA or other filamentous fungi. We also analyzed a subset of isavuconazole-treated patients who had available isavuconazole plasma concentrations and serial GMI to evaluate the pharmacokinetic/pharmacodynamic (PK/PD) relationship with a goal to identify a drug-exposure target that can be used for individual patients.
METHODS
Study Design
Patients from the SECURE trial (NCT00412893), a phase 3, randomized, double-blind study comparing isavuconazonium sulfate to voriconazole for the treatment of invasive mold disease caused by Aspergillus species or other filamentous fungi, were eligible [16]. The evaluation of the use of GM as a biomarker was prespecified as an exploratory objective. Patients from the modified intent-to-treat (all patients receiving study drug with proven or probable invasive mold infections, as determined by an independent blinded data review committee) population who had a positive baseline serum GMI (optical density ≥0.5) plus 1 or more postbaseline (>day 7) GMIs were included. Patients were eligible for the PK/PD analysis if plasma samples were collected to enable an estimate of isavuconazole plasma exposure.
Eligibility criteria for the main trial are detailed elsewhere [16]. Patients randomized to the isavuconazole group received the prodrug, isavuconazonium sulfate, as a loading regimen intravenously (IV) over days 1 and 2 at a dose of 372 mg (equivalent to 200 mg of isavuconazole) every 8 hours, followed by a maintenance dose of 372 mg once daily, given either IV or orally. Voriconazole patients received 6 mg/kg IV twice daily on day 1 followed by 4 mg/kg IV twice daily on day 2. From day 3 onward, voriconazole was administered as an IV infusion (4 mg/kg twice daily) or orally (200 mg twice daily). The patients were treated for up to 84 days.
Outcome Measurements
The primary efficacy outcome measure was all-cause mortality through day 42. Secondarily, overall response at the end of therapy (EOT) as evaluated by the data review committee was assessed. Successful overall response was defined as a composite of complete or partial clinical and radiological response, plus a mycological response of eradication or presumed eradication. The response criteria are detailed elsewhere [16].
GM Measurements
Protocol-defined serum samples were drawn at screening, on days 1, 2, 14, 28, 42, 84, and at EOT. Samples drawn on an ad hoc basis were included. In total, 1347 GMI values were available with 114 around day 7 (±1 day) (Supplementary Figure 1). A central laboratory analyzed protocol-defined samples, while ad hoc samples were analyzed locally. Specimens were stored at –20°C until shipped to the central laboratory (Eurofins Global Central Laboratory, Chantilly, Virginia) and assayed using Platelia Aspergillus enzyme immunoassay from Bio-Rad Laboratories according to the manufacturer’s instructions. The total interassay variability, according to the instructions, ranged from 6.8% to 29.2% for samples.
Pharmacokinetics
Blood samples were collected on treatment days 7, 14, and 42 and at EOT. Collection was targeted 24 hours after the start of the infusion or the oral dose the previous day (ie, trough concentration). Full 24-hour profiles were obtained from a subset of 8 patients. After collection, samples were processed immediately and stored at −80°C until shipment to the central research laboratory. Isavuconazole (BAL4815) concentrations in plasma samples were measured retrospectively using a validated liquid chromatography–tandem mass spectrometry method as previously described [17]. Voriconazole concentrations were not measured.
PK/PD Analysis
A population PK/PD model was fitted to the data to describe the relationship between dose, drug exposure, and the time-course of GMI. Pmetrics was used for all model fitting [18]. Modeling proceeded in a stepwise fashion because a stable solution could not be obtained when the PK and PD were co-modeled. Both 1- and 2-compartment PK models that included an absorptive compartment were initially examined and distinguished [17]. The Bayesian posterior estimates for each patient’s PK were obtained. These PK parameter values were then fixed for each patient to enable their PD to be estimated. Subsequently, the mean Bayesian posterior estimates were used to calculate the area under the concentration-time curve (AUC) for each patient. The average AUC was calculated by dividing the AUC for the entire dosing duration by the total number of days on therapy. Because minimum inhibitory concentration (MIC) was not available, we used a newly described PD index (AUC:EC50) to estimate drug exposure in individual patients [19]. The EC50 is the concentration of drug that causes half-maximal antifungal killing. The relationship between AUC:EC50 and the terminal GM was described using an inhibitory sigmoid maximum effect (Emax) model.
Statistical Analysis
The risk of death through day 42 as a function of the change in GMI at days 7 and 14 was estimated using a Cox proportional hazards model. The overall response by EOT was analyzed using a logistic regression model for change in GMI. Not all individuals had GMI measured at each day; therefore, to calculate the above GMI changes, the GMI value of each individual per day was predicted from a joint event-time and longitudinal GMI model. Mortality through day 42 and overall response at the EOT included time to death and time to dropout, respectively, as the event-time outcome in each joint model. Dropout was defined if overall response was assessed prior at 84 days. Joint models are effective methods compared with classical linear mixed models to assess longitudinal GMI data. Two data sources of data are modeled simultaneously so that the underlying dependence between the longitudinal GMI and event-time is explicitly acknowledged [20–22]. The joint model consisted of a linear model with individual-level intercept, slope, and quadratic terms in the random-effects model for the time course of GMI and Cox proportional hazard model for the event-time and took the form:
where are longitudinal GMI at time , is the covariate for treatment (1 = isavuconazole; 0 = voriconazole) with regression parameter β2, β0 and β2 are population-level (fixed) intercept and slope parameters, respectively, are the corresponding random effects, ε accounts for measurement error in the time course of GMI, γ allows the association between GMI and event-time, and is the hazard rate of event. We used joineR library in R language to fit joint models.
For both assessments, we analyzed the total population primarily and then compared the 2 treatment groups. Prior to the analysis, the baseline comorbidities were reviewed to evaluate for any imbalances between the isavuconazole and voriconazole populations.
RESULTS
Overview of Patient Characteristics
The original study included 527 patients, with 185 intent-to-treat patients (71%) having positive baseline serum GMI [16]. Of these, 158 modified intent-to-treat patients (79 isavuconazole-treated and 79 voriconazole-treated) were available. Seventy-eight of the isavuconazole-treated patients with plasma concentrations were eligible for the PK/PD subanalysis. The patient demographics and baseline characteristics are summarized in Table 1. The isavuconazole-treated patients included numerically more patients with hematological malignancies, allogeneic hematopoietic stem cell transplant, use of T-cell immunosuppressants, use of corticosteroids, and neutropenia at baseline. The median white blood cell count at baseline was lower (0.3 vs 0.4 × 109 cells/L; P = .0152); however, this difference is not likely clinically relevant. Baseline median GMI in the isavuconazole group was significantly lower (0.8 vs 1.2; P = .0011). Except for a significant difference in gender (P = .0022), the remaining demographics were similar between the 2 treatment groups. The efficacy outcomes for the 2 groups were comparable to the primary study analyses [16]. However, the numerical differences in the outcomes were reflective of the higher number of immunosuppressed patients in the isavuconazole group (Table 2).
Table 1.
Patient Demographics and Characteristics at Baseline
| Characteristic | Isavuconazole (n = 79) | Voriconazole (n = 79) | Total (N = 158) |
P Value (χ2 Test) |
|---|---|---|---|---|
| Sex | ||||
| Male | 36 (46) | 56 (71) | 92 (58) | .0022 |
| Female | 43 (54) | 23 (29) | 66 (42) | |
| Race | ||||
| White | 67 (85) | 56 (71) | 123 (78) | |
| Asian | 11 (14) | 23 (29) | 34 (22) | |
| Other | 1 (11) | … | 1 (11) | |
| Age, y, mean ± SD | 52.4 ± 16.1 | 50.7 ± 15.2 | 51.5 ± 15.7 | |
| Weight, kg, mean ± SD | 68.4 ± 14.6 | 69.9 ± 14.6 | 69.2 ± 14.6 | |
| Underlying disease | ||||
| Hematological malignancy | 77 (97) | 70 (89) | 147 (93) | .0563 |
| Active malignancy | 60 (76) | 59 (75) | 119 (75) | 1.0 |
| Allogeneic HSCT | 27 (34) | 19 (24) | 46 (30) | .2203 |
| Baseline neutropenia | 62 (78) | 55 (70) | 117 (74) | .2762 |
| T-cell immunosuppressants | 40 (51) | 34 (43) | 74 (47) | .4254 |
| Use of corticosteroids | 15 (19) | 11 (14) | 26 (16) | .5198 |
| Baseline GMI, median (range) | 0.8 (0.1–6.2) | 1.2 (0.5–9.7) | 1.0 (0.1–9.7) | .0018 |
| Duration of therapy, d | ||||
| Mean ± SD | 50.2 ± 31.0 | 51.6 ± 31.6 | 50.9 ± 31.2 | |
| Min-Max | 5–85 | 2–88 | 2–88 | |
| Median | 53 | 53 | 53 | |
Data are presented as No. (11) unless otherwise indicated.
Abbreviations: d, day; GMI, galactomannan index; HSCT, hematopoietic stem cell transplantation; SD, standard deviation; y, year.
Table 2.
All-Cause Mortality Through Day 42 and Overall Outcome at the End of Therapy
| Mortality and Outcome | Isavuconazole (n = 79) | Voriconazole (n = 79) | Total (N = 158) | 95% CI | P Value |
|---|---|---|---|---|---|
| All-cause mortality through day 42 | 16 (20%) | 12 (15%) | 28 (17.7%) | −8.1% to 20.3% | .5320 |
| Successful overall response at EOT | 26 (33%) | 30 (38%) | 56 (35.4%) | −21.2% to 11.1% | .6178 |
Abbreviations: CI, confidence interval; EOT, end of therapy.
Change in GMI by Survival Status Through Day 42
The joint event-time longitudinal model predicted the day 7 GMI very closely compared to measured values in the subset of subjects with available data (Pearson correlation coefficient = 0.90; P = .0001) (Supplementary Figure 2A–E), increasing confidence about the associations between day 7 GMI change and outcomes, even if day 7 GMI was not measured in all patients.
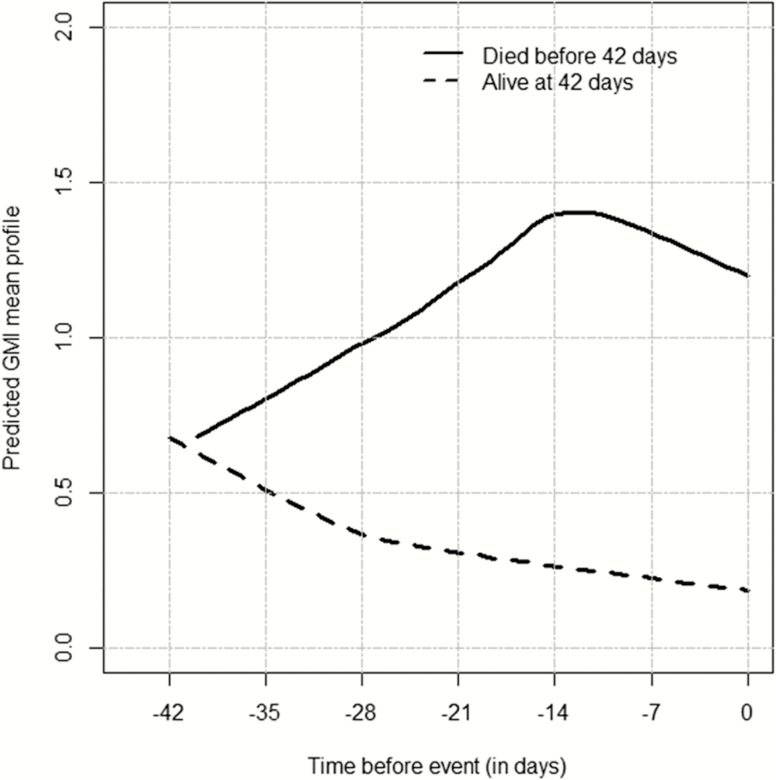
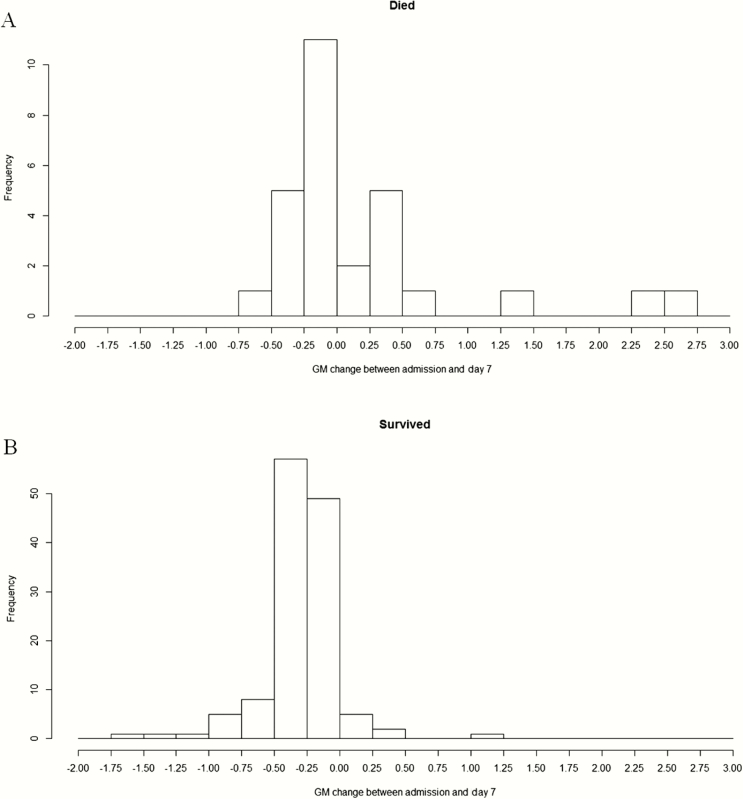
GMI steadily increased for the patients who died. The values for the patients who survived remained below 1.0, steadily declining during therapy (Figure 1). Changes in GMI by day 7 (ie, model-predicted day 7 GMI minus baseline GMI) for the patients who died (Figure 2A) were more positive than for those who survived (Figure 2B). A 3- and 1.7-fold increase in the risk of death was found for each unit increase in GMI from baseline to day 7 (hazard ratio [HR], 3.008; 95% confidence interval [CI], 1.993–4.541; P < .0001) and day 14 (HR, 1.69; 95% CI, 1.368–2.087; P < .0001), respectively.
Figure 1.
Predicted galactomannan index (GMI) mean profile from the joint model with treatment as a covariate in the longitudinal component for the overall population. Time = day 0 is the time when a patient died (cases) or had last follow-up alive (controls). GMI values were predicted at each day before event (death/alive). Negative values correspond to days before event.
Figure 2.
Change in galactomannan (GM) index at day 7 from baseline for patients who died (11) and survived (11) (overall population).
If GMI was treated categorically, an increase in GMI by day 7 of >0.25 GMI units from baseline increased the overall risk of death nearly 10-fold higher than those patients with GMI changes ≤0.25 at day 7. The change in GMI of >0.25 from baseline to day 14 predicted an overall 8-fold risk of death (HR, 8.37; 95% CI, .120–3.748; P < .0001).
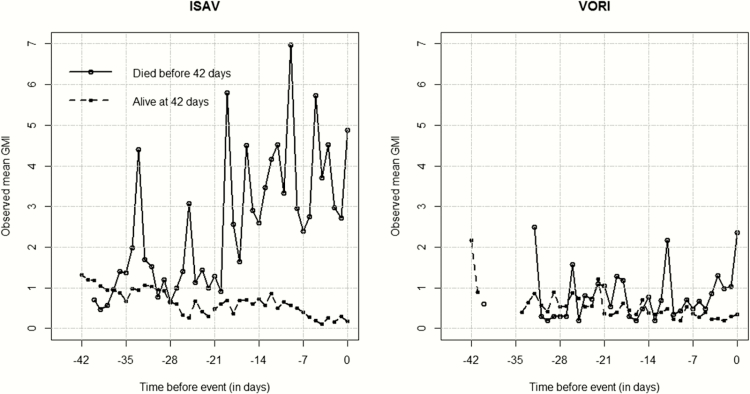
Because of differences in the underlying comorbidities between the 2 treatment groups, we focused the analysis on the total population of patients rather than compare the 2 treatment groups. In patients who died, the GMI was higher among isavuconazole-treated patients (Figure 3). Neutropenia was assessed using a linear mixed-effects model for the longitudinal measures of GMI with an interaction term between neutropenia and drug arms. No prominent differences were found between drugs, neutropenic status, and GMI over time (P = .1127).
Figure 3.
Observed galactomannan index (GMI) mean profile for each treatment group (isavuconazole [ISAV] and voriconazole [VORI]) in the longitudinal component. Time = day 0 is the time when a patient died (cases) or had last follow-up alive (controls). Mean GMI values were computed at each day before event (death/alive). Negative values correspond to days before event.
Change in GMI by Overall Response at the End of Therapy
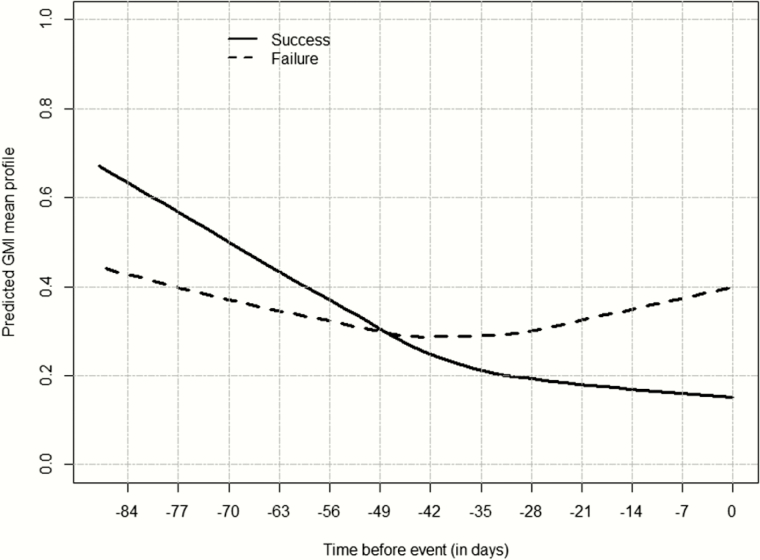
The GMI at the EOT was also higher in patients who failed therapy compared to those with a successful overall response (Figure 4). For GMI increases of 1 unit from baseline to day 7, the odds of successful overall response were reduced by half. Conversely, if the GMI decreased 1 unit by day 7, the success rate more than doubled (odds ratio, 2.154; 95% CI, 1.173–3.955). If the GMI decreased by >0.25 units, the successful overall response was increased by approximately 43% compared to that if GMI increased, although this change was not significant (P = .0704). By day 14, the success rate decreased by 35.5% for every unit increase in GMI (P = .0318). If the GMI decreased by >0.25 units, the success rate increased by approximately 21% compared to that if GMI increased, although this change was not significant (P = .2360).
Figure 4.
Predicted mean galactomannan index (GMI) profile at the end of therapy (EOT) for patients with a successful overall response (black line) and those who failed treatment (dashed line). Time 0 represents the assessment day (EOT) (overall population).
PK/PD Characteristics of Isavuconazole-Treated Patients
Seventy-eight isavuconazole-treated patients with positive baseline GMI had plasma concentrations and serial GMIs during therapy available for the PK/PD analysis. A 1-compartment model with an absorptive compartment fit the data well. Visual inspection of the observed vs predicted concentration values was acceptable. The coefficient of determination (r2) of predictions based on median Bayesian posterior parameter values for each subject was 0.829 and estimates of bias and imprecision were also acceptable (0.164 and 1.04, respectively).
The Bayesian posterior parameter estimates from the population PK model were calculated for each patient and included as fixed covariates in the data file. The fit of the linked PK/PD model to the data was good (Supplementary Data). The coefficient of determination of the linear regression of observed-predicted values (r2) after the Bayesian step was 0.859. Visual inspection of the observed-vs-predicted concentrations were acceptable and measures of bias and imprecision were reasonable (–0.155 and 0.328, respectively). The parameter estimates from the final model for PK and PD are summarized in Supplementary Table 1.
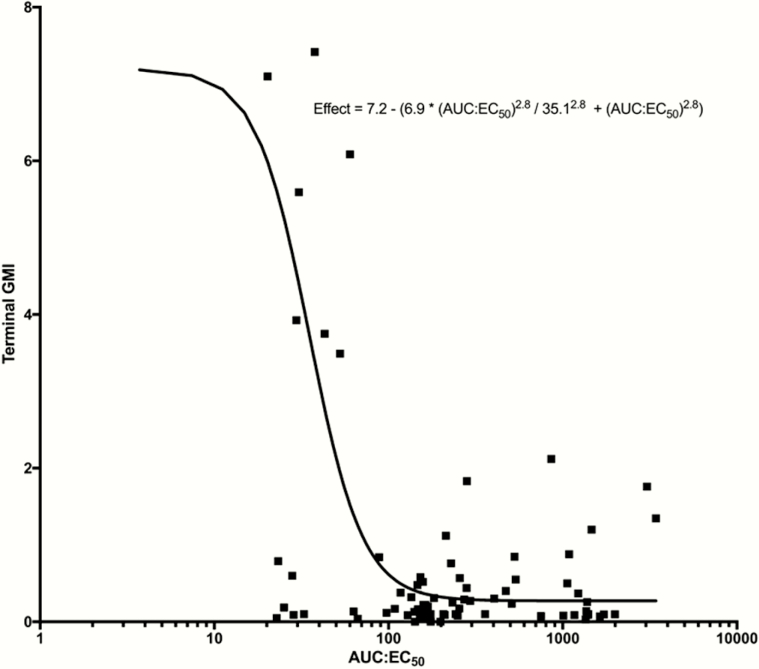
The relationship of the exposure to the predicted GMI at the EOT is shown in Figure 5. An AUC:EC50 of 108.6 or more resulted in a GMI <0.5. No relationship between change in GMI by day 7 or GMI at the EOT and average exposure (AUCave) was demonstrated, consistent with previous analyses [23].
Figure 5.
Inhibitory sigmoid maximum effect (Emax) curve demonstrating the pharmacokinetic/pharmacodynamic relationship of isavuconazole area under the concentration-versus-time curve over half-maximal effective concentration (AUC:EC50) and galactomannan index (GMI) values at the end of treatment (terminal GMI). Logistic regression resulted in an r2 of 0.237. Solid line represents the sigmoid curve fit of the model to the data, black squares represent the observed GMI values for each patient, and GMI negative value is included as a dotted line.
DISCUSSION
We demonstrated that early changes in GMI (within 1 week) are significantly linked to clinical response and mortality. An increase in GMI after 1 week of therapy of >0.25 GMI units is associated with an approximate 10-fold increased risk of death compared to patients who had no change from baseline, decreased GMI, or had an increase of <0.25 units. Based on the Platelia GM assay performance, 0.25 units is within the range of interassay variability. However, our results suggest that this threshold of response by day 7 is predictive of clinical response and mortality. Moreover, for any 1-unit increase in GMI baseline to day 7, the risk of mortality increases 3-fold. These results can now be used in a prospective study examining GM for directing antifungal therapy. If by the end of week 1 of treatment the GMI is increasing and is >0.25 above the baseline value, treatment could be modified.
All-cause mortality in SECURE was 19.4% [16]. In this subset, the rate was similar (17.7%); however, the number of deaths was just over a quarter of the number in the original study (28 vs 100 patients). Even with smaller numbers, the P values describing the significance of GMI change by day 7 in predicting outcome are quite low, suggesting that attributable mortality associated with IA as per increasing GMI is relatively high.
This analysis is consistent with previous studies in which serial GMI measurements were correlated with patient outcomes. A meta-analysis of >20 studies showed that a negative GMI within 1 week of outcome assessment is highly correlated with survival and successful outcome (alive or death without findings of aspergillosis at autopsy) [14]. Most reports have consistently reported that increasing GMI during therapy correlates with unsatisfactory clinical responses or mortality [12, 13, 15, 24–27]. In contrast to another analysis of a clinical trial where a correlation was found for OR but not for 12-week survival [26], we report that a change in GMI by the end of week 1 was significantly higher for patients who died through day 42 and in patients who had a failure in overall response at the EOT. In our study, a GMI change of >0.25 at 2 weeks was significantly associated with mortality but not overall response. In each analysis, the association of GMI changes by day 14 was not as strong as day 7. As per Chai et al [26], status of the underlying disease and comorbidities are increasingly powerful determinants of the final outcome with the passage of time.
Together with clinical care of patients with IA, these findings will be helpful for designing and assessing clinical trials of new anti-Aspergillus agents in GM-positive patients. An increasing GMI of >0.25 after the first week of therapy could be validated as an early marker of ultimate clinical failure and trigger a change in therapy, the addition of another agent, or an increase in dosage. A GMI <0.5 of >2 weeks’ duration was highly correlated with successful response and this has been proposed as a potential clinical trial endpoint [12]. We did not find a specific GMI cutoff value that linked with outcome at the end of week 1. Rather, our analyses suggested that early stabilization of GMI was highly correlated with an increased likelihood of successful outcome and survival, suggesting that an individual patient may not require absolute negativity of their GMI early in therapy to achieve ultimate therapeutic success.
For the subset of patients with isavuconazole concentrations, we estimated the AUC:EC50 that results in a negative GMI at the EOT. We were unable to define a relationship between traditional measures of drug exposures (eg, minimum concentration [Cmin] and AUC) and either clinical response or survival [28]. Our inability to identify a threshold of exposure (eg, AUCave) linked with response (change in GMI) could be because the regimen used in the trial provided exposures above those where thresholds are detectable (ie, drug exposures had already elicited maximal antifungal responses).
We used a newly described PD index of AUC:EC50 for our analysis because the MIC is rarely available for patients with IA. The EC50 is the estimated in vivo drug concentration that is required for half maximal antifungal killing. The EC50 requires some skills in PK/PD modeling and is estimated directly from the data rather than in the laboratory. It is an in vivo MIC where the patient declares what drug concentrations are required to adequately treat his or her individual disease. The AUC:EC50 provides an alternative way of evaluating the exposure-response relationships in the absence of a MIC for the invading pathogen, provided a biomarker, such as GMI, exists to determine the response of the organism to the drug concentrations.
Beyond drug, factors such as impaired host function, persistent neutropenia, dosage and duration of corticosteroids, immunosuppressive therapies, graft-vs-host disease, and concomitant infections (eg, cytomegalovirus viremia) could drive poor outcomes. These factors cannot be accounted for in model equations. Thus, the results should be interpreted carefully.
In conclusion, our analyses suggest that early trends in GMI are highly predictive of the ultimate therapeutic outcome. Increases in GMI by the end of the first week after therapy could trigger a change in treatment. Further validation is required to determine if the results hold. Such information may improve the likelihood of improved clinical responses and survival for a disease for which therapeutic outcomes remain persistently suboptimal.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. Isavuconazole was co-developed by Astellas Pharma Global Development, Inc and Basilea Pharmaceutica International Ltd.
Financial support. This work was supported by Astellas Pharma Global Development, Inc. W. W. H. is supported by a National Institute of Health Research Clinician Scientist Fellowship. M. N. received support from the National Institutes of Health (grant numbers R01 HD070886 and R01 GM068968).
Potential conflicts of interest. L. L. K. and M. L. are employees of Astellas Pharma Global Development, Inc. J. M. has received personal fees from Astellas, Gilead Sciences, MSD, and Pfizer and grants from Gilead Sciences, MSD, and Pfizer. W. W. H. has received grants and personal fees from Astellas and personal fees from Basilea during the conduct of the study; has received grants from Pfizer, AiCuris, and F2G; and has received personal fees from F2G, Gilead, Nordic Pharma, Mayne Pharma, and Pulmocide. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Schmiedel Y, Zimmerli S. Common invasive fungal diseases: an overview of invasive candidiasis, aspergillosis, cryptococcosis, and Pneumocystis pneumonia. Swiss Med Wkly 2016; 146:w14281. [DOI] [PubMed] [Google Scholar]
- 2. Patterson T, Thompson GR, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63:e1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leroux S, Ullmann AJ. Management and diagnostic guidelines for fungal diseases in infectious diseases and clinical microbiology: critical appraisal. Clin Microbiol Infect 2013; 19:1115–21. [DOI] [PubMed] [Google Scholar]
- 4. Lanternier F, Lortholary O. Liposomal amphotericin B: what is its role in 2008? Clin Microbiol Infect 2008; 14(suppl 4):71–83. [DOI] [PubMed] [Google Scholar]
- 5. Enoch DA, Idris SF, Aliyu SH, Micallef C, Sule O, Karas JA. Micafungin for the treatment of invasive aspergillosis. J Infect 2014; 68:507–26. [DOI] [PubMed] [Google Scholar]
- 6. Herbrecht R, Maertens J, Baila L, et al. Caspofungin first-line therapy for invasive aspergillosis in allogeneic hematopoietic stem cell transplant patients: an European Organisation for Research and Treatment of Cancer study. Bone Marrow Transplant 2010; 45:1227–33. [DOI] [PubMed] [Google Scholar]
- 7. Dolton MJ, McLachlan AJ. Voriconazole pharmacokinetics and exposure-response relationships: assessing the links between exposure, efficacy and toxicity. Int J Antimicrob Agents 2014; 44:183–93. [DOI] [PubMed] [Google Scholar]
- 8. Vermeulen E, Lagrou K, Verweij PE. Azole resistance in Aspergillus fumigatus: a growing public health concern. Curr Opin Infect Dis 2013; 26:493–500. [DOI] [PubMed] [Google Scholar]
- 9. Jeans AR, Howard SJ, Al-Nakeeb Z, et al. Pharmacodynamics of voriconazole in a dynamic in vitro model of invasive pulmonary aspergillosis: implications for in vitro susceptibility breakpoints. J Infect Dis 2012; 206:442–52. [DOI] [PubMed] [Google Scholar]
- 10. Petraitis V, Petraitiene R, Moradi PW, et al. Pharmacokinetics and concentration-dependent efficacy of isavuconazole for treatment of experimental invasive pulmonary aspergillosis. Antimicrob Agents Chemother 2016; 60:2718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al-Nakeeb Z, Petraitis V, Goodwin J, Petraitiene R, Walsh TJ, Hope WW. Pharmacodynamics of amphotericin B deoxycholate, amphotericin B lipid complex, and liposomal amphotericin B against Aspergillus fumigatus. Antimicrob Agents Chemother 2015; 59:2735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nouér SA, Nucci M, Kumar NS, Grazziutti M, Barlogie B, Anaissie E. Earlier response assessment in invasive aspergillosis based on the kinetics of serum Aspergillus galactomannan: proposal for a new definition. Clin Infect Dis 2011; 53:671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woods G, Miceli MH, Grazziutti ML, Zhao W, Barlogie B, Anaissie E. Serum Aspergillus galactomannan antigen values strongly correlate with outcome of invasive aspergillosis: a study of 56 patients with hematologic cancer. Cancer 2007; 110:830–4. [DOI] [PubMed] [Google Scholar]
- 14. Miceli MH, Grazziutti ML, Woods G, et al. Strong correlation between serum Aspergillus galactomannan index and outcome of aspergillosis in patients with hematological cancer: clinical and research implications. Clin Infect Dis 2008; 46:1412–22. [DOI] [PubMed] [Google Scholar]
- 15. Maertens J, Buvé K, Theunissen K, et al. Galactomannan serves as a surrogate endpoint for outcome of pulmonary invasive aspergillosis in neutropenic hematology patients. Cancer 2009; 115:355–62. [DOI] [PubMed] [Google Scholar]
- 16. Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 2016; 387:760–9. [DOI] [PubMed] [Google Scholar]
- 17. Kovanda LL, Desai AV, Lu Q, et al. Isavuconazole population pharmacokinetic analysis using nonparametric estimation in patients with invasive fungal disease (results from the VITAL Study). Antimicrob Agents Chemother 2016; 60:4568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 2012; 34:467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huurneman LJ, Neely M, Veringa A, et al. Pharmacodynamics of voriconazole in children: further steps along the path to true individualized therapy. Antimicrob Agents Chemother 2016; 60:2336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostatistics 2000; 1:465–80. [DOI] [PubMed] [Google Scholar]
- 21. Ibrahim JG, Chu H, Chen LM. Basic concepts and methods for joint models of longitudinal and survival data. J Clin Oncol 2010; 28:2796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Asar Ö, Ritchie J, Kalra PA, Diggle PJ. Joint modelling of repeated measurement and time-to-event data: an introductory tutorial. Int J Epidemiol 2015; 44:334–44. [DOI] [PubMed] [Google Scholar]
- 23. Desai A, Kovanda L, Hope W, et al. Exposure–response analysis of isavuconazole in patients with disease caused by Aspergillus species or other filamentous fungi. In: European Conference of Clinical Microbiology and Infectious Diseases, Copenhagen, Denmark, 2015. [Google Scholar]
- 24. Boutboul F, Alberti C, Leblanc T, et al. Invasive aspergillosis in allogeneic stem cell transplant recipients: increasing antigenemia is associated with progressive disease. Clin Infect Dis 2002; 34:939–43. [DOI] [PubMed] [Google Scholar]
- 25. Chai LY, Kullberg BJ, Johnson EM, et al. Early serum galactomannan trend as a predictor of outcome of invasive aspergillosis. J Clin Microbiol 2012; 50:2330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chai LY, Kullberg BJ, Earnest A, et al. Voriconazole or amphotericin B as primary therapy yields distinct early serum galactomannan trends related to outcomes in invasive aspergillosis. PLoS One 2014; 9:e90176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neofytos D, Railkar R, Mullane KM, et al. Correlation between circulating fungal biomarkers and clinical outcome in invasive aspergillosis. PLoS One 2015; 10:e0129022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Desai A, Kovanda L, Kowalski D, Townsend R, Mujais S, Bonate P. Exposure-safety analysis of isavuconazole in patients from SECURE study with disease caused by Aspergillus species or other filamentous fungi. In: 55th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, 2015. Vol. A-019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.