Abstract
AIM
To examine the normal morphology of the epiligament tissue of the knee medial collateral ligament (MCL) in humans.
METHODS
Several samples of the mid-substance of the MCL of the knee joint from 7 fresh human cadavers (3 females and 4 males) were taken. Examination of the epiligament tissue was conducted by light microscopy and photomicrography on semi-thin sections of formalin fixed paraffin-embedded blocks that were routinely stained with haematoxylin and eosin, Mallory stain and Van Gieson’s stain. Electron microscopy of the epiligament tissue was performed on ultra-thin sections incubated in 1% osmium tetroxide and contrasted with 2.5% uranyl acetate, lead nitrate, and sodium citrate.
RESULTS
The current light microscopic study demonstrated that the epiligament of the MCL consisted of fibroblasts, fibrocytes, adipocytes, neuro-vascular bundles and numerous multidirectional collagen fibers. In contrast, the ligament body was poorly vascularised, composed of hypo-cellular fascicles which were formed of longitudinal groups of collagen fibers. Moreover, most of the vessels of the epiligament-ligament complex were situated in the epiligament tissue. The electron microscopic study revealed fibroblasts with various shapes in the epiligament substance. All of them had the ultrastructural characteristics of active cells with large nuclei, well developed rough endoplasmic reticulum, multiple ribosomes, poorly developed Golgi apparatus, elliptical mitochondria and oval lysosomes. The electron microscopy also confirmed the presence of adipocytes, mast cells, myelinated and unmyelinated nerve fibers and chaotically oriented collagen fibers.
CONCLUSION
Significant differences exist between the normal structure of the ligament and the epiligament whose morphology and function is to be studied further.
Keywords: Knee, Epiligament, Knee medial collateral ligament, Electron Microscopy, Humans, Microscopy, Photomicrography
Core tip: The epiligament of the medial collateral ligament of the human knee is an important enveloping supporting structure of the ligament proper containing fibroblasts, fibrocytes, adipocytes, mast cells, and neurovascural bundles in a network of collagen fibres that is not limited to the surface of the ligament but also pervades it, as the endoligament, thus providing the cellular elements and blood vessels that participate in the ligament’s nutrition and during the process of healing.
INTRODUCTION
The medial collateral ligament (MCL) of the knee joint, also known as tibial collateral ligament (TCL), is an often injured ligamentous structure of the knee joint[1-3]. Ninety percent of knee ligament injuries involve the MCL or the anterior cruciate ligament (ACL)[4]. The incidence of this injury has increased in recent years and presents a commonly encountered problem in modern sports medicine[5,6]. Most injuries result from a valgus force on the knee from direct contact or with cutting manoeuvres, namely when athletes place a foot in a stable position and then rapidly change the direction of movement[7]. The popularity of sports such as football, skiing and ice hockey has also contributed to the increased incidence of MCL injuries[8]. According to the model of Warren and Marshall, the medial knee is divided into the following three layers: Superficial (I), intermediate (II), and deep (III)[9]. The superficial layer (I) consists of the deep crural fascia which invests the sartorius and quadriceps and continues into the deep fascia of the lower extremity, where it covers the gastrocnemius and the popliteal fossa. Layer II, or the intermediate layer, includes the superficial MCL (sMCL) and medial patellofemoral ligament (MPFL). Layer III is the deep layer and comprises the joint capsule and the deep MCL (dMCL). The superficial and dMCL have similar functions and act as the primary supporting structures of the medial side of the knee[5,10], therefore injuries to these structures merit due attention and adequate treatment[1]. The healing of ligaments after injury is associated with scar tissue formation rather than regeneration[11-14].
The structure of the MCL has been studied extensively, however, very little is known about the thin layer of connective tissue adherent to this ligament, termed the epiligament (EL) [epi-(Greek-on or upon); ligament (Latin-ligare, to bind)]. In 1990, Bray et al[15] described the epiligament as a “surrounding adherent connective tissue removed simultaneously with the ligament but which was grossly distinguishable from ligament tissue proper”. Our previous studies on the MCL in rat knee models led to the conclusion that the EL tissue plays a key role in the healing of the ligament tissue after injury[13,16,17]. According to Georgiev and Vidinov[18-20] and Georgiev et al[12,13,16,21,22] the EL is a donor of fibroblasts, progenitor cells and blood vessels, which proliferate and migrate towards the body of the ligament through the endoligament during the process of ligament recovery. Fibroblasts in the EL tissue normally produce collagen types I, III, V, fibronectin (FN) and matrix metalloproteinases-2 and -9 (MMP-2, -9) which are essential for the normal functioning of the ligament and their synthesis is increased in order to promote adequate repair after injury[13,16,17,21,23]. Therefore, detailed knowledge of the morphology and function of the EL during physiological conditions and post injury is important in deepening our knowledge with regard to ligament healing and may thus lead to proposal of better treatment options in the future. There is plentiful literature data concerning the role of the EL during MCL healing in rats, however its normal anatomy in humans has not been studied yet. In line with this, the aim of this study is to investigate the normal morphology of the MCL EL in humans for the first time in the literature, through light and electron microscopy and to compare it to the ligament substance.
MATERIALS AND METHODS
Several samples of the mid-substance of the MCL of the knee joint of 7 cadavers (3 females and 4 males) were taken from the fresh human cadavers available at the Department of Anatomy, Histology and Embryology at the Medical University of Sofia. The study was approved by the Medical Legal Office, the Local Ethics Committee and the Institutional Review Board.
After skin incision, the overlying connective tissue was dissected to expose the knee’s MCL. The MCL and the external surface of the surrounding EL were precisely dissected and then the pieces were immediately fixed in formalin (Merck Catalogue No. 1040031000) for light microscopy or in 3% glutaraldehyde (Merck Catalogue No. 354400) for 2 h for electron microscopy.
Light microscopic study protocol
After fixation, the samples were embedded in paraffin and cut into semi-thin sections that were stained routinely with HE (Haematoxylin Merck Catalogue No. 105 1741000; Eosin Merck Catalogue No. 1170811000), Mallory stain and Van Gieson’s stain. Photomicrographs of representative fields of the light microscopy staining were obtained using Olympus CX 21 microscope fitted with an Olympus C5050Z digital camera (Olympus Optical Co, Ltd).
Electron microscopic study protocol
After fixation, the tissues were rinsed several times with 0.1% phosphate buffer (Merck Catalogue No. 146 5920006) to remove the fixative solution and were incubated in 1% osmium tetroxide (Merck Catalogue No. 1245050500) for 2 h. Then the pieces were dehydrated in EtOH (50%, 70%, 95%, 100%) (Merck Catalogue No. 1009835000) and treated for 30 min with a 2:1 mixture of propylene oxide (Merck Catalogue No. 807027) and epon. The pieces were embedded in Durcupan (Fluka, Buchs, Switzerland). Afterwards, all slices were processed with a dissectional microscope and cut by an ultramicrotome (LKB, Stockholm-Bromma, Sweden). The EL regions were identified on semi-thin sections. Ultrathin sections (60 nm thick) were taken only from the MCL EL and then both were contrasted with 2.5% uranyl acetate, lead nitrate, and sodium citrate. We used a Hitachi 500 electron microscope.
RESULTS
Normal morphology of the MCL EL: Light microscopy
The light microscopic study revealed that human MCL EL is markedly distinctive from the ligament substance and confirmed our previous observations in rats. The external surface of the MCL EL was comprised of fibroblasts, fibrocytes, adipocytes, mast cells and neuro-vascular bundles as well as numerous multidirectional collagen fibres (Figure 1). The EL was relatively rich in blood vessels (Figure 1A). In contrast to the EL, the ligament tissue was poorly vascularised and composed of uniform fascicles that were formed of longitudinally aligned groups of collagen fibres. Each fascicle appeared hypo-cellular and the scarce cells were interspersed between bundles of collagen fibres. Unlike in the ligament, the collagen fibres in the EL of the midportion of the MCL were quite similar in diameter and were positioned in bundles with various orientation.
Figure 1.

Normal morphology of the medial collateral ligament epiligament tissue in humans. A and B: Normal morphology of the MCL EL tissue. Haematoxylin and eosin stain, × 200. EL: Epiligament; L: Ligament; red arrows: Adipocytes; arrows: Vessels in the EL tissue; C and D: Normal morphology of the MCL EL tissue. Mallory stain, × 200. EL: Epiligament; L: Ligament; arrows: Vessels in the EL tissue; arrow head: The EL extending into the endoligament; E and F: Normal morphology of the MCL EL tissue. Van Gieson’s stain, × 200. EL: Epiligament; L: Ligament; red arrows: Adipocytes; arrow head: The EL extending into the endoligament; MCL: Medial collateral ligament.
Normal morphology of the MCL EL: Electron microscopy
The electron microscopy revealed the presence of various types of fibroblasts in the EL: Spindle-shaped, spinous-shaped, elongated and fibroblasts with irregular shape. They had large nuclei, well developed rough endoplasmic reticulum, multiple ribosomes, poorly developed Golgi complex, individual elliptical mitochondria and oval, individually located lysosomes (Figure 2A-C). The electron microscopy also manifested the presence of adipocytes and mast cells (Figure 1F). The mast cells had well-presented nuclei with peripheral heterochromatin. The cytoplasm contained the specific round or oval granules. The granules were always enclosed by a membrane and separated from other granules by cytoplasmic septa. The matrix of each granule was homogeneous and electron-dense. The electron microscopy revealed that the adipocytes had large lipid droplets which pushed the rest of cytoplasm at the periphery of the cell. The nuclei of the adipocytes were eccentrically located.
Figure 2.
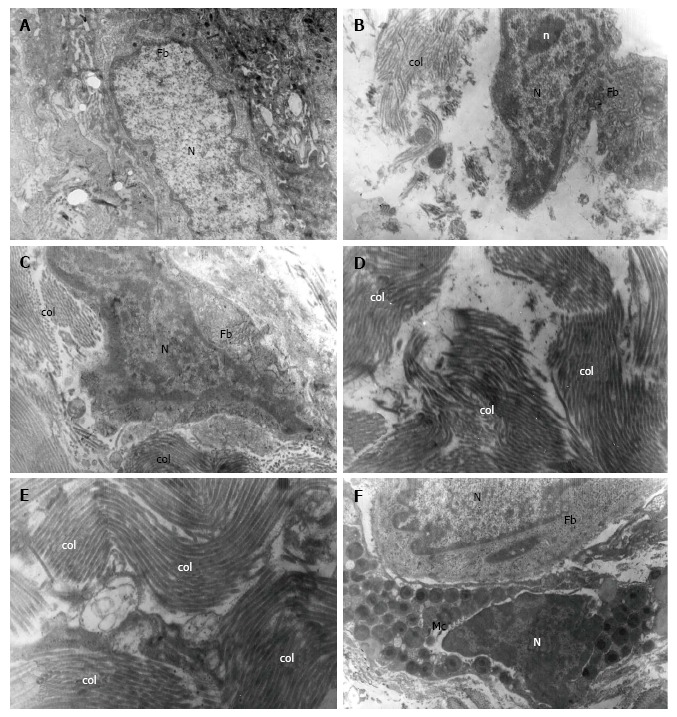
Normal morphology of the medial collateral ligament epiligament tissue in human. A-C: Electron micrograph of a fibroblast (Fb) and its nucleus (N). Mitochondria, lysosomes and rough endoplasmic reticulum are visible in the cytoplasm of the Fb; in the extracellular matrix numerous collagen fibers (col) are presented × 7000; D and E: Electron micrograph of EL collagen fibers (col) in the extracellular matrix oriented in different directions × 7000, × 9000; F: Electron micrograph of a fibroblast (Fb) and its nucleus (N) and a mast cell (Mc) with numerous granules and its nucleus (N), × 9000.
Collagen fibres in the EL of the midportion of the MCL were quite similar in diameter and were organized in bundles with various orientation, unlike the parallel pattern of distribution of collagen fibres in the ligament (Figure 2D and E). Again, chaotically oriented small groups of collagen fibres were observed. Both myelinated and unmyelinated nerve fibres were detected.
DISCUSSION
The ligament is built of connective tissue, which comprises two main elements-connective tissue cells and extracellular matrix[11,24]. Collagen fibres in the ligaments are organized in longitudinal groups and form fascicles[11,24,25]. The thin layer of connective tissue separating these fascicles is known as endoligament and is related to another connective tissue structure, containing more blood vessels, which envelops the entire ligament and is known as epiligament[12,13,16-18]. In rabbits, Chowdhury et al[26] (1991) examined the external surface of the MCL EL and described two types of cells - spinous-shaped adipocytes and fibroblasts. It is fibroblasts that produce collagen fibers and thus are responsible for the formation of scar tissue[26]. In rats, Georgiev et al[12,13,16,17] showed the external portion of the MCL EL to consist of fibroblasts, fibrocytes, adipocytes, neurovascular bundles, and a number of collagen fibres, oriented in varying directions. These cells are located among bundles of collagen fibres. Georgiev et al[22] also described the ultrastructural characteristics of the different types of fibroblasts in the EL of the lateral collateral ligament (LCL) in rat knees. In terms of shape, they described spindle-shaped fibroblasts, small elongated fibroblasts and fibroblasts with irregular shape. All of these cells were characterized by the presence of a large nucleus with prominent nucleoli, well-developed rough endoplasmic reticulum and numerous ribosomes. These ultrastructural characteristics led Georgiev et al[22] to conclude that fibroblasts in the EL might take part in the differentiation, phagocytosis and collagen synthesis, possibly thus playing a role in the regeneration of the ligament after injury, which has also been proposed by other authors[11]. Moreover, fibroblasts in the EL may proliferate and migrate through the endoligament into the ligament proper[18,27].
Other rarely observed types of cells are mast cells which have an oval shape and numerous granules with homogeneous density[22]. Adipocytes are organized in cellular lobuli, enveloped by thing connective tissue fibres and represent the building blocks of white adipose tissue[24]. According to Chowdhury et al[26] adipocytes synthesize, process and store lipids and thus participate in nutrition and confine specific storage and protective functions to the EL.
In humans, our light microscopic and ultrastructural study confirmed the aforementioned characteristics of the EL tissue and its constituent cells. On light microscopy, we noted the existence of fibroblasts, fibrocytes, adipocytes, neuro-muscular bundles and numerous multidirectional collagen fibres. This greatly resembled the structure of the EL observed in rats[22]. Also, we observed that the main cytological features of the EL were closely related to those in the synovium. This provides further support to the theory that the EL is a specialised form of synovium[11,28]. Electron microscopy revealed a great variety of fibroblasts in terms of shape - spindle-shaped, spinous-shaped, elongated and irregularly-shaped, which confirmed earlier results in rats[22]. We found an abundance of structures in their cytoplasm, namely a well-developed rough endoplasmic reticulum and multiple ribosomes, which supports the hypothesis that fibroblasts play a key role in the ligament nutrition and healing after injury[11,22].
As in the rat and the rabbit, the EL tissue in humans appears to contain a relative abundance of blood vessels[12,24,26,29,30]. Blood vessels in the EL are randomly dispersed in an amorphous structure, built of loose connective tissue[26,30]. They branch extensively, forming anastomotic networks of interconnected vessels[29,30]. Blood vessels in the EL are often accompanied by nerve bundles, but apparently not all blood vessels are organized in a neurovascular bundle[15,26,30].
The healing of ligaments after injury is associated with scar tissue formation rather than regeneration, which shows common mechanisms to the healing processes in other soft tissue structures[31-34]. According to Frank et al[32] injury location has an impact on ligament healing. The MCL heals much better and faster than the ACL of the knee joint. This is most likely due to the specific characteristics of the EL, located above the MCL. Georgiev and Vidinov[18-20], Georgiev et al[13,16,17,21,22] and Lo et al[29], claim that the EL may be the primary donor of connective tissue cells participating in the scar formation as part of the process of ligament healing. Fibroblasts are not static cells and as such can migrate from the EL to the healing ligament[12,13,21,22,26]. According to Chamberlain et al[27], ligament injuries stimulate the migration of various cell types from the EL, including neutrophils and cells in the process of mitosis up to the fifth day after injury, which proves that there is a bilateral cooperation between the EL and the ligament with regard to adequate healing of the ligament.
In conclusion, this study illustrates in detail the normal morphology of the MCL EL in humans and demonstrates its difference from the structure of the ligament tissue for the first time. The electron microscopic study reveals the specific characteristics of the various types of cells in the EL and supports the hypothesis that fibroblasts in particular, together with the abundant blood vessels are essential for the nutrition and healing of the MCL.
ACKNOWLEDGMENTS
The authors would like to express their most sincere gratitude and to pay their respect to all the men and women who donated their bodies for the purpose of scientific research.
COMMENTS
Background
The epiligament has relatively recently been shown to be a distinct structure enveloping ligaments in mammals and to be the main donor of cells and blood vessels for ligament nutrition and healing not only at its periphery but also within its substance where it penetrates as a ramified network - the endoligament.
Research frontiers
Previous research was performed on rat and rabbit models yielding consistent results.
Innovations and breakthroughs
This is the first light microscopic and ultrastructural study of the epiligament in humans showing it to be structurally, and possibly functionally, similar to that of other mammals.
Applications
Improving the understanding of the biology of the epiligament tissue might further the development and fine-tuning of treatment modalities after ligament injuries.
Terminology
Epiligament: A connective tissue structure enveloping the ligaments and containing cells and blood vessels necessary for the nutrition and healing of the ligament; Endoligament: The ramifications of the epiligament within the ligament substance.
Peer-review
The content is clear and definite, level of structure is logical and accurate. The research method is scientific and reasonable. The article is well-written.
Footnotes
Institutional review board statement: The study was approved by the Medical University of Sofia Institutional Review Board.
Institutional animal care and use committee statement: No animals were analysed during this study.
Conflict-of-interest statement: The authors declare that there is no conflict of interest regarding the publication of this article.
Data sharing statement: No additional data are available.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Bulgaria
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: December 25, 2016
First decision: February 17, 2017
Article in press: March 13, 2017
P- Reviewer: Anand A, Luo XH S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Fetto JF, Marshall JL. Medial collateral ligament injuries of the knee: a rationale for treatment. Clin Orthop Relat Res. 1978;132:206–218. [PubMed] [Google Scholar]
- 2.Najibi S, Albright JP. The use of knee braces, part 1: Prophylactic knee braces in contact sports. Am J Sports Med. 2005;33:602–611. doi: 10.1177/0363546505275128. [DOI] [PubMed] [Google Scholar]
- 3.Phisitkul P, James SL, Wolf BR, Amendola A. MCL injuries of the knee: current concepts review. Iowa Orthop J. 2006;26:77–90. [PMC free article] [PubMed] [Google Scholar]
- 4.Woo SL, Abramowitch SD, Kilger R, Liang R. Biomechanics of knee ligaments: injury, healing, and repair. J Biomech. 2006;39:1–20. doi: 10.1016/j.jbiomech.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Wijdicks CA, Griffith CJ, Johansen S, Engebretsen L, LaPrade RF. Injuries to the medial collateral ligament and associated medial structures of the knee. J Bone Joint Surg Am. 2010;92:1266–1280. doi: 10.2106/JBJS.I.01229. [DOI] [PubMed] [Google Scholar]
- 6.Georgiev GP, Stokov L. Acute medial collateral ligament and associated medial structures injuries of the knee: current concepts review. Bulg J Orthop Trauma. 2011;48:7–12. [Google Scholar]
- 7.Indelicato PA. Isolated Medial Collateral Ligament Injuries in the Knee. J Am Acad Orthop Surg. 1995;3:9–14. doi: 10.5435/00124635-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Kim PD, Ahmad CS, Levine WN. Medial collateral ligament injuries of the knee: current treatment concepts. Curr Rev Musculoskelet Med. 2008;1:108–113. doi: 10.1007/s12178-007-9016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren LF, Marshall JL. The supporting structures and layers on the medial side of the knee: an anatomical analysis. J Bone Joint Surg Am. 1979;61:56–62. [PubMed] [Google Scholar]
- 10.LaPrade MD, Kennedy MI, Wijdicks CA, LaPrade RF. Anatomy and biomechanics of the medial side of the knee and their surgical implications. Sports Med Arthrosc. 2015;23:63–70. doi: 10.1097/JSA.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 11.Lo IK, Ou Y, Rattner JP, Hart DA, Marchuk LL, Frank CB, Rattner JB. The cellular networks of normal ovine medial collateral and anterior cruciate ligaments are not accurately recapitulated in scar tissue. J Anat. 2002;200:283–296. doi: 10.1046/j.1469-7580.2002.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgiev GP, Landzhov B, Dimitrova IN, Slavchev S, Malinova L, Kartelov Y, Ankova D, Ovtscharoff W. Light microscopic and immunohistochemical study of the medial collateral ligament epiligament in rat knee. Compt Rend Acad Bulg Sci. 2015;68:95–100. [Google Scholar]
- 13.Georgiev GP, Landzhov B, Dimitrova IN, Slavchev S, Malinova L, Ovtscharoff W. Immunohistochemical study during early healing of the medial collateral ligament epiligament in rat knee model. Compt Rend Acad Bulg Sci. 2015;68:655–660. [Google Scholar]
- 14.Landzhov B, Georgiev GP, Brainova I. The epiligament-the main donor of cells and vessels during healing of the collateral ligaments of the knee. Anat Physiol. 2015;4:1–6. [Google Scholar]
- 15.Bray RC, Fisher AW, Frank CB. Fine vascular anatomy of adult rabbit knee ligaments. J Anat. 1990;172:69–79. [PMC free article] [PubMed] [Google Scholar]
- 16.Georgiev GP, Landzhov B, Dimitrova IN, Malinova L, Ovtscharoff W. Expression of fibronectin during early healing of the medial collateral ligament epiligament in rat knee model. Compt Rend Acad Bulg Sci. 2016;69:639–644. [Google Scholar]
- 17.Georgiev GP, Iliev A, Landzhov B, Dimitrova IN, Kotov G, Malinova L, Ovtscharoff W. Localization of matrix metalloproteinase-2 in injured medial collateral ligament epiligament in rat knee. Compt Rend Acad Bulg Sci. 2017;70:273–278. [Google Scholar]
- 18.Georgiev GP, Vidinov NK. Investigation of the epiligament morphology of the lateral collateral ligament during postnatal development in a rat knee model. Compt Rend Acad Bulg Sci. 2009;62:1473–1478. [Google Scholar]
- 19.Georgiev GP, Vidinov NK. Electron and light microscopic study of the epiligament of the lateral collateral ligament in a rat knee joint during early postnatal development. J Biomed Clin Res. 2009;2:166–168. [Google Scholar]
- 20.Georgiev GP, Vidinov NK. Epiligament changes after injury of the knee lateral collateral ligament in rat. J Biomed Clin Res. 2009;2:96–98. [Google Scholar]
- 21.Georgiev GP, Kinov P, Rashev P, Sapundzhiev E, Vidinov NK. Changes in the distribution of fibrillar collagens during early healing of the lateral collateral ligament epiligament tissue in rat knee model. Compt Rend Acad Bulg Sci. 2010;63:761–766. [Google Scholar]
- 22.Georgiev GP, Vidinov NK, Kinov PS. Histological and ultrastructural evaluation of the early healing of the lateral collateral ligament epiligament tissue in a rat knee model. BMC Musculoskelet Disord. 2010;11:117. doi: 10.1186/1471-2474-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iliev A, Georgiev GP, Dimitrova IN, Kotov G, Malinova L, Rashev P, Landzhov B. Expression of matrix metalloproteinase-2 and 9 in the medial collateral ligament epiligament in rat knee. Acad Anat Int. 2016;2:44–48. [Google Scholar]
- 24.Junqueira LC, Carneiro J, Kelley RO. New York - Toronto: Lange Medical Books/McGraw-Hill; 1998. Basic Histology. In Connective tissue. 9th edition; pp. 89–117. [Google Scholar]
- 25.Bray RC, Salo PT, Lo IK, Ackermann P, Rattner JB, Hart DA. Normal ligament structure, physiology and function. Sports Med Arthrosc Rev. 2005;13:127–135. [Google Scholar]
- 26.Chowdhury P, Matyas JR, Frank CB. The “epiligament” of the rabbit medial collateral ligament: a quantitative morphological study. Connect Tissue Res. 1991;27:33–50. doi: 10.3109/03008209109006993. [DOI] [PubMed] [Google Scholar]
- 27.Chamberlain CS, Crowley E, Vanderby R. The spatio-temporal dynamics of ligament healing. Wound Repair Regen. 2009;17:206–215. doi: 10.1111/j.1524-475X.2009.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Key JA. The reformation of synovial membrane in the knees of rabbits after synovectomy. J Bone Joint Surg. 1925;7:793–813. [Google Scholar]
- 29.Lo IK, Marchuk LL, Leatherbarrow KE, Frank CB, Hart DA. Collagen fibrillogenesis and mRNA levels in the maturing rabbit medial collateral ligament and patellar tendon. Connect Tissue Res. 2004;45:11–22. doi: 10.1080/03008200490278070. [DOI] [PubMed] [Google Scholar]
- 30.Bray RC, Rangayyan RM, Frank CB. Normal and healing ligament vascularity: a quantitative histological assessment in the adult rabbit medial collateral ligament. J Anat. 1996;188(Pt 1):87–95. [PMC free article] [PubMed] [Google Scholar]
- 31.Breuls RG, Klumpers DD, Everts V, Smit TH. Collagen type V modulates fibroblast behavior dependent on substrate stiffness. Biochem Biophys Res Commun. 2009;380:425–429. doi: 10.1016/j.bbrc.2009.01.110. [DOI] [PubMed] [Google Scholar]
- 32.Frank C, Woo SL, Amiel D, Harwood F, Gomez M, Akeson W. Medial collateral ligament healing. A multidisciplinary assessment in rabbits. Am J Sports Med. 1983;11:379–389. doi: 10.1177/036354658301100602. [DOI] [PubMed] [Google Scholar]
- 33.Frank CB, Hart DA, Shrive NG. Molecular biology and biomechanics of normal and healing ligaments--a review. Osteoarthritis Cartilage. 1999;7:130–140. doi: 10.1053/joca.1998.0168. [DOI] [PubMed] [Google Scholar]
- 34.Frank C, Shrive N, Hiraoka H, Nakamura N, Kaneda Y, Hart D. Optimisation of the biology of soft tissue repair. J Sci Med Sport. 1999;2:190–210. doi: 10.1016/s1440-2440(99)80173-x. [DOI] [PubMed] [Google Scholar]


