Summary
The milk processing industry is one of the world’s staple industries, thus the treatment possibilities of dairy effluents have been attracting more and more attention. The purpose of the paper is to review contemporary research on dairy wastewater. The origin, categories, as well as liquid by-products and general indicators of real dairy wastewater are described. Different procedures applied for dairy wastewater management are summarised. Attention is focused on in-factory treatment technologies with the emphasis on biological processes. Aerobic and anaerobic methods with both their advantages and disadvantages are discussed in detail. Consecutive anaerobic and aerobic systems are analysed, too. Finally, future research niches are identified.
Key words: dairy wastewater, wastewater composition, whey, biological treatment
Introduction
The dairy industry includes the transformation of raw milk into pasteurised and sour milk, yoghurt, hard, soft and cottage cheese, cream and butter products, ice cream, milk and whey powders, lactose, condensed milk, as well as various types of desserts (1–6). The general distinctions among these foods are due to the reuse of non- -fat milk and whey (a by-product in cheese manufacturing) and the evaporation of the free water from the coagulum as well as from milk and whey powders (5). With the rapid industrialisation observed in the last century (4) and the growing rate of milk production (around 2.8% per annum), dairy processing is usually considered the largest industrial food wastewater source, especially in Europe (1–3, 7). Moreover, in around 50% of the world’s whey production, especially concerning acid whey, it is untreated prior to disposal (8–10). The effluents originating from various production technologies are not discharged simultaneously, thus forming a stream with wide qualitative and quantitative variations (4). Notwithstanding the differences in composition, attributable to the manufactured product and technological operations (11, 12), dairy effluents are distinguished by their relatively increased temperature, high organic content (13–15) and a wide pH range, which requires special purification in order to eliminate or reduce environmental damage (1). Treatments of dairy wastewaters include the application of mechanical, physicochemical and biological methods. Mechanical treatment is necessary to equalise volumetric and mass flow changes. It also reduces parts of the suspended solids. Physicochemical processes are effective in the removal of emulsified compounds, but reagent addition increases water treatment costs. Another disadvantage is the very low elimination of soluble chemical oxygen demand (COD). Therefore, biological wastewater treatment systems are preferred due to the highly biodegradable contaminants (7, 11, 16, 17).
The purpose of the paper is to review the data on the basic composition and treatment possibilities of milk- -processing effluents. Their origin and major characteristics are summarised. Various methods for wastewater utilisation are discussed. Finally, suggestions for future research are made.
Dairy Wastewater Characteristics
Wastewater volume
Water plays a key role in milk processing. It is used in every step of the technological lines, including cleaning and washing, disinfection, heating and cooling. Water requirements are huge (14).
The bulk of wastewater comes from manufacturing processes (6). Contaminated water, including sanitary activities, reaches 50–80% of the total water consumed in the dairy factory, whereas the remaining 20–50% is conditionally clean (6, 18). It has been estimated that the amount of wastewater is approx. 2.5 times higher than that of processed milk in units of volume. The amount and characteristics of the wastewater depend largely on the factory size, applied technology, effectiveness and complexity of clean-in-place (CIP) methods, good manufacture practices (GMP), etc. (3, 4). However, the introduction of GMP can reduce the world’s wastewater mean volume from 0.5–37 to 0.5–2 m3 of effluent per m3 of processed milk (3, 19). Nowadays, the designed volumetric load is 1 m3 of effluent per tonne of manufactured milk (4).
In dairy plants, the great fluctuations in wastewater quality and quantity are very problematic because each milk product needs a separate technological line (1, 5). This results in the change of dairy effluent composition with the start of a new cycle in the manufacturing process, which impedes the work of in-factory wastewater treatment plants. Furthermore, intensive effluent volumetric variations in time are commonly observed. Daily and hourly changes are the consequence of washing the equipment and floors as the final step in every process cycle. Seasonal variations can be attributed to a higher dairy plant load in summer than in winter (20). One way of explaining hourly homogeneity is by coefficients in the range of 1.4–2.0 (6). The diurnal inequality coefficient depends on the seasonal character of dairy processing, varying from 1.5 for 2- and 3-shift work in summer to 2.6 for winter shifts. The actual concentration of polluting dairy effluents varies widely depending on the profile and capacity of the company, the production technology, the type of equipment used, the degree of wastewater reuse, the loss of raw materials, waste management, etc. (1, 18). A major factor in the volumetric loading of dairy wastewater treatment plants are the immediate discharges produced in the cleaning of tank trucks, pipelines or equipment at the end of each cycle. In such cases, the effluent volumes are higher than those of manufactured milk (4, 21). On average, wastewater discharge is 70% of the amount of the fresh water used at the plant (6).
Dairy processing effluents mostly include milk or milk products lost in the technological cycles (spilled milk, spoiled milk, skimmed milk and curd pieces); starter cultures used in manufacturing; by-products of processing operations (whey, milk and whey permeates); contaminants from the washing of milk trucks, tanks, cans, equipment, bottles and floors; reagents applied in CIP procedures, cooling of milk and milk products, for sanitary needs, in equipment damage or operational problems; and various additives introduced in manufacturing (13, 18, 22, 23). Milk loss in wastewater is around 0.5–2.5% of processed milk, but it can increase to 3–4% (20).
Wastewater categories
The wide variety of dairy products presupposes the existence of many wastewater types. However, three major categories can be outlined according to their origin and composition (1), explained in the following chapters.
Processing water
Processing water is formed in the cooling of milk in special coolers and condensers, as well as condensates from the evaporation of milk or whey. Milk and whey drying produces vapours which form the cleanest effluent after condensation although it may contain volatile substances as well as milk or whey droplets from evaporators (6). In general, processing waters lack pollutants and, after minimal pretreatment, they can be reused or discharged together with stormwater (1). Water reusage is possible for installations that are not in direct contact with derived products. Typical applications include hot water and steam production as well as membrane cleaning. The water from the cooling of products during pasteurisation after the last rinse of bottles and condensates generated in vacuum installations from secondary vapours can be utilised for room cleaning, lawn irrigation, etc. (6, 18).
Cleaning wastewater
Cleaning wastewater usually comes from washing equipment which is in direct contact with milk or dairy products. It also includes milk and product spillage, whey, pressing and brine, CIP effluents or equipment malfunction and even operational errors. Over 90% of organic solids in effluents come from milk and manufacturing residues: cheese pieces, whey, cream, water from separation and clarification, starter cultures, yoghurt, fruit concentrates or stabilisers. These effluents are in large quantities and are highly polluted, thus requiring further treatment.
Sanitary wastewater
Sanitary wastewater is found in lavatories, shower rooms, etc. Sanitary wastewater is similar in composition to municipal wastewater and is generally piped directly to sewage works (1, 6, 21). It can be used as nitrogen source for unbalanced dairy effluents before a secondary aerobic treatment (1, 12).
Additionally, the by-products of manufacturing processes, such as whey, milk and whey permeates, can also be grouped separately if they are collected individually from other wastewater streams (12, 24, 25).
The main pollutant in milk processing wastewater is whey due to its high organic and volumetric load. It represents about 85–95% of the milk volume and 55% of the milk components. Whey consists of carbohydrates (4–5%), mostly lactose. Proteins and lactic acid amount to less than 1%, fats to around 0.4–0.5%, while salts vary from 1 to 3% (2, 15, 26). Whey is produced mainly in cheese manufacturing, and its volume depends on the productivity of cheese and the type of processed milk – bovine, goat, sheep, etc. (2, 9, 27). On the basis of milk casein coagulation procedures, whey can be categorised as cheese whey and second cheese whey. Cheese whey is a by-product in the production of hard, semi-hard and soft cheese, after the addition of rennin to milk. Mild enzyme action produces sweet whey with a pH=6–7 (2, 28). Second cheese whey is a by-product in cottage cheese production after milk has been fermented, or curdled, with organic or mineral acids. Due to strong acid conditions, whey develops an acidic taste, while the average pH value rarely exceeds 5. Scientific literature also discusses casein whey whose composition is very close to that of second cheese whey (2). Sweet and acid whey also differ in mineral and protein content (9, 29).
During cheese manufacturing, cheese whey wastewater is produced as well. Its volume and composition change with respect to the type of produced cheese, the applied technology, the milk type and the environment. Cheese whey wastewater originates in the addition of surplus cheese whey and second cheese whey to washing effluents. Nevertheless, its contamination level is lower than that of cheese whey (2, 30).
Cheese whey waste streams are valuable sources of different compounds (protein, lactose, mineral elements) and are utilised in the manufacture of various products, such as lactic acid, single-cell protein, baker’s yeast, starter cultures, fermented whey drinks, enzymes, antibiotics, organic acids, vitamins, food gums, etc. Nevertheless, it should be taken into account that whey or whey product recovery results in new waste streams which also need to be treated although such effluents are less polluted than whey and their organic loading is comparable to other dairy wastewater (4, 9, 31, 32).
Milk and whey permeates are by-products in cheese manufacturing; they are produced during milk and whey ultrafiltration, respectively. Their solid content is lower; they are rich in soluble compounds, over 80% of which is lactose (24, 25, 33).
Dairy wastewater consists of complex constituents (11, 34). Knowing the composition of milk and milk products, we can estimate better the wastewater contaminant loading (Table 1) (22). Although milk manufacturing produces waste streams analogous to milk and dairy product loss, every process gives an effluent unique in volume and composition (4).
Table 1. Composition of different milk products.
| Product | w(dry matter) | w(fat) | w(protein) | w(lactose) | COD | BODu | Reference |
|---|---|---|---|---|---|---|---|
| % | % | % | % | g/kg | g/kg | ||
| Whole milk | 11.5–12.5 | 3–4 | 3.3 | 4.8 | 192.9–218.6 | 135.5–156.2 | (18) |
| Skimmed milk | 8.3–8.47 | 0.02–0.06 | 3.3 | 4.7–4.9 | 112–115.3 | 72.4–75.1 | (18) |
| Buttermilk | 7.7–8 | 0.4–0.86 | 3 | 4 | 104.5–111.9 | 72.4–75.1 | (18) |
| Cheese whey | 6–6.2 | 0.05–0.2 | 0.75–1.0 | 4.5–4.8 | 72–77 | 51.6–55.9 | (9, 18) |
| Second cheese whey | 5.7 | < 0.01 | 0.3 | 4.6 | – | – | (9) |
| Casein whey | 6.1 | <0.01 | 0.5 | 4.7 | – | – | (9) |
| Cream | 40.4–43 | 33–35 | 2 | 3 | 871–936.5 | 695–747 | (18) |
| Dried whey permeate | 95.2 | – | 5.9 (as N) | 83 | 1034.3 | – | (25) |
| Delactosed permeate | 23.5–25 | – | 2.6–3.7 | 14–16 | – | – | (9) |
COD=chemical oxygen demand, BODu=ultimate biological oxygen demand
Dairy wastewater volumetric and flow rates (depending on the production capacity and work shifts), as well as pH and total suspended solids (TSS) content (as a consequence of applied CIP methods) affect the efficiency of wastewater treatment management (1). It is important to know the quantity of the milk to be pasteurised, how much milk is processed into cheese and whether the entire obtained whey is discharged in wastewater or part of it is processed and reused (6). Contaminant concentrations in wastewater can be determined by using Eq. 1:
where C is contaminant concentration in wastewater (g/m3), L is the loss of milk and milk products in different technological production cycles expressed in proportional units (m3 or t), C1, C2 and Cn are contaminant concentration per unit of milk or milk product loss (g/t), and N1, N2 and Nn are wastewater discharge per unit of milk or generated milk products (m3/t) (18).
Dairy wastewater composition
Milk processing effluents have an increased temperature and large variations in pH, TSS, biological oxygen demand (BOD), COD, total nitrogen (TN), total phosphorus (TP) and fat, oil and grease (FOG) (1, 3, 7, 13, 15, 35). There is little information on industrial-scale dairy effluent composition (1, 11). The information on the general characteristics of dairy wastewater is shown in Table 2.
Table 2. Composition of milk processing effluents.
| Milk processing effluent | Active reaction (pH) |
γ/(g/L) | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BOD5 | COD | FOG | TS | TSS | TN | TP | Alkalinity as CaCO3 |
|||
| Mixed dairy | 4–11 | 0.24–5.9 | 0.5–10.4 | 0.02–1.92 | 0.71–7 | 0.06–5.80 | 0.01–0.66 | 0–0.6 | 0.32–1.2 | (1,3–7,9,11,13,14, 17,18,20,22,23,35) |
| Milk reception | 7.18 | 0.8 | 2.54 | 1.06 | – | 0.65 | – | – | – | (20) |
| Dairy/sewage=7:3 | 9.1±6.7 | 1.08–2.81 | 2.04–4.73 | 0.24–0.29 | – | 0.53–1.13 | – | 0.02–0.03 | – | (13) |
| Fluid milk | 5–9.5 | 0.5–1.3 | 0.95–2.4 | – | – | 0.09–0.45 | – | – | – | (11) |
| Yoghurt | 4.53 | – | 6.5 | – | – | – | – | – | – | (16) |
| Butter | 12.08 | 0.22–2.65 | 8.93 | 2.88 | – | 0.7–5.07 | – | – | – | (6, 20) |
| Ice cream | 5.1–6.96 | 2.45 | 5.2 | – | 3.9 | 3.1 | – | 0.014 | 0.22 | (3, 25) |
| Cheese | 3.38–9.5 | 0.59–5 | 1–63.3 | 0.33–2.6 | 1.92–53.2 | 0.19–2.5 | 0.018–0.83 | 0.005–0.28 | – | (1, 6, 11, 18, 20, 23) |
| Cottage cheese | 7.83 | 2.6 | 17.65 | 0.95 | – | 3.38 | – | – | – | (20) |
| Cheese whey | 3.92–6.5 | 27–60 | 50–102.1 | 0.9–14 | 55–70.9 | 1.27–22.15 | 0.2–1.76 | 0.12–0.53 | – | (2, 8, 10, 11, 22, 23, 26, 27) |
| Hard cheese whey | 5.8 | 9.48 | 73.45 | 0.99 | – | 7.15 | – | – | – | (20) |
| Soft cheese whey | 5.35 | 26.77 | 58.55 | 0.49 | – | 8.31 | – | – | – | (20) |
| Cottage cheese whey | 4.5 | – | 79 | – | 68 | – | 2 | – | – | (2) |
| Cheese whey wastewater | 4.6 | 35 | – | 0.8 | – | – | – | 0.64 | – | (1) |
| Whey processing effluent | 5–9 | 0.59–1.21 | 1.07–2.18 | – | – | 0.08–0.44 | – | – | – | (33) |
| Milk permeate | 5.55–6.52 | – | 52.94–57.46 | – | 11.61–15.39 | 1.94–3.4 | 0.3–0.4 | 0.35–0.45 | 2.5 | (3, 15, 24) |
| Condensate | 8.3 | – | – | – | – | – | 0.0006 | 0.0001 | – | (23) |
| Washing wastewater | 10.37 | 3.47 | 14.64 | 3.11 | – | 3.82 | – | – | – | (20) |
BOD5=biological oxygen demand for 5 days, COD=chemical oxygen demand, FOG=fat, oil and grease, TS=total solids, TSS=total suspended solids, TN=total nitrogen, TP=total phosphorus
Typically, dairy wastewater is white in colour (whey is yellowish-green) and has an unpleasant odour and turbid character (2, 12, 36).
With annual temperatures of 17–25 °C, dairy waste streams are warmer than municipal wastewater (10–20 °C), which results in faster biological degradation compared to sewage treatment plants (37). The average temperatures of industrial dairy effluents range from 17–18 °C in winter and 22–25 °C in summer (6). Using the Arrhenius equation, the biodegradation rates and oxygen consumption can be predicted to be 1.5 times higher in summer than in winter (37). The design winter temperature of 15 °C is adopted for this type of wastewater due to the utilisation of hot water for washing and cleaning of equipment (6, 18).
A crucial requirement for biological treatment of dairy wastewater is their pH value between 6 and 9 (37). Milk and butter factories have effluents with active reaction close to neutral (pH=6.8–7.4). In plants where a certain amount of whey is discharged, the pH of the effluent is reduced to below 6.2. In cheese manufacturing, sweet whey is slightly acidic, with pH=5.9–6.6, while mineral acid coagulation gives an acidic whey with pH=4.3–4.6 (6, 27). The sharp increase in the short-term pH of the total flow of up to 10–10.5 is attributable to the discharge of alkaline cleaning solutions. The prolonged exposure of wastewater to anaerobic conditions (in the sewer network with sumps) causes liquid acidification by lactic acid fermentation that leads to a decrease in pH (18).
Although dairy wastewaters have low concentrations of settleable solids, they may clog sewage pipes. Most of the suspension enters the initial stage of equipment cleaning. The bulk of the sediment (90%) of organic matter is usually of protein origin, namely particles of solid milk processing (pieces of cheese, coagulated milk, cheese, curd fines, milk film or flavouring agents, etc.) and other impurities (soil or sand) that get into the sewage system during equipment washing or packaging (12, 18). Formation of protein and fat deposits on the inside of the pipes requires periodic cleaning with appropriate chemical or bacterial preparations. The main advantage in the application of such bacteria is that they continue acting in the next stages of wastewater treatment, increasing the purification effect (38). The highest amount of total solids (TS) has been reported in whey, with negligible amount of volatiles (3). Fats in dairy industry effluents are found in trace amounts in the form of emulsions with a droplet diameter of 1–10 µm (6). During homogenisation, the size of milk fat globules is reduced to 1–2 µm. The obtained stable emulsion, when passing into dairy effluents, affects the mechanical wastewater treatment system due to its difficult separation (4). Thus, fats remaining in cheese whey wastewater can produce an undesired flotation, which results in the washout of active sludge during biological processes (2). In the production of high-fat products (cream, sour cream and butter), larger fat globules are extracted from the milk, due to their coalescence and enlargement, as well as the degradation of the protein shell. That is why fat impurities in the wastewater from these productions are significantly different in type and concentration and their elimination by settling is more efficient than in other dairy effluents. The FOG concentration in the wastewater from dairy plants specialised in the production of high-fat products is 0.2–0.4 g/L although higher values (up to 2.88 g/L in a butter factory have been reported) (18). In the wastewater from other dairy plants, it usually does not exceed 0.1 g/L (18).
Due to their high organic content, represented mainly by rapidly assimilable carbohydrates and slowly degradable proteins (20) and lipids, dairy wastewater is characterised by high BOD and COD values varying from 0.1 to 100 g/L (3, 11, 27, 39). It is known that there is a direct relationship between the ultimate 20-day BOD (BODu) and COD values in dairy wastewater, as shown in Eq. 2:
It should be taken into account that such a logical connection cannot be made between a 5-day BOD (BOD5) and BODu, and between BOD5 and COD. Therefore, BOD5 value of dairy waste streams is not an objective indicator of organic pollution (18). Nevertheless, many authors use the BOD5 value of dairy wastewater in the BOD5/COD ratio. For dairy effluents this ratio varies between 0.4 and 0.8 (10, 12, 20, 24). However, it should be determined separately in every particular case (40) and, since dairy wastewater is industrial, the BOD analysis should be conducted with selected microbial consortia, instead of traditional seeding material in order to achieve reliable results (41).
The highest whey COD and BOD5 concentrations have been reported to be between 60–80 and 30–50 g/L, respectively. About 90% of BOD and COD loading is caused by lactose, while protein removal contributes to only around 12% of the whey COD reduction. High lactose solubility increases soluble COD part, which is removed mostly by biological units. Like whey, milk and whey permeates have high COD load because they are rich in lactose, which excludes the possibility for a direct discharge in water bodies (15, 24). Cheese whey wastewater also has increased concentrations of organic matter, the values varying significantly: 0.8–77 g/L of COD and 0.6–16 g/L of BOD5. The lower lactose concentration reported is due to the fermentation in anaerobic conditions that leads to a lower initial pH and casein precipitation and odour production from the obtained butyric acid (2).
The time-consuming BOD analysis requires the application of faster methods that determine aerobically digestible organic matter in dairy wastewater. Many authors show that COD fractionation combined with the calculation of respirometric oxygen uptake rate is a good alternative method for the determination of wastewater biodegradability (42–44). However, results were obtained only for mixed dairy wastewater (45–47), while the information on single manufacturing processes is insufficient (48). Total organic carbon (TOC) calculation also includes organic carbonaceous fractions. It gives immediate results and can be used for online measurements. However, the TOC-BOD relationship should be estimated first (49). There is no available scientific data for the online TOC application or the TOC-BOD relationship in dairy wastewater treatment.
Every milk effluent has notably different TN and TP concentrations (3). Nitrogen exists mainly in the form of amino groups from milk proteins. Other nitrogenous compounds are also detected: urea, uric acids, and NH4+, NO2¯ and NO3¯ ions (11, 27). Small quantities of nitrogen ammonium salts originating from ammonia compressors can also be found (18). Phosphorus compounds are mostly inorganic, phosphate (PO43–) and diphosphate (P2O74–), but they can also be present in organic form (11). Total nitrogen content in the wastewater from urban dairies, dairy and butter plants is 4.2–6% and that from cheese factories 3.7% of the BOD5. The phosphorus concentration is in the 0.6–0.7% range of the BOD5. The reported TN and TP values demonstrate an increased eutrophication risk in water receivers. Their concentrations are sufficient for normal biological treatment processes and the respective growth of bacteria involved in the oxidation of dairy wastewater impurities. However, cheese effluents lack in nitrogen for proper aerobic biological treatment due to the following C/N/P ratio of approx. 200:3.5:1 but can easily be treated anaerobically (50). During biological treatment of cheese factory wastewater, nitrification is less intense than in other dairy industry wastewater treatment facilities because of the lower BOD5/N ratio (2, 12, 18).
Dairy effluents are characterised by very low alkalinity (approx. 2.5 g/L expressed as CaCO3 in milk permeate), thus bringing about a potential for rapid acidification and increased reagent costs for pH maintenance during purification (15, 24).
The high salinity of industrial dairy effluents causes a non-volatile suspended solid content increase in the primary and secondary sludge. Inorganic impurities in dairy wastewater are represented by Na+, K+, Ca2+ and Cl¯ ions, with their highest amounts in cheese and cottage cheese production (0.46–10%), mostly NaCl and KCl (>50%) as well as Ca3(PO4)2, where salt is added in advance. Increased Na+ amounts indicate the application of alkaline cleaning agents in milk factories. The amount of Ca2+ in acidic whey is twice as high as that in sweet whey (2, 12). The presence of chlorides in dairy wastewater is due to the addition of salt in the production of brine and cooling liquors, and the Cl¯ concentration in fresh water and milk. Cl¯ concentration in dairy wastewater reaches 0.8–1 g/L but the average value range is 0.15–0.2 g/L.
The additional wastewater pollution due to the used cleaning solutions, additives and other products which enter the drainage pipes should be taken into account (4, 18). CIP methods produce wastewater streams at 12- or 24-hour time intervals, while sanitisers are used if the dairy factory has been shut down for more than 96 h. Thus, wastewater pH will change widely depending on the cleaning program applied (23). Different chemical solutions can be used in accordance with the installation type, water hardness, etc. (4). The cleaning agents applied in CIP procedures affect principally the effluent pH (mineral and organic acids), contributing less than 10% to BOD5 and COD loading and increasing amounts of water for cleaning and disinfection (up to 30% of total water flow rate). Most of the applied chemicals are very toxic to microorganisms in secondary treatment units. NaOH and HCl increase the mineral scale (1), while HNO3, quaternary ammonium surfactants, and detergents containing H3PO4 and P influence TN and TP loading, which leads to an accelerated eutrophication of the environment if not treated properly (6). Due to the above-mentioned environmental problems, the trend is to apply more HNO3 instead of the less desirable H3PO4 although the latter is a better cleaner the application of which will not be reduced in the future. The cleaning solutions utilised in CIP procedures are hot (64–82 °C), which causes a temperature increase in the resulting effluents (4). Strong oxidants or bleaches (NaOCl and ClO2) are applied for sanitising installations. Cl-containing bleaching agents can produce dangerous organochlorides which pollute dairy effluents. Enzymes as well as surfactants are the chemicals preferred for cool surface cleaning and cause fewer negative environmental problems (6). In minor doses, the following substances can also be found: NH3, Na3PO4, HCl, HOCH2COOH, Na2SiO3, hydraulic oil, propylene glycol, emulsifiers, antifoaming agents, sodium azide and chloramphenicol (4, 51).
Dairy Wastewater Treatment
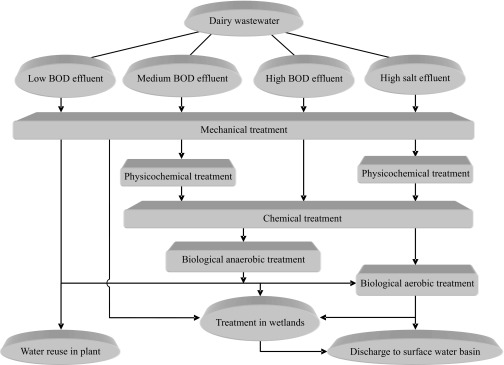
Dairy manufacturing has a strong impact on the environment, producing large volumes of wastewater with high organic and nutrient loading and extreme pH variations. This requires the application of effective and cheap wastewater treatment procedures which ensure fresh water preservation (1, 4). There are various dairy effluent treatment strategies (Fig. 1), which are described in the following paragraphs.
Fig. 1.
Dairy wastewater treatment options, adapted from (19)
Discharge in nature without treatment
It is not recommended that raw dairy wastewater be discharged directly into water bodies because this would lead to different pollution problems, including rapid dissolved O2 depletion due to the high organic loading, which results in anaerobic conditions, the release of volatile toxic substances, aquatic life destruction and subsequent environmental damage. Higher water temperatures decrease O2 solubility and increase biota sensitivity (1).
Treatment in wetlands
Wetland systems use natural processes that include self-supported microbial communities to improve wastewater treatment (52). The simple construction and the lack of sludge recycling make them preferable for dairy effluent utilisation in developing communities (52, 53). The main drawbacks of their application include the need for a large surface area, the potential risks for surface and groundwater pollution, the presence of dangerous volatile substances and the presence of insects. The easy exploitation of the systems counteracts with the complexity of the biological processes, which exceeds that of other treatment systems applied in wastewater purification. Also problematic is the generation of Fe3+, Mn3+ and Ca2+ ions. They precipitate and reduce bed permeability with time. As a result, anaerobic conditions prevail and the NH3 removal is limited (1).
Generally, dairy wastewater is treated in wetlands under aerobic conditions. Five days are enough for an 85% BOD5 reduction in aerobic ponds with milk wastes at 20 °C, while high-load dairy wastewater is treated mostly in facultative wetlands (1). In a butyl-covered lagoon, processing effluent was biodegraded at 35 °C, with the organic loading rate (OLR), expressed as COD, of 1.5 kg/(m3·day), neutral pH and a hydraulic retention time (HRT) of 1–2 days. However, a polishing step in aerated pond is necessary to achieve 99% total COD reduction (1, 4). A surface-flow wetland was applied to utilise 2.65 m3/day of milkhouse wastewater with OLR, expressed as BOD5, of 7.3 g/(m2·day) (54). The results showed high TSS, BOD5, TP and total Kjeldahl nitrogen (TKN) biodegradation with respective values of 94, 85, 68 and 53%. Despite the fact that the lagoon produced NH3, its outflow concentrations gradually declined over time. Most of the nitrogen was stored in biomass, while denitrification had a minor role (<1%) (54). Clarified effluents needed more BOD5 reduction to meet water discharge standards (55). Cheese wastewater with OLR, expressed as COD, of 5.5 kg/(m3·day) was consistently treated in a grease trap, an upflow anaerobic sludge blanket (UASB) reactor-type pond, aerobic pond and a final wetland with water hyacinth (56). A high quality effluent was obtained: BOD5, COD, TSS, FOG, organic N and total coliforms were reduced by more than 90%, except for the phosphorus from PO43– (with a decrease of only 62%).
Purification in urban or in-factory wastewater treatment plant
In-plant effluent treatment is the most common strategy for dairy wastewater purification (1). Typically, it includes mechanical, physicochemical, chemical and biological methods.
Mechanical treatment
Mechanical treatment removes suspended solids from wastewater. Conventional mechanical procedures reduce insufficiently the organic load because of the low settleable solid concentration in dairy wastewater (5). Nevertheless, the faster the wastewater is screened, the better, due to less TSS biodegradation and a low soluble COD increase (1).
High variations of dairy effluents can bring about an instability of the subsequent treatment facilities. Adequate equalisation will smooth the fluctuations in the flow, organic loading, pH and temperature, neutralise residual cleaning agents and completely destroy excess oxidisers. In practice, a 24-hour flow pattern at the highest load can be effectively handled by effluent equalisation for at least 6–12 h with a basin dimension from 25 to 50% of the total effluent volume (1, 6).
Physicochemical treatment
Physicochemical treatment destroys and reduces milk fat and protein colloids in the dairy wastewater (4). FOG removal is a major problem in the plants producing unskimmed milk, in milk and whey separation, cheese and butter production, as well as milk bottling. Skimmed milk production rarely creates such problems.
Animal fat is solid at room temperature due to the high levels of saturated fatty acids in its composition. Milk fat is no exception. This physical state, combined with the low density of the fat allows its easy removal from the surface of wastewater (6, 57). If the equalisation unit precedes the FOG trap, a temperature drop will heighten the risk of high fat accumulation on the top of the liquid. Otherwise, the equalisation unit must have a sufficient volume to collect the peak effluent flow. In general, flow balancing is followed by FOG removal. Increased wastewater temperatures can reduce fat separation ability (1, 5). Dissolved air flotation is more effective because it reduces organic loading via protein and fat colloid destabilisation with coagulants (Al2(SO4)3, FeCl3 and FeSO4) and flocculants. Nevertheless, this method requires expensive, synthetic chemicals which causes environmental problems and removes soluble matter to a lesser extent (58). The resulting scum is very hard to dewater and it is not recommended to mix it with activated sludge. Scum must be treated properly before disposal (1, 4). If inorganic and synthetic chemicals are replaced by biopolymers (carboxymethyl cellulose (CMC) or chitosan), processed sludge can be used as an animal food ingredient (59, 60).
According to some authors (60–62) natural coagulation in dairy wastewater can be achieved with the application of certain lactic acid bacteria. These bacteria ferment soluble lactose to lactic acid, which denatures milk proteins in the wastewater. In combination with CMC, the total COD was reduced by 65–78%, while reduction of 49–82% was obtained when chitosan was used (60). At an initial 5 g/L of COD, over 0.01 g/L of proteins and 0.7–0.8 g/L of sugars, 75, >90 and 10–25% of COD were removed, respectively (61).
Chemical treatment
Chemical treatment removes mostly colloids and soluble contaminants from milk processing effluents. It includes reagent oxidation or pH correction. During cheese wastewater reaction with FeSO4 and H2O2, up to 80% of fat (initial concentration of 1.93 g/L) is removed (63). Extreme pH values of dairy wastewater below 6.5 and above 10 can increase the corrosion of pipes and be highly detrimental to microbiological assemblages in biological processes. Therefore, they should be corrected to reduce side effects. If a dissolved air flotation (DAF) unit is used, then the pH control is a necessary step to achieve optimal coagulant conditions (64). However, coagulants work best at an acidic pH, which requires a second pH adjustment to a neutral value before biological treatment (65). It is very suitable to collect independently used CIP solutions and outflow them constantly during the whole wastewater plant exploitation (1, 4).
Biological treatment
One of the most reliable methods for dairy effluent purification is biological removal. Such methods can assimilate all dairy wastewater components but they mostly utilise soluble compounds and small colloids. These processes have not been fully studied. Moreover, because of their unlimited adaptation potential, they can be jointly used in various sequences to meet certain component biodegradation requirements (1, 7). Biological treatment has two main branches depending on oxygen requirements: aerobic and anaerobic processes (66).
Aerobic processes. Nowadays, most dairy wastewater treatment plants are aerobic although they have been less efficient, mainly due to filamentous growth and rapid acidification caused by high lactose levels and low water buffer capacity, respectively (4, 12, 67). Problems generally encountered with activated sludge processes are bulking and foaming, which diminish sludge settling, Fe3+ and CO32– precipitation, additional biomass production as well as poor activity at low temperatures. It takes a few months for the sludge adaptation before full operational capacity is reached. Nitrogen from NH3 is easily degraded. Phosphorus removal is less effective and relies on environmental conditions. Aerobic bacteria are less useful in colloid utilisation when compared to anaerobic bacteria. The heightened O2 depletion (>3 kg of O2 per kg of BOD5) requires large energy demands during the aerobic treatment of concentrated dairy wastewater (>2 g of COD per L) (1, 4). Plug flow systems are better than complete-mix processes since they are less sensitive to high organic load problems like bulking sludge, etc. (21). Commonly, dairy effluent OLR, expressed as BOD5, should be less than 0.28–0.30 kg/m3. To enhance biological removal, a proper pretreatment or adequate wastewater dilution should be applied (1, 68).
Aerobic biological systems give a very positive response during synthetic dairy wastewater treatment with 4 g/L of COD and 1 g/L of TKN at pH=11.5, with over 96% of degradation being achieved in a continuous mode (69). An artificial effluent similar to milk powder and butter processing wastewater was treated in an anaerobic- -anoxic-oxic system at HRT of 7 days and a nominal sludge age of 20 days (70). The process was characterised by sludge bulking due to the growth of filamentous bacteria (Sphaerotilus natans, Type 0411 and Haliscomenobacter hydrossis). TN removal remained unchanged at 66% without the improvement in the sludge volume index. TP depended on the anoxic selector relative dimensions (from 49 to 20%) and a respective nitrate rise in the effluent. Nevertheless, more than 90% of COD reduction was achieved.
Aerobic filters are applied to a lesser extent in the treatment of high-strength dairy effluents rich in FOG. High fat and heavy biofilm blockage are possible, which results in biomass loss, filter fouling and corresponding reduction in productivity (1).
The sequencing batch reactor (SBR) is preferred in dairy wastewater treatment because of its various loading capabilities and effluent flexibility. A traditional technology with free sludge flocs is mostly applied. The purification of milk effluents is given by Britz et al. (1). COD was reduced by 91–97, TS by 63, volatile solids (VS) by 66, TKN by 75, and TN by 38%. However, mechanical treatment had to be applied first. Another study shows the aerobic SBR as an excellent example of the combination of activated sludge granulation with dairy effluent treatment (71). Granulation stability is limited by nutrient concentration in the wastewater, while effluent quality depends on the need for preliminary sludge settling, usually 0.25–0.5 HRT. Up to 90% of total COD, 80% of TN and 67% of TP were reached in an 8-hour cycle and 50% volume exchange ratio. The results were obtained after fully activated sludge granulation and consecutive biomass sedimentation. The soluble effluent COD was reduced to 125 mg/L. Industrial effluents are more difficult to treat than synthetic ones. The lower maximum OLRs also reduced the SBR granular sludge efficiency (17). In a bench-scale SBR, raw industrial dairy wastewater was treated with Lactobacillus casei TISTR 1500 (62). Microaerobic conditions maintained in the SBR allow for biomass accumulation in large amounts, leading to 85% lactose reduction via rapid fermentation and subsequent protein coagulation by 90%. As a consequence, 70% of COD degradation can be achieved. Around 2.67 times higher OLR was achieved in two laboratory aerobic SBRs treated with a mixed landfill and dairy effluent than in traditional SBR processes (71). The best BOD5 removal mode was reached at OLR, expressed as BOD5, of 0.8 kg/(m3·day) per a 10- -day HRT. The application of flexible fibre as an activated sludge carrier increases the laboratory SBR reliability and it is possible to treat dairy effluents at very high OLRs. At OLR, expressed as COD, of 0.4 kg/(m3·day), COD was degraded by more than 89% and up to 97% at OLR, expressed as COD, of 2.74 kg/(m3·day) (72). Membrane technologies are successfully applied in the treatment of low-load dairy effluents in an SBR. A high BOD removal (over 97%) and TSS-free wastewater are obtained. Due to low influent loading, TN removal reaches 96% by means of assimilation only. TP elimination reaches only 80% after system optimisation due to the limited excess sludge disposal (73).
Moving bed biofilm reactor (MBBR) shows very high performance when applied to dairy wastewaters: OLR increases dozens of times compared to conventional activated sludge systems. A milk processing effluent was treated in a MBBR with biomass developed on FLOCOR- -RMP® particles (Henderson Plastics Ltd, Norfolk, UK) (74). At OLR, expressed as COD, of 5 kg/(m3·day), more than 80% of total COD degradation was achieved in almost half-order kinetics with partial substrate penetration. TN was decreased by 13.3–96.2%. The small reactor volume and the high OLR encompass process applications including plant renovation and the introduction of new, limited-space treatment facilities (74). A novel MBBR with free-floating plastic elements (with a density slightly less than 1.0 kg/m3) may give 85 and 60% COD reduction at OLRs of 12 and 21.6 kg/(m3·day), respectively. On the basis of test results, we can say that the MBBR should be very suitable for the treatment of dairy industry effluents (75).
Good results can be reached in a membrane bioreactor during the treatment of an ice-cream factory effluent with 13.3 kg/m3 of COD, 6.5 kg/m3 of BOD5 at a temperature of 25 °C. Both indicators are reduced by over 95%, while TKN is decreased by more than 96 and TP by 80%. Under aerobic conditions, the indigenous microflora composed of lactic acid bacteria may reach over 109 CFU/mL, which will downgrade CIP-induced alkaline pH variations (76).
Various alternatives for aerobic treatment of dairy effluents are also used. Pure oxygen is another possibility in the biodegradation of milk wastewater. Oxygen can be applied directly in the homogenisation tank during a traditional physicochemical treatment and stable operation is achieved under a broad initial COD and TSS range. This modification improves effluent quality and reduces process costs. Such oxygen injection systems can replace the expensive anaerobic treatment and are naturally safer (77). Cheese whey can also be successfully utilised as a cheap medium for edible mushroom cultivation. Some authors report the growth of Ganoderma lucidum on protein-free cheese whey. The best soluble COD utilisation was achieved at pH=4.6 and 27.1 °C, while the maximum mycelial yield of 0.35 mg per mg of soluble COD removed was obtained at pH=4.2 and 28.5 °C (78). Although there is information on edible fungal growth, dairy wastewater utilisation has not been studied from a COD point of view (79–82).
Cheese whey effluents can be treated successfully in municipal wastewater treatment plants. Factories with onsite treatment technologies should collect sanitary wastewater independently from processing effluents and discharge them directly into municipal wastewater treatment plants. Nevertheless, such a treatment option can lead to operational problems with secondary treatment units (1, 12). Periodic sludge bulking is possible and is caused by intermittent high soluble COD levels in the receiving sewage plant.
Anaerobic processes. Anaerobic systems are more suitable for the direct utilisation of high-strength dairy wastewater and are more cost-effective than aerobic processes. If properly operated, these systems do not produce unpleasant odours (1, 4). The major problems of anaerobic dairy wastewater treatment include long start-up periods due to complex substrate degradation, preliminary biomass adaptation prior to protein and fat utilisation, fast drop in pH and a resultant inhibition of methane production (as a consequence of the high concentration of easily fermentable lactose and low substrate alkalinity), sludge disintegration by fats in the form of triglyceride emulsions and subsequent biomass flotation, presence of inhibitory compounds (long-chain fatty acids, K+ and Na+ ions), inability of ammonia biodegradation and phosphorus removal, careful management, increased sensitivity to various OLRs and shock loadings, etc. Notwithstanding the little information on industrial-scale anaerobic plants utilising cheese whey, more than 75% COD removal and around 10 kg/(m3·day) of OLRs, expressed as COD, are achieved. The degree of biodegradation depends on the HRT applied (4, 12, 22, 83–85).
Milk processing effluents are predominantly treated in conventional one-phase systems: upflow anaerobic sludge blanket (UASB) reactor and anaerobic filter (AF) are most commonly applied (4). UASB reactors have been used in industrial dairy wastewater treatment for more than 20 years. They are suitable for treatment of overloaded effluents with COD higher than 42 g/L (86). Laboratory scale UASB reactors utilising whey permeates in a continuous regime have been designed (87). Kinetic coefficients using the Monod equation are determined per HRT of 0.4–5 days and an initial wastewater COD of 10.4–0.2 g/L (87). It was shown by a comparative study of the possibility of using flocculent sludge and the effect of different HRTs (6–16 h) on the anaerobic UASB reactor behaviour applied to dairy wastewater treatment that nearly 80% of protein mineralisation, soluble COD and volatile fatty acid degradation as well as over 60% fat removal can be reached at an HRT of at least 12 h and an OLR, expressed as COD, of less than 2.5 g/(L·day) (88). Biomass granulation was also achieved in the UASB reactor within 60–70 days. Of all the elements studied, only Ca2+ ions had any significant effect (89). When treating a synthetic ice-cream effluent in the UASB reactor, TOC was reduced by 86% at an HRT of 18.4 h, with the highest OLR, expressed as TOC, reaching 3.06 kg/(m3·day) (1). High FOG degradation is also possible in an UASB reactor. A couple of bench-scale UASB reactors were successfully employed during the utilisation of a synthetic milk effluent rich in FOG (0.2, 0.6 and 1 g/L) (90). Enzymatic pre-hydrolysis contributed to 8% more COD removal at the highest FOG concentration (90). Cheese effluents are degraded in UASB reactors in laboratory tests and on an industrial scale. A laboratory-scale UASB reactor utilising a cheese factory effluent eliminates around 90% of effluents at an OLR, expressed as COD, of 31 g/(L·day) (91). Organic loads, expressed as COD, over 45 g/(L·day) perform worse (70–80% only). Moreover, chemicals are needed to support a constant pH. Short-shock OLR during operation increases sludge granulation, improving stability in reactor performance. The results of the laboratory tests on an industrial level have been confirmed (1), improving them by 6% per 10% higher load. A full-plant UASB reactor can be applied in cheese factory wastewater treatment. With an initial COD of 33 g/L, HRT of 16 h and OLR, expressed as COD, of 49.5 kg/(m3·day), 86% degradation can be reached. During the utilisation of an industrial effluent from Edam cheese, butter and milk production, a full-scale UASB reactor can be applied, the COD being decreased by 70% (1).
Dairy effluents with a low TSS can be successfully utilised in AFs in an all-scale range. The COD decreased by between 60 and 98% at a HRT of 12–48 h and an OLR, expressed as COD, of 1.7–20 kg/(m3·day) (1). A large specific surface of the filter media creates a precondition for higher biomass accumulation which is less affected by shear stress. A five-time higher load than with the non- -porous filler under the same conditions is achieved. It has been reported that with a couple of mesophilic upflow AFs utilising a milk bottling effluent, the reactor with the porous packing performed better (OLR, expressed as COD, of 21 kg/(m3·day)) than the same reactor with non-porous packing (OLR, expressed as COD, of 4 kg/(m3·day)), which is influenced by shear stress to a greater extent (92). Different temperature regimes can be analysed during the treatment of dairy wastewater in laboratory upflow AFs. At 12.5, 21 and 30 °C and HRT of 4 days on average, the COD removal in each reactor amounted to 92, 85 and 78%, respectively (93). An AF was used to treat ice-cream wastewater in a comparative study with contact process, UASB reactor and fluidised bed bioreactor (FBB) (94). The data showed a COD removal of 67, 80 and 50% at OLR, expressed as COD, of 6, 1 and 2 kg/(m3·day) and 60% of total COD removal, at OLR, expressed as COD, of 2–4 kg/(m3·day). All reactors had a poor biomass retention resulting from FOG loading. An upflow AF performed better, which allowed its full-scale installation in the manufacturing process (94). An upflow AF has been claimed to be unsuitable for the anaerobic digestion of very dilute dairy wastewaters (95). In fact, continuous stirred-tank (CSTR), UASB and baffled reactors also cause problems although experimental data show that the baffled reactor performs better with an OLR, expressed as VS, of 0.117–1.303 g/(L·day) and HRTs between 18.8 and 2 days.
Although a CSTR is a good option for scientific research of complete-mix systems (96), it is difficult to use it on an industrial scale because of HRT restrictions. Such reactors were studied with a cheese effluent consisting of wash water/whey ratio of 4:1 with 17 g/L of COD. However, problems with sludge loss arise if the HRT drops to below 9 days (1).
Milk processing effluents can be treated in hybrid systems too (4). An anaerobic contact digester may reach a COD degradation of over 80–95% under mesophilic conditions. The main disadvantage is the difficult sludge settlement. However, the technology is applied worldwide in dairy plants although it is quite old (1). A laboratory-scale experiment analysed the kinetic performance of anaerobic synthetic ice-cream effluent at 37 °C applying the Monod and Contois equations at an HRT range between 2.99 and 7.45 days. A better explanation of the kinetic coefficients can be achieved in the final pilot-scale plant since it allows variations in the initial substrate concentration (97).
Anaerobic packed-bed bioreactor (PBB) can be successfully applied for dairy wastewater treatment of various organic loads. A downflow PBB was used for treating deproteinised cheese whey with 59 g/L of COD (1). At OLR, expressed as COD, of 12.5 kg/(m3·day), the system decreased the COD to 90–95% at HRT of 2–2.5 days. The influent pH was around 2.9, while the pH in the reactor was almost neutral. Good results were obtained in a pilot--scale plant with an up-flow anaerobic PBB (98). The initial cheese whey COD was 59.4 g/L. A 16-hour HRT was enough to reach 99.4% of lactose conversion. Whey wastewater was degraded to 89% in an anaerobic MBBR at (35±2) °C per 1-day HRT and an OLR, expressed as COD, of 11.6 kg/(m3·day) (99). The cheese whey was decomposed in a laboratory PBB with a polyethylene carrier. The highest COD reduction was achieved at a 3.5-day HRT with OLR, expressed as COD, of 3.8 kg/(m3·day) and biogas production of 0.42 m3 per kg of COD per day (1). The mesophilic anaerobic fluidized-bed bioreactor system degraded 5.2 g/L of COD in the ice-cream wastewater to 94.4% at 35 °C, OLR, expressed as COD, of 15.6 kg/(m3·day) and HRT of 8 h. Under shock loading, the return to steady-state conditions was possible within 6–16 h (100). The fluidized-bed bioreactor was used to treat a low-load milk effluent with 0.2–0.5 g/L of COD. At an 8-hour HRT, 80% of COD was removed (1).
Membrane applications in anaerobic systems are good options for improved effluent filtration combined with a higher concentration and an effective differentiation between HRT and solids retention time. A completely mixed anaerobic microfiltration membrane reactor system was used on cheese whey high in COD (63 g/L) (1). More than 99% of organic matter was utilised when HRT was 7.5 days, which allowed authors to upgrade the studies from the pilot plant to a full-scale demonstration. The application of the ultrafiltration system made it possible to achieve a higher biomass retention for more efficient wastewater treatment.
Different temperature conditions have been tested in order to reach a higher COD anaerobic removal. The psychrophilic anaerobic operation in some laboratory hybrid reactors, utilising whey effluents with low (COD of 1 kg/m3) and high (COD of 10 kg/m3) load, showed a better COD performance when the OLR reached 70–80% in the first reactor (at OLRs, expressed as COD, of 0.5–1.3 kg/(m3·day), in a 20–12 °C range) and more than 90% in the second (at OLRs, expressed as COD, up to 13.3 kg/(m3·day), in a 20–14 °C range) (101). If the high-load reactor was operated at 12 °C, COD removal decreased to 50–60% and biogranule decomposition started. These side effects could be eliminated via an OLR reduction down to 6.6 kg/(m3·day). However, dairy wastewater has higher average temperature, which makes it possible to apply high-load wastewater treatment technologies (6, 18). Another study showed that mesophilic conditions ((36±1) °C) generate more H2 compared to thermophilic ones ((55±1) °C) during the treatment of cheese whey wastewater, with 9.2 and 8.1 mmol of H2 per g of COD, respectively. The specific H2 production was 4.6 times higher at 36 than at 55 °C (102).
Separated-phase systems are preferred from technological point of view. They have the highest organic loading and shortest HRT compared to other anaerobic digesters. The consecutive acidogenic-methanogenic phase division of anaerobic digestion is suitable for the treatment of dairy wastewater with an unbalanced composition (high C:N ratios which acidify very quickly). In such separated-phase systems, the acidogenic reactor has a major role as it supplies short-chain volatile fatty acids which can be easily fermented to CH4 in the methanogenic reactor. The easily utilisable lactose requires a shorter HRT and a smaller volume of the acidogenic reactor than the methanogenic digester (1, 4, 103). Such a system was used to treat a dairy effluent with 50 kg/m3 of COD and pH=4.5. The COD was decreased by 72% at 35 °C and the following operating conditions: OLR, expressed as COD, of 50 and 9 kg/(m3·day), when HRT was only 1 and 3.3 days in the acidogenic and the methanogenic reactors, respectively (1). The CSTR was the preferable model for the acidogenic phase. In a 9-month operation study, a two- -phase anaerobic reactor comprising an acidogenic-phase CSTR and a methanogenic-phase upflow AF was used to treat dairy waste streams (104). The effluent COD was reduced by 90% and the BOD5 by 95%, while an OLR, expressed as COD, of 5 kg/(m3·day) and a 2-day HRT were obtained. The H2 and subsequent CH4 production from fresh cheese whey were achieved in a CSTR, at 35 °C and HRT of 1 day. The mixed liquor was consequently fermented to CH4 in a baffled bioreactor, operated at HRTs of 20, 10 and 4.4 days. At the lowest HRT, the COD reduction reached 94% (105). An acidogenic CSTR and a final methanogenic upflow AF were used to utilise cheese whey. The results showed that a maximum acidogenesis of up to 50%, with the same OLR (expressed as COD) range (0.5–2 g per mixed liquor suspended solids per day) could be achieved at an HRT of 24 h. The effluent was fed subsequently to the upflow AF where the initial soluble COD was decreased by 90% during HRT of 4 days (106). A two-stage hybrid UASB reactor, filled respectively with polyurethane foam and polyvinyl chloride rings in each phase, was supposed to exceed other anaerobic methods in the treatment of dairy effluents. The combined COD removal in the reactor in a stable equilibrium (10.7 to 19.2 kg/(m3·day)) changed from 97 to 99% (39). Anaerobic rotating biological contact reactors are also discussed in the literature for anaerobic separate phase treatment (1). Carrier incorporation into anaerobic reactors for biomass support greatly increases their specific activity. Depending on the operating temperature, dairy wastewater can be treated in a two-phase separation. The basic configuration presupposes that thermophilic acidogenesis is followed by mesophilic methanogenesis. The information on these processes in the literature is scarce (107–109). An experiment compared two couples of anaerobic SBRs working at the following temperatures: the first couple (thermophilic-mesophilic system) at 55–35 °C and the second (mesophilic-mesophilic system) at 35–35 °C. At an OLR, expressed as VS, varying between 2–4 g/(L·day), the thermophilic-mesophilic system performs better (VS removal rate of 43.8–44.1% when HRT is 3 days and 37.1–38.9% when HRT is 6 days) than the mesophilic-mesophilic system (VS removal rate of 29.3– 30.2% when HRT is 3 days and 26.1–29.1% when HRT is 6 days). The overall improved performance showed that the thermophilic-mesophilic system with respect to total coliform reduction, TSS removal and biogas production, is preferable to the mesophilic-mesophilic SBR couple. Despite that, higher energy consumption during the thermophilic phase should be taken into account from an economical point of view (84). During a set of experiments, a high-temperature-based technology including acetic and butyric acid fermentation followed by CH4 production achieved 116% COD reduction and 43% CH4 biosynthesis, thus performing better than single- -phased processes (110).
Combined (anaerobic-aerobic) processes. Since an anaerobic technology reduces mostly C-containing contaminants and has a weaker effect on nutrient removal, it needs to be considered as only a preliminary step which must be polished. This can be achieved by incorporating a local aerobic step or, occasionally, by directly discharging anaerobic effluent into the municipal wastewater treatment plants (4).
A mixed dairy wastewater was purified on a full- -scale level in consecutive UASB reactor and aerobic denitrification steps. When 95% COD removal was achieved, the produced CH4 was sufficient to cover the plant energy requirements (1).
SBR great flexibility makes it an adequate post-aerobic step in combined dairy wastewater treatment. A new downflow-upflow hybrid reactor containing downflow pre-acidification and upflow methanation chambers was designed to treat high-load cheese wastewater at an average OLR, expressed as COD, of 10 g/(L·day). COD (98%) was converted into biogas, while the discharged soluble COD reached 1 g/L. The process was maintained at stable pH values without chemical addition. After treatment in the SBR, more than 90% of COD, nitrogen from NH3 and TP were removed (32). Wastewaters from raw milk quality laboratories, containing milk preservatives (sodium azide or chloramphenicol), were utilised in an industrial-scale plant with an AF and SBR. Influent FOG were completely treated in the anaerobic step without biomass washout for more than 2 years of operation, the COD decrease being more than 90% at an OLR, expressed as COD, of 5–6 kg/(m3·day). However, alkali had to be added to reduce the critical pH drop. The outgoing stream from the anaerobic process was polished in SBR until the final COD dropped to 200 mg/L and the TN to less than 10 mg/L (52).
The consecutive anaerobic-aerobic technology was used to purify reconstituted whey wastewater in a single reactor at low oxygen concentration and 20 °C. Maximum COD removal of (98±2) % was reached at total cycle time of 4 days and OLR, expressed as COD, of 0.78 g/(L·day). In accordance with specific biomass activity, trophic differentiation can be seen in the system: methanogens predominantly live at the bottom of the bulk liquid, while acidogens inhabit suspended flocs. When the soluble O2 rose to 0.5 mg/L during the aerobic phase, the COD was reduced to (88±3) % in a 2-day total cycle time at 1.55 kg/(m3·day) (111).
Conclusions
The discontinuous manufacturing process and high production heterogeneity in milk processing make it hard to outline the general dairy wastewater characteristics. Nevertheless, it can be concluded that dairy factories are large water consumers and therefore produce unstable waste streams with increased temperatures, variable pH values, high COD, BOD, FOG, N and P concentrations in combination with inhibiting cleaning agents and strong fluctuations in all factors. However, there is little information on the composition of wastewater streams from certain dairy industry branches, such as the production of yoghurt and whey products, which require more attention in future research.
Conventional aerobic activated sludge systems and percolating filters are not appropriate for dairy wastewater treatment. The high soluble COD values in wastewater account for the vast filamentous growth, which obstructs proper treatment and plant management. The application of immobilised biofilm technologies offers the opportunity to treat concentrated wastewater. MBBR are promising systems. However, many studies should be performed on other dairy wastewater streams, such as high FOG effluents, acid whey, etc.
High organic contamination levels create conditions for the preference of anaerobic digestion over aerobic processes in dairy wastewater utilisation although anaerobic treatment rarely produces clear streams. This necessitates the development of novel, more effective fermentation technologies to deal with high-strength dairy effluents. Insufficient information on temperature-phased anaerobic biodegradation paves the way for new research on dairy wastewater management. A major problem in the anaerobic fermentation of dairy wastewater is ammonia, known for its toxicity if generated in high concentrations. Research can contribute a lot to the anaerobic ammonium oxidation application in the treatment of anaerobic effluents from dairy manufacturing for an improved nitrogen removal.
The consecutive combination of fermentative and oxygen processes may be a solution for appropriate milk processing wastewater treatment. However, innovative and more compact equipment should be designed to meet the challenges associated with wastewater treatment limitations and water-quality requirements. Moreover, the replacement of outdated equipment with new machines needs to be supported by more, real-case studies, which will help us understand better dairy wastewater treatment.
References
- 1.Britz JT, van Schalwyk C, Hung YT. Treatment of dairy processing wastewaters. In: Wang LK, Hung YT, Lo HH, Yapijakis C, editors. Waste treatment in the food processing industry. Boca Raton, FL, USA: CRC Press; 2006. pp.1–25. [Google Scholar]
- 2.Carvalho F, Prazeres AR, Rivas J. Cheese whey wastewater: characterization and treatment. Sci Total Environ. 2013;445-446:385–96. 10.1016/j.scitotenv.2012.12.038 [DOI] [PubMed] [Google Scholar]
- 3.Karadag D, Köroğlu OE, Ozkaya B, Cakmakci M. A review on anaerobic biofilm reactors for the treatment of dairy industry wastewater. Process Biochem. 2015;50:262–71. 10.1016/j.procbio.2014.11.005 [DOI] [Google Scholar]
- 4.Nadais MHGAG, Capela MIAPF, Arroja LMGA, Hung YT. Anaerobic treatment of milk processing wastewater. In: Wang LK, Tay JH, Tay STL, Hung YT, editors. Handbook of environmental engineering, vol. 11. Environmental bioengineering. New York, NY, USA: Humana Press, Springer; 2010. pp. 555–618. http://dx.doi.org/ 10.1007/978-1-60327-031-1_17 [DOI] [Google Scholar]
- 5.Rosenwinkel KH, Austermann-Haun U, Meyer H. Industrial wastewater sources and treatment strategies. Dairy industry. In: Rehm HJ, Reed G, Pühler A, Stadler P, editors. Environmental processes I, vol. 11a. Biotechnology. Weinheim, Germany: Wiley-VCH; 1999. pp. 208–9. [Google Scholar]
- 6.Tsachev T. Dairy industry wastewater treatment. In: Industrial wastewater treatment. Sofia, Bulgaria: State Publishing House Technique; 1982. pp. 239–41 (in Bulgarian). [Google Scholar]
- 7.Cristian O. Characteristics of the untreated wastewater produced by food industry. An Univ Oradea Fasc Prot Med. 2010;15:709–14. Available from http://protmed.uoradea.ro/facultate/anale/protectia_mediului/2010/im/29.%20Onet%20Cristian%201.pdf [Google Scholar]
- 8.Najafpour GD, Hashemiyeh BA, Asadi M, Ghasemi MB. Biological treatment of dairy wastewater in an upflow anaerobic sludge-fixed film bioreactor. Am-Euras J Agric Environ Sci. 2008;4:251–7. [Google Scholar]
- 9.Pesta G, Meyer-Pittroff R, Russ W. Utilization of whey. In: Oreopoulou W, Russ W, editors. Utilization of by-products and treatment of waste in the food industry. New York, NY, USA: Springer; 2007. pp. 193–205. http://dx.doi.org/ 10.1007/978-0-387-35766-9_10 [DOI] [Google Scholar]
- 10.Saddoud A, Hassaïri I, Sayadi S. Anaerobic membrane reactor with phase separation for the treatment of cheese whey. Bioresour Technol. 2007;98:2102–8. 10.1016/j.biortech.2006.08.013 [DOI] [PubMed] [Google Scholar]
- 11.Demirel B, Yenigun O, Onay TT. Anaerobic treatment of dairy wastewaters: a review. Process Biochem. 2005;40:2583–95. 10.1016/j.procbio.2004.12.015 [DOI] [Google Scholar]
- 12.Prazeres AR, Carvalho F, Rivas J. Cheese whey management: a review. J Environ Manage. 2012;110:48–68. 10.1016/j.jenvman.2012.05.018 [DOI] [PubMed] [Google Scholar]
- 13.Tawfik A, Sobhey M, Badawy M. Treatment of a combined dairy and domestic wastewater in an up-flow anaerobic sludge blanket (UASB) reactor followed by activated sludge (AS system). Desalination. 2008;227:167–77. 10.1016/j.desal.2007.06.023 [DOI] [Google Scholar]
- 14.Sarkar B, Chakrabarti PP, Vijaykumar A, Kale V. Wastewater treatment in dairy industries – possibility of reuse. Desalination. 2006;195:141–52. 10.1016/j.desal.2005.11.015 [DOI] [Google Scholar]
- 15.Farizoglu B, Keskinler B, Yildiz E, Nuhoglu A. Simultaneous removal of C, N, P from cheese whey by jet loop membrane bioreactor (JLMBR). J Hazard Mater. 2007;146:399–407. 10.1016/j.jhazmat.2006.12.051 [DOI] [PubMed] [Google Scholar]
- 16.Un UT, Ozel E. Electrocoagulation of yoghurt industry wastewater and the production of ceramic pigments from the sludge. Separ Purif Tech. 2013;120:386–91. 10.1016/j.seppur.2013.09.031 [DOI] [Google Scholar]
- 17.Schwarzenbeck N, Borges JM, Wilderer PA. Treatment of dairy effluents in an aerobic granular sludge sequencing batch reactor. Appl Microbiol Biotechnol. 2005;66:711–8. 10.1007/s00253-004-1748-6 [DOI] [PubMed] [Google Scholar]
- 18.Schifrin SM, Ivanov GV, Mishukov BG, Feodanov YuA. Wastewaters from dairy industry. In: Arkhangelskaya EP, editor. Wastewater treatment of meat and dairy industry. Moscow, Russia: Light and Food Industry; 1981. pp. 11–9 (in Russian). [Google Scholar]
- 19.Environment Protection Authority (EPA). Environmental guidelines for the dairy processing industry. Melbourne, Australia; 1997. Available from: www.epa.vic.gov.au/~/media/Publications/570.pdf.
- 20.Janczukowicz W, Zieliński M, Dębowski M. Biodegradability evaluation of dairy effluents originated in selected sections of dairy production. Bioresour Technol. 2008;99:4199–205. 10.1016/j.biortech.2007.08.077 [DOI] [PubMed] [Google Scholar]
- 21.Rosenwinkel KH, Austermann-Haun U, Meyer H. Industrial wastewater sources and treatment strategies. In: Jördening HJ, Winter J, editors. Environmental biotechnology: concepts and applications. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2005. pp. 69–70. [Google Scholar]
- 22.Doble M, Kumar A. Treatment of waste from food and dairy industries. In: Biotreatment of industrial effluents. Burlington, VT, USA: Elsevier Butterworth-Heinemann; 2005. pp. 183–5. http://dx.doi.org/ 10.1016/B978-075067838-4/50018-X [DOI] [Google Scholar]
- 23.Watkins M, Nash D. Dairy factory wastewaters, their use on land and possible environmental impacts – a mini review. Open Agric J. 2010;4:1–9. 10.2174/1874331501004010001 [DOI] [Google Scholar]
- 24.Wang S, Rao NC, Qui R, Moletta R. Performance and kinetic evaluation of anaerobic moving bed biofilm reactor for treating milk permeate from dairy industry. Bioresour Technol. 2009;100:5641–7. 10.1016/j.biortech.2009.06.028 [DOI] [PubMed] [Google Scholar]
- 25.Yang P, Zhang R, McGarvey JA, Benemann JR. Biohydrogen production from cheese processing wastewater by anaerobic fermentation using mixed microbial communities. Int J Hydrogen Energy. 2007;32:4761–71. 10.1016/j.ijhydene.2007.07.038 [DOI] [Google Scholar]
- 26.Gannoun H, Khelifi E, Bouallagui H, Touhami Y, Hamdi M. Ecological clarification of cheese whey prior to anaerobic digestion in upflow anaerobic filter. Bioresour Technol. 2008;99:6105–11. 10.1016/j.biortech.2007.12.037 [DOI] [PubMed] [Google Scholar]
- 27.Venetsaneas N, Antonopoulou G, Stamatelatou K, Kornaros M, Lyberatos G. Using cheese whey for hydrogen and methane generation in a two-stage continuous process with alternative pH controlling approaches. Bioresour Technol. 2009;100:3713–7. 10.1016/j.biortech.2009.01.025 [DOI] [PubMed] [Google Scholar]
- 28.Okigbo LM, Richardson GH, Brown RJ, Ernstrom CA. Interactions of calcium, pH, temperature and chymosin during milk coagulation. J Dairy Sci. 1985;68:3135–42. 10.3168/jds.S0022-0302(85)81218-2 [DOI] [PubMed] [Google Scholar]
- 29.Chatzipaschali A, Stamatis AG. Biotechnological utilization with a focus on anaerobic treatment of cheese whey: current status and prospects. Energies. 2012;5:3492–525. 10.3390/en5093492 [DOI] [Google Scholar]
- 30.Potter N, Hotchkiss J. Waste solids upgrading and treatment. In: Food science. New York, NY, USA: Springer; 2012. pp. 526–7. http://dx.doi.org/ 10.1007/978-1-4615-4985-7 [DOI] [Google Scholar]
- 31.Göblös S, Portörő P, Bordás D, Kálmán M, Kiss I. Comparison of the effectivities of two-phase and single-phase anaerobic sequencing batch reactors during dairy wastewater treatment. Renew Energy. 2008;33:960–5. 10.1016/j.renene.2007.06.006 [DOI] [Google Scholar]
- 32.Malaspina F, Stante L, Cellamare CM, Tilche A. Cheese whey and cheese factory wastewater treatment with a biological anaerobic-aerobic process. Water Sci Technol. 1995;32:59–72. 10.1016/0273-1223(96)00139-4 [DOI] [Google Scholar]
- 33.Geilman WG, Schmitd D, Herfurth-Kennedy C, Path J, Cullor J. Production of an electrolyte beverage from milk permeate. J Dairy Sci. 1992;75:2364–9. 10.3168/jds.S0022-0302(92)77996-X [DOI] [PubMed] [Google Scholar]
- 34.Borja R, Banks CJ. Anaerobic digestion of ice-cream wastewater: a comparison of single and two-phase reactor systems. Bull Environ Contam Toxicol. 1995;54:466–71. 10.1007/BF00195122 [DOI] [PubMed] [Google Scholar]
- 35.Venkata, Mohan S, Babu VL, Sarma PN. Effect of various pretreatment methods on anaerobic mixed microflora to enhance biohydrogen production utilizing dairy wastewater as substrate. Bioresour Technol. 2008;99:59–67. 10.1016/j.biortech.2006.12.004 [DOI] [PubMed] [Google Scholar]
- 36.Quasim W, Mane AV. Characterization and treatment of selected food industrial effluents by coagulation and adsorption techniques. Water Resour Ind. 2013;4:1–12. 10.1016/j.wri.2013.09.005 [DOI] [Google Scholar]
- 37.Water Environment Federation. Temperature. Chapter 17. Characterization and sampling of wastewater. In: Operation of municipal wastewater treatment plants, no. 11. New York, NY, USA: WEF Press; 2007. p. 5. Available from: http://www. wefnet.org/mopnew/Operation_of_Municipal_Wastewater_Treatment_Plants/Chapter%2017%20Revised_6th%20Edition.pdf.
- 38.Cammarota MC, Freire DMG. A review on hydrolytic enzymes in the treatment of wastewater with high oil and grease content. Bioresour Technol. 2006;97:2195–210. 10.1016/j.biortech.2006.02.030 [DOI] [PubMed] [Google Scholar]
- 39.Kotoupas A, Rigas F, Chalaris M. Computer-aided process design, economic evaluation and environmental impact assessment for treatment of cheese whey wastewater. Desalination. 2007;213:238–52. 10.1016/j.desal.2006.03.611 [DOI] [Google Scholar]
- 40.Chapter 22. Dairy effluent. In: Dairy processing handbook. Pully, Switzerland: Tetra Pak International SA; 2016. Available from: http://www.dairyprocessinghandbook.com/chapter/dairy-effluent.
- 41.Dhall P, Siddiqi TO, Ahmad A, Kumar R, Kumar A. Restructuring BOD:COD ratio of dairy milk industrial wastewaters in BOD analysis by formulating a specific microbial seed. ScientificWorldJournal. 2012;2012:105712. 10.1100/2012/105712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahman MS, Islam MA, Habib S, Sarker J. Measuring biodegradability of industrial wastewater by a low-cost differential respirometer. Res J Eng Sci. 2013;2:1–4. [Google Scholar]
- 43.Mecler H, Dold PL, Jones RM, Bye CM, Takacs I, Stensel HD, et al. OUR for readily biodegradable COD utilization. Chapter 7.3. OUR response in batch test. Readily biodegradable COD. In: Methods for wastewater characterization in activated sludge modeling. Aleksandria, VA, USA: Water Environment Foundation (WERF)/London, UK: IWA Publishing; 2003. p. 7. [Google Scholar]
- 44.Orhon D, Ҫokgör EU. COD fractionation in wastewater characterization – the state of the art. J Chem Technol Biotechnol. 1997;68:283–93. [DOI] [Google Scholar]
- 45.Leonard AM. Activated sludge treatment of dairy processing wastewaters: the role of selectors for the control of sludge bulking [PhD Thesis]. Palmerston North, New Zealand: Massey University; 1996. [Google Scholar]
- 46.Hayet C, Saida BA, Youssef T, Hédi S. Study of biodegradability for municipal and industrial Tunisian wastewater by respirometric technique and batch reactor test. Sustain Environ Res. 2016;26:55–62. 10.1016/j.serj.2015.11.001 [DOI] [Google Scholar]
- 47.Ubay, Çokgör E, Sözen S, Insel G. Orhon. Respirometric evaluation of biodegradation characteristics of dairy wastewater for organic carbon removal. Environ Technol. 2009;30:1169–76. 10.1080/09593330903144041 [DOI] [PubMed] [Google Scholar]
- 48.Loperena L, Saravia V, Murro D, Ferrari MD, Lareo C. Kinetic properties of a commercial and a native inoculum for aerobic milk fat degradation. Bioresour Technol. 2006;97:2160–5. 10.1016/j.biortech.2005.09.032 [DOI] [PubMed] [Google Scholar]
- 49.Method 5310. Total organic carbon (TOC). Part 5000. Aggregate organic constituents. In: Eaton AD, Clesceri LS, Greenberg AE, Franson MAH, editors. Standard methods for the examination of water and wastewater. Washington DC, USA: American Public Health Association; 1998. [Google Scholar]
- 50.Henze M, Harremoës P. Anaerobic treatment of wastewaters in fixed film reactors – a literature review. Water Sci Technol. 1983;15:1–101. [Google Scholar]
- 51.Omil F, Garrido JM, Arrojo B, Méndez R. Anaerobic filter reactor performance for the treatment of complex dairy wastewater at industrial scale. Water Res. 2003;37:4099–108. 10.1016/S0043-1354(03)00346-4 [DOI] [PubMed] [Google Scholar]
- 52.Ibekwe AM, Grieve CM, Lyon SR. Characterization of microbial communities and composition in constructed dairy wetland wastewater effluent. Appl Environ Microbiol. 2003;69:5060–9. 10.1128/AEM.69.9.5060-5069.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haberl R, Perfler R, Mayer H. Constructed wetlands in Europe. Water Sci Technol. 1995;32:305–15. 10.1016/0273-1223(95)00631-1 [DOI] [Google Scholar]
- 54.Newman JM, Clausen JC, Neafsey JA. Seasonal performance of a wetland constructed to process dairy milkhouse wastewater in Connecticut. Ecol Eng. 1999;14:181–98. 10.1016/S0925-8574(99)00028-2 [DOI] [Google Scholar]
- 55.91/271/EEC. Council directive of 21 May 1991 concerning urban waste water treatment. Off J Eur Commun. 1991. Available from: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/ ?uri=CELEX:31991L0271&from=EN.
- 56.Monroy OH, Vázquez FM, Derramadero JC, Guyot JP. Anaerobic-aerobic treatment of cheese wastewater with national technology in Mexico: The case of “El Sauz”. Water Sci Technol. 1995;32:149–56. 10.1016/0273-1223(96)00149-7 [DOI] [Google Scholar]
- 57.Vaclavik VA, Christian EV. Composition of dietary fats and oils. Chapter 12. Fat and oil products. In: Heldman DR, editor. Essentials of food science. New York, NY, USA: Springer; 2008. p. 284. [Google Scholar]
- 58.Gupta BS, Ako JE. Application of guar gum as a flocculant aid in food processing and potable water treatment. Eur Food Res Technol. 2005;221:746–51. 10.1007/s00217-005-0056-4 [DOI] [Google Scholar]
- 59.Selmer-Olsen E, Ratnaweera HC, Pehrson R. A novel treatment process for dairy wastewater with chitosan produced from shrimp-shell waste. Water Sci Technol. 1996;34:33–40. 10.1016/S0273-1223(96)00818-9 [DOI] [Google Scholar]
- 60.Dyrset N, Selmer-Olsen E, Havrevoll Ø, Rathaweera H, Storrø I, Birkeland SE. Feed supplement recovered from dairy wastewater by biological and chemical pretreatment. J Chem Technol Biotechnol. 1998;73:175–82. [DOI] [Google Scholar]
- 61.Seesurichan P. Dairy wastewater treatment by lactic acid bacteria [PhD Thesis]. Bangkok, Thailand: Kasetsart University; 1997 (in Thai). [Google Scholar]
- 62.Seesuriyachan P, Kuntiya A, Sasaki K, Techapun C. Biocoagulation of dairy wastewater by Lactobacillus casei TISTR 1500 for protein recovery using micro-aerobic sequencing batch reactor (micro-aerobic SBR). Process Biochem. 2009;44:406–11. 10.1016/j.procbio.2008.12.006 [DOI] [Google Scholar]
- 63.Vlyssides AG, Tsimas ES, Barampouti EMP, Mai ST. Anaerobic digestion of cheese dairy wastewater following chemical oxidation. Biosyst Eng. 2012;113:253–8. 10.1016/j.biosystemseng.2012.09.001 [DOI] [Google Scholar]
- 64.Shammas N, Wang L, Hahn H. Costs of flotation. Flotation technology. Chapter 4. Fundamentals of wastewater flotation. In: Wang L, Shammas N, Selke W, Aulenbach D, editors. Handbook of environmental engineering, vol. 12. New York, NY, USA: Humana Press; 2010. p. 150. http://dx.doi.org/ 10.1007/978-1-60327-133-2 [DOI] [Google Scholar]
- 65.Adams CE Jr, Aulenbach DB, Bollyky JL, Boyd JL, Buchanan RD, Burns DE, et al. Chemical sludge production. Chapter 7. Wastewater treatment. In: Liu DHF, Liptak BG, editors. Environmental engineer’s handbook. New York, NY, USA: CRC press LLC; 1999. p. 762. [Google Scholar]
- 66.Grady L Jr, Daigger G, Lim H. 1.3.1 Suspended growth bioreactors. In: Grady LCP Jr, Daigger GT, Lim HC, editors. Biological wastewater treatment. New York, NY, USA: Marcel Dekker Inc.; 1999. pp. 10–6. [Google Scholar]
- 67.Banu JR, Anandan S, Kaliappan S, Yeom IT. Two-stage anaerobic treatment of dairy wastewater using HUASB with PUF and PVC carrier. Biotechnol Bioprocess Eng; BBE. 2007;12:257–64. 10.1007/BF02931101 [DOI] [Google Scholar]
- 68.Rivas J, Prazeres AR, Carvalho F. Aerobic biodegradation of precoagulated cheese whey wastewater. J Agric Food Chem. 2011;59:2511–7. 10.1021/jf104252w [DOI] [PubMed] [Google Scholar]
- 69.Carta-Escobar F, Pereda-Marín J, Álvarez-Mateos P, Romero-Guzmán F, Durán-Barrantes MM, Barriga-Mateos F. Aerobic purification of dairy wastewater in continuous regime: Part I: Analysis of the biodegradation process in two reactor configurations. Biochem Eng J. 2004;21:183–91. 10.1016/j.bej.2004.06.007 [DOI] [Google Scholar]
- 70.Donkin MJ, Russell JM. Treatment of a milk powder/butter wastewater using the AAO activated sludge configuration. Water Sci Technol. 1997;36:79–86. 10.1016/S0273-1223(97)00644-6 [DOI] [Google Scholar]
- 71.Neczaj E, Kacpzak M, Kamizela T, Lach J, Okoniewska E. Sequencing batch reactor system for the co-treatment of landfill leachate and dairy wastewater. Desalination. 2008;222:404–9. 10.1016/j.desal.2007.01.133 [DOI] [Google Scholar]
- 72.Abdulgader M, Yu QJ, Zinatizadeh A, Williams P. Biological treatment of milk processing wastewater in a sequencing batch flexible fibre biofilm reactor. Asia-Pac J Chem Eng. 2009;4:698–703. 10.1002/apj.320 [DOI] [Google Scholar]
- 73.Bae TH, Han SS, Tak TM. Membrane sequencing batch reactor system for the treatment of dairy industry wastewater. Process Biochem. 2003;39:221–31. 10.1016/S0032-9592(03)00063-3 [DOI] [Google Scholar]
- 74.Andreottola G, Foladori P, Ragazzi M, Villa R. Dairy wastewater treatment in a moving bed biofilm reactor. Water Sci Technol. 2002;45:321–8. [PubMed] [Google Scholar]
- 75.Rusten B, Ødegaard H, Lundar A. Treatment of dairy wastewater in a novel moving bed biofilm reactor. Water Sci Technol. 1992;26:703–11. [Google Scholar]
- 76.Scott JA, Smith KL. A bioreactor coupled to a membrane to provide aeration and filtration in ice-cream factory wastewater remediation. Water Res. 1997;31:69–74. 10.1016/S0043-1354(96)00234-5 [DOI] [Google Scholar]
- 77.Martín-Rilo S, Coimbra RN, Martín-Villacorta J, Otero M. Treatment of dairy industry wastewater by oxygen injection: performance and outplay parameters from the full scale implementation. J Clean Prod. 2015;86:15–23. 10.1016/j.jclepro.2014.08.026 [DOI] [Google Scholar]
- 78.Lee H, Song M, Hwang S. Optimizing bioconversion of deproteinated cheese whey to mycelia of Ganoderma lucidum. Process Biochem. 2003;38:1685–93. 10.1016/S0032-9592(02)00259-5 [DOI] [Google Scholar]
- 79.Gothwal R, Gupta A, Kumar A, Sharma S, Alappat BJ. Feasibility of dairy waste water (DWW) and distillery spent wash (DSW) effluents in increasing the yield potential of Pleurotus flabellatus (PF 1832) and Pleurotus sajor-caju (PS 1610) on bagasse. 3 Biotech. 2012;2:249–57. 10.1007/s13205-012-0053-9 [DOI] [Google Scholar]
- 80.Wu X. Effects of whey permeate medium on mushroom mycelia composition and antioxidant properties [PhD Thesis]. Logan, UT, USA: Utah State University; 2008. [Google Scholar]
- 81.Mukhopadhyay R, Chatterjee S, Chatterjee BP, Guha AK. Enhancement of biomass production of edible mushroom Pleutorus sajor-caju grown in whey by plant growth hormones. Process Biochem. 2005;40:1241–4. 10.1016/j.procbio.2004.05.006 [DOI] [Google Scholar]
- 82.Asada C, Okumura R, Sasaki C, Nakamura Y. Acceleration of Hericium erinaceum mycelial growth in submerged culture using yogurt whey as an alternative nitrogen source. Adv Biosci Biotechnol. 2012;3:828–32. 10.4236/abb.2012.37103 [DOI] [Google Scholar]
- 83.Dugba PN, Zhang R. Treatment of dairy wastewater with two-stage anaerobic sequencing batch reactor systems – thermophilic versus mesophilic operations. Bioresour Technol. 1999;68:225–33. 10.1016/S0960-8524(98)00156-4 [DOI] [Google Scholar]
- 84.Fang H, Yu H. Effect of HRT on mesophilic acidogenesis of dairy wastewater. J Environ Eng. 2000;126:1145–8. 10.1061/(ASCE)0733-9372(2000)126:12(1145) [DOI] [Google Scholar]
- 85.Perle M, Kimchie S, Shelef G. Some biochemical aspects of the anaerobic degradation of dairy wastewater. Water Res. 1995;29:1549–54. 10.1016/0043-1354(94)00248-6 [DOI] [Google Scholar]
- 86.Gavala HN, Kopsinis H, Skiadas IV, Stamatelatou K, Lyberatos G. Treatment of dairy wastewater using an upflow anaerobic sludge blanket reactor. J Agric Eng Res. 1999;73:59–63. 10.1006/jaer.1998.0391 [DOI] [Google Scholar]
- 87.Borja R, Banks CJ. Kinetics of an upflow anaerobic sludge blanket reactor treating ice-cream wastewater. Environ Technol. 1994;15:219–32. 10.1080/09593339409385423 [DOI] [Google Scholar]
- 88.Nadais H, Capela I, Arroja L, Duarte A. Treatment of dairy wastewater in UASB reactors inoculated with flocculent biomass. Water SA. 2005;31:603–7. 10.4314/wsa.v31i4.5151 [DOI] [Google Scholar]
- 89.Goodwin JAS, Wase DAJ, Forster CF. Anaerobic digestion of ice-cream wastewaters using the UASB process. Biol Wastes. 1990;32:125–44. 10.1016/0269-7483(90)90077-6 [DOI] [Google Scholar]
- 90.Leal MCMR, Freire DMG, Cammarota MC. SanťAnna Jr GL. Effect of enzymatic hydrolysis on anaerobic treatment of dairy wastewater. Process Biochem. 2006;41:1173–8. 10.1016/j.procbio.2005.12.014 [DOI] [Google Scholar]
- 91.Gutiérrez JLR, Encina PAG, Fdz-Polanco F. Anaerobic treatment of cheese-production wastewater using a UASB reactor. Bioresour Technol. 1991;37:271–6. 10.1016/0960-8524(91)90194-O [DOI] [Google Scholar]
- 92.Anderson GK, Kasapgil B, Ince O. Comparison of porous and non-porous media in upflow anaerobic filters when treating dairy wastewater. Water Res. 1994;28:1619–24. 10.1016/0043-1354(94)90229-1 [DOI] [Google Scholar]
- 93.Viraraghavan T, Kikkeri SR. Effect of temperature on anaerobic filter treatment of dairy wastewater. Water Sci Technol. 1990;22:191–8. [Google Scholar]
- 94.Hawkes FR, Donnelly T, Anderson GK. Comparative performance of anaerobic digesters operating on ice-cream wastewater. Water Res. 1995;29:525–33. 10.1016/0043-1354(94)00163-2 [DOI] [Google Scholar]
- 95.Chen TH, Shyu WH. Performance of four types of anaerobic reactors in treating very dilute dairy wastewater. Biomat Bioeng. 1996;11:431–40. 10.1016/S0961-9534(96)00038-4 [DOI] [Google Scholar]
- 96.Usack JG, Spirito CM, Angenent LT. Continuously-stirred anaerobic digester to convert organic wastes into biogas: system setup and basic operation. J Vis Exp. 2012;65:e3978. 10.3791/3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu WC, Thayanithy K, Forster CF. A kinetic study of the anaerobic digestion of ice-cream wastewater. Process Biochem. 2002;37:965–71. 10.1016/S0032-9592(01)00310-7 [DOI] [Google Scholar]
- 98.Najafpour GD, Tajallipour M, Komeili M, Mohammadi M. Kinetic model for an up-flow anaerobic packed bed bioreactor: dairy wastewater treatment. Afr J Biotechnol. 2009;8:3590–6. [Google Scholar]
- 99.Rodgers M, Zhan XM, Dolan B. Mixing characteristics and whey wastewater treatment of a novel moving anaerobic biofilm reactor. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2004;39:2183–93. 10.1081/ESE-120039383 [DOI] [PubMed] [Google Scholar]
- 100.Borja R, Banks CJ. Response of an anaerobic fluidized bed reactor treating ice-cream wastewater to organic, hydraulic, temperature and pH shocks. J Biotechnol. 1995;39:251–9. 10.1016/0168-1656(95)00021-H [DOI] [Google Scholar]
- 101.McHugh S, Collins G, O’Flaherty V. Long-term, high-rate anaerobic biological treatment of whey wastewaters at psychrophilic temperatures. Bioresour Technol. 2006;97:1669–78. 10.1016/j.biortech.2005.07.020 [DOI] [PubMed] [Google Scholar]
- 102.Azbar N, Dokgöz FT, Keskin T, Eltem R, Korkmaz KS, Gezgin Y, et al. Comparative evaluation of bio-hydrogen production from cheese whey wastewater under thermophilic and mesophilic anaerobic conditions. Int J Green Energy. 2009;6:192–200. 10.1080/15435070902785027 [DOI] [Google Scholar]
- 103.Lo KV, Liao PH. Two-stage anaerobic digestion of cheese whey. Biomass. 1986;10:319–22. 10.1016/0144-4565(86)90005-3 [DOI] [Google Scholar]
- 104.Ince O. Performance of a two-phase anaerobic digestion system when treating dairy wastewater. Water Res. 1998;32:2707–13. 10.1016/S0043-1354(98)00036-0 [DOI] [Google Scholar]
- 105.Antonopoulou G, Stamatelatou K, Venetsaneas N, Kornaros M, Lyberatos G. Biohydrogen and methane production from cheese whey in a two-stage anaerobic process. Ind Eng Chem Res. 2008;47:5227–33. 10.1021/ie071622x [DOI] [Google Scholar]
- 106.Yilmazer G, Yenigün O. Two-phase anaerobic treatment of cheese whey. Water Sci Technol. 1999;40:289–95. 10.1016/S0273-1223(99)00397-2 [DOI] [Google Scholar]
- 107.Beux S, Nunes E, Barana AC. Effect of temperature on two- -phase anaerobic reactors treating slaughterhouse wastewater. Braz Arch Biol Technol. 2007;50:1061–72. 10.1590/S1516-89132007000700017 [DOI] [Google Scholar]
- 108.Fernández-Rodríguez J, Pérez M, Romero LI. Semicontinuous temperature-phased anaerobic digestion (TPAD) of organic fraction of municipal solid waste (OFMSW). Comparison with single-stage processes. Chem Eng J. 2016;285:409–16. 10.1016/j.cej.2015.10.027 [DOI] [Google Scholar]
- 109.Ke S, Shi Z, Fang HHP. Applications of two-phase anaerobic degradation in industrial wastewater treatment. Int J Environ Pollut. 2005;23:65–80. 10.1504/IJEP.2005.006396 [DOI] [Google Scholar]
- 110.Yang K, Yu Y, Hwang S. Selective optimization in thermophilic acidogenesis of cheese-whey wastewater to acetic and butyric acids: partial acidification and methanation. Water Res. 2003;37:2467–77. 10.1016/S0043-1354(03)00006-X [DOI] [PubMed] [Google Scholar]
- 111.Frigon JC, Breton J, Bruneau T, Moletta R, Guiot SR. The treatment of cheese whey wastewater by sequential anaerobic and aerobic steps in a single digester at pilot scale. Bioresour Technol. 2009;100:4156–63. 10.1016/j.biortech.2009.03.077 [DOI] [PubMed] [Google Scholar]