Summary
Early and effective long-term control of HIV-1 replication in perinatal infection leads to exceedingly low concentrations of circulating HIV-1–infected cells in adolescence.
Keywords: perinatal HIV-1 infection, HIV-1 DNA decay, early ART.
Abstract
Background.
Early antiretroviral therapy (ART) limits proviral reservoirs, a goal for human immunodeficiency virus type 1 (HIV-1) remission strategies. Whether this is an immediate or long-term effect of virologic suppression (VS) in perinatal infection is unknown.
Methods.
We quantified HIV-1 DNA longitudinally for up to 14 years in peripheral blood mononuclear cells (PBMCs) among 61 perinatally HIV-1–infected youths in the Pediatric HIV/AIDS Cohort Study who achieved VS at different ages. Participants in group 1 (n = 13) were <1 year of age and in group 2 (n = 48) from 1 through 5 years of age at VS. Piecewise linear mixed-effects regression models assessed the effect of age at VS on HIV-1 DNA trajectories during VS.
Results.
In the first 2 years following VS, HIV-1 DNA levels decreased by –0.25 (95% confidence interval [CI], –.36 to –.13) log10 copies/million PBMCs per year and was faster with early VS by age 1 year compared with after age 1 (–0.50 and –0.15 log10 copies/million PBMCs per year, respectively). Between years 2 and 14 from VS, HIV-1 DNA decayed by –0.05 (95% CI, –.06 to –.03) log10 copies/million PBMCs per year and was no longer significantly different between groups. The estimated mean half-life of HIV-1 DNA from VS was 15.9 years and was shorter for group 1 compared to group 2 at 5.9 years and 18.8 years, respectively (P = .09). Adjusting for CD4 cell counts had no effect on decay estimates.
Conclusions.
Early effective, long-term ART initiated from infancy leads to decay of HIV-1–infected cells to exceedingly low concentrations desired for HIV-1 remission strategies.
The early establishment of latent human immunodeficiency virus type 1 (HIV-1) reservoirs, primarily in the long-lived, resting memory CD4+ T cells [1–4], precludes HIV-1 cure, with viremic rebound generally occurring within 2–4 weeks of stopping combination antiretroviral therapy (ART), and irrespective of prior ART duration [5–7]. Emerging data have shown that a small reservoir size achieved through early ART can promote sustained virologic control in the absence of ART (HIV-1 remission) in a small subset of infected individuals [8–11] and is also predictive of delayed time to virologic rebound off ART [12, 13].
Perinatal HIV-1 infection is distinct from adult infection with respect to widespread implementation of early treatment, as timing of infection can be established [14]. We and others have shown that early ART from infancy with sustained virologic suppression (VS) is associated with persistence of low concentrations of HIV-1–infected cells in the circulation as measured by HIV-1 DNA copies per million peripheral blood mononuclear cells (PBMCs) [15, 16]. Among early-treated children surviving to adolescence (median age, 12.6 years), 79% had circulating HIV-1 DNA concentrations of <10 copies per million PBMCs [15]. Similar reports of low HIV-1–infected cell concentrations following early ART were also reported in cross-sectional studies of Thai and South African children treated from early infancy, in support of the effects of early ART in perinatal infection to limit HIV-1 reservoirs [17, 18]. We performed a longitudinal study to examine the kinetics of HIV-1–infected cell concentrations during VS in perinatally HIV-1–infected (PHIV+) children and youth who differed by their ages of virologic control after ART initiation.
MATERIALS AND METHODS
Study Population
We studied PHIV+ children and youth enrolled in the Adolescent Master Protocol (AMP) of the Pediatric HIV/AIDS Cohort Study (PHACS), a prospective cohort study of the outcomes of perinatal HIV infection in the United States. Between March 2007 and December 2009, 451 infected youths from 15 study sites were enrolled if they were born to HIV-infected mothers, were 7–16 years of age, and had complete medical history of ART use, plasma HIV RNA viral loads (pVLs), and lymphocyte subset measurements since birth. The AMP was approved by the institutional review board at the Harvard T.H. Chan School of Public Health and at each participating site. Written informed consent was obtained from each participant’s parent or legal guardian. Assent was obtained from child participants according to local institutional review board guidelines.
To be eligible for this analysis, participants must have confirmed VS after ART initiation and by 5 years of age, have maintained VS until the data freeze date of 1 July 2012, and have >1 PBMC specimen. Only samples with detectable HIV-1 DNA using the pol primer during at least 1 study visit were included in the analysis. ART was defined as a regimen including at least 3 antiretroviral drugs from at least 2 drug classes. Confirmed VS was defined as achieving 2 consecutive pVLs <400 copies/mL after ART initiation; maintenance of VS was defined as maintaining pVL <400 copies/mL during the study, although single measures of pVL ≥400 copies/mL, in between pVL measurements <400 copies/mL, were allowed.
Quantification of HIV-1 DNA
Genomic DNA was isolated from PBMCs with the Qiagen Blood Midi kit (Qiagen, Valencia, California) and HIV-1 DNA quantified using a slight modification of a previously published droplet digital polymerase chain reaction method [19, 20]. The modification involved a change in the restriction enzyme used to digest the genomic DNA, which was with XbaI (New England BioLabs, Ipswich, Massachusetts) rather than BsaJI. The restriction digest was carried out at 37°C for 1 hour, followed by inactivation at 65°C for 20 minutes. HIV-1 DNA concentrations were determined from 4 replicate reactions containing 1000 ng total of genomic DNA with primers targeting HIV-1 pol (HBX2: 2536, 2662 [19, 20]). For samples with low DNA concentration (<200 ng/µL), 8 replicate reactions containing 500 ng total of genomic DNA were run. The number of cells analyzed was determined by the concentration of the housekeeping gene RRP30. All assays were performed with equal numbers of negative controls containing genomic DNA from the MOLT-4 cell line and no template controls, along with 2 positive control reactions. The average limit of detection for the HIV-1 pol assay was 2.5 copies/million PBMCs (interquartile range, 1.5–2.7) for the 402 samples tested.
Statistical Analyses
Sociodemographic, HIV-1–specific characteristics (ART, CD4, pVL), and PBMC specimen characteristics were compared by the age at VS using either Fisher exact test or Wilcoxon rank-sum test as appropriate. Locally weighted scatterplot smoothing (LOESS) plots were used to first obtain a graphical summary of the relationship between time since VS and HIV-1 DNA concentrations, for all study participants and then stratified by age at VS. Piecewise linear mixed-effects regression models were then fit to estimate HIV-1 DNA trajectories following VS for all study participants and by age at VS. To estimate trajectories by age at VS, age at VS as well as interaction terms between age at VS and the slope parameters were added to the model. A knot was placed at year 2 from VS, based on visual inspection of the LOESS plots and prior knowledge on changes in immune population at this age. Model-based mean PBMC-associated HIV-1 DNA load during VS and half-lives were calculated. Fisher permutation test was used to calculate P values for comparison of these estimates by age at VS group. In further analyses, HIV-1 DNA concentrations were normalized for the number of CD4+ T cells. This was done by dividing the original outcome of PBMC-associated HIV-1 DNA by the proportion of PBMCs that were CD4+ T cells.
RESULTS
Study Participants
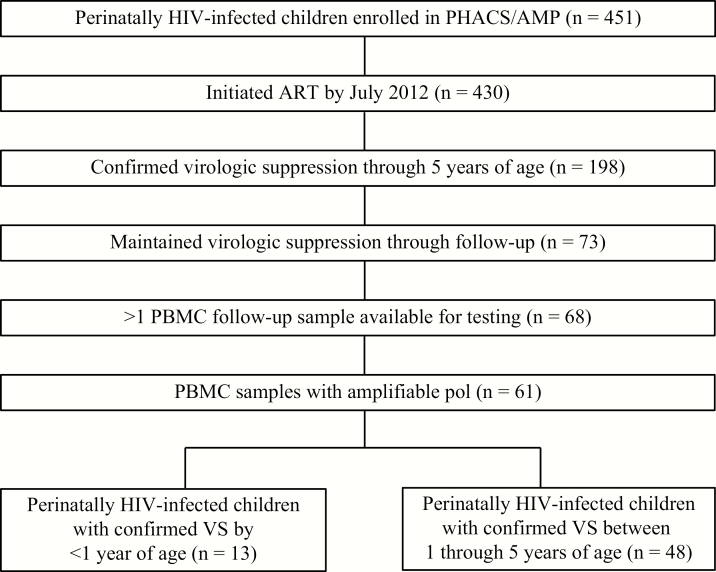
Of the 451 HIV-1–infected children and adolescents enrolled in PHACS/AMP, 430 initiated ART by July 2012, 198 achieved confirmed VS by 5 years of age, and 73 maintained VS throughout follow-up (Figure 1). Five participants had no or only a single PBMC sample available for testing. Seven participants were also excluded from analyses because HIV-1 DNA–positive cells were not detected in PBMC samples from all time points tested; failure to detect HIV-1 in these participants with known HIV-1 infection is likely due to primer mismatch as none of the samples tested detected HIV-1–positive cells. The remaining 61 study participants were stratified into 2 groups based on the age at VS: Group 1 (n = 13) achieved VS by age 1 and group 2 (n = 48) achieved VS after age 1 (between 1 through 5 years of age). The median duration of VS throughout the study period was 10.1 years. A median of 9 and 5.5 PBMC samples were available per study participant in groups 1 and 2, through a median follow-up of 11.9 and 9.5 years during VS, respectively. The median age at last study visit, defined as the date of the last specimen analyzed for HIV-1 DNA, was 12.6 years for both groups.
Figure 1.
Derivation of study population in the Pediatric HIV/AIDS Cohort Study (PHACS) Adolescent Master Protocol (AMP) cohort. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; PBMC, peripheral blood mononuclear cell; VS, virologic suppression.
Participant Characteristics
Group 1 initiated ART at a median of 2.1 months of age, and achieved VS at a median of 5.9 months after starting ART, while group 2 initiated ART at 1.7 years and achieved VS within 10.2 months (Table 1). At ART initiation, the pVL distribution was not significantly different between groups 1 and 2 (median, 5.7 log10 copies/mL and 5.0 log10 copies/mL, respectively; P = .08). Similarly, the distribution for CD4 percentage at ART initiation was not significantly different across groups (median, 31.5% and 28%, respectively; P = .19). Participants had an average of 3.7 pVL measures per year during VS follow-up. The majority had either zero (44%) or 1 (33%) pVL ≥400 copies/mL during VS follow-up; 9 participants had 2 pVL measures ≥400 copies/mL, 3 had 3 pVLs ≥400 copies/mL, and 2 had 5 pVLs ≥400 copies/mL during VS follow-up. The median pVL among the 57 observed measures ≥400 copies/mL (2.5% of 2298 total pVL measures) was 807 copies/mL.
Table 1.
Patient Characteristics
| Age at Virologic Suppression | P Valuea | |||
|---|---|---|---|---|
| Characteristic | All (N = 61) | <1 y (Group 1; n = 13) | 1 to 5 y (Group 2; n = 48) | |
| Female sex, No. (11) | 31 (51) | 6 (46) | 25 (52) | .76b |
| Year of birth | 1998 (1996–2000) | 1998 (1997–1999) | 1998 (1996–2000) | .37c |
| Received ARV prophylaxisd | 14 (23) | 5 (38) | 9 (19) | .15b |
| No. of ART regimens before suppressive ART | ||||
| Median | 0 (0–1) | 0 (0–0) | 0 (0–1.5) | .12c |
| Median duration, y | 0 (0.0–0.5) | 0 (0–0) | 0 (0.0–1.0) | .08c |
| ART regimen (NRTIs plus:) | ||||
| PI alone | 45 (74) | 9 (69) | 36 (75) | .58b |
| PI + NNRTI | 8 (13) | 1 (11) | 7 (15) | |
| NNRTI alone | 8 (13) | 3 (23) | 5 (10) | |
| Age at ART initiation, y | 1.2 (0.3–2.6) | 0.2 (0.1–0.3) | 1.7 (0.8–3.4) | <.001c |
| pVL at ART initiation, copies/mL | 5.2 (4.7–5.5) | 5.7 (5.0–6.3) | 5.0 (4.7–5.4) | .08c |
| CD4% at ART initiation | 28 (21.8–39.0) | 31.5 (26.5–41.0) | 28 (21–38) | .19c |
| Age at first confirmed VS, y | 2.9 (1.3–4.7) | 0.7 (0.6–0.8) | 3.6 (2.6–4.9) | <.001c |
| Time from ART initiation to VS, y | 0.7 (0.4–1.9) | 0.5 (0.3–0.6) | 0.9 (0.4–2.8) | .01c |
| Age at last visit, y | 12.6 (10.9–14.6) | 12.6 (11.8–14.0) | 12.6 (10.4–15.5) | .87c |
| CD4 count at last visit | 822 (617–1186) | 866 (553–1220) | 816 (651–1131) | .58c |
| CD4% at last visit | 38.1 (35.8–43.0) | 40 (37.0–41.6) | 38 (35–43) | .55c |
| CD4/CD8 ratio at last visit | 1.4 (1.1–1.7) | 1.4 (1.1–1.5) | 1.4 (1.1–1.7) | .57c |
| Duration of ART through last visit, y | 11.5 (8.8–13.2) | 12.4 (11.7–13.7) | 11.1 (8.3–12.9) | .02c |
| Time from confirmed VS to last visit, y | 10.1 (7.5–12.2) | 11.9 (11.4–13.4) | 9.5 (6.9–11.7) | .002c |
| Age at first analyzed PBMCs, y | 4.2 (1.6–6.8) | 0.6 (0.2–1.8) | 4.8 (2.7–7.4) | <.001c |
| Analyzed PBMCs per person during VS | 6 (4–9) | 9 (7–11) | 5.5 (3.5–8) | .01c |
Continuous variables are reported as median (interquartile range); dichotomous variables are expressed as frequency (11).
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PBMC, peripheral blood mononuclear cell; PI, protease inhibitor; pVL, plasma viral load; VS, virologic suppression.
aStatistical test comparing groups (<1 y and 1 to 5 y) by age at VS.
bFisher exact test.
cWilcoxon test.
dProphylaxis is defined as the first ART a given participant was on during life that also must have been both initiated and completed during the first 2 months of life.
Decay Dynamics of Cell-Associated HIV-1 DNA With Long-term ART
Total HIV-1 DNA
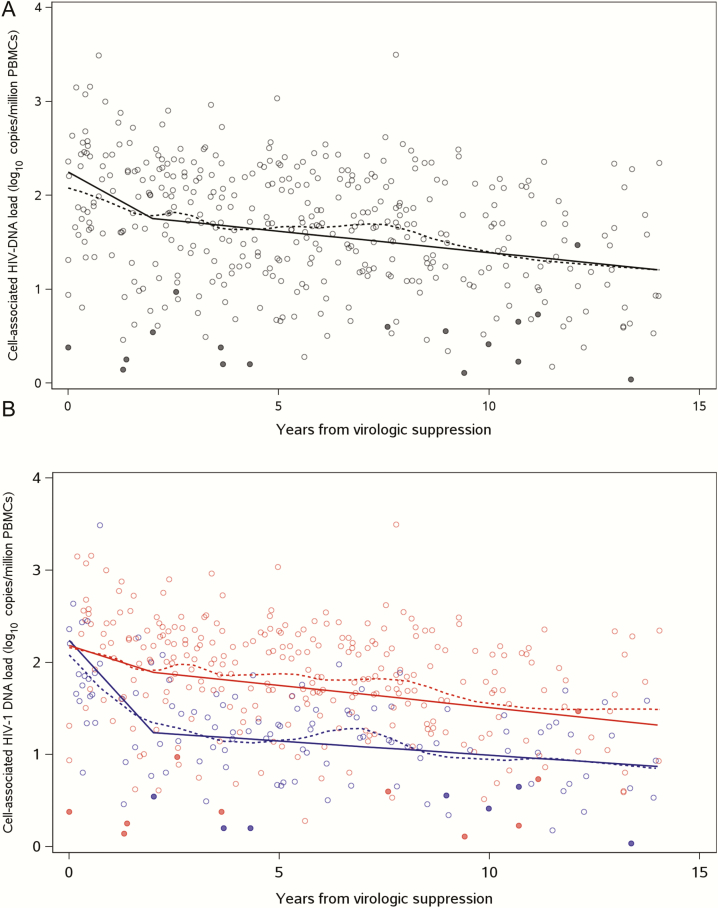
Among the 61 study participants, the estimated mean HIV-1 DNA concentration at the time of VS was 2.25 log10 copies/million PBMCs. During the first 2 years from the time of VS, HIV-1 DNA decreased by 3.2-fold at a rate of –0.25 (95% confidence interval [CI], –.36 to –.13) log10 copies/million PBMCs per year (Figure 2 and Table 2). Between years 2 and 14 from VS, the decrease in HIV-1 DNA concentrations was slower, at –0.05 (95% CI, –.06 to –.03) log10 copies/million PBMCs per year. The estimated mean half-life of HIV-1 DNA from the time of VS was 16 years. With 10 years of VS, the estimated mean HIV-1 DNA concentration was 0.86 log lower than the concentration at the time of VS with a mean concentration of 1.39 (95% PI, .57–2.06) log10 copies/million PBMCs (Table 3).
Figure 2.
Decay of human immunodeficiency virus type 1 (HIV-1) DNA in peripheral blood mononuclear cells (PBMCs) following virologic suppression (VS) overall (11) and by age at VS (11). Piecewise linear mixed-effects model of HIV-1 DNA decay in overall study population (solid black line) and in those who achieve VS by 1 year of age (solid blue line) and by 1 to 5 years of age (solid red line). Dashed lines represent fitted locally weighted scatterplot smoothing curve. Closed circles represent DNA levels below limit of detection.
Table 2.
Estimated Peripheral Blood Mononuclear Cell–Associated HIV-1 DNA Trajectories Over Years Since Virologic Suppression
| Age at Virologic Suppression | P Valuea | |||
|---|---|---|---|---|
| Trajectory | All (N = 61) | <1 y (n = 13) | 1 to 5 y (n = 48) | |
| Interceptb (95% CI) | 2.25 (1.98–2.51) | 2.24 (1.69–2.79) | 2.18 (1.93–2.43) | .86 |
| Slope/y (0–2 y) (95% CI) | −0.25 (−.36 to −.13) | −0.50 (−.73 to −.27) | −0.15 (−.23 to −.07) | .005 |
| Slope/y (2–14 y) (95% CI) | −0.05 (−.06 to −.03) | −0.03 (−.06 to −.00) | −0.05 (−.07 to −.03) | .35 |
Abbreviations: CI, confidence interval; HIV-1, human immunodeficiency virus type 1.
aDifference between group slopes (<1 y and 1 to 5 y) by age at virologic suppression.
bIntercept defined as the estimated HIV-1 DNA concentrations at the time of virologic suppression.
Table 3.
Estimated Peripheral Blood Mononuclear Cell –Associated HIV-1 DNA Concentrations Following Virologic Suppression
| Age at Virologic Suppression | P Valuea | |||
|---|---|---|---|---|
| Time Since VS | All (N = 61) | <1 y (n = 13) | 1 to 5 y (n = 48) | |
| Start of VS (95% PI) | 2.25 (1.02–3.17) | 2.24 (1.14–3.35) | 2.18 (.72–3.08) | .87 |
| Year 2 (95% PI) | 1.75 (.71–2.50) | 1.24 (.49–1.90) | 1.89 (.81–2.53) | .01 |
| Year 5 (95% PI) | 1.62 (.65–2.36) | 1.15 (.51–1.67) | 1.75 (.75–2.37) | .001 |
| Year 7 (95% PI) | 1.53 (.61–2.24) | 1.09 (.52–1.60) | 1.65 (.68–2.27) | <.001 |
| Year 10 (95% PI) | 1.39 (.57–2.06) | 0.99 (.51–1.54) | 1.51 (.66–2.12) | <.001 |
DNA concentrations reported as log10 copies/million peripheral blood mononuclear cells.
Abbreviations: HIV-1, human immunodeficiency virus type 1; PI, prediction interval; VS, virologic suppression.
aDifference between groups (<1 y and 1 to 5 y) by age at VS.
There was no difference in the estimated mean HIV-1 DNA concentrations at VS between groups 1 and 2 (2.24 and 2.18 log10 copies/million PBMCs, respectively; P = .86) (Figure 2 and Table 2). The decline in HIV-1 DNA concentrations during the first 2 years from VS was significantly faster in group 1 (–0.50 [95% CI, –.73 to –.27] log10 copies/million PBMCs per year) compared with group 2 (–0.15 [95% CI, –.23 to –.07] log10 copies/million PBMCs per year) (P = .005). This corresponded to a net decrease of 10-fold and 2-fold in HIV-1 DNA concentrations in the first 2 years of VS in groups 1 and 2, respectively. Between years 2 and 14 from VS, there was ongoing decay of HIV-1 DNA in both groups; however, the decay estimates were no longer significantly different between groups 1 and 2 (–0.03 [95% CI, –.06 to –.00] log10 copies/million PBMCs per year and –0.05 [95% CI, –.07 to –.03] log10 copies/million PBMCs per year, respectively) (P = .35). The estimated mean half-life of HIV-1 DNA from VS was shorter for group 1 compared with group 2 at 5.9 years and 18.8 years, respectively (P = .09). With 10 years of VS, the estimated mean HIV-1 DNA concentration reached a substantially lower level in group 1 at 0.99 (95% prediction interval, .51–1.54) log10 copies/million PBMCs compared with group 2 at 1.51 (95% prediction interval, .66–2.12) log10 copies/million PBMCs (P < .001; Table 3).
Normalization for Changes in CD4+ T Cells
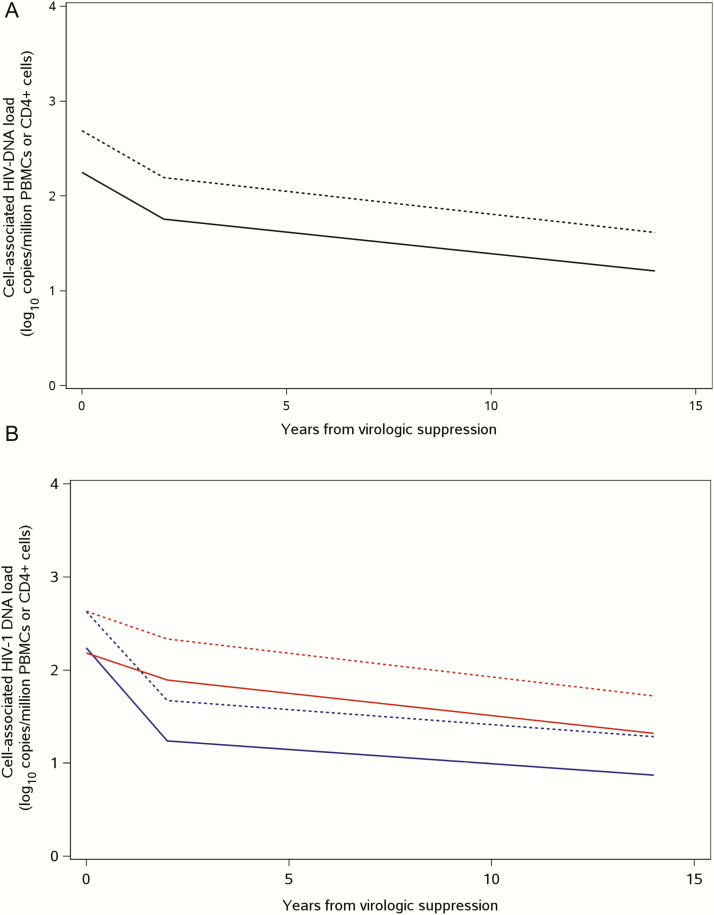
As CD4 reconstitution of immune cells following VS can have a dilutional effect on HIV-1–infected cell concentrations, we examined the effects of normalizing for the number of CD4+ T cells on the decay estimates. Essentially, the same decay kinetics of HIV-1 DNA was observed with normalization for CD4+ T-cell count (Figure 3 and Table 4). At the time of VS, HIV-1 DNA concentration was estimated to be 2.63 log10 copies/million CD4 cells in both groups and, as expected, was about 3-fold higher than in total PBMCs. Similar rates and differences in the decay of HIV-1 as a function of age at virologic control were found, with faster clearance in group 1 compared with group 2 in the first 2 years from VS (P = .005), and no difference between the 2 groups for 2–14 years of VS (P = .29).
Figure 3.
Decay of human immunodeficiency virus type 1 (HIV-1) DNA in peripheral blood mononuclear cells (PBMCs) following virologic suppression (VS) overall (11) and by age at VS (11), normalized for CD4 cell count. Piecewise linear mixed-effects model of HIV-1 DNA decay in overall study population (black line) and in those who achieve VS by 1 year of age (blue line) and by 1 to 5 years of age (red line). HIV-1 proviral DNA decay by PBMCs (solid lines) and CD4 percentage (dashed lines).
Table 4.
Estimated CD4-Associated HIV-1 DNA Trajectories Over Years Since Virologic Suppression
| Age at Virologic Suppression | P Valuea | |||
|---|---|---|---|---|
| Trajectory | All (N = 61) | <1 y (n = 13) | 1 to 5 y (n = 48) | |
| Intercept (95% CI) | 2.69 (2.43–2.94) | 2.63 (2.13–3.13) | 2.63 (2.38–2.88) | .99 |
| Slope/y (0–2 y) (95% CI) | −0.25 (−.36 to −.14) | −0.48 (−.69 to −.26) | −0.15 (−.23 to −.07) | .005 |
| Slope/y (2–14 y) (95% CI) | −0.05 (−.06 to −.03) | −0.03 (−.06 to −.00) | −0.05 (−.07 to −.03) | .29 |
DNA concentrations reported as log10 copies/million CD4 cells.
Abbreviations: CI, confidence interval; HIV-1, human immunodeficiency virus type 1.
aDifference between groups (<1 y and 1 to 5 y) by age at virologic suppression.
DISCUSSION
This is the first study to report on the long-term decay dynamics of HIV-1–infected cells in perinatal infection with VS, as well as the difference in decay parameters by age at virologic control. These findings are pertinent for the timing of ART initiation in pediatric HIV-1 infection with relevance to achieving HIV-1 remission or posttreatment control of HIV-1 replication in absence of ART. We found that, in contrast to HIV-1–infected adults, sustained control of virus replication leads to substantial decreases in HIV-1–infected cells over childhood through adolescence, to reach the unprecedented low concentrations (mean of 1.39 log10 copies per million PBMCs at 10 years after VS) desirable for viral remission strategies. This finding was consistent irrespective of age at VS.
Importantly, however, earlier age at VS (by 1 year of age) was associated with larger decreases in the HIV-1–infected cell concentrations in the first 2 years following VS as compared to when virologic control occurred later (between 1 through 5 years of age; estimated mean decay rate of –0.50 log10 copies/million PBMCs per year compared with –0.15 log10 copies/million PBMCs per year, respectively). This difference in decay between the 2 groups could not be explained by differences in starting concentrations of HIV-1–infected cells, as there was no difference in the HIV-1–infected cell concentration at the time of VS, when first phase decay would have been completed. Similarly, differences in immune reconstitution between infants and young children or lymphoid expansion from somatic growth did not appear to contribute to the finding as adjustment for the percentages of CD4+ T cells did not alter the decay slopes. Together, these findings suggest qualitative differences in the types of reservoir cells generated as a function of age and duration of uncontrolled virus replication in perinatal infection, specifically the memory CD4+ T-cell reservoir with estimated half-lives ranging from 88 months to 277 months for transitional and T-memory stem cells, respectively [21]. Importantly, this difference is likely not due to preservation of HIV-1–specific immune responses through earlier therapy, as HIV-specific immune responses are largely undetectable with early control of HIV-1 replication in infancy [22–24].
It is established that HIV-1–infected cells decrease by about 10-fold from the pre-ART concentrations during the first year of ART in both perinatal [20, 25, 26] and adult [27, 28] infection, reflective of clearance of shorter-lived, infected CD4+ T cells. In infected adults treated during chronic infection, the concentration of HIV-1–infected cells in the peripheral blood plateaus after 4 years on ART to a median of 2.84 log10 copies per million PBMCs, with no additional decay through a decade or more of ART [27]. Whether the steady state of persistence of HIV-1–infected cells occurs earlier than 4 years of ART is unknown, as this time point is influenced by the timing of sample collection in the study [27]. Likewise, in children initiating ART during chronic infection (median, 9 years of age), the concentration of HIV-1–infected cells stabilizes at 1.82 log10 copies per million PBMCs after 4 years of ART [29]. This is consistent with the long-term stability of HIV-1–infected cells despite effective ART.
However, ART initiation during acute infection may influence the long-term stability of HIV-1–infected cells through alterations in the types of memory CD4+ T-reservoir cells that are generated [11, 30]. A recent study comparing the size of the HIV-1 reservoir in adults treated during acute or chronic infection for a decade or more showed that ART during acute infection resulted in significantly smaller reservoir size compared with ART in chronic infection [8]. Our results showing faster HIV-1 decay and smaller reservoir size among children with VS by 1 year of age are consistent with these findings, implying that there are differences in the mechanisms of HIV-1 persistence when ART is started during the acute vs chronic stages of the infection.
Several small cross-sectional studies in perinatally infected children have reported on the observation of fairly low concentrations of HIV-1–infected cells at 8–15 years of age in children on effective ART from early infancy [18, 31]. The extent to which this was a consequence of early restriction in HIV-1–infected cells is unknown. This is the first longitudinal study to examine HIV-1–infected cell decay over the time course of ART. Here, we show that the low-infected cell concentrations observed with early treatment is a longer-term effect of sustained control of HIV-1 replication from infancy with ART initiation at <3 months of age, rather than an immediate restriction in HIV-1–infected cell concentrations.
Importantly, despite the similarities in the initial concentrations of infected cells generated during acute HIV-1 infection in adult and perinatal infection [20, 28, 32], and the ongoing decay of HIV-1–infected cells during a decade or more of early ART in infected adults [32], the HIV-1–infected cell concentrations achieved with long-term ART were 15-fold lower in early-treated infants compared with early-treated adults. This difference highlights unique aspects of HIV-1–infected cell clearance and persistence in perinatal infection, especially with early and effective long-term treatment, for which processes such as slower development of long-term memory T-cell responses and homeostatic proliferation, along with lymphoid expansion and immune reconstitution from thymic output, may be responsible but require further investigation.
Our study was limited by the lack of availability of PBMCs before ART to directly estimate first-phase decay of infected cells with ART. However, we were able to previously demonstrate [20] that a high concentration of HIV-1–infected cells are present by 2 months of age, with similar decay in the first 2 years of ART, as observed in HIV-1–infected adults [28]. In addition, studies to explain the mechanisms of persistence of proviral genomes—specifically, distribution of proviral genomes by memory CD4+ T-cell subsets [33] and integration site analyses [34, 35]—were not feasible, but warrant further investigation. Last, given that >90% of HIV-1 DNA is replication defective [36], the true size of the latent, replication-competent HIV-1 reservoir that reestablishes viremia when ART is stopped was not evaluable from the sample repository, although recent data suggest that these defective genomes can express viral proteins and may be targets for immune clearance [37]. However, HIV-1 DNA has recently been shown to serve as a predictor of posttreatment control and time to virologic rebound in the context of treatment during acute HIV-1 infection in adults [13, 38].
In conclusion, the observation that HIV-1–infected cell concentrations decline to remarkably low levels in the peripheral circulation of perinatally infected preadolescent children and youth has important implications for novel treatment strategies aimed at HIV-1 remission and cure. With 10 years of effective virologic control, the proviral reservoir size was 3-fold lower in the early-suppressed group compared with the later-suppressed group. Understanding the unique properties of HIV-1 persistence in perinatal infection, as identified in this study, and its implications for virologic remission, is critical for advancing the field of HIV-1 remission and cure research toward improved ART-sparing approaches for HIV-1 control in this population.
MEMBERS OF THE PEDIATRIC HIV/AIDS COHORT STUDY (PHACS)
The following institutions, clinical site investigators, and staff participated in conducting PHACS AMP and AMP Up in 2015, in alphabetical order: Ann and Robert H. Lurie Children’s Hospital of Chicago: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynnette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Mahboobullah Baig, Anna Cintron; Children’s Diagnostic and Treatment Center: Ana Puga, Sandra Navarro, Patricia A. Garvie, James Blood; Children’s Hospital, Boston: Sandra K. Burchett, Nancy Karthas, Betsy Kammerer; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Molly Nozyce; Rutgers–New Jersey Medical School: Arry Dieudonne, Linda Bettica; St Christopher’s Hospital for Children: Janet S. Chen, Maria Garcia Bulkley, Latreaca Ivey, Mitzie Grant; St Jude Children’s Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University School of Medicine: Margarita Silio, Medea Gabriel, Patricia Sirois; University of California, San Diego: Stephen A. Spector, Kim Norris, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Juliana Darrow, Emily Barr, Paul Harding; University of Miami: Gwendolyn Scott, Grace Alvarez, Anai Cuadra.
Notes
Acknowledgments. We thank the children and families for their participation in Pediatric HIV/AIDS Cohort Study (PHACS), and the individuals and institutions involved in the conduct of PHACS.
Disclaimer. The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health (NIH) or the US Department of Health and Human Services.
Financial support. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases (NIAID), the Office of AIDS Research, the National Institute of Mental Health (NIMH), the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) (Principal Investigator [PI]: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104) (PI: Russell Van Dyke; Co-PIs: Kenneth Rich, Ellen Chadwick; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson). This study was also supported by grants to D. P. from the NIH (RO1 HD080474) and from the Johns Hopkins University Center for AIDS Research (P30AI094189). Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the NIH/NIAID under award numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC), with co-funding from the NICHD and NIMH.
Potential conflicts of interest. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
for the Pediatric HIV/AIDS Cohort Study (PHACS):
Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter, William Shearer, Mary Paul, Norma Cooper, Lynnette Harris, Murli Purswani, Mahboobullah Baig, Anna Cintron, Ana Puga, Sandra Navarro, Patricia A. Garvie, James Blood, Sandra K. Burchett, Nancy Karthas, Betsy Kammerer, Andrew Wiznia, Marlene Burey, Molly Nozyce, Arry Dieudonne, Linda Bettica, Janet S. Chen, Maria Garcia Bulkley, Latreaca Ivey, Mitzie Grant, Katherine Knapp, Kim Allison, Megan Wilkins, Midnela Acevedo-Flores, Heida Rios, Vivian Olivera, Margarita Silio, Medea Gabriel, Patricia Sirois, Stephen A. Spector, Kim Norris, Sharon Nichols, Elizabeth McFarland, Juliana Darrow, Emily Barr, Paul Harding, Gwendolyn Scott, Grace Alvarez, and Anai Cuadra
References
- 1. Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 1997; 387:183–8. [DOI] [PubMed] [Google Scholar]
- 2. Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997; 278:1295–300. [DOI] [PubMed] [Google Scholar]
- 3. Persaud D, Pierson T, Ruff C, et al. A stable latent reservoir for HIV-1 in resting CD4(11) T lymphocytes in infected children. J Clin Invest 2000; 105:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siliciano JD, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003; 9:727–8. [DOI] [PubMed] [Google Scholar]
- 5. Davey RT, Jr, Bhat N, Yoder C, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A 1999; 96:15109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rothenberger MK, Keele BF, Wietgrefe SW, et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci U S A 2015; 112:E1126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klein N, Sefe D, Mosconi I, et al. ; Paediatric European Network for Treatment of AIDS 11 Trial Team The immunological and virological consequences of planned treatment interruptions in children with HIV infection. PLoS One 2013; 8:e76582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malatinkova E, De Spiegelaere W, Bonczkowski P, et al. Impact of a decade of successful antiretroviral therapy initiated at HIV-1 seroconversion on blood and rectal reservoirs. Elife 2015; 4:e09115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laanani M, Ghosn J, Essat A, et al. ; Agence Nationale de Recherche sur le Sida PRIMO Cohort Study Group Impact of the timing of initiation of antiretroviral therapy during primary HIV-1 infection on the decay of cell-associated HIV-DNA. Clin Infect Dis 2015; 60:1715–21. [DOI] [PubMed] [Google Scholar]
- 10. Hocqueloux L, Prazuck T, Avettand-Fenoel V, et al. Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS 2010; 24:1598–601. [DOI] [PubMed] [Google Scholar]
- 11. Sáez-Cirión A, Bacchus C, Hocqueloux L, et al. ; ANRS VISCONTI Study Group Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013; 9:e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hill AL, Rosenbloom DI, Fu F, Nowak MA, Siliciano RF. Predicting the outcomes of treatment to eradicate the latent reservoir for HIV-1. Proc Natl Acad Sci U S A 2014; 111:13475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hurst J, Hoffmann M, Pace M, et al. Immunological biomarkers predict HIV-1 viral rebound after treatment interruption. Nat Commun 2015; 6:8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rainwater-Lovett K, Luzuriaga K, Persaud D. Very early combination antiretroviral therapy in infants: prospects for cure. Curr Opin HIV AIDS 2015; 10:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Persaud D, Patel K, Karalius B, et al. ; Pediatric HIV/AIDS Cohort Study Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr 2014; 168:1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McManus M, Mick E, Hudson R, et al. ; PACTG 356 Investigators Early combination antiretroviral therapy limits exposure to HIV-1 replication and cell-associated HIV-1 DNA levels in infants. PLoS One 2016; 11:e0154391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Zyl GU, Bedison MA, van Rensburg AJ, Laughton B, Cotton MF, Mellors JW. Early antiretroviral therapy in South African children reduces HIV-1-infected cells and cell-associated HIV-1 RNA in blood mononuclear cells. J Infect Dis 2014; 212:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ananworanich J, Puthanakit T, Suntarattiwong P, et al. ; HIV-NAT 194 Study Group Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS 2014; 28:1015–20. [DOI] [PubMed] [Google Scholar]
- 19. Strain MC, Lada SM, Luong T, et al. Highly precise measurement of HIV DNA by droplet digital PCR. PLoS One 2013; 8:e55943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uprety P, Chadwick EG, Rainwater-Lovett K, et al. Cell-associated HIV-1 DNA and RNA decay dynamics during early combination antiretroviral therapy in HIV-1-infected infants. Clin Infect Dis 2015; 61:1862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee GQ, Lichterfeld M. Diversity of HIV-1 reservoirs in CD4+ T-cell subpopulations. Curr Opin HIV AIDS 2016; 11:383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scott ZA, Chadwick EG, Gibson LL, et al. ; PACTG (Pediatric AIDS Clinical Trial Group) 345 Investigators Infrequent detection of HIV-1-specific, but not cytomegalovirus-specific, CD8(11) T cell responses in young HIV-1-infected infants. J Immunol 2001; 167:7134–40. [DOI] [PubMed] [Google Scholar]
- 23. Luzuriaga K, McManus M, Catalina M, et al. Early therapy of vertical human immunodeficiency virus type 1 (HIV-1) infection: control of viral replication and absence of persistent HIV-1-specific immune responses. J Virol 2000; 74:6984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goulder PJ, Lewin SR, Leitman EM. Paediatric HIV infection: the potential for cure. Nat Rev Immunol 2016; 16:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Rossi A, Walker AS, De Forni D, Gibb DM; Paediatric European Network for Treatment of AIDS (PENTA) Biphasic decay of cell-associated HIV-1 DNA in HIV-1-infected children on antiretroviral therapy. AIDS 2002; 16:1961–3. [DOI] [PubMed] [Google Scholar]
- 26. Saitoh A, Hsia K, Fenton T, et al. Persistence of human immunodeficiency virus (HIV) type 1 DNA in peripheral blood despite prolonged suppression of plasma HIV-1 RNA in children. J Infect Dis 2002; 185:1409–16. [DOI] [PubMed] [Google Scholar]
- 27. Besson GJ, Lalama CM, Bosch RJ, et al. HIV-1 DNA decay dynamics in blood during more than a decade of suppressive antiretroviral therapy. Clin Infect Dis 2014; 59:1312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strain MC, Little SJ, Daar ES, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis 2005; 191:1410–8. [DOI] [PubMed] [Google Scholar]
- 29. Zanchetta M, Walker S, Burighel N, et al. Long-term decay of the HIV-1 reservoir in HIV-1-infected children treated with highly active antiretroviral therapy. J Infect Dis 2006; 193:1718–27. [DOI] [PubMed] [Google Scholar]
- 30. Ananworanich J, Schuetz A, Vandergeeten C, et al. ; RV254/SEARCH 010 Study Group Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 2012; 7:e33948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Zyl GU, Bedison MA, van Rensburg AJ, Laughton B, Cotton MF, Mellors JW. Early antiretroviral therapy in South African children reduces HIV-1-infected cells and cell-associated HIV-1 RNA in blood mononuclear cells. J Infect Dis 2015; 212:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hocqueloux L, Avettand-Fènoël V, Jacquot S, et al. ; AC32 (Coordinated Action on HIV Reservoirs) of the Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS) Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother 2013; 68:1169–78. [DOI] [PubMed] [Google Scholar]
- 33. Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009; 15:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maldarelli F, Wu X, Su L, et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 2014; 345:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Simonetti FR, Sobolewski MD, Fyne E, et al. Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc Natl Acad Sci U S A 2016; 113:1883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ho YC, Shan L, Hosmane NN, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013; 155:540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Imamichi H, Dewar RL, Adelsberger JW, et al. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc Natl Acad Sci U S A 2016; 113:8783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williams JP, Hurst J, Stöhr W, et al. ; SPARTAC Trial Investigators HIV-1 DNA predicts disease progression and post-treatment virological control. Elife 2014; 3:e03821. [DOI] [PMC free article] [PubMed] [Google Scholar]