Abstract
AIM
To establish a reversible porcine model of acute liver failure (ALF) and treat it with an artificial liver system.
METHODS
Sixteen pigs weighing 30-35 kg were chosen and administered with acetaminophen (APAP) to induce ALF. ALF pigs were then randomly assigned to either an experimental group (n = 11), in which a treatment procedure was performed, or a control group (n = 5). Treatment was started 20 h after APAP administration and continued for 8 h. Clinical manifestations of all animals, including liver and kidney functions, serum biochemical parameters and survival times were analyzed.
RESULTS
Twenty hours after APAP administration, the levels of serum aspartate aminotransferase, total bilirubin, creatinine and ammonia were significantly increased, while albumin levels were decreased (P < 0.05). Prothrombin time was found to be extended with progression of ALF. After continuous treatment for 8 h (at 28 h), aspartate aminotransferase, total bilirubin, creatinine, and ammonia showed a decrease in comparison with the control group (P < 0.05). A cross-section of livers revealed signs of vacuolar degeneration, nuclear fragmentation and dissolution. Concerning survival, porcine models in the treatment group survived for longer times with artificial liver system treatment (P < 0.05).
CONCLUSION
This model is reproducible and allows for quantitative evaluation of new liver systems, such as a bioartificial liver. The artificial liver system (ZHJ-3) is safe and effective for the APAP-induced porcine ALF model.
Keywords: Hepatic failure, Acetaminophen, Artificial liver, Acute liver failure, Liver-assisted device
Core tip: This is an article about an artificial liver system that was used to treat an acetaminophen (APAP)-induced porcine acute liver failure (ALF) model. An APAP porcine ALF model was developed and treated with an artificial liver system. The artificial liver system (ZHJ-3) improved serum biochemistry levels and extended porcine survival times significantly. This study concluded that the APAP-induced model is reproducible and allows for quantitative evaluation of new liver systems, such as a bioartificial liver, for the support of hepatic failure in humans. The artificial liver system (ZHJ-3) is safe and effective for the APAP-induced porcine ALF model.
INTRODUCTION
Acute liver failure (ALF) is defined as sudden and severe liver dysfunction with rapid development of coagulopathy, encephalopathy, and multi-organ failure[1,2]. The mortality due to liver failure remains exceedingly high, and liver transplantation is still the only effective treatment to improve survival[3,4]. However, liver transplantation is hampered by the severe shortage of donor organs[5]. A large animal model of ALF, which simulates the human clinical syndrome, has a predictable time course to death but also has the potential for reversal of liver failure[6].
Animal models simulating ALF are not only needed to study the underlying poorly understood pathophysiological mechanisms but are also important for the evaluation of new liver support systems prior to introduction in clinical studies. A devascularization surgical model of ALF is one of the most common animal models used for evaluating liver support systems[7]. Terblanche and Hickman[8] suggested the following criteria that an ideal model should satisfy: (1) the induced hepatic failure should be potentially reversible; (2) liver damage should be reproducible; (3) selective liver damage should occur that leads to death from liver failure during an interval similar to that seen clinically; (4) death should occur sufficiently long enough from the insult to provide a suitable therapeutic window; (5) a large animal should be used to make possible the use of therapies applicable to humans; and (6) the toxin should present minimal risks to laboratory personnel.
Acetaminophen (APAP) is one of the most widely used analgesics, with few side effects when taken at therapeutic doses[9]. An overdose of APAP, taken either accidentally or deliberately, induces hepatotoxicity leading to severe hepatic damage, which may cause ALF[10]. Currently, APAP overdose is the leading cause of ALF in the United States and Europe[11,12]. Newsome et al[13] induced ALF in pigs by maintaining steady blood concentrations of APAP without fatal methemoglobinemia. Thiel et al[14] used jejunal administration of APAP, and Lee et al[15] successfully made an APAP-induced ALF porcine model.
In this study, we developed an APAP-induced porcine model of ALF. Using this animal model, we evaluated the safety and efficacy of a liver support system (ZHJ-3), a type of bioartificial liver system which incorporates many functions. The aims of the present were: (1) to establish a stable and efficient APAP-induced ALF porcine model; and (2) to evaluate the safety and efficacy of ZHJ-3.
MATERIALS AND METHODS
Animals
Sixteen 6-7-mo-old healthy Tibet mini pigs (weight, 30-35 kg) obtained from the Animal Center of Southern Medical University [Certificate of Conformity: SCXK (Guangdong) 2011-0015, complying with the GB 14925-94 proposal for all experimental pigs] were used in this study. All the animals received humane care and the study protocols were in compliance with the animal care guidelines established by Southern Medical University. The study was approved by the Institutional Review Board of the Second Affiliated Hospital of Southern Medical University, Guangzhou, China (No. ZJYY-2014-GDEK-003).
Animal manipulation
Experimental pigs were on preoperative fasting for 10 h and only water was provided. Xylazine hydrochloride injection (0.15-0.2 mL/kg) was used as an intramuscular injection for anesthesia. Using paraffin oil as a lubricant, a stomach tube was inserted orally to the scheduled length and then fixed along the mouth. The preliminary stomach tube was fixed and checked to determine whether the tube was in front of the mouth. Then, the gastric juice was extracted after the tube was located in the stomach, and tape used to fix the tube on the cheek. Pigs were thus maintained under general anesthesia, which was induced with xylazine hydrochloride (0.5-1.0 mL/kg), and maintained with intravenous propofol (0.2 mL/kg per hour). Omeprazole (40 mg) was given intravenously to prevent gastric ulceration, and cefoperazone (20 mg/kg intravenous) was given as a preventive antibiotic throughout the perioperative period to prevent infection. A 5 Fr catheter (Guangdong Baihe Medical Technology Co., Ltd.) was inserted into the jugular vein for fluid and drug administration and for measuring hemodynamic parameters. A double lumen catheter (12 Fr) (Guangdong Baihe Medical Technology Co., Ltd., Guangzhou, China) was placed into the femoral vein for artificial liver treatment.
ALF model establishment
ALF was induced with APAP tablets, which were dissolved in 50 mL of normal saline and administered via the oro-duodenal feeding tube. A loading dose of 0.3 g/kg APAP was given initially, followed by a maintenance dose per hour until the 12 h. The maintenance APAP dose was 3.0 g, which aimed to keep arterial methemoglobin concentrations at 1%-5% of the total hemoglobin. All the pigs were divided into two groups randomly, with eleven animals in a treatment group and five in a control group.
Supportive care
Fluid replacement solutions were given at 5 mL/kg per hour via the jugular vein throughout the whole research period: sodium lactate Ringer’s injection, 0.9% sodium chloride and 5% glucose were given at the beginning of the study for maintaining the electrolyte balance, acid-base balance, and glucose level. Colloid solution Voluven (hydroxyethyl starch 130/0.4 sodium chloride injection) was given intravenously starting at 1 mL/kg per hour. Human albumin (25%) was given intravenously when albumin levels dropped to less than 10 g/L.
Artificial liver therapy
Artificial liver treatment was started 20 h after APAP administration and continued for 8 h. Heparin was given to animals in the treatment group by continuous infusion. APTT was monitored for adjusting the rate of heparin infusion. The blood pump flow was adjusted to 50 mL/min, and ultrafiltration rate was set at 20%. Normal saline (200 mL) was injected into the tube every 2 h to prevent the filter and tubing from clogging.
End of study
The study end point was death for the control group animals. For the treatment group, animals were sacrificed with intravenous pentobarbital 5 d after induction of ALF.
Biochemical index assessment
Blood samples were collected before the administration of APAP (0 h) and at fixed intervals (every 2 h after administration of APAP) to observe aspartate transaminase (AST), albumin (Alb), total bilirubin (Tbil), ammonia (NH3), creatinine (Cr), and prothrombin time (PT) until the animals died.
Histological examination
Tissue specimens were fixed in 4% paraformaldehyde solution for 24 h to 36 h, decalcified by 10% EDTA, sliced and paraffin embedded, which was followed by step-by-step alcohol dehydration. Then, the changes were observed by hematoxylin and eosin staining under a light microscope. For electron microscopy, liver tissue was immediately placed in a 0.1 mol/L dimethyl mixture of sodium arsenate buffer in 2.5% glutaraldehyde and fixed at 4 °C for 2 h. Then, the tissue was washed three times with 0.1 mol/L sodium dimethyl arsenic acid buffer and fixed with 1% osmic acid for 2 h.
Animal care and use statement
The animals which lived at the end of the experiment were euthanized by barbiturate overdose for tissue collection.
Statistical analysis
The mean ± SD of the analyzed parameters obtained before, during, and after the administration of APAP were compared with a t-test. A P-value < 0.05 was considered significant.
RESULTS
Vital signs of pigs
No Tibet mini pigs with ALF died during the treatment. There was no divulgation in the piping of ZHJ-3 and no significant hemorrhage, allergy, or other adverse reactions found in the pigs during the whole study process. The heart rate, oxyhemoglobin saturation (SPO2), respiration rate, and artery blood pressure kept stable in the treatment (Table 1).
Table 1.
Vital signs of pigs at each time point (mean ± SD)
| Time (h) | Heart rate (per min) | Respiratory rate (per min) | SPO2 (%) | Arterial pressure (mmHg) |
| 0 | 84 ± 4 | 25 ± 13 | 96 ± 2 | 103 ± 4 |
| 1 | 79 ± 4 | 21 ± 3 | 91 ± 7 | 100 ± 5 |
| 2 | 74 ± 4 | 23 ± 5 | 94 ± 5 | 101 ± 9 |
| 3 | 80 ± 5 | 21 ± 3 | 96 ± 2 | 99 ± 6 |
| 4 | 89 ± 8 | 24 ± 6 | 93 ± 7 | 96 ± 11 |
| 5 | 91 ± 7 | 21 ± 4 | 92 ± 3 | 91 ± 7 |
| 6 | 79 ± 4 | 19 ± 3 | 94 ± 5 | 98 ± 5 |
| 7 | 76 ± 7 | 20 ± 3 | 95 ± 2 | 100 ± 2 |
| 8 | 83 ± 5 | 23 ± 6 | 92 ± 5 | 97 ± 6 |
Serum biochemical parameters
We observed a rapid reduction in animal food intake from 12 h after APAP administration, and as shown in Table 2 and Figure 1, the serum AST level increased with the progression of ALF. The levels of NH3, Tbil, and Cr were significantly increased after APAP administration in comparison with their levels before administration of APAP (P < 0.05). However, serum albumin levels after APAP administration were significantly decreased (P < 0.05). PT was found to be increased with increasing times of APAP administration. The 8 h treatment after APAP administration (i.e., 28 h) caused a significant decrease in AST, Tbil, NH3 and Cr levels than the control group animals (P < 0.05; Figure 1). This indicated that the APAP-induced porcine model of ALF was established successfully.
Table 2.
Effect of acetaminophen administration on serum biochemical parameters
| Indicator |
Before administration |
20 h after administration |
28 h after administration |
|||
| Treatment | Control | Treatment | Control | Treatment | Control | |
| AST (U/L) | 37.8 ± 5.5 | 44.8 ± 6.5 | 460.6 ± 22.9a | 410.2 ± 13.5a | 108.8 ± 13.2c | 522.1 ± 29.5 |
| NH3 (g/dL) | 26.9 ± 4.1 | 37.4 ± 5.6 | 134.1 ± 11.1a | 149.1 ± 8.3a | 96.7 ± 16.9c | 170.3 ± 23.9 |
| Tbil (μmol/L) | 22.4 ± 1.9 | 24.5 ± 1.1 | 97.8 ± 15.3a | 105.5 ± 6.1a | 66.7 ± 12.4c | 170.2 ± 37.8 |
| Cr (μmol/L) | 56.1 ± 4.4 | 55.9 ± 7.6 | 175.7 ± 19.5a | 166 ± 10.3a | 119.4 ± 10.4c | 263.1 ± 9.1 |
| ALB (g/L) | 35.9 ± 2.1 | 31.7 ± 0.9 | 22.2 ± 3.2a | 20.4 ± 2.8a | 21.4 ± 1.3 | 19 ± 1 |
| PT (s) | 11.6 ± 3.2 | 13 ± 3.0 | 60.8 ± 9.3a | 70.2 ± 4.9a | 73.0 ± 7.7 | 85.8 ± 9.2 |
P < 0.05, 20 h after administration vs before administration;
P < 0.05, treatment vs control, 28 h after administration.
Figure 1.
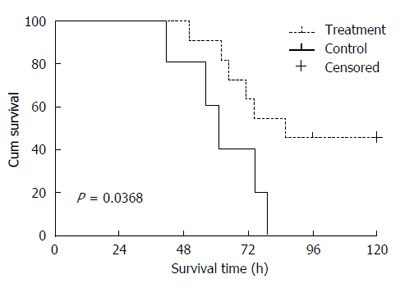
Survival curves of the porcine models in the treatment and control groups.
Histological observation
In the ALF control group, the livers were slightly enlarged, soft, and blunt in the edges, and showed smooth liver capsules, mottled surface and scattered pinpoint-like to rice-like bleeding points. There was dark-red congestion on the cut surface of livers with yellow chyliform necrotic tissues. Cross sections of the livers showed blood congestion, and the rest of the livers were red or brownish gray in color. Hepatocytes showed diffuse swelling and were sinusoidal. After APAP administration, the hepatic ultrastructure was observed by electron microscopy, which revealed degranulation of rough endoplasmic reticulum, swelling mitochondria with obscure mitochondrial crista, some dissolved mitochondria, abnormal nuclei with congested chromatin and intranuclear pseudoinclusions, and a significant decrease of intracellular bile canaliculi (Figure 2).
Figure 2.
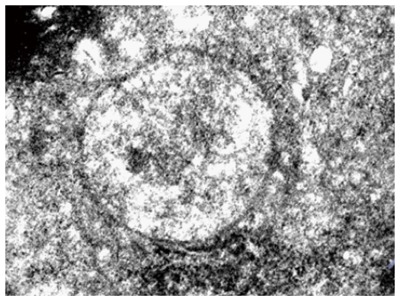
Hepatocyte swelling, nuclear fragmentation, and dissolution. Electron microscopy, × 10800.
Statistical analysis
In the treatment group, five of the eleven porcine models survived with an average survival duration of 84 h, while the five animals in the control group died, with an average survival time of 61 h. The artificial liver support system (ZHJ-3) prolonged the survival time significantly (P < 0.05; Figure 3). Of the five survival porcine models, one died at 123 h and the other four survived for more than 5 d and were sacrificed.
Figure 3.
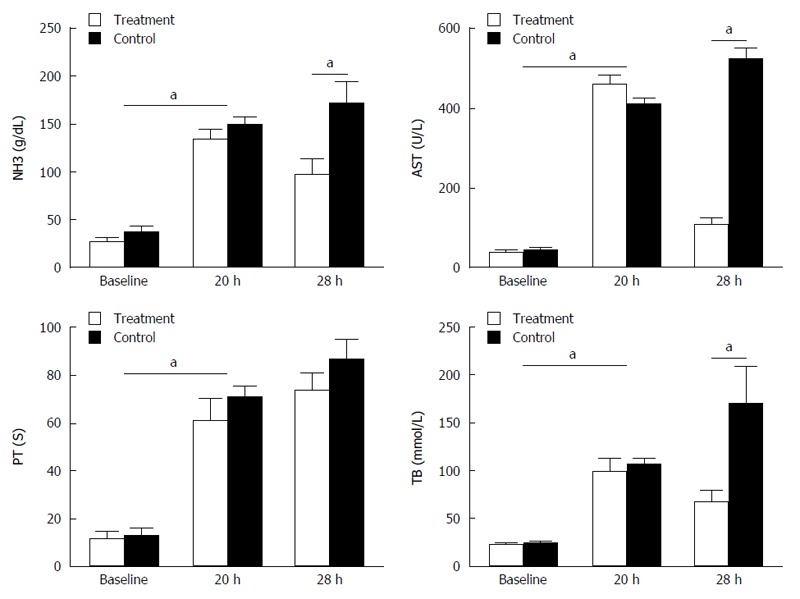
Changes in serum biochemical parameters before (baseline) and 20 h after administration of acetaminophen. Serum AST, Tbil, NH3, and Cr levels were increased, while Alb levels showed a decrease. Prothrombin time was prolonged. About 28 h after administration of acetaminophen, AST, Tbil, NH3 and Cr remained elevated while Alb remained decreased. But in the bioartificial liver system group, AST, Tbil, NH3 and Cr decreased significantly in comparison with the control group. aP < 0.05 vs control group.
DISCUSSION
Artificial liver support systems are devised to provide a transitional treatment for ALF patients awaiting compatible organs. For the pre-clinical evaluation of artificial liver support systems, a stable and reproducible large animal ALF model was proposed[16-18].
APAP is generally considered a safe drug for pain relief or fever reduction, but the incidence rate of toxicity leading to ALF has been steadily increasing[19]. Previous attempts to establish a standardized APAP intoxication model[13-15,20] were unsuccessful. Thiel et al[20] administered APAP directly into the upper jejunum via an implanted catheter, and this route of administration was affected by anesthesia, laparotomy and jejunotomy. Lee et al[15] used an oro-duodenal feeding tube that was placed for APAP dosing without any surgery. The way a gastric tube is used for APAP injection not only avoids a complex operation but also facilitates basic anesthesia intubation, thus greatly facilitating the induction of hepatic failure. Therefore, we used an oro-duodenal tube to administer APAP. Splanchnic blood flow and the volume, composition and pH of alimentary secretions are known to alter the rate of APAP absorption in experimental animals[21], but they do not influence the controllability of APAP intoxication. This, along with the intensive support provided, produced a reproducible clinically relevant model of ALF. However, the current model might be criticized for testing APAP intoxication in a less clinically applicable situation because animals underwent general anesthesia and pretreatment with antibiotics[22].
The treatment with this artificial liver system improved survival by supporting the detoxification function of the liver and the kidney, thereby interrupting the vicious cycles of elevated toxin levels and the resultant worsening of multiple organ failure. The artificial liver system improved the levels of liver biochemical indexes, such as AST and Tbil in the ALF animals, but the treatment resulted in a significant decrease in albumin levels, resulting likely from the lack of bioreactor hepatocytes[23-25]. In cirrhotic patients, albumin has been shown to be a highly effective plasma expander[26,27]. Albumin infusion began when albumin levels dropped to less than 10 g/L to avoid marked hemodynamic instability. The albumin dosing regimen used in this research was based on a previous pilot study by Tympa et al[28].
Artwohl et al[29] and Henne-Bruns et al[30] realized that APAP-dependent toxic side effects produced major complications in their studies. APAP plasma level-adapted intoxication was used to minimize complications. Significant methemoglobinemia was prevented by adjusting APAP dosing to a percentage of methemoglobin; once methemoglobin exceeded 1%, serum APAP concentrations were > 300 mg/L, and the APAP dose could be reduced. Thiel et al[14] noted that a toxic plasma APAP range between 300 mg/L and 450 mg/L produced a reproducible large animal model of ALF.
Therefore, the APAP-induced porcine model of ALF is a reproducible way to test the artificial liver system, and this system (ZHJ-3) can improve the porcine biochemical levels and extend the survival time.
The major limitation of this study was the small number of animals with ALF, which may lead to incorrect conclusions.
COMMENTS
Background
Acute liver failure (ALF) is a sudden loss of hepatic function, and its mortality remains exceedingly high. The study was aimed to establish a simple and reversible pig model of acetaminophen (APAP)-induced ALF to evaluate the effectiveness and safety of an artificial liver system (ZHJ-3). The study concluded that the APAP-induced model is reproducible and can allow for quantitative evaluation of new technologies, such as a bioartificial liver, for the support of hepatic failure in humans.
Research frontiers
There have been many studies on acute liver failure. But there has been few previous reported studies of an artificial liver system for treating acute liver failure in an APAP-induced porcine model.
Innovations and breakthroughs
In this study, APAP administration using a stomach tube is a simple and reproducible way to develop a porcine ALF model. The artificial liver system significantly reduced serum biochemistry levels and extended animal survival times.
Applications
The study concluded that the APAP-induced model is reproducible and can allow quantitative evaluation of new technologies, such as a bioartificial liver, for the support of hepatic failure in humans.
Peer-review
This is a very interesting paper about the treatment of an APAP-induced porcine model using an artificial liver system. The most important innovation of this study is APAP administration using a stomach tube. It is a simple and reproducible way to develop a porcine ALF model. In addition, it demonstrated that the artificial liver system (ZHJ-3) significantly reduced serum biochemistry levels and extended animal survival times.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Zhujiang Hospital Institutional Review Board.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Guangdong Province [IACUC protocol number: SCXK (Guangdong) 2011-0015].
Conflict-of-interest statement: The authors declare no conflicts of interest.
Data sharing statement: No additional data are available.
Peer-review started: December 6, 2016
First decision: December 29, 2016
Article in press: March 20, 2017
P- Reviewer: Berg T, Tsoulfas G S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Brown KM, Moore BT, Sorensen GB, Boettger CH, Tang F, Jones PG, Margolin DJ. Patient-reported outcomes after single-incision versus traditional laparoscopic cholecystectomy: a randomized prospective trial. Surg Endosc. 2013;27:3108–3115. doi: 10.1007/s00464-013-2914-7. [DOI] [PubMed] [Google Scholar]
- 2.Whitehouse T, Wendon J. Acute liver failure. Best Pract Res Clin Gastroenterol. 2013;27:757–769. doi: 10.1016/j.bpg.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Chan AC, Fan ST, Lo CM, Liu CL, Chan SC, Ng KK, Yong BH, Chiu A, Lam BK. Liver transplantation for acute-on-chronic liver failure. Hepatol Int. 2009;3:571–581. doi: 10.1007/s12072-009-9148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Grady JG. Acute liver failure. Postgrad Med J. 2005;81:148–154. doi: 10.1136/pgmj.2004.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525–2534. doi: 10.1056/NEJMra1208937. [DOI] [PubMed] [Google Scholar]
- 6.Newsome PN, Plevris JN, Nelson LJ, Hayes PC. Animal models of fulminant hepatic failure: a critical evaluation. Liver Transpl. 2000;6:21–31. doi: 10.1002/lt.500060110. [DOI] [PubMed] [Google Scholar]
- 7.Fourneau I, Pirenne J, Roskams T, Yap SH. An improved model of acute liver failure based on transient ischemia of the liver. Arch Surg. 2000;135:1183–1189. doi: 10.1001/archsurg.135.10.1183. [DOI] [PubMed] [Google Scholar]
- 8.Terblanche J, Hickman R. Animal models of fulminant hepatic failure. Dig Dis Sci. 1991;36:770–774. doi: 10.1007/BF01311235. [DOI] [PubMed] [Google Scholar]
- 9.Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clin Mol Hepatol. 2012;18:249–257. doi: 10.3350/cmh.2012.18.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray KF, Hadzic N, Wirth S, Bassett M, Kelly D. Drug-related hepatotoxicity and acute liver failure. J Pediatr Gastroenterol Nutr. 2008;47:395–405. doi: 10.1097/MPG.0b013e3181709464. [DOI] [PubMed] [Google Scholar]
- 11.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 12.Lee WM. Acetaminophen-related acute liver failure in the United States. Hepatol Res. 2008;38 Suppl 1:S3–S8. doi: 10.1111/j.1872-034X.2008.00419.x. [DOI] [PubMed] [Google Scholar]
- 13.Newsome PN, Henderson NC, Nelson LJ, Dabos C, Filippi C, Bellamy C, Howie F, Clutton RE, King T, Lee A, et al. Development of an invasively monitored porcine model of acetaminophen-induced acute liver failure. BMC Gastroenterol. 2010;10:34. doi: 10.1186/1471-230X-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiel C, Thiel K, Etspueler A, Schenk T, Morgalla MH, Koenigsrainer A, Schenk M. Standardized intensive care unit management in an anhepatic pig model: new standards for analyzing liver support systems. Crit Care. 2010;14:R138. doi: 10.1186/cc9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KC, Palacios Jimenez C, Alibhai H, Chang YM, Leckie PJ, Baker LA, Stanzani G, L Priestnall S, Mookerjee RP, Jalan R, et al. A reproducible, clinically relevant, intensively managed, pig model of acute liver failure for testing of therapies aimed to prolong survival. Liver Int. 2013;33:544–551. doi: 10.1111/liv.12042. [DOI] [PubMed] [Google Scholar]
- 16.Lee KU, Zheng LX, Cho YB, Kim KH, Ha J, Suh KS, Jung SE. An experimental animal model of fulminant hepatic failure in pigs. J Korean Med Sci. 2005;20:427–432. doi: 10.3346/jkms.2005.20.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieuwoudt M, Kunnike R, Smuts M, Becker J, Stegmann GF, Van der Walt C, Neser J, Van der Merwe S. Standardization criteria for an ischemic surgical model of acute hepatic failure in pigs. Biomaterials. 2006;27:3836–3845. doi: 10.1016/j.biomaterials.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Carraro A, Gringeri E, Calabrese F, Violi P, Brolese A, Zanus G, Boccagni P, Valente ML, Bassi D, D’Amico F, et al. A new experimental model of isolated perfused pig liver to support acute hepatic failure. Transplant Proc. 2007;39:2028–2030. doi: 10.1016/j.transproceed.2007.05.070. [DOI] [PubMed] [Google Scholar]
- 19.Simpson KJ, Bates CM, Henderson NC, Wigmore SJ, Garden OJ, Lee A, Pollok A, Masterton G, Hayes PC. The utilization of liver transplantation in the management of acute liver failure: comparison between acetaminophen and non-acetaminophen etiologies. Liver Transpl. 2009;15:600–609. doi: 10.1002/lt.21681. [DOI] [PubMed] [Google Scholar]
- 20.Thiel C, Thiel K, Etspueler A, Morgalla MH, Rubitschek S, Schmid S, Steurer W, Königsrainer A, Schenk M. A reproducible porcine model of acute liver failure induced by intrajejunal acetaminophen administration. Eur Surg Res. 2011;46:118–126. doi: 10.1159/000323411. [DOI] [PubMed] [Google Scholar]
- 21.Diamond L, Doluisio JT, Crouthamel WG. Physiological factors affecting intestinal drug absorption. Eur J Pharmacol. 1970;11:109–114. doi: 10.1016/0014-2999(70)90261-x. [DOI] [PubMed] [Google Scholar]
- 22.Al-Chalabi A, Matevossian E, V Thaden AK, Luppa P, Neiss A, Schuster T, Yang Z, Schreiber C, Schimmel P, Nairz E, et al. Evaluation of the Hepa Wash® treatment in pigs with acute liver failure. BMC Gastroenterol. 2013;13:83. doi: 10.1186/1471-230X-13-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Kjaergard LL, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for liver failure: a Cochrane Hepato-Biliary Group Protocol. Liver. 2002;22:433–438. doi: 10.1034/j.1600-0676.2002.01554.x. [DOI] [PubMed] [Google Scholar]
- 24.Naruse K, Tang W, Makuuch M. Artificial and bioartificial liver support: a review of perfusion treatment for hepatic failure patients. World J Gastroenterol. 2007;13:1516–1521. doi: 10.3748/wjg.v13.i10.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rademacher S, Oppert M, Jörres A. Artificial extracorporeal liver support therapy in patients with severe liver failure. Expert Rev Gastroenterol Hepatol. 2011;5:591–599. doi: 10.1586/egh.11.59. [DOI] [PubMed] [Google Scholar]
- 26.Fernández J, Navasa M, Garcia-Pagan JC, G-Abraldes J, Jiménez W, Bosch J, Arroyo V. Effect of intravenous albumin on systemic and hepatic hemodynamics and vasoactive neurohormonal systems in patients with cirrhosis and spontaneous bacterial peritonitis. J Hepatol. 2004;41:384–390. doi: 10.1016/j.jhep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Sagi SV, Mittal S, Kasturi KS, Sood GK. Terlipressin therapy for reversal of type 1 hepatorenal syndrome: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2010;25:880–885. doi: 10.1111/j.1440-1746.2009.06132.x. [DOI] [PubMed] [Google Scholar]
- 28.Tympa A, Nastos C, Defterevos G, Papalois A, Kalimeris K, Kostopanagiotou G, Vassiliou I, Smyrniotis V, Arkadopoulos N. Effects of intraperitoneal albumin on systemic and cerebral hemodynamics in a swine model of acute liver failure. J Invest Surg. 2011;24:129–133. doi: 10.3109/08941939.2011.557143. [DOI] [PubMed] [Google Scholar]
- 29.Artwohl JE, Henne-Bruns D, Carter E, Cera LM. Acetaminophen toxicosis: a potential model for acute liver failure in swine. Vet Hum Toxicol. 1988;30:324–328. [PubMed] [Google Scholar]
- 30.Henne-Bruns D, Artwohl J, Broelsch C, Kremer B. Acetaminophen-induced acute hepatic failure in pigs: controversical results to other animal models. Res Exp Med (Berl) 1988;188:463–472. doi: 10.1007/BF01852004. [DOI] [PubMed] [Google Scholar]


