Abstract
AIM
To assess the role of ultrasonography of submandibular glands (SGs) in the diagnosis of type 1 autoimmune pancreatitis (AIP).
METHODS
Thirty-seven patients who were definitively diagnosed with type 1 AIP according to the international consensus diagnostic criteria (ICDC) for AIP at our institution between December 1990 and April 2016 were retrospectively reviewed. Findings by physical examination, ultrasonography, and scintigraphy of SGs were analyzed to reach a diagnosis based on the ICDC for AIP. The efficacy of corticosteroid treatment in the resolution of hypoechoic lesions in SGs was also evaluated by assessment with ultrasonography before and after treatment in 18 cases.
RESULTS
The sensitivity of multiple hypoechoic lesions in SGs by ultrasonography for the diagnosis of sialadenitis in type 1 AIP (84%) was higher than that of physical examination (46%), scintigraphy (28%), and SGs thickness (49%). Ultrasonographic evidence of hypoechoic lesions in SGs improved the definitive diagnosis of sialadenitis and type 1 AIP by the ICDC criteria in 11 (30%) and 2 (5.4%) cases, respectively. Multiple hypoechoic lesions in SGs were resolved or disappear by corticosteroid administration in 14 of 16 cases with hypoechoic lesions in SGs, whereas the ultrasonographic findings in the remaining 2 cases with hypoechoic lesions in SGs and the 2 cases with homogenous SG parenchyma remained unchanged after corticosteroid administration.
CONCLUSION
SG ultrasonography to detect multiple hypoechoic lesions might be useful for type 1 AIP diagnosis by improving diagnostic accuracy together with the ICDC sialadenitis criteria.
Keywords: Autoimmune pancreatitis, Ultrasonography, Submandibular glands, International consensus diagnostic criteria, Sialadenitis
Core tip: We previously reported that multiple hypoechoic lesions in submandibular glands (SGs) were a specific marker of autoimmune pancreatitis (AIP). In this study, we aimed to clarify the significance of hypoechoic lesions in SGs by ultrasonography in AIP diagnosis using the international consensus diagnostic criteria (ICDC). Ultrasonographic evidence of hypoechoic lesions in SGs improved the definitive diagnosis of sialadenitis and type 1 AIP by the ICDC criteria in 11 (30%) and 2 (5.4%) cases, respectively. SG ultrasonography to detect multiple hypoechoic lesions might be useful for type 1 AIP diagnosis by improving diagnostic accuracy together with the ICDC sialadenitis criteria.
INTRODUCTION
Autoimmune pancreatitis (AIP) is a distinct form of pancreatitis characterized by periductal inflammation. Recent studies revealed that AIP might manifest as two distinct types: type 1 and 2 AIP[1]. Typical radiological and clinical findings of type 1 AIP are swelling of the pancreas, diffuse or segmental narrowing of the main pancreatic duct, high serum levels of immunoglobulin G4 (IgG4), extrapancreatic lesions with abundant infiltration of IgG4-positive plasma cells, and responsiveness to corticosteroid therapy[1-3]. In contrast, type 2 AIP, also known as idiopathic duct-centric chronic pancreatitis, is a pancreas-specific disease characterized by granulocytic epithelial lesions and is not associated with IgG4. The international consensus diagnostic criteria (ICDC) for AIP was implemented in 2011 for systematic diagnosis of this complicated disease based on five cardinal features: imaging of parenchyma and duct, serology, other organ involvement (OOI), histology of the pancreas, and response to corticosteroid therapy. Each feature is categorized as level 1 or 2 according to its specificity and reliability[1].
Type 1 AIP is frequently associated with extrapancreatic lesions such as sclerosing cholangitis, sclerosing cholecystitis, sclerosing sialadenitis, sclerosing dacryoadenitis, retroperitoneal fibrosis, and pseudotumors. Histological examination revealed that these lesions are similar to those observed in the pancreas of type 1 AIP patients and include dense fibrosis and abundant infiltration of IgG4-positive plasma cells and lymphocytes[4,5]. Among these extrapancreatic lesions, sclerosing cholangitis, and retroperitoneal fibrosis are considered as level 1 findings according to the ICDC for AIP, whereas sclerosing sialadenitis and renal involvement are considered as level 2 findings. Sclerosing sialadenitis is a feature of Mikulicz’s disease that manifests as bilateral, painless, and symmetrical swelling of the parotid and submandibular glands (SGs)[6]. Incidentally, SGs are near the body surface and can easily be evaluated by ultrasonography. However, the ultrasonographic characteristics of SGs have been documented only in a small group of nine type 1 AIP patients[7], whereas the diagnostic criteria for sialadenitis are not well-documented in ICDC.
We previously reported that multiple hypoechoic lesions in SGs were a specific marker of AIP that could be objectively diagnosed by ultrasonography[8]; however, neither the significance of these lesions in the diagnosis of sialadenitis and AIP nor their implication in IgG4-related diseases are known. In this study, we aimed to clarify the significance of hypoechoic lesions in SGs by ultrasonography in AIP diagnosis.
MATERIALS AND METHODS
Patients and diagnosis
The medical files of 47 patients who were definitively diagnosed with type 1 AIP according to the ICDC for AIP[1] between December 1990 and April 2016 at our institution were retrospectively reviewed, and 37 patients who were evaluated by SG ultrasonography were included in this study. This retrospective study was approved by the ethics committee of Yamanashi University Hospital, which waived the requirement for written informed consent because the study was a retrospective data analysis, with appropriate consideration given to patient risk, privacy, welfare, and rights.
Ultrasonographic examination of submandibular glands
Ultrasonographic examination of SGs was performed with a ProSound α10® (Aloka, Tokyo, Japan) at a frequency of 7.5 MHz. The thickest part of each SG was measured and its characteristic features were analyzed by two ultrasonography experts to reach a diagnosis. SG findings were classified into homogenous and multiple hypoechoic lesions based on ultrasonography (Figure 1), as previously reported[8]. We previously showed that multiple hypoechoic lesions in SGs were specific to type 1 AIP compared with controls[8].
Figure 1.
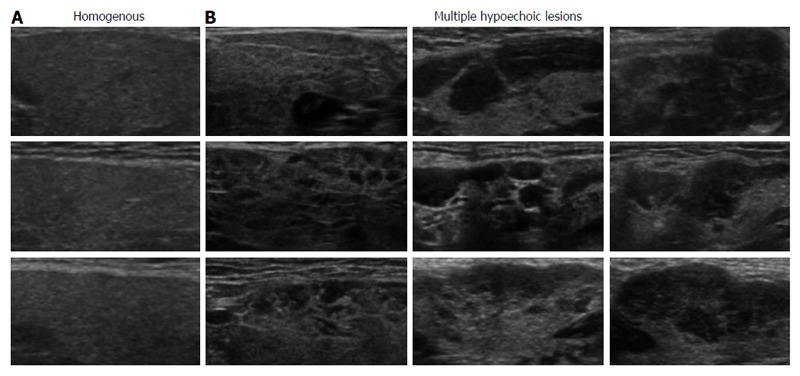
Ultrasonographic classification of echogenicity in submandibular glands. A: Homogenous echogenicity in submandibular glands with normal parenchyma; B: Multiple hypoechoic lesions detected only in patients with type 1 autoimmune pancreatitis.
Statistical analysis
Quantitative data were expressed as means ± SD and continuous data were expressed as medians and ranges. Two-group comparisons were conducted by Paired t-test. P values < 0.05 were considered statistically significant.
RESULTS
Classification of submandibular glands by ultrasonography
Characteristics of patients included in this retrospective study are shown in Table 1. All patients (n = 37) underwent ultrasonographic evaluation of SGs and received the definitive diagnosis of type 1 AIP by the ICDC criteria. In agreement with our previous report, categorization of the ultrasonographic findings of SGs into homogenous (Figure 1A) and multiple hypoechoic lesions (Figure 1B) achieved the objective diagnosis of AIP in 100% of patients harboring multiple hypoechoic lesions[8].
Table 1.
Patient characteristics n (%)
| Characteristics | Value (n = 37) |
| Female sex | 22 (60) |
| Age, median (range) | 68 (48-81) |
| Enlargement of the pancreas | |
| Diffuse | 21 (57) |
| Segmental | 16 (43) |
| Irregular narrowing of the MPD by ERP | |
| Diffuse | 23 (62) |
| Segmental | 7 (19) |
| NA | 7 (19) |
| Serum IgG4 (mg/dL), median (range) | 462 (3-2870) |
MPD: Main pancreatic duct; NA: Not available; ERP: Endoscopic retrograde pancreatography.
Efficacy of corticosteroid therapy
We next evaluated the efficacy of corticosteroid therapy on multiple hypoechoic lesions in SGs to indirectly elucidate whether these lesions were associated with infiltrating IgG4-positive cells. The upper panels in Figure 2A and 2B show the presence of multiple hypoechoic lesions in SGs by ultrasonography of cases A and B, respectively, before corticosteroid therapy, whereas the lower panels of ultrasonographic SG images reveal obscured hypoechoic lesions after corticosteroid administration in these 2 cases. Table 2 summarizes the changes in thickness and echogenicity of SGs after corticosteroid administration in all cases included the study. Average SG thickness decreased from 16.1 mm before treatment to 13.5 mm after corticosteroid administration (P = 0.002). Homogenous and multiple hypoechoic lesions in SGs were found in two and 12 cases, respectively, before corticosteroid administration. The lesions with homogenous echogenicity in 2 cases remained unchanged after corticosteroid administration. After corticosteroid administration, multiple hypoechoic lesions in one and 13 cases became homogenous or obscured, respectively, whereas 2 cases with multiple hypoechoic lesions remained unchanged.
Figure 2.
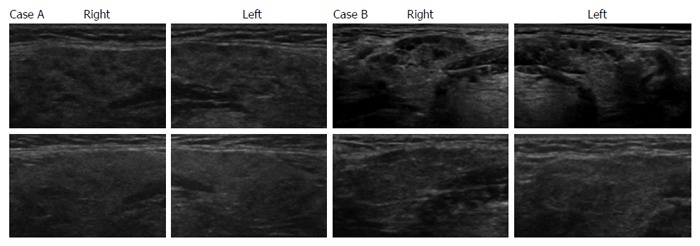
Ultrasonographic findings of echogenicity in submandibular glands before and after corticosteroid administration. Ultrasonography of bilateral submandibular glands in two cases (A and B) are shown. Upper panels show submandibular glands with multiple hypoechoic lesions before corticosteroid administration and lower panels show submandibular glands with resolved hypoechoic lesions after corticosteroid administration.
Table 2.
Impact of corticosteroid administration on ultrasonographic findings in submandibular glands
| Pre-Tx (n = 18) | Post-Tx (n = 18) | P value | |
| Thickness of SGs (mm), mean ± SD | 16.1 ± 4.2 | 13.5 ± 3.5 | 0.002 |
| Echogenicity of SGs | |||
| Homogenous | 2 | 3 | |
| Multiple hypoechoic lesions | 16 | 2 | |
| Obscured hypoechoic lesions | 13 |
SGs: Submandibular glands; pre/post-Tx: Pre/post-treatment by corticosteroids.
Diagnostic specificity and sensitivity
The diagnostic specificity of the ultrasonographic detection of multiple hypoechoic lesions in SGs of AIP patients was compared with those achieved by physical examination of SGs, gallium-67 scintigraphy, and measurement of SG thickness. As shown in Table 3, ultrasonographic detection of multiple hypoechoic lesions in SGs exhibited high diagnostic sensitivity (84%), whereas diagnostic sensitivities were lower for physical SG examination (46%), gallium-67 scintigraphy (28%), measurement of SG thickness (49%), and multiple hypoechoic lesions in parotid glands (14%). Compared with the control cases including patients diagnosed with pancreatic cancer or chronic pancreatitis, a remarkably high diagnostic specificity for AIP was achieved with multiple hypoechoic lesions in SGs (100%, data not shown), in agreement with our previous report[8].
Table 3.
Difference in sensitivity among diagnostic methods for sialadenitis n (%)
| Examined cases | Positive examination | |
| SG swelling on physical examination | 33 | 15 (46) |
| Ga accumulation in SGs by scintigraphy | 25 | 7 (28) |
| Increased thickness in SGs by US (≥ 15 mm) | 37 | 18 (49) |
| Multiple hypoechoic lesions in SGs by US | 37 | 31 (84) |
| Multiple hypoechoic lesions in parotid glands by US | 36 | 5 (14) |
Ga: Gallium-67; SG: Submandibular glands; US: Ultrasonography.
Impact of multiple hypoechoic lesions in diagnoses of sialadenitis and autoimmune pancreatitis
Among a total of 37 cases with AIP included in this study, IgG4-related sclerosing cholangitis and retroperitoneal fibrosis, both defined as level 1 OOI, were found in 4 and 5 cases, respectively; both organs were involved in 1 case (Table 4). Level 2 OOI was present in 18 cases. Specifically, level 2 findings of sialadenitis and renal involvement were found in 20 and 4 cases, respectively, whereas both organs were involved in 21 cases (Table 4). When the presence of multiple hypoechoic lesions in SGs was used as a diagnostic criterion for sialadenitis, the number of cases with level 2 OOI increased from 18 to 25, based on the increase in the number of cases with sialadenitis from 20 to 31 (Table 4).
Table 4.
Other organ involvement
| n | |
| Level 1 findings | |
| IgG4-related SC | 4 |
| Retroperitoneal fibrosis | 5 |
| Level 2 findings | |
| Enlarged salivary glands | 20 |
| Sialadenitis by SGUS | 31 |
| Renal involvement | 4 |
| Final diagnosis of OOI | |
| Level 1 | 8 |
| Level 2 | 18 |
| Level 2 by SGUS | 25 |
SC: Sclerosing cholangitis; SGUS: Ultrasonography of submandibular glands; OOI: Other organ involvement.
Classification of the diagnosis of type 1 AIP based on the ICDC criteria is shown in Table 5. Type 1 AIP diagnosis was achieved in 1, 21, 8, and 7 cases by the ICDC criteria of histology, typical imaging, indeterminate imaging, and response to corticosteroid treatment, respectively. All but three cases were definitively diagnosed as type 1 AIP by not including findings of OOI as a diagnostic criterion, whereas the remaining three cases required the presence of OOI to reach the definitive diagnosis of type 1 AIP. Furthermore, the AIP diagnosis in two of these 3 cases required the presence of multiple hypoechoic lesions in SGs.
Table 5.
Classification of primary diagnosis for type 1 autoimmune pancreatitis
| Primary basis for diagnosis | Final diagnosis | Definitive diagnosis without OOI | Definitive diagnosis with OOI | Definitive diagnosis with SGUS |
| Histology | 1 | 1 | ||
| Typical imaging | 21 | 19 | 1 | 1 |
| Indeterminate imaging | 8 | 8 | ||
| Response to steroid | 7 | 6 | 1 |
OOI: Other organ involvement; SGUS: Ultrasonography of submandibular glands.
DISCUSSION
This study demonstrated that diagnostic accuracy of type 1 AIP might be improved by the adoption of ultrasonographic findings of multiple hypoechoic lesions in SGs for the diagnosis of sialadenitis. In addition, our findings indicated that hypoechoic lesions in SGs responded to corticosteroid administration, suggesting that these lesions might represent infiltrated foci of IgG4-positive plasma cells in SGs.
The diagnosis of AIP remains a challenge due to the difficulty in distinguishing it from pancreatic cancer, especially in patients with segmental/localized type AIP. The diagnostic criteria for AIP using a combination of histological, serological, radiological, and clinical features has been established in several countries. The ICDC for AIP[1], first reported in 2011, enabled the systematic diagnosis of this complicated disease. We were able to assess OOI for the objective diagnosis of sclerosing cholangitis and retroperitoneal fibrosis based on their characteristic radiological and histological findings; however, the definitive diagnosis of sialadenitis and renal involvement can be challenging due to the lack of definitive diagnostic criteria and reliable methods for evaluation, except for the measurement of swelling by subjective methods, as outlined in the ICDC for AIP.
Mikulicz’s disease, also known as IgG4-related sclerosing sialadenitis, presents with symmetrical swelling of the lacrimal and parotid glands, as well as the SGs, and is frequently associated with type 1 AIP[9]. The definitive diagnosis of Mikulicz’s disease requires (1) persistent symmetrical swelling of the lacrimal and parotid glands, as well as the SGs; and (2) either elevated serum IgG4 level (> 135 mg/dL) or marked IgG4-positive plasma cell infiltration into the lacrimal and salivary gland tissues[6]. The only other study on SG ultrasonography for AIP reported the presence of hypoechoic areas in SGs in eight patients with Mikulicz’s disease[7]. Importantly, these findings were distinct from those of Sjögren’s syndrome, the other type of sialadenitis[10]. Our previous study clearly demonstrated that hypoechoic lesions in SGs were objectively associated with type 1 AIP but not with PDC[8]. Furthermore, the diagnostic sensitivity of SG ultrasonography for IgG4-related sclerosing sialadenitis was higher than that of the ICDC diagnostic criteria for sialadenitis with symmetrical enlargement of salivary glands.
Ultrasonographic findings of sialadenitis can potentially improve the diagnostic sensitivity and specificity of type 1 AIP. Moreover, definite diagnosis of sialadenitis by ultrasonography as a level 1 finding might render response to corticosteroid treatment an unnecessary criterion for the diagnosis of type 1 AIP in certain cases. Corticosteroid response can also be utilized to differentiate type 1 AIP from PDC[11]; however, it is not included in the Japanese diagnostic criteria for type 1 AIP[12] as certain pancreatic malignancies such as malignant lymphoma respond to corticosteroid therapy, which might lead to misdiagnosis. Additionally, corticosteroid therapy is considered a somewhat aggressive approach due to associated adverse effects. In the current study of 37 cases with type 1 AIP, sialadenitis and level 2 OOI were identified in 11 and 7 additional cases, respectively, by appending the ultrasonographic findings of SGs to the currently adopted diagnostic criteria. Furthermore, 2 cases could not be definitively diagnosed as type 1 AIP by the ICDC criteria without adopting ultrasonographic findings of SGs, as shown in Table 5; however, the definite diagnosis of type 1 AIP in these cases was achieved with other diagnostic criteria such as the Japanese diagnostic criteria for type 1 AIP[12]. In addition, the inclusion of hypoechoic SG lesions by ultrasonography as a level 1 finding allowed for the exclusion of corticosteroid response as a diagnostic criterion for type I AIP in four of a total of 37 cases in the current study. Therefore, sialadenitis diagnosis by ultrasonography should be considered as a level 1 finding as part of the ICDC criteria, given that the ultrasonographic evidence of hypoechoic lesions in SGs is a specific and objective diagnostic finding in type 1 AIP patients.
This study has several limitations inherent to the retrospective design and the small number of cases in the study cohort.
In conclusion, the findings of the current study provided evidence for the utility of SG ultrasonography in the diagnosis of type 1 AIP, specifically based on the presence of multiple hypoechoic lesions in SGs. Our findings suggested that SG ultrasonography might improve the diagnostic accuracy in type 1 AIP when used in combination with the ICDC criteria for sialadenitis.
COMMENTS
Background
The authors previously reported that multiple hypoechoic lesions in submandibular glands were a specific marker of autoimmune pancreatitis (AIP) that could be objectively diagnosed by ultrasonography; however, neither the significance of these lesions in the diagnosis of sialadenitis and AIP nor their implication in immunoglobulin G4 (lgG4)-related diseases are known.
Research frontiers
Ultrasonographic findings of multiple hypoechoic lesions in submandibular glands improve the diagnostic sensitivity and accuracy of both sialadenitis and type 1 AIP.
Innovations and breakthroughs
This study demonstrated that diagnostic accuracy of type 1 AIP might be improved by the adoption of ultrasonographic findings of multiple hypoechoic lesions in submandibular glands (SGs) for the diagnosis of sialadenitis. In addition, these findings indicated that hypoechoic lesions in SGs responded to corticosteroid administration, suggesting that these lesions might represent infiltrated foci of IgG4-positive plasma cells in SGs.
Applications
The authors findings suggested that ultrasonography of submandibular glands might improve the diagnostic accuracy in type 1 AIP when used in combination with the international consensus diagnostic criteria criteria for sialadenitis.
Peer-review
This paper clarified the significance of hypoechoic lesions in submandibular glands by ultrasonography in AIP diagnosis using the international consensus diagnostic criteria.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This retrospective study was approved by the ethics committee of Yamanashi University Hospital.
Informed consent statement: The ethics committee waived the requirement for written informed consent because the study was a retrospective data analysis, with appropriate consideration given to patient risk, privacy, welfare, and rights.
Conflict-of-interest statement: The authors report no financial conflicts of interest.
Data sharing statement: No additional data are available.
Peer-review started: December 27, 2017
First decision: February 10, 2017
Article in press: April 21, 2017
P- Reviewer: Iida T, Liao KF, Manenti A S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
References
- 1.Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–358. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 2.Okazaki K, Uchida K, Miyoshi H, Ikeura T, Takaoka M, Nishio A. Recent concepts of autoimmune pancreatitis and IgG4-related disease. Clin Rev Allergy Immunol. 2011;41:126–138. doi: 10.1007/s12016-010-8214-2. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Chari S, Smyrk TC, Deshpande V, Klöppel G, Kojima M, Liu X, Longnecker DS, Mino-Kenudson M, Notohara K, et al. Autoimmune pancreatitis (AIP) type 1 and type 2: an international consensus study on histopathologic diagnostic criteria. Pancreas. 2011;40:1172–1179. doi: 10.1097/MPA.0b013e318233bec5. [DOI] [PubMed] [Google Scholar]
- 4.Deshpande V, Chicano S, Finkelberg D, Selig MK, Mino-Kenudson M, Brugge WR, Colvin RB, Lauwers GY. Autoimmune pancreatitis: a systemic immune complex mediated disease. Am J Surg Pathol. 2006;30:1537–1545. doi: 10.1097/01.pas.0000213331.09864.2c. [DOI] [PubMed] [Google Scholar]
- 5.Taguchi M, Aridome G, Abe S, Kume K, Tashiro M, Yamamoto M, Kihara Y, Nakamura H, Otsuki M. Autoimmune pancreatitis with IgG4-positive plasma cell infiltration in salivary glands and biliary tract. World J Gastroenterol. 2005;11:5577–5581. doi: 10.3748/wjg.v11.i35.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Himi T, Takano K, Yamamoto M, Naishiro Y, Takahashi H. A novel concept of Mikulicz’s disease as IgG4-related disease. Auris Nasus Larynx. 2012;39:9–17. doi: 10.1016/j.anl.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu M, Moriyama M, Okamura K, Kawazu T, Chikui T, Goto TK, Ohyama Y, Nakamura S, Yoshiura K. Sonographic diagnosis for Mikulicz disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:105–113. doi: 10.1016/j.tripleo.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 8.Takano S, Sato T, Fukasawa M, Shindo H, Takahashi E, Yokota Y, Kadokura M, Enomoto N. Hypoechoic Lesions in Submandibular Glands Are Diagnostic Markers of Type 1 Autoimmune Pancreatitis. Pancreas. 2016;45:e8–e9. doi: 10.1097/MPA.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 9.Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Sumida T, Mimori T, Tanaka Y, et al. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol. 2012;22:1–14. doi: 10.1007/s10165-011-0508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu M, Okamura K, Yoshiura K, Ohyama Y, Nakamura S. Sonographic diagnosis of Sjögren syndrome: evaluation of parotid gland vascularity as a diagnostic tool. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:587–594. doi: 10.1016/j.tripleo.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Moon SH, Kim MH, Park DH, Hwang CY, Park SJ, Lee SS, Seo DW, Lee SK. Is a 2-week steroid trial after initial negative investigation for malignancy useful in differentiating autoimmune pancreatitis from pancreatic cancer? A prospective outcome study. Gut. 2008;57:1704–1712. doi: 10.1136/gut.2008.150979. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki K, Uchida K, Koyabu M, Miyoshi H, Takaoka M. Recent advances in the concept and diagnosis of autoimmune pancreatitis and IgG4-related disease. J Gastroenterol. 2011;46:277–288. doi: 10.1007/s00535-011-0386-x. [DOI] [PubMed] [Google Scholar]


