Abstract
AIM
To clarify the role of neoadjuvant concurrent chemoradiotherapy (NACCRT) followed by surgical resection for localized or locally advanced perihilar cholangiocarcinoma (CCA).
METHODS
We retrospectively reviewed 57 patients who underwent surgical resection with or without NACCRT for perihilar CCA; 12 patients received NACCRT and 45 patients did not received NACCRT. Patients with locally advanced perihilar CCA requiring NACCRT were defined as follows: (1) a mass involving unilateral branches of the portal vein or hepatic artery with insufficient volume of the anticipated remnant lobe; or (2) an infiltrating mass in the main portal vein that was too long for reconstruction, identified at preoperative staging.
RESULTS
The median disease-free survival (DFS) durations of the neoadjuvant and non-neoadjuvant CCRT groups were 26.0 and 15.1 mo, respectively (P = 0.91). The median overall survival (OS) durations of the neoadjuvant and non-neoadjuvant CCRT groups were 32.9 and 27.1 mo, respectively (P = 0.26). The NACCRT group showed a downstaging tendency compared to the non-NACCRT group as compared with the tumor stage confirmed by histological examination after surgery and the tumor stage confirmed by imaging test at the time of diagnosis (P = 0.01).
CONCLUSION
NACCRT does not prolong DFS and OS in localized or locally advanced perihilar CCA. However, NACCRT may allow tumor downstaging and improve tumor resectability.
Keywords: Klatskin tumor, Locally advanced, Survival rate, Neoadjuvant therapy, Chemoradiotherapy
Core tip: This study is a retrospective study to clarify the role of neoadjuvant concurrent chemoradiotherapy (NACCRT) followed by surgical resection for locally advanced perihilar cholangiocarcinoma (CCA). The median disease free survival (DFS) durations of the neoadjuvant and non-neoadjuvant group were 26.0 and 15.1 mo, respectively (P = 0.91). The median overall survival (OS) durations of the neoadjuvant and non-neoadjuvant groups were 32.9 and 27.1 mo, respectively (P = 0.26). However, the NACCRT group showed a downstaging tendency compared to the non-NACCRT group (P = 0.01). This paper will be helpful in the treatment plan for patients with locally advanced perihilar cholangiocarcinoma, especially NACCRT.
INTRODUCTION
Cholangiocarcinoma (CCA) is a group of tumor that arises from the intrahepatic, perihilar, or distal extrahepatic bile duct epithelial lining[1]. Among these diseases, perihilar CCA, commonly called Klatskin tumor, which develops from the right and/or left hepatic ducts at or near the biliary confluence is the most common, and accounts for around 70% of all CCA cases[2]. With advances in surgical technique, including aggressive liver resection, the indications of curative surgery for perihilar CCA have been broadened[3]. However, many cases are regarded as unresectable or locally advanced without distant metastasis. The common causes of unresectability for locally advanced perihilar CCA are anatomic proximity to major vessels, including the hepatic artery or portal vein, and combined atrophic or cirrhotic changes in the affected segment of the liver.
Recently, neoadjuvant concurrent chemoradiotherapy (NACCRT) followed by liver transplantation has shown promising clinical outcomes in selected cases of perihilar CCA[4]. However, liver transplantation cannot be used as the standard treatment for all cases of locally advanced perihilar CCA because of the risk of recurrences, as well as a shortage of organ donors. Nevertheless, NACCRT is a promising therapeutic option for locally advanced perihilar CCA considering the meaningful outcomes of NACCRT in liver transplantation and borderline resectable pancreatic cancer[4,5].
Herein, we analyzed the clinical outcomes of NACCRT with curative resection of perihilar CCA in locally advanced stage.
MATERIALS AND METHODS
Patient population
From January 2004 to December 2013, 98 patients with perihilar CCA underwent surgery at Severance Hospital, Yonsei University College, Korea. Of the 98 patients, 12 with locally advanced non-metastatic perihilar CCA received NACCRT. Patients with locally advanced perihilar CCA requiring NACCRT in this study were defined as follows: (1) a mass involving unilateral branches of the portal vein or hepatic artery with insufficient volume of the anticipated remnant lobe; or (2) an infiltrating mass in the main portal vein that was too long for reconstruction, identified at preoperative staging. Twelve patients had Bismuth type III or IV and TNM stage III or IV disease. Of the remaining 86 patients, 45 had the same disease stage as those who received NACCRT. In this study, patients with locally advanced perihilar CCA who received NACCRT were defined as the NACCRT group. Of the 57 patients, 31 underwent biopsy at the time of diagnosis and 26 did not undergo biopsy. Among the 31 patients who underwent biopsy, 13 were diagnosed as adenocarcinoma and the remaining 18 were suspected of cancer. In analysis of the tissues obtained from the operation, 55 patients were adenocarcinoma. Of 57 patients, 2 patients with no remnant cancer cell were adenocarcinoma in the biopsy performed at the time of diagnosis. In result, all of the 57 patients were adenocarcinoma, which was confirmed by biopsy performed at diagnosis or surgery. These patients received NACCRT followed by surgery, and adjuvant treatment as needed. Patients with locally advanced perihilar CCA who did not receive NACCRT were defined as the non-NACCRT group. They underwent surgery immediately after diagnosis and received adjuvant treatment as needed. Patients who needed biliary decompression at the time of diagnosis had undergone procedures such as endoscopic retrograde biliary drainage (ERBD) or percutaneous transhepatic biliary drainage (PTBD).
Clinical, radiologic, histopathologic, and survival data were retrieved up to December 2015 from a database at Severance Hospital, and were analyzed retrospectively. All of the laboratory data checked at the time of diagnosis and clinical stages, such as Bismuth classification and TNM stage before surgery, pathologic TNM stage, resection margin of surgical specimen, and microvascular/perineural invasions after surgery were analyzed in both groups. In the NACCRT group, the chemotherapy regimen, total radiation dose, complications during NACCRT, and response to NACCRT were analyzed.
This retrospective study was reviewed and approved by the institutional review board of Severance Hospital (IRB 2016-2480-001) and was conducted in accordance with the Declaration of Helsinki. Patient consent was waived owing to the retrospective nature of the study.
Neoadjuvant concurrent chemoradiotherapy regimen
In this study, 12 patients received NACCRT. Of 12 patients, 5 patients received 5-fluorouracil (5-FU; 450 mg/m2 per day, D1-4) and leucovorin (20 mg/m2 per day, D1-4) with external beam radiotherapy (1.8 Gy per day to a total dose of 50.4 Gy or 45 Gy). They received an average of 3.2 cycles of 5-FU/leucovorin. Of 12 patients, 5 patients received gemcitabine (1000 mg/m2 per day, D1, 8, 15, 22) with external beam radiotherapy (1.8 Gy per day to a total dose of 50.4 Gy or 45 Gy). They received an average of 1.6 cycles of gemcitabine. Of 12 patients, 1 patient received gemcitabine (1000 mg/m2 on D1, 8, 15, 22) and cisplatin (70 mg/m2 on D1) with external beam radiotherapy (1.8 Gy per day to a total dose of 50.4 Gy). He received one cycle of gemcitabine/cisplatin. Of 12 patients, 1 patient received Tegfur/Uracil (UFT; daily) with external beam radiotherapy (1.8 Gy per day to a total dose of 45 Gy).
Follow-up duration
The survival of all 57 patients was analyzed. In cases of death, the date of death was investigated. In cases of survival or follow-up loss, the last follow-up date was investigated. Overall survival (OS) was defined from the date of diagnosis to the date of death, or to the date of the last follow-up. Disease free survival (DFS) was defined from the operation date to the date when recurrence was confirmed using imaging.
Statistical analysis
Continuous variables were summarized and reported as means with standard deviations or medians and interquartile ranges. Categorical variables were summarized and reported as frequencies and percentages. Statistical analyses were performed with a t test or χ2 test, as appropriate. Significance was defined at a two-sided P-value of < 0.05. Survival analyses for DFS and OS were performed using the Kaplan-Meier method. Univariate and multivariate analyses were performed using the Cox proportional hazards model. Analyses were performed using the SPSS statistics software version 21 (IBM Corporation, Armonk, NY)
RESULTS
The baseline characteristics of patients are listed in Table 1. The median age of patients in the neoadjuvant group was lower than that of the non-neoadjuvant group (neoadjuvant group, 59.0 years vs non-neoadjuvant group, 67.0 years; P = 0.02). However, when the patients were divided by age using 60 years as a cut-off, the numbers of patients above and below 60 years of age were similar in the 2 groups [neoadjuvant group, 6 (50.0%) vs non-neoadjuvant group, 35 (77.8%), P = 0.08]. There were no significant differences in sex or follow-up durations between the 2 groups [sex (male patient)]; neoadjuvant group, 8 (66.7%) vs non-neoadjuvant group, 24 (53.3%), P = 0.41; follow-up durations, neoadjuvant group, 31.9 mo vs non-neoadjuvant group, 26.4 mo, P = 0.27. Total bilirubin levels (neoadjuvant group, 2.3 vs non-neoadjuvant group, 6.4; P < 0.05) was higher in the non-neoadjuvant group. However albumin and carbohydrate antigen 19-9 (CA 19-9) levels were no significant difference between the 2 groups. There was no difference in the distribution of Bismuth type and TNM stage between the 2 groups because they were selected. All CCAs included in this analysis were histologically adenocarcinoma. Five cases were classified as undetermined for tumor differentiation, as shown in Table 1. Of these 5 cases, 2 surgical specimens had no detectable cancer cells and 3 pathologic records of tumor differentiation were not recorded. There was a difference in the distribution of tumor differentiation between the 2 groups, and the proportion of well-differentiated tumors in the non-NACCRT group was higher (P < 0.01). Of the patients who underwent adjuvant treatment after surgery, there were 5 (41.7%) in the NACCRT group and 27 (60.0%) in the non-NACCRT group (P = 0.26). Tumor recurrence occurred in 10 patients (83.3%) in the NACCRT group and in 31 patients (68.9%) in the non-NACCRT group (P = 0.48). Moreover, post-operative site recurrence was the most common in each group [NACCRT group, 3 (30.0%); non-NACCRT group, 11 (35.5%)].
Table 1.
Patient characteristics n (%)
| Neoadjuvant chemoradiotherapy (n = 12)1 | Non-neoadjuvant chemoradiotherapy (n = 45)1 | P value2 | |
| Demographic variables | |||
| Age (yr) | 59.0 (48.25, 62.75) | 67.0 (60.00, 69.00) | 0.02 |
| Age ≥ 60 | 6 (50.0) | 35 (77.8) | 0.08 |
| Sex (Male) | 8 (66.7) | 24 (53.3) | 0.41 |
| Follow-up duration (mo) | 31.9 (19.8, 53.8) | 26.4 (14.5, 42.1) | 0.27 |
| Laboratory variables | |||
| Albumin (g/dL) | 3.75 (3.450, 4.075) | 3.60 (3.200, 3.850) | 0.32 |
| Total bilirubin (mg/dL) | 2.3 (0.60, 8.45) | 6.4 (2.35, 11.40) | < 0.05 |
| CA 19-9 (U/mL) | 181.8 (27.08, 1452.50) | 210.0 (71.35, 976.00) | 0.74 |
| Cancer-related variables | |||
| Bismuth classification | |||
| IIIA | 2 (16.7) | 21 (46.7) | 0.10 |
| IIIB | 3 (25.0) | 2 (4.4) | 0.06 |
| IV | 7 (58.3) | 22 (48.9) | 0.56 |
| Pre-op AJCC 7th stage | |||
| IIIA (T3N0M0) | 8 (66.7) | 23 (51.1) | 0.34 |
| IIIB (T1-3N1M0) | 2 (16.7) | 17 (37.8) | 0.30 |
| IVA (T4N0-1M0) | 1 (8.3) | 3 (6.7) | > 0.99 |
| IVB (T1-4N2M0) | 1 (8.3) | 2 (4.4) | 0.52 |
| Tumor differentiation | < 0.01 | ||
| Well | 0 (0) | 11 (24.4) | |
| Moderately | 7 (58.3) | 29 (64.4) | |
| Poorly | 0 (0) | 5 (11.1) | |
| Undetermined | 5 (41.7) | 0 (0) | |
| Post-operative adjuvant treatment | 5 (41.7) | 27 (60.0) | 0.26 |
| Recurrence | 10 (83.3) | 31 (68.9) | 0.48 |
| OP site | 3 (30.0) | 11 (35.5) | |
| Liver | 2 (20.0) | 3 (9.7) | |
| Distant organ | 3 (30.0) | 9 (29.0) | |
| Carcinomatosis | 2 (20.0) | 8 (25.8) | |
Continuous variables were denoted median (Q1, Q3) and categorical variables were denoted number (%);
P values were determined using a Mann-Whitney test for continuous variables and a χ2 test (Fisher’s exact test, Pearson and Mantel-Haenszel χ2 test) for categorical variables. CA19-9: Carbohydrate antigen 19-9; AJCC: American Joint Committee on Cancer.
The DFS estimates for the 2 treatment groups are shown in Figure 1. The median DFS durations of the NACCRT and non-NACCRT groups were 26.0 and 15.1 mo, respectively (P = 0.91). The OS estimates for the 2 treatment groups are shown in Figure 2. The median OS duration of the NACCRT and non-NACCRT groups were 32.9 and 27.1 mo, respectively (P = 0.26).
Figure 1.
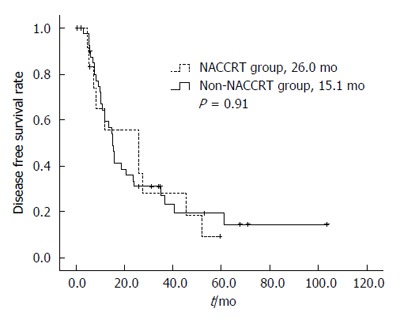
Disease-free survival rate curves of the 2 treatment groups. DFS rate was performed with using the Kaplan-Meier method.
Figure 2.
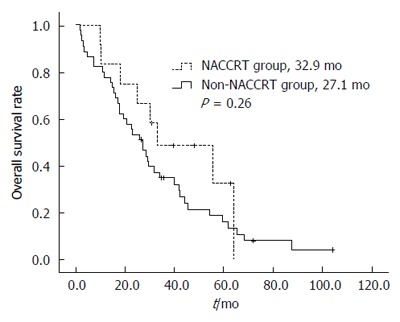
Overall survival rate curves of the 2 treatment groups. OS rate was performed with using the Kaplan-Meier method.
Various chemotherapy regimens were used for NACCRT, as listed in Table 2. Of the 12 patients, 5 (41.7%) received 5-fluorouracil + leucovorin, 5 (41.7%) received gemcitabine, and 1 of the remaining 2 patients received gemcitabine + cisplatin and the other 1 tegafur/uracil (UFT). All 12 patients received 3-dimensional (3D) conformal radiotherapy using CCRT. The median dose of radiotherapy was 5040 cGy (range, 4545 to 5040). Three (25%) patients showed toxicities related to CCRT. The most common toxicities were nausea and vomiting (66.7%). No treatment-related deaths occurred. Two (66.7%) patients showed neutropenia and anemia higher than grade II, which delayed the treatment. When abdominal computed tomography (CT) scans were compared before and after NACCRT, and confirmed using the Response Evaluation Criteria in Solid Tumors (RECIST) 2.0, 7 (58.3%) out of 12 patients had a partial response (PR) and 5 (41.7%) out of 12 patients had a stable disease (SD).
Table 2.
Summary of neoadjuvant chemoradiotherapy n (%)
| Neoadjuvant chemoradiotherapy (n = 12) | |
| Regimen of chemotherapy | |
| 5-fluorouracil + leucovorin | 5 (41.7) |
| Gemcitabine | 5 (41.7) |
| Gemcitabine + cisplatin | 1 (8.3) |
| Tegfur/Uracil (UFT) | 1 (8.3) |
| Total dose of radiotherapy (cGy) | 5040 (4545, 5040)1 |
| Response in follow-up image | |
| Partial response | 7 (58.3) |
| Stable disease | 5 (41.7) |
| Complete response in pathology | 2 (16.7) |
| Downstaging of TNM stage | 4 (33.3) |
| Toxicity | |
| ≥ Grade II | 2 (16.7) |
1Continuous variables were denoted median (Q1, Q3) and categorical variables were denoted number (%).
Table 3 shows the efficacy of NACCRT. The NACCRT group showed a decrease in tumor stage after surgery compared with preoperative tumor stage [downstaging in the NACCRT group, 11 (91.7%) vs downstaging in the non-NACCRT group, 23 (51.1%), P = 0.01]. Ten patients (83.3%) in the NACCRT group and 30 (64.4%) in the non-NACCRT group underwent margin-negative resection (R0 resection) (P = 0.32). Microvascular invasion [NACCRT group, 6 (50.0%) vs non-NACCRT group, 29 (64.4%), P = 0.51] and lymph node metastasis [NACCRT group, 3 (25.0%) vs non-NACCRT group, 25 (55.6%), P = 0.06] showed no significant difference between the 2 treatment groups. Perineural invasion was more frequent in the non-NACCRT group [NACCRT group, 7 (58.3%) vs non-NACCRT group, 44 (97.8%), P < 0.01]. Moreover, there were 2 patients (16.7%) with perioperative complications in the NACCRT group and 11 (24.4%) in the non-NACCRT group (P = 0.71). Of the 2 patients in the NACCRT group, 1 (50.0%) experienced small bowel obstruction due to post-operative adhesion and 1 (50.0%) experienced wound seroma. Of the 11 patients in the non-NACCRT group who experienced postoperative complications, 4 (36.4%) developed septic shock due to wound infection, 2 (18.2%) experienced wound infection, 1 (9.1%) had wound dehiscence, 1 (9.1%) had a catheter infection, 1 (9.1%) developed septic shock due to aspiration pneumonia, 1 (9.1%) had a liver abscess, and 1 (9.1%) experienced liver failure. However, there was no death due to perioperative complications in both 2 groups.
Table 3.
Efficacy of neoadjuvant chemoradiotherapy n (%)
| Neoadjuvant chemoradiotherapy (n = 12) | Non-neoadjuvant chemoradiotherapy (n = 45) | P value1 | |
| Alteration of TNM stage before and after surgery | 0.01 | ||
| Upstaging | 0 (0) | 11 (24.4) | |
| No change | 1 (8.3) | 11 (24.4) | |
| Downstaging | 11 (91.7) | 23 (51.1) | |
| R0 resection | 10 (83.3) | 30 (66.7) | 0.32 |
| Surgical pathology | |||
| Microvascular invasion | 6 (50.0) | 29 (64.4) | 0.51 |
| Perineural invasion | 7 (58.3) | 44 (97.8) | < 0.01 |
| Lymph node metastasis | 3 (25.0) | 25 (55.6) | 0.06 |
| Perioperative complications | 2 (16.7) | 11 (24.4) | 0.71 |
P values were determined using a Mann-Whitney test for continuous variables and a χ2 test (Fisher’s exact test, Pearson and Mantel-Haenszel χ2 test) for categorical variables.
DISCUSSION
Several studies have already reported the role of NACCRT in CCA. Some studies have shown the role of neoadjuvant therapy in allowing downstaging surgery for unresectable cancer. McMasters et al[6] described 9 patients, 5 with perihilar and 4 with distal common bile duct CCA, who were treated with preoperative chemoradiotherapy. Of the 9 patients, 3 experienced a pathologically complete response. The rate of margin-negative resection was 100% for the preoperative chemoradiotherapy group compared with 54% for the surgery alone group. Moreover, there was no patient who experienced any significant treatment-related complications during preoperative treatment. Nelson et al[7] identified a cohort of 12 patients out of 45 with both proximal and distal extrahepatic CCAs who underwent NACCRT. Of these 12 patients, 10 had initially unresectable disease and 2 had resectable disease. As a result, 11 out of the 12 patients in the neoadjuvant group underwent R0 resection, and 3 achieved a complete pathological response.
As described above, the potential advantages of neoadjuvant chemoradiotherapy include the delivery of therapy with an intact vascular supply, downstaging of borderline resectable tumors, a reduction of tumor seeding and locoregional dissemination during surgery, and selection of patients for surgery according to their response to therapy[8].
In our study, the response evaluation of NACCRT through comparison abdominal CT scans was 7 patients PR and 5 patients SD. Furthermore, 2 (16.7%) out of 12 patients had a pathologically complete response. The follow-up image, which is usually performed and evaluated not only by the patients in this study but also by the usual CCRT, was performed one month after the completion of NACCRT. In two cases, the main lesions treated with CCRT were observed as residual mass due to post-CCRT inflammation and evaluated as partial response and stable disease through RECIEST. As another result of our study, 4 out of 12 patients achieved tumor downstaging due to downgrade of T stage by receiving NACCRT. Furthermore, there was a tendency of downstaging in the NACCRT group compared to the non-NACCRT group. To evaluate the tendency to downgrade the stage of the two groups, we compared the clinical stage at diagnosis and the postoperative pathological stage. Obviously, the clinical TNM stage and pathological TNM stage may be different. Even considering this point, the NACCRT group tends to downstage more than the non-NACCRT group. If a large number of data is analyzed, this tendency can be confirmed more clearly. There was no significant difference in the performance of margin-negative resection between the 2 treatment groups [NACCRT group, 10 (83.3%) vs non-NACCRT group, 30 (66.7%); P = 0.32]. Reduction of metastasis to lymph nodes was expected to be an advantage of neoadjuvant therapy; however, it did not show a meaningful result [NACCRT group, 3 (25.0%) vs non-NACCRT group, 25 (55.6%), P = 0.06].
In addition, in our study, NACCRT did not show any results that could ultimately improve the cure rate by lowering the cancer stage to an operable state (OS of the NACCRT group, 32.9 mo vs OS of the non-NACCRT group, 29.8 mo, P = 0.26). Similar results were also found in other studies[6,7]. In particular, the aim to reduce recurrence through locoregional control, one of the objectives of NACCRT, was not seen in this analysis (DFS of the NACCRT group, 26.0 mo vs DFS of the non-NACCRT group, 15.1 mo, P = 0.91). Locoregional recurrence or distant recurrence occurs in spite of margin-negative resection, which has already been confirmed through other studies and highlights the need for adjuvant treatment[9,10]. In our study, we analyzed predicting variables for DFS and OS (Tables 4 and 5). The postoperative adjuvant treatment group showed a decreased risk compared with non-postoperative adjuvant treatment about DFS; however, there was no statistical significance (HR = 0.78, 95%CI: 0.42-1.45, P = 0.43). In addition, adjuvant therapy was performed with lymph node metastasis or margin-positive resection. Of the neoadjuvant group, 5 patients received chemotherapy after surgery for adjuvant therapy. Of the regimens for adjuvant therapy, 3 patients received Fluorouracil/Cisplatin and 2 patients received Gemcitabine/Cisplatin. Of the non-neoadjuvant group, 19 patients received chemotherapy, 5 patients received radiotherapy and 3 patients received concurrent chemoradiotherapy after surgery for adjuvant therapy. Of the regimens for adjuvant therapy, 6 patients received Fluorouracil/Cisplatin, 3 patients received Gemcitabine/Cisplatin, 3 patients received Gemcitabine, 2 patients received UFT, 1 patient received Tegafur (TS-1)/cisplatin, 1 patient received Tegfur/uracil (UFT)/Cisplatin, 1 patient received Fluorouracil/Carboplatin and 1 patient received Fluorouracil/leucovorin/oxaliplatin (FOLFOX).
Table 4.
Univariate analysis for identifying the risk factor for disease free survival and overall survival
|
Disease-free survival |
Overall survival |
|||
| HR(95%CI) | P value1 | HR (95%CI) | P value1 | |
| Male | > 1.00 (0.54-1.87) | < 1.00 | 1.34 (0.75-2.41) | 0.33 |
| Age < 60 | 1.20 (0.61-2.36) | 0.59 | 0.86 (0.46-1.61) | 0.63 |
| Bismuth type | ||||
| IIIA | 1.00 | 1.00 | ||
| IIIB | 1.95 (0.70-5.42) | 0.20 | 1.07 (0.31-3.63) | 0.92 |
| IV | > 1.00 (0.52-1.95) | 0.99 | 0.84 (0.47-1.51) | 0.56 |
| Pre-op AJCC 7th stage | ||||
| IIIA | 1.00 | 1.00 | ||
| IIIB | 0.86 (0.44-1.66) | 0.64 | 0.88 (0.46-1.68) | 0.70 |
| IVA | 0.55 (0.13-2.37) | 0.42 | 0.70 (0.21-2.34) | 0.57 |
| IVB | 2.51 (0.56-11.19) | 0.23 | 2.43 (0.71-8.25) | 0.16 |
| Serum total bilirubin | 0.76 (0.95-1.04) | 0.76 | 1.01 (0.97-1.06) | 0.66 |
| Serum total bilirubin ≥ 3 | 1.21 (0.64-2.28) | 0.56 | 1.40 (0.77-2.53) | 0.27 |
| Serum CA19-9 | 1.01 (> 1.00-1.01) | < 0.01 | > 1.00 (1.00-1.01) | 0.13 |
| Serum CA19-9 ≥ 300 | 3.28 (1.68-6.41) | < 0.01 | 2.58 (1.39-4.78) | < 0.01 |
| Variation of CA19-9 after NACCRT | 1.07 (0.98-1.15) | 0.12 | 1.02 (0.95-1.10) | 0.61 |
| Received NACCRT | 1.05 (0.51-2.15) | 0.91 | 0.65 (0.30-1.39) | 0.27 |
| Performed R0 resection | 1.01 (0.50-2.04) | 0.98 | 0.61 (0.33-1.15) | 0.12 |
| Tumor differentiation | ||||
| Well | 1.00 | 1.00 | ||
| Moderately | 2.30 (0.95-5.55) | 0.06 | 1.34 (0.63-2.83) | 0.45 |
| Poorly | 1.25 (0.15-10.47) | 0.84 | 3.66 (1.08-12.33) | 0.04 |
| Indeterminate | 3.30 (0.92-11.88) | 0.07 | 1.21 (0.37-3.97) | 0.75 |
| Microvascular invasion | 1.37 (0.73-2.56) | 0.33 | 1.89 (1.02-3.51) | 0.04 |
| Perineural invasion | 0.58 (0.22-1.49) | 0.25 | 1.35 (0.53-3.45) | 0.53 |
| Lymph node metastasis | 1.01 (0.54-1.86) | 0.98 | > 1.00 (0.56-1.78) | 0.99 |
| Received adjuvant treatment | 0.78 (0.42-1.45) | 0.43 | 0.77 (0.43-1.36) | 0.36 |
P values were determined with a univariate Cox proportional hazards regression test. AJCC: American Joint Committee on Cancer; CA19-9: Carbohydrate antigen 19-9; NACCRT: Neoadjuvant concurrent chemoradiotherapy.
Table 5.
Multivariate analysis for identifying the risk factor for disease-free survival and overall survival
|
Disease-free survival |
Overall survival |
|||
| HR (95%CI) | P value1 | HR (95%CI) | P value1 | |
| Serum CA19-9 ≥ 300 | 3.28 (1.68-6.41) | < 0.01 | 2.66 (1.40-5.06) | < 0.01 |
| R0 resection | 0.47 (0.24-0.90) | 0.02 | ||
P values were determined using a multivariate Cox proportional hazards regression test. CA19-9: Carbohydrate antigen 19-9.
Other predicting variable for DFS and OS in our study was serum CA19-9 level. Serum CA19-9 level is a well-known prognostic marker of CCA[11,12]. In addition, there is a conclusion that CA19-9 is the independent risk factor for recurrence after curative resection in biliary tract cancer[13] as well as the staging system including preoperative CA19-9[14]. These results including our study suggest that CA19-9 is sufficient marker for predicting prognosis as well as marker for tracking cancer recurrence. However, the value of CA19-9 is known to be influenced by cholestasis[15], and it is known that the higher the pre-treatment value, the worse the prognosis[14,16]. Therefore, we conducted a univariate and multivariate analysis using a Cox proportional hazard model with a category of more than 3 mg/dL of total bilirubin (bilirubin ≥ 3) and more than 300 U/mL of CA19-9 (CA19-9 ≥ 300) based on the results of Kim et al[15]. In univariate analysis, CA19-9 ≥ 300 was identified as risk factor for DFS and OS. In addition, CA19-9 ≥ 300 was analyzed as an independent risk factor for DFS and OS in multivariate analysis including the effect of bilirubin ≥ 3. Although the outcome of surgery in the high CA19-9 group is known to be poor[17], the relationship between high CA19-9 and perioperative complications was not statistically significant in this study (P = 0.33; not listed in the table). However, the change of CA19-9 by NACCRT was not a predicting variable for DFS and OS (DFS: HR = 1.07, 95%CI: 0.98-1.15, P = 0.12 and OS: HR = 1.02, 95%CI, 0.95-1.10, P = 0.61). Another risk factor for OS in multivariate analysis was R0 resection (HR = 0.47, 95%CI: 0.24-0.90, P = 0.02). In univariate analysis, the effect of R0 resection on OS was not statistically significant, but it was confirmed as independent predictive variable in multivariate analysis. In fact, several studies have suggested R0 resection as the most important factor affecting prognosis[18-20].
Our study had some limitations that are inherent to a retrospectively designed study. There may be limitations in representing the effect of NACCRT in the NACCRT group because of the small number of patients and the variety of regimens used as NACCRT. For a more statistically significant comparison, we also considered the propensity match score. However, the number of study groups and control groups are small and limited. Controlling the two groups under similar conditions to compare only the use of NACCRT is expected that the number of subjects to be compared will be reduced and the statistical meaning will be insufficient. Nevertheless, the advantage of this study is that we set up a control group consisting of patients with disease of a Bismuth type and TNM stage similar to the locally advanced disease to determine the efficacy of NACCRT.
NACCRT is thought to affect the downstaging of locally advanced stage perihilar CCA, which may increase the possibility of surgery. However, this analysis did not show an expected increase in DFS or OS as the possibility of surgery increased. In conclusion, NACCRT may be a feasible downstaging method to allow surgery, or an option as a bridging therapy during preparatory procedures for extended hepatectomy, such as portal vein embolization. In addition, systemic treatment, such as adjuvant chemotherapy, is also required for patients with perihilar CCA who do not have distant metastasis.
COMMENTS
Background
The only treatment for cholangiocarcinoma, including Klatskin tumor, is known as surgery. Although the development of surgical techniques improves the chances of surgery for Klatskin tumors, it is still impossible for the masses involved in the main vessels to be operated. If locally advanced stage Klatskin tumor can be expected to have effects such as downstaging through neoadjuvant treatment, the possibility of surgery will be increased and the extension of disease free survival (DFS) or overall survival (OS) will be considered. In this study, they investigated whether neoadjuvant concurrent chemoradiotherapy affects DFS and OS in locally advanced stage Klatskin tumor.
Research frontiers
The efficacy of neoadjuvant concurrent chemoradiotherapy in biliary tract cancer has not been established. Few prior studies have shown that neoadjuvant treatment can be used for downstaging and that it has been effective in survival by controlling regional extension. The results of this study contribute to clarifying the role of neoadjuvant concurrent chemoradiotherapy in locally advanced stage Klatskin tumor.
Innovations and breakthroughs
In this study, neoadjuvant concurrent chemoradiotherapy showed tendency of downstaging to operable state in locally advanced stage Klatskin tumor. This result is similar to few previous studies. However, as the probability of operation increased, there was no prolongation of disease free survival and overall survival. The median DFS durations of the neoadjuvant and non-neoadjuvant CCRT groups were 26.0 and 15.1 mo, respectively (P = 0.91). The median OS durations of the neoadjuvant and non-neoadjuvant CCRT groups were 32.9 and 27.1 mo, respectively (P = 0.26).
Applications
This study suggests that neoadjuvant concurrent chemoradiotherapy is thought to affect the downstaging of locally advanced stage Klatskin tumor, which may increase the possibility of surgery. However, this analysis did not show an expected increase in disease free survival or overall survival as the possibility of surgery increased.
Terminology
Klatskin tumor: Cancer that develops in cells that line the bile ducts in the liver, where the right and left ducts meet. Neoadjuvant therapy: treatment given as a first step to shrink a tumor before the main treatment, which is usually surgery, is given. Concurrent chemoradiotherapy: The use of chemotherapy delivered concurrently with radiation.
Peer-review
The article reviewed the retrospective case-control study of the outcome of the treatment with addition of neoadjuvant by chemoradiotherapy in comparison with treatment without neoadjuvant for locally advanced perihilar cholangiocarcinoma. Benefit of neoadjuvant concurrent chemoradiotherapy for locally advanced perihilar cholangiocarcinoma.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The institutional review board of Severance Hospital approves this study (IRB 2016-2480-001).
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: We have no financial relationships to disclose.
Data sharing statement: No additional data are available.
Peer-review started: February 8, 2017
First decision: February 23, 2017
Article in press: April 12, 2017
P- Reviewer: Kukongviriyapan V, Liu XF, Ramia JM S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512–522. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuhaus P, Jonas S, Bechstein WO, Lohmann R, Radke C, Kling N, Wex C, Lobeck H, Hintze R. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–18; discussion 819. doi: 10.1097/00000658-199912000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, Botha JF, Mezrich JD, Chapman WC, Schwartz JJ, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88–98.e3; quiz e14. doi: 10.1053/j.gastro.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barugola G, Partelli S, Crippa S, Capelli P, D’Onofrio M, Pederzoli P, Falconi M. Outcomes after resection of locally advanced or borderline resectable pancreatic cancer after neoadjuvant therapy. Am J Surg. 2012;203:132–139. doi: 10.1016/j.amjsurg.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 6.McMasters KM, Tuttle TM, Leach SD, Rich T, Cleary KR, Evans DB, Curley SA. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am J Surg. 1997;174:605–608; discussion 605-68;. doi: 10.1016/s0002-9610(97)00203-1. [DOI] [PubMed] [Google Scholar]
- 7.Nelson JW, Ghafoori AP, Willett CG, Tyler DS, Pappas TN, Clary BM, Hurwitz HI, Bendell JC, Morse MA, Clough RW, et al. Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2009;73:148–153. doi: 10.1016/j.ijrobp.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spitz FR, Abbruzzese JL, Lee JE, Pisters PW, Lowy AM, Fenoglio CJ, Cleary KR, Janjan NA, Goswitz MS, Rich TA, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997;15:928–937. doi: 10.1200/JCO.1997.15.3.928. [DOI] [PubMed] [Google Scholar]
- 9.Jarnagin WR, Ruo L, Little SA, Klimstra D, D’Angelica M, DeMatteo RP, Wagman R, Blumgart LH, Fong Y. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689–1700. doi: 10.1002/cncr.11699. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa S, Ikai I, Fujii H, Hatano E, Shimahara Y. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg. 2007;31:1256–1263. doi: 10.1007/s00268-007-9001-y. [DOI] [PubMed] [Google Scholar]
- 11.Abd ElWahab M, El Nakeeb A, El Hanafy E, Sultan AM, Elghawalby A, Askr W, Ali M, Abd El Gawad M, Salah T. Predictors of long term survival after hepatic resection for hilar cholangiocarcinoma: A retrospective study of 5-year survivors. World J Gastrointest Surg. 2016;8:436–443. doi: 10.4240/wjgs.v8.i6.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai WK, Lin JJ, He GH, Wang H, Lu JH, Yang GS. Preoperative serum CA19-9 levels is an independent prognostic factor in patients with resected hilar cholangiocarcinoma. Int J Clin Exp Pathol. 2014;7:7890–7898. [PMC free article] [PubMed] [Google Scholar]
- 13.Chung MJ, Lee KJ, Bang S, Park SW, Kim KS, Lee WJ, Song SY, Chung JB, Park JY. Preoperative serum CA 19-9 level as a predictive factor for recurrence after curative resection in biliary tract cancer. Ann Surg Oncol. 2011;18:1651–1656. doi: 10.1245/s10434-010-1529-7. [DOI] [PubMed] [Google Scholar]
- 14.Bergquist JR, Ivanics T, Storlie CB, Groeschl RT, Tee MC, Habermann EB, Smoot RL, Kendrick ML, Farnell MB, Roberts LR, et al. Implications of CA19-9 elevation for survival, staging, and treatment sequencing in intrahepatic cholangiocarcinoma: A national cohort analysis. J Surg Oncol. 2016;114:475–482. doi: 10.1002/jso.24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HJ, Kim MH, Myung SJ, Lim BC, Park ET, Yoo KS, Seo DW, Lee SK, Min YI. A new strategy for the application of CA19-9 in the differentiation of pancreaticobiliary cancer: analysis using a receiver operating characteristic curve. Am J Gastroenterol. 1999;94:1941–1946. doi: 10.1111/j.1572-0241.1999.01234.x. [DOI] [PubMed] [Google Scholar]
- 16.Chung YJ, Choi DW, Choi SH, Heo JS, Kim DH. Prognostic factors following surgical resection of distal bile duct cancer. J Korean Surg Soc. 2013;85:212–218. doi: 10.4174/jkss.2013.85.5.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo N, Murakami Y, Uemura K, Sudo T, Hashimoto Y, Sasaki H, Sueda T. Elevated perioperative serum CA 19-9 levels are independent predictors of poor survival in patients with resectable cholangiocarcinoma. J Surg Oncol. 2014;110:422–429. doi: 10.1002/jso.23666. [DOI] [PubMed] [Google Scholar]
- 18.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, Youssef BA M, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517; discussion 517-9. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakeeb A, Tran KQ, Black MJ, Erickson BA, Ritch PS, Quebbeman EJ, Wilson SD, Demeure MJ, Rilling WS, Dua KS, et al. Improved survival in resected biliary malignancies. Surgery. 2002;132:555–563; discission 563-564. doi: 10.1067/msy.2002.127555. [DOI] [PubMed] [Google Scholar]
- 20.Cheng QB, Yi B, Wang JH, Jiang XQ, Luo XJ, Liu C, Ran RZ, Yan PN, Zhang BH. Resection with total caudate lobectomy confers survival benefit in hilar cholangiocarcinoma of Bismuth type III and IV. Eur J Surg Oncol. 2012;38:1197–1203. doi: 10.1016/j.ejso.2012.08.009. [DOI] [PubMed] [Google Scholar]


