Abstract
A systematic review of the published literature (from January 2000 to January 2016) to ascertain the safety of, and patient satisfaction with, the aesthetic use of abobotulinumtoxinA was conducted. In addition to the licensed indications, other special populations were considered for discussion. The potential impact of neutralizing antibodies and systemic toxicity were also addressed. A total of 364 papers were screened and 86 were found to be relevant to the population, intervention(s), and outcomes stipulated in the protocol. The safety and patient satisfaction data from these publications are discussed in this review.
Level of Evidence: 2

There is a wealth of evidence for the safety of botulinum toxin A (BoNT-A) in aesthetic use, which has led to its licensing in the temporary improvement in the appearance of moderate to severe glabellar lines associated with procerus and corrugator muscle activity in adult patients <65 years of age.1
The aim of this article was to provide an up-to-date review of the published safety data for BoNT-A, with a focus on abobotulinumtoxin-A (ABO), in the treatment of glabellar lines, other areas of the face, and scar optimization, and to examine key safety issues such as neutralizing antibodies, use in pregnancy, and systemic toxicity. In addition, the data supporting patient satisfaction were also addressed.
METHODS
A systematic review protocol was prepared. In January 2016, in keeping with the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines,2 we searched the PubMed and Cochrane databases for literature published between January 2000 and January 2016 to address 6 key questions. These questions are not listed in order of any importance and each received equal weight in the review process.
-
1. What is the clinical evidence for the safety of ABO in aesthetic procedures?
a. Dosage by area and concentration (based on dilution)
b. Injection points (identification of target positions—the neuromuscular junctions), which will differ based on individual patient anatomy
c. Dilution/reconstitution volume, which will differ by site of injection/injector
d. Technique according to region of injection
e. Repeated treatment according to region
-
2. What is the clinical evidence for intradermal safety of botulinumtoxinA in scar optimization?
a. In prevention of hypertrophic or keloid scars (reduction in postsurgical inflammation)
b. In postoperative healing (reduction of wound tension)
-
3. What is the evidence for neutralizing antibody formation as a result of ABO treatment?
a. Evidence for possible nonresponse: patients who have stopped responding or suddenly respond very weakly
4. What is the clinical experience for use of ABO treatment in patients who are breastfeeding or are subsequently found to be pregnant?
-
5. What is the clinical evidence for systemic toxicity as a result of ABO treatment?
a. Central effects of toxins, neural plasticity
6. What is the evidence for patient satisfaction with ABO for aesthetic procedures?
The following keywords were used when searching the literature databases: abobotulinum, abobotulinumtoxin, abobotulinumtoxinA, adverse event, aesthetic, anaphylaxis, antibody, asymmetry, birth, blepharoptosis, botulinum, breastfeeding, bruising, conception, contraception, cutaneous infection, dilution, diplopia, dosage, Dysport, ecchymosis, ectropion, edema, entropion, fetal, fetus, flu-like, Headache, hyperesthesia, immune, immunogenicity, immunological, inflammation, injection site pain, keloid, loss of response, maternal, neurological, neutralizing, neutralizing antibody, nursing, postoperative, pregnancy, pregnant, ptosis, repeat, repeated, safety, scar, side effect, strabismus, surgery, surgical, systemic, systemic toxicity, toxicity, urticaria, wound.
A total of 364 papers were identified and initially screened by Dr Vanessa Lane and 86 were found to be relevant to the population, intervention(s), and outcomes. These papers were reviewed by the authors (J.L.C. and N.S). Disagreements were resolved through discussion and an independent review of the papers was performed by Dr Andy Pickett.
RESULTS
What is the Clinical Evidence for the Safety of AbobotulinumtoxinA in Aesthetic Procedures?
Many studies have investigated the safety of BoNT-A as a class. In 2014, a systematic review on the safety of BoNT-A (ABO, incobotulinumtoxinA [INCO], and onabotulinumtoxinA [ONA]) for aesthetic use was performed, which included data from 35 clinical studies including 8787 individuals. Of these studies, 13 (37%) assessed ABO, 1 (3%) assessed INCO, and 21 (60%) assessed ONA.3 The overall incidence of adverse events (AEs) for all botulinum toxin preparations was not found to be significantly different between experimental and placebo groups, and AEs were reported as being mostly mild and transient in nature.3 The most common AEs were blepharoptosis (2.5%), brow ptosis (3.1%), and eye sensory disorders (3%) in the upper face, and lip asymmetries and imbalances in the lower face (6.9%). In a 2015 systematic review, eyelid edema was also reported in 1.4% of patients, with a greater risk for BoNT-A-induced eyelid edema reported in Asian populations than Caucasian populations (3.1% vs 0.7%).4
Headache, injection site pain, edema, and bruising have been reported and occur independently of the area being treated, with no statistical difference compared with placebo injection. This suggests that these AEs may be related to the injection procedure and could be avoided by improving injection technique.5-7
There are no reported long-term AEs related to the aesthetic use of ABO, INCO, or ONA.8
Studies that specifically investigated ABO have reported a good overall safety profile, with most AEs being minor and related to the trauma of injection.9,10 There are substantial safety data relating to the treatment of glabellar lines.11 However, safety data relating to the treatment of the forehead, lateral canthal lines (“crow’s feet”), and other anatomical areas are less well documented.10
Glabellar Lines
The safety of ABO for the treatment of glabellar lines has been demonstrated in 5 Phase III studies,12-15 a 24-month extension trial,16 and a 36-month extension trial.17 These clinical trials combined included over 4500 individuals who received up to 7 cycles of treatment. There were no reported serious treatment-emergent AEs (TEAEs) deemed possibly or probably related to treatment.17,18Table 1 summarizes the TEAEs (>1% incidence) reported in the safety population for ABO.18
Table 1.
Summary of Data Supporting the Safety of AbobotulinumtoxinA From 5 Phase III Clinical Trials Investigating its Use in the Treatment of Glabellar Lines: The Number of Patients With Treatment-Emergent Adverse Events With >1% Incidence Reported in the Safety Population. Reproduced With permission From Aesthetic Surgery Journal, Mark Rubin et al18
| TEAEs no. of patients (%) | Rubin et al 15 | Kane et al 13 | Brandt et al 12 | Moy et al 14 | Cohen et al 16 | |||
|---|---|---|---|---|---|---|---|---|
| ABO n = 311 | ABO n = 544 | Placebo n = 272 | ABO n = 105 | Placebo n = 53 | ABO n = 1200 | ABO n = 1415 | ||
| Fixed dose n = 1390 | Variable dose n = 715 a | |||||||
| No. of patients with any TEAEs | 207 (67) | 168 (31) | 75 (28) | 49 (47) | 21 (40) | 880 (73) | 818 (59) | 260 (36) |
| Nasopharyngitis | 38 (12) | 15 (3) | 6 (2) | 12 (11) | 6 (11) | 153 (13) | 168 (12) | 22 (3) |
| Headache | 42 (14) | 19 (3) | 8 (3) | 10 (10) | 4 (8) | 178 (15) | 81 (6) | 30 (4) |
| Eyelid ptosis | 6 (2) | 13 (2) | 1 (<1) | 3 (3) | 0 (0) | 45 (4) | 30 (2) | 10 (<1) |
| Blepharospasm | 3 (1) | 1 (<1) | 0 (0) | 1 (1) | 2 (4) | 15 (1) | 12 (<1) | 1 (<1) |
| Injection site pain | 12 (4) | 2 (<1) | 4 (1) | 4 (4) | 2 (4) | 83 (7) | 50 (4) | 10 (1) |
| Injection site bruising | 16 (5) | 4 (1) | 0 (0) | 0 (0) | 1 (2) | 72 (6) | 30 (2) | 7 (1) |
| Upper respiratory tract infection | 6 (2) | 10 (2) | 4 (1) | 1 (1) | 0 (0) | 82 (7) | 67 (5) | 15 (2) |
| Sinusitis | 14 (5) | 6 (1) | 3 (1) | 2 (2) | 0 (0) | 92 (8) | 84 (6) | 9 (1) |
| Influenza | 10 (3) | 5 (<1) | 0 (0) | 2 (2) | 2 (4) | 44 (4) | 32 (2) | 5 (<1) |
ABO, abobotulinumtoxinA; TEAE, treatment-emergent adverse event. aPatients who received both fixed- and variable-dose treatments were counted in both groups; therefore, the total number of patients in the fixed- and variable-dose groups is greater than the total number in the study.
The treatment of glabellar lines with ABO was well tolerated, and the safety profile of ABO was comparable to that of placebo in terms of type, frequency, severity, and relatedness of TEAEs for fixed-dose and variable-dose treatment regimens, as well as for single and repeat-dose treatment.18 Each of these Phase III studies represented a different aspect of the safety profile of ABO.
Rubin et al compared 311 patients who received different cycles of open-label treatments before entering the double-blinded, randomized treatment phase.15 In this study involving open-label treatment, the incidence of TEAEs did not increase over 2 to 3 treatment cycles (Table 1).
Kane et al investigated the safety of variable dosing in 816 individuals randomized in a 2:1 ratio to ABO (50, 60, 70, or 80 U) or placebo. Only slightly more TEAEs were reported with ABO compared with placebo and these differences were not statistically significant (31% vs 28%, respectively) (Table 1).13 In the cardiovascular subanalysis within this study (n = 79), there was no QT/QTc prolongation observed, where QT interval is a measure of time between the Q wave and the T wave in the heart's electrical cycle. It is widely known as QT and QTc refers to the corrected QT.
Brandt et al assessed the safety of a single treatment of ABO in 105 patients. TEAEs occurred in a similar proportion of patients in the treatment (49/105, 47%) and placebo (21/53, 40%) groups.12 The most frequently occurring TEAEs were injection site reaction, injection site pain, nasopharyngitis, and headache. The majority of TEAEs were considered not related, or unlikely to be related, to study treatment. The incidence of eyelid ptosis in the ABO group was 3/105 (2.9%) compared with 0/53 (0%) in the placebo group, and all of the reports were mild and resolved without sequelae (Table 1).
Moy et al found that up to 5 cycles of treatment with 50 units of ABO in 1200 patients were well tolerated.14 The results revealed no evidence of cumulative safety issues over the course of 13 months (Table 1).
Cohen et al assessed the long-term cumulative safety of ABO in both fixed-unit and variable-dosing settings in an extension study of patients from the 4 Phase III trials.16 Over 24 months, 1415 patients underwent open-label retreatment with ABO. Patients were retreated with 50 units or a variable dose of 50, 60, 70, or 80 units based on sex and muscle mass. Some 932 patients (66%) experienced at least 1 AE. The rate of TEAEs was similar in both the fixed- and variable-dose groups and most were rated mild (70%) or moderate (20%). The majority (87%) of AEs were considered not related, or unlikely to be related, to study treatment. The incidence of all TEAEs and related TEAEs remained relatively constant or decreased over repeat cycles of ABO treatment. ABO was readministered with a minimum of 85 days between treatments, and then only if glabellar lines returned to a moderate or severe level. The repeat-dose studies summarized in the article showed no evidence of cumulative safety issues despite the fact that the majority of the patients received more than 50 units of ABO (Table 1).
Schlessinger et al carried out a long-term extension trial of 36 months’ duration, which enrolled patients from the 4 Phase III trials, to assess the safety and efficacy of repeat injections with ABO for the treatment of glabellar lines.17 The final results of the extension study in 1415 patients demonstrated that multiple cycles of ABO treatment over 24 months were well tolerated and effective for the correction of glabellar lines, with no evidence of cumulative safety problems over the 3 year study period. The AEs that patients experienced were typically mild to moderate in severity, and the majority of reported TEAEs were judged to be unlikely or not related to treatment.
In repeated-treatment studies of ABO, the incidence of TEAEs was the highest in the first cycle and decreased in subsequent treatment cycles.19 Approximately 91% of 945 patients treated with ABO (median total dose/session = 100 units) over 3 to 5 consecutive cycles experienced no AEs. The glabella was the area treated most frequently (93.9%), with the majority (81.5%) of patients also receiving treatment in other areas of the face. AEs decreased with repeat treatments, occurring in 39/945 patients (4.1%) in the first treatment cycle and 11/553 (2.0%) in the fifth treatment cycle. Ptosis was restricted to 0.85% of patients.
A 2010 clinical overview by Rzany et al examined the safety of ABO for the treatment of glabellar lines in 11 clinical studies, which involved a total of 4649 patients and 12,844 treatments (including the 5 pivotal studies already described).20 It was concluded that ABO demonstrated good overall safety during these studies. Most of the TEAEs were considered unrelated to the treatment and the most frequent treatment-related TEAEs again included headache and injection site reaction. Furthermore, the majority of treatment-related TEAEs were mild in intensity and resolved without additional treatment.20 The percentage of patients that reported eyelid ptosis was low: <3% in all single-treatment studies and <4% in all repeated-treatment studies. TEAEs reported by >3% of patients included headaches (most common), injection site reaction, injection site pain, nasopharyngitis, and upper respiratory tract infection, and generally occurred to a similar degree among patients receiving ABO and placebo.13,21,22
Other Areas of the Face (Forehead and Crow’s Feet)
Although ABO is commonly used in many facial areas, the US Food and Drug Administration (FDA) approval is specifically only for the glabella at this time. The lack of a large pivotal trial to support FDA approval of an indication other than the glabella reflects the limited published safety data available for the use of ABO in other facial areas.
The reported AEs in 20 patients receiving ABO treatment for severe frontalis lines at maximum elevation were bruising and occasional headache. Although the results were based on 20 treated subjects, the use of bilateral frontalis comparisons resulted in a statistical N = 40.23 However, injection of any BoNT-A into the frontalis muscles (forehead) may worsen brow ptosis, and injecting the lower part of the forehead should be avoided for this reason.8,10,24 This is particularly the case for patients who depend on their lateral frontalis to elevate their brows in an effort to avoid accentuating dermatochalasis.
AEs associated with the use of any of the BoNT-A formulations to soften the appearance of lateral canthal rhytides (crow’s feet) have included bruising, diplopia, asymmetric smile, and lid ptosis.8
In a double-blind, placebo-controlled, dose-ranging study including 218 patients, Ascher et al demonstrated the safety of ABO at doses ranging between 15 U and 45 U for the treatment of lateral crow’s feet.25 The findings have been confirmed by more recent open-label and retrospective studies of several hundred patients.26,27
Impact of Multiple Injection Sites
The use of multiple vs single injection sites does not appear to impact safety outcomes for ABO treatment. A study comparing 1 vs 3 injection sites of ABO in the treatment of lateral crow’s feet in 40 patients showed no difference in AEs between the 2 sides.27 This finding confirmed the results of a previous retrospective analysis that found when using a total dose of ABO the same for each side of the face, 1 injection point had similar safety outcomes to 3 separate injection points.26
Hexsel et al compared the safety of different doses of ABO administered to the entire face simultaneously.28 Ninety patients were enrolled with at least 2 indications for ABO treatments on each third of the face (upper, middle, and lower). Patients were randomized into 3 groups, with a predefined total dose range of ABO (group 1, 120-165 U; group 2, 166-205 U; and group 3, 206-250 U). No statistically significant difference was identified between the 3 dose groups for AEs. The incidence of AEs was low and events were mainly related to the injections and included erythema (67%), bruising (27%), edema (13%), pain, pruritus and burning (2.4%), and some bleeding (4.7%). Only 1 patient (1.2%) reported a sensation of heaviness in the eyelids. This symptom was transitory and did not lead to eyelid ptosis. These results suggested that concomitant injections of ABO in various facial areas could be performed with no increased safety concerns.
Field of Effect
If the dose is increased, the area of effectiveness (otherwise known as the field of effect) will increase, and by this, the final effect will also potentially increase.29 The field of effect of a BoNT-A formulation is a function of the active process of “spread” during injection and the subsequent passive process of diffusion afterward.30,31,32 The field of effect depends on a number of variables (including: injection volume; total dose; depth, angle, and rate of injection; anatomic area; desired degree of effect; and patient-specific factors)33 but today, the effect of dose on diffusion is considered to be the key factor influencing the field of effect and comparable results have been demonstrated between equal doses of different BoNT-A products.34
AEs related to the field of effect have been infrequently observed in large, randomized controlled trials (RCTs), as reflected by the low incidences of eyelid ptosis.10 When unwanted effects such as ptosis do occur, they can often be traced back to lack of injector experience or poor technique, which results in subsequent spread followed by diffusion to musculature adjacent to the site of injection.35
Diffusion within the tissues is slower and not dependent on the injection technique,30 although volume might play a role in the initial spread of the toxin from the original site of injection.36 A recent study investigating the field of effect in 10 patients found a larger field of effect (ie, mean wrinkle reduction, 794.1 vs 486.6 mm2) when a larger reconstitution volume (and hence injection volume) was used.36 Electromyography studies show that BoNT-A can diffuse up to 3 cm from the point of injection, making accurate identification and injection of target muscles essential to achieving the desired outcome.37
What is the Clinical Evidence for Safety of Injection of BoNT-A in Scar Optimization?
BoNT-A is an ideal biochemical agent that allows near-total elimination of muscle pull on the healing facial wound. The goal of therapy in patients with scars has been to eliminate dynamic tension on the healing tissues, to both improve wound healing and minimize scarring for optimal aesthetic results.38 Hypertrophic scarring in a range of areas of the body (for example, face, neck, chest, back, earlobes, and buttocks) can be associated with physical deformities, restricted range of motion, pain, and pruritus.39 In these patients, BoNT-A could, in addition to relaxing muscle tension, affect the cell cycle distribution of fibroblasts derived from the hypertrophic scar.39 BoNT-A can also be considered as an adjunctive treatment for cutaneous lacerations.38
One key factor that determines the final aesthetic appearance of a cutaneous scar is the tension that acts on the wound edges during the healing phase.38 Dynamic tension caused by local muscle pull may be addressed by denervating the muscles pulling on a wound through chemoimmobilization.38 BoNT-A allows near-complete elimination of dynamic muscle tension on the healing wound.38
There are no published data for the use of ABO in the treatment of scars. The safety profile of other BoNT-A formulations for scar optimization is demonstrated in several clinical studies as summarized below.
In a 2014 study, there were no complications reported when ONA was used to improve scar quality in 30 patients undergoing cleft lip scar revision surgery.40 Ziade et al also found that early injections of ONA improved the scar quality of facial wounds in 11 patients, with no safety issues reported.41 These safety findings confirm those of an earlier study of 19 patients with persistent hypertrophic scars who were treated at a scar clinic using a Chinese BoNT-A formulation.39 In this study, BoNT-A was found to decrease the volume of hypertrophic scars, with injection site pain being the only reported AE. A 2006 study of 40 patients with “ugly” scars of the face found that when ONA was used during revision surgery to minimize tension on healing wound edges, 90% of patients had an improved outcome, and no untoward effects of BoNT-A on wound healing were reported.42
What is the Evidence for Neutralizing Antibody Formation as a Result of AbobotulinumtoxinA Treatment?
As with any therapeutic protein, BoNT-A may be regarded as foreign by the host and therefore has the potential to induce at least some type of immune response, particularly with repeated administration. ABO consists of the 150 kDa neurotoxin and a set of neurotoxin-associated complexing proteins.43 Complexing proteins (consisting of several hemagglutinins and nontoxin nonhemagglutinin protein) may increase the risk of neutralizing antibody formation, which may cause secondary treatment failure, particularly if frequent injections are required.43,44 However, this has not been demonstrated clinically in any trial. Although the core neurotoxin is initially in the complex with neurotoxin-associated proteins (as for ABO and ONA, but not INCO), the complex dissociates almost immediately upon dilution in the vial as it encounters the neutral pH of the physiological solution.
In addition to the presence of complexing proteins, a number of other factors may impact the immunogenicity of BoNT-A. These include product-related factors (such as the manufacturing process—through causing an inactive toxin protein content to be formed—and the antigenic protein load) and treatment-related factors (such as the overall toxin dose, booster injections, and prior exposure),43 with the most important factors believed to be the inactive toxin protein load per effective dose and the frequency of exposure.45-49 However, antibody titers required to cause clinical resistance to BoNT-A have not been defined and immune responses can differ between patients.43 The reported prevalence of neutralizing antibodies and treatment failure is variable and may be attributed to study design, administered doses, indication, assay methodology, timing of serum sample testing, and treatment history,50 and in some patients, the formation of antibodies may have no effect on treatment.
Not all immune responses preclude BoNT-A therapy from being clinically effective.43 Only antibodies that bind BoNT-A in a manner that neutralizes the biological activity sufficiently will attenuate its effect at the neuromuscular junction.43 Thus, the formation of antibodies may have no effect on treatment or may result in partial or complete clinical unresponsiveness to BoNT-A.48,51 Furthermore, patient expectation may lead to a subjective nonresponse to previously effective BoNT-A treatment.52
The development of neutralizing antibodies is more common in therapeutic indications, where doses tend to be much larger.43,49,53-57 Lower doses of BoNT-A preparations are used in the aesthetic field, but as treatment indications require repeated injections, individuals may be considered to be at risk for the formation of neutralizing antibodies and secondary nonresponsiveness.43,49,53-57 Data have emerged that show that complexing proteins, and in particular hemagglutinins, can trigger an immune response against BoNT-A, however some of the data are questionable.43,49,53-57 The published literature in this area is sparse, and more data are needed to determine the true prevalence of resistance in the aesthetic field as well as the nature of the neutralizing antibodies.49
With respect to patients receiving ABO for aesthetic treatment, clinical studies have found no confirmed evidence of neutralizing antibodies.12,14,22 Furthermore, a large (n = 1554) integrated review of ABO clinical trials found only 5 patients who had a positive screening result for neutralizing antibodies using a radioimmunoprecipitation-competition assay protocol.58 However, none of the positive findings were confirmed upon additional testing with the gold standard mouse protection assay, a highly specific bioassay that tests for neutralizing BoNT-A antibodies by determining the ability of sera to prevent the death of mice given a lethal dose of BoNT-A.58 In addition, all patients with a positive screening result were clinical responders to treatment. These findings suggest that repeated injections of ABO with the recommended doses did not induce the formation of neutralizing antibodies under the study settings, and demonstrate a limited risk of secondary nonresponse.20
The development of neutralizing antibodies to ABO after injection for aesthetic use (either alone or with other BoNT-A formulations) has been reported in 7 case studies including 11 patients. Dressler et al reported on 2 patients in whom neutralizing antibodies to ABO developed after injection for aesthetic use, resulting in secondary treatment failure.56 The details of the 2 cases are summarized in Table 2.
Table 2.
Case Studies of Patients Developing Neutralizing Antibodies After ABO Injection for Aesthetic Use56
| Parameter | Patients receiving ABO | |
|---|---|---|
| Case 1 | Case 2 | |
| Aesthetic indication | Hyperkinetic skin lines in the glabellar, forehead, and bilateral periocular regions | Hyperkinetic skin lines in the glabellar, and forehead regions |
| Previous BoNT-A therapy | None | None |
| Average interinjection intervals, days | 87 | 119 |
| Minimal interinjection intervalsa, days | 14 | 15 |
| ABO single dose size, mean, MU | 82 | 68 |
| Occurrence of complete secondary treatment failure | After: 10 injection series. Treatment time: 25 months | After: 5 injection series. Treatment time: 16 months |
| Mouse HDA (mU/mL) (at time of complete secondary treatment failure) | 7.0 | >10 |
ABO, abobotulinumtoxinA; HDA, hemidiaphragm assay. aThe minimal interinjection intervals in these 2 cases were 14 and 15 days, thus constituting “booster injections” currently not recommended.
Torres et al reported on 5 case studies in which neutralizing antibodies to BoNT-A (including ABO) developed after injection for aesthetic use, resulting in secondary treatment failure.49 The patients in this report all had a declining clinical response to BoNT-A over several injection sessions. They developed secondary treatment failure and tested positive for neutralizing antibodies using the mouse phrenic nerve hemidiaphragm assay, suggesting that the cause of the therapy failure was neutralizing antibodies to BoNT-A. The details of the cases are summarized in Table 3.
Table 3.
Five Case Studies of Patients Developing Neutralizing Antibodies After Botulinum Toxin Type A Injection for Aesthetic Use49
| Parameter | Patients receiving BoNT-A | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Aesthetic indication | Upper face | Various sites over face | Hyperhidrosis | Various sites over face | Upper face |
| BoNT-A therapy | ONA No response so switched to ABO after 1 treatment | ABO for 13 months Declining response so switched to ONA for 3 months, then ABO for 4 weeks, then INCO after 9 months | 3 treatments of ONA with declining duration of treatment effect (from 5 to 2-3 months) Switched to ABO (duration of effect 1.5 months) | ABO over period of 8 years | 3 treatments of ABO over 2 years |
| Duration of treatment effect | 3 months | ~13 months | Declining from 5 months to 1.5 months | For first 3 years: 6-8 months Thereafter <3 months | Initially 6 months, then 2 months and then no response |
| Presence of neutralizing antibodies confirmed mouse phrenic nerve HDA | Yes (low positive) | Yes | Yes | Yes (high positive) | Yes (high positive) |
ABO, abobotulinumtoxinA; HDA, hemidiaphragm assay; INCO, incobotulinumtoxinA; ONA, onabotulinumtoxinA.
The absence of any substantial numbers of patients reported in the literature strongly indicates that the development of antibody resistance to aesthetic use of BoNT-A products is highly unlikely.30
What is the Clinical Experience for Use of AbobotulinumtoxinA Treatment in Patients Who Are Breastfeeding or Are Subsequently Found to be Pregnant?
Only a few case studies dealing with the issue of safety of ABO in human pregnancy have been reported. There are no controlled trial data and it is unlikely that such studies can be conducted to resolve this issue.59
Newman et al reported on clinical ONA treatment during pregnancy in a patient treated with BoNT-A for cervical dystonia.60 The patient was injected during 4 full-term pregnancies with doses ranging from 600 U to 1200 U per pregnancy, without any effect on the pregnancy outcome.
In a survey of physicians who frequently used ONA, pregnancy outcomes were analyzed in patients who were pregnant at the time of BoNT-A injection.61 Twelve physicians reported injecting BoNT-A in 16 pregnant women, mostly during the first trimester. Only 1 patient, who had a history of spontaneous abortions, suffered a miscarriage. Another woman had a therapeutic abortion. All other pregnancies continued to full term and there were no fetal malformations.
De Oliveira Monteiro reported on 2 women who were injected in the early first trimester but had uneventful pregnancies with no untoward effects on the fetus.62
What is the Clinical Evidence for Systemic Toxicity as a Result of AbobotulinumtoxinA Treatment?
Distant spread of toxin effect is the unintended extension of BoNT-A’s effect into areas not adjacent to the injection site. It is associated with higher-dose indications and may cause symptoms such as unexpected loss of muscle strength or weakness, blurred vision, and eyelid ptosis;63 and, rarely, a life-threatening illness with symptoms consistent with botulism.64
After focal injection for aesthetic use, BoNT-A should not be present at measurable levels in the peripheral blood, and studies have demonstrated this point and shown that administration of the recommended amounts at each treatment does not lead to systemic effects.65-67 Only a minuscule quantity of BoNT-A is used for aesthetic purposes and there have been no reports of distant spread of any of the approved BoNT-A preparations in normal healthy adults.11,64 In a systematic review of 11 RCTs on the use of BoNT-A in facial aesthetics dating from 1977 to 2009, Gadhia et al concluded that the use of BoNT-A treatment had few or no AEs in the immediate vicinity of the injections and no systemic effects.7
There is no evidence from long-term clinical use in humans that BoNT-A injected into peripheral muscles, skin, or other tissues causes clinically detectable effects in the central nervous system.68,69
Despite the safety of the BoNT-A products shown in these studies, the potential safety outcomes of distant spread of toxin effect are serious.64 In 2009, the FDA required all manufacturers of approved and marketed BoNT-A products to update their labeling to include a boxed warning describing postmarketing safety data on distant spread of toxin effect.63 The warning applies equally to therapeutic and aesthetic uses of BoNT products. As presented in the Dysport US prescribing information, “the risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses, including spasticity in children, and in approved indications, cases of spread of effect have been reported at doses comparable to or lower than the maximum recommended total dose.”
What is the Evidence for Patient Satisfaction With AbobotulinumtoxinA for Aesthetic Procedures?
Measures of Patient Satisfaction
A range of measures are used to evaluate patient satisfaction and specific outcomes that are important to patients receiving aesthetic BoNT-A treatment.
Likert-type scales, such as the Facial Lines Treatment Satisfaction Questionnaire, which ranges from “very dissatisfied” to “very satisfied” may be used to rate patient satisfaction.70 Another instrument is the Facial Line Outcome questionnaire, which assesses specific outcomes such as self-perception of age, perception of attractiveness, and the extent to which facial lines result in looking tired, stressed, or angry when that does not coincide with the way the patient feels.71 Patients’ attitudes on beauty and body may be surveyed using scales of the Freiburg Questionnaire on Aesthetic Dermatology and Cosmetic Surgery and the Freiburg Life Quality Assessment core version.35
The FACE-Q is a new patient-reported outcome instrument that can be used for measuring patient perceptions of aesthetic facial procedures.72-74 The developers of the FACE-Q identified 4 domains of patient satisfaction: appearance appraisal, AEs, process of care, and quality of life. Each of these domains has a series of individual surveys to assess, for example, appearance appraisal of skin or process of care of information. Physicians trying to assess the patient-perceived efficacy of an aesthetic intervention can handpick the qualities for their analysis metrics. The ability to tailor the FACE-Q content to specific aspects of facial anatomy, as well as to specific qualities of patient satisfaction, makes the FACE-Q instrument important to evidence-based medicine studies of aesthetic facial interventions.
Adherence, which is likely to be strongly influenced by patient satisfaction with outcome, may also be used as a measure of patient satisfaction.35 Adherence with a therapeutic regimen is reflected in the choices patients make about continuing with a treatment plan and in their selection of specific products and procedures.35
Patient Satisfaction With Botulinum Toxin A for Aesthetic Procedures
A 2008 comprehensive review and meta-analyses of 23 clinical studies including over 1500 patients found that patient-reported satisfaction with ABO or ONA treatment in aesthetic indications was consistently and significantly high, ranging from >65% to >90%, depending on facial area treated, dose, assessment, and other treatment specifics.75 Treatment also significantly improved patient self-perceptions and reduced perceived age relative to current age by approximately 5 years.75
An RCT including 125 patients who received either ONA or placebo for glabellar lines reported high satisfaction rates.76 The proportion of patients who were satisfied with the treatment for their glabellar lines in the BoNT-A group remained at ≥75% for up to 120 days after treatment.
In another study, patients were given a questionnaire, which included demographic details, details of any cosmetic treatment they had received recently and historically, and a copy of the Irritability-Depression-Anxiety Scale.77 The latter provided 3 measures of mood based on 3 distinguishable elements (irritability, depression, and anxiety). The questionnaire also asked patients to provide a percentage value measure of their attractiveness now and prior to the treatment they had just received.77 BoNT-A treatment to the forehead was found to result in a more positive mood in a study of 25 white female patients.77 Those who had received BoNT-A had a significantly more positive mood than those who had not, which was displayed mainly by lower anxiety and depression scores. As patients felt equally attractive after either treatment, an increase in attractiveness could not explain the difference in mood. This supported the study hypothesis that the paralysis of the corrugator (frown) muscles that made negative facial expressions impossible, meant that negative moods were harder to maintain. The lack of the negative mood feedback from the facial muscles was concluded to result in these women feeling happier.
A retrospective chart review in a private aesthetic surgery practice setting used retention rates to evaluate patient satisfaction in patients treated with ONA.78 Retention rates were between 70% and 76% in 60 patients who received ONA injections, suggesting a high level of patient satisfaction.78
A 2014 study by Xie et al showed that in 252 ONA patients treated for masseter hypertrophy (504 masseter muscles), the overall patient satisfaction rate was 95.9%.79
Dayan et al analyzed patient-reported outcomes from 2 Phase III RCTs for ONA treatment of crow’s feet lines in 445 patients.80 In these trials, treated patients experienced significantly greater psychological improvement and age-related impact, improved perception of crow’s feet line appearance, and treatment satisfaction compared with placebo.
Patient Satisfaction With AbobotulinumtoxinA for Aesthetic Procedures
In 2015, Molina et al reported a multicenter, prospective, noninterventional large-scale observational study in 525 patients in France, Germany, Spain, and the United Kingdom to assess the level of patient satisfaction 3 weeks and 4 months after the treatment of glabellar lines with ABO.81 Approximately half of the patients (252; 47.9%) had not previously received ABO in the glabellar complex, while 266 patients (50.6%) had received another BoNT-A product in their glabellar region on average 12.7 months prior to enrollment in the present study. Two different satisfaction questionnaires were used, focusing on the results after treatment and the duration of the treatment, respectively.
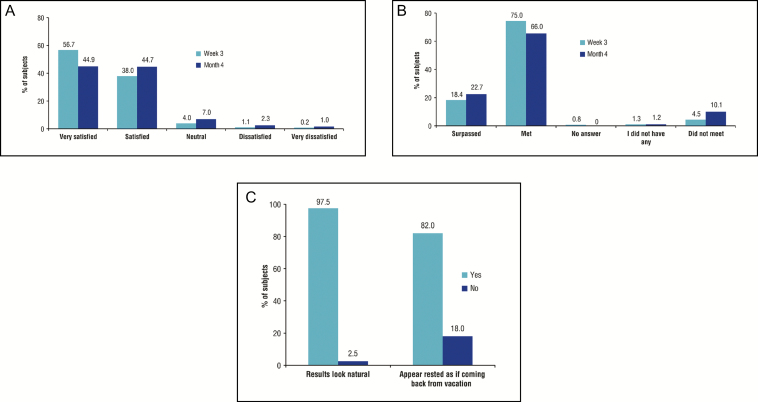
A high level of satisfaction was observed at both time points, with 94.7% and 89.6% of patients being satisfied or very satisfied with the aesthetic outcome at week 3 and month 4, respectively (Figure 1). Patients were highly satisfied with the treatment regardless of their sex or severity of glabellar lines at baseline, whether or not they received a touch-up injection, and whether or not they were naive to BoNT-A treatment. Of the patients who had been treated previously with another product, 51.2% considered the results obtained in the present study with ABO to be better. Major reasons for satisfaction included the positive aesthetic outcome, a natural appearance, a rested look, and comfort of injection. The overall high level of satisfaction with treatment corresponded to a more positive self-perception after the treatment. At 3 weeks after the treatment, 82.0% of patients said they appeared rested, 97.5% considered the result looked natural, and 75.9% felt more attractive. Although 20.0% of patients felt they looked older than their age before any treatment was given, only 0.4% of patients at week 3 and 0.8% at month 4 still thought so. Patients also felt the treatment brought them “harmony,” “self-esteem/confidence,” or “youth.” This study demonstrated that treatment of glabellar lines with BoNT-A resulted in a high level of patient satisfaction and corresponded to a more positive self-perception 4 months after injection.
Figure 1.
Patient satisfaction regarding (A) aesthetic outcome, (B) the results compared with expectations, and (C) posttreatment appearance. Reproduced from Molina et al.81 Patient satisfaction after the treatment of glabellar lines with Botulinum toxin type A (Speywood Unit): a multi-centre European observational study, © 2014 Galderma. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons, Ltd. on behalf of European Academy of Dermatology and Venereology.
Other studies have indicated that patient satisfaction with ABO for aesthetic procedures is generally high and correlates with the efficacy of treatment.82-84 In a retrospective multicountry study of repeated ABO treatments in the upper face involving 945 patients, patient satisfaction with the effect of treatment ranged from 96.0% to 98.9% between treatment cycles.19 A similar high level of satisfaction was noted by physicians, ranging from 88.0% to 94.0% between treatment cycles.
A 2011 retrospective 2-phase study of 185 patients treated with ABO for dynamic facial line reduction over an 8-month period evaluated the efficacy of and patient satisfaction with ABO injections.85 For the first phase of this study, ABO was administered at a concentration of 10 U/0.1 mL saline. During the second phase, the product was administered at a concentration of 12 U/0.1 mL saline. During each phase, dynamic rhytides in the following sites were treated: crow’s feet, depressor anguli oris, frontalis, glabella, nasalis, mentalis, and platysmal bands. Combinations of treatment sites varied according to individual patient need. Overall, the majority of patients were satisfied with ABO (70.9% in the first phase and 68% in the second phase).
The quality of life and satisfaction of patients treated with full-face injections of variable doses of ABO was investigated by Hexsel et al in a Phase IV RCT.86 Ninety patients, mostly women, were randomized into 3 groups, with predefined total dose ranges of ABO, varying from 120 U to 250 U. The World Health Organization Quality of Life (WHOQOL)-BREF questionnaire and a Satisfaction and Self-Assessment Questionnaire (SSQ) were completed by the patients up to 6 months after treatment. The WHOQOL-BREF questionnaire is composed of 26 questions that contemplate 4 different domains (physical, psychological, social relationships, and environmental).87 The SSQ included 9 questions for patients to assess their wrinkles, beauty, harmony, and symmetry of their face. The full-face approach using variable doses of ABO led to significant improvements in patient quality of life, self-assessment about their image, and level of satisfaction compared with baseline. For the physical domain in WHOQOL-BREF a statistical difference was observed between baseline and at 4 weeks (P = 0.036). There was no difference between groups for mean grades regarding number of wrinkles, beauty, harmony, and symmetry. However, there was a significant difference in the mean grades for between visits (P < 0.001) for all at 4 weeks. Patient opinions also showed an improvement in their self-image up to 4 months after treatment according to the self-grading.
In 2016, Chang et al used FACE-Q to assess patient satisfaction before and after BoNT-A treatment of glabellar lines.88 A total of 20 of the 57 female patients who were eligible for the analysis received ABO injections, and the remaining patients received ONA (n = 18) or INCO (n = 19). Patient satisfaction with the overall appearance of their face increased by 18% in patients treated with ABO, which was not statistically significantly different (P = 0.33) from that of the other BoNT-A groups (ONA 29%, INCO 36%). Patients were more satisfied with how old they looked following BoNT-A injection, believing that they looked on average 5.6 years younger following any BoNT-A formulation injection. The study also determined that patient satisfaction with BoNT-A did not correlate with patient age, skin color, or degree of skin wrinkling, although there was an inverse trend for patient satisfaction, advancing age, and degree of glabellar strain.
The outcomes of these studies support other data that demonstrate a positive patient-perceived effect of ABO neuromodulation on facial appearance.
DISCUSSION
This systematic review focused on key questions regarding the evidence for the safety of BoNT-A in aesthetic use. This identified 86 papers (including several clinical trials [open and randomized] and previous systematic literature reviews) that met the predefined objectives. A potential limitation of this review was its focus primarily on ABO, limiting its ability to draw conclusions regarding the safety of all BoNT-A formulations. It was also limited to PubMed and Cochrane databases. It would be interesting to see if further conclusions could be drawn by conducting a meta-analysis of the safety data, providing a further and more robust analysis of the available studies. Overall this review provides more up to date information in this field, enhancing the current understanding and providing key information to clinicians when assessing the best treatment options for their patients.
CONCLUSION
The adverse event profile for ABO has been shown to be comparable to placebo and other formulations of BoNT-A across a number of indications. In aesthetic use, neither the incidence/impact of neutralizing antibodies nor systemic toxicity has been demonstrated in clinical studies of ABO for aesthetic use. ABO treatment for aesthetic indications is associated with consistently and significantly high levels of patient satisfaction, which correlates with the efficacy of treatment.
Disclosures
Dr Cohen has served as a clinical investigator for Leo, Syneron-Candela, Neothetics, MELA Sciences, Evolus/Alpheon, Suneva, and Croma; and has received honoraria as a clinical investigator/consultant or speaker from Allergan, Valeant, Merz/Ulthera/Cellfina, Galderma, Sciton, and Thermi/Almirall. Dr Scuderi declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
This publication was supported by Galderma Laboratories, LP (Fort Worth, TX), who funded the development of this supplement. Editorial assistance was funded by Galderma Laboratories, LP and provided by Vanessa Lane and MedSense, Ltd. (High Wycombe, UK). The authors did not receive compensation for writing the manuscripts.
Acknowledgements
The authors would like to acknowledge the support of Dr Vanessa Lane, who assisted in the writing of this paper, MedSense, Ltd. (High Wycombe, UK), and Wendy Findlay for additional editorial support, funded by Galderma. The authors would like to thank Dr Andy Pickett (Toxin Science Limited, Wrexham, UK; and the Botulinum Research Center, Institute of Advanced Sciences, Dartmouth, MA, USA) who provided an independent review of the paper as required.
REFERENCES
- 1. Dysport, Dysport Prescribing Information. 2016. https://www.dysport.com/pdfs/Dysport_Full_Prescribing_Information.pdf Accessed July 11, 2016. [Google Scholar]
- 2. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cavallini M, Cirillo P, Fundarò SP, et al. Safety of botulinum toxin A in aesthetic treatments: a systematic review of clinical studies. Dermatol Surg. 2014;40(5):525-536. [DOI] [PubMed] [Google Scholar]
- 4. Chang YS, Chang CC, Shen JH, Chen YT, Chan KK. Nonallergic eyelid edema after botulinum toxin type a injection: case report and review of literature. Medicine (Baltimore). 2015;94(38):e1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carruthers JD, Glogau RG, Blitzer A; Facial Aesthetics Consensus Group Faculty Advances in facial rejuvenation: botulinum toxin type a, hyaluronic acid dermal fillers, and combination therapies—consensus recommendations. Plast Reconstr Surg. 2008;121(5 Suppl):5S-30S. [DOI] [PubMed] [Google Scholar]
- 6. Redaelli A. Medical rhinoplasty with hyaluronic acid and botulinum toxin A: a very simple and quite effective technique. J Cosmet Dermatol. 2008;7(3):210-220. [DOI] [PubMed] [Google Scholar]
- 7. Gadhia K, Walmsley AD. Facial aesthetics: is botulinum toxin treatment effective and safe? A systematic review of randomised controlled trials. Br Dent J. 2009;207(5):E9; discussion 216-E9; discussion 217. [DOI] [PubMed] [Google Scholar]
- 8. Gendler E, Nagler A. Aesthetic use of BoNT: options and outcomes. Toxicon. 2015;107(Pt A):120-128. [DOI] [PubMed] [Google Scholar]
- 9. Klein AW, Carruthers A, Fagien S, Lowe NJ. Comparisons among botulinum toxins: an evidence-based review. Plast Reconstr Surg. 2008;121(6):413e-422e. [DOI] [PubMed] [Google Scholar]
- 10. Maas C, Kane MA, Bucay VW, et al. Current aesthetic use of abobotulinumtoxinA in clinical practice: an evidence-based consensus review. Aesthet Surg J. 2012;32(1 Suppl):8S-29S. [DOI] [PubMed] [Google Scholar]
- 11. Dessy LA, Fallico N, Mazzocchi M, Scuderi N. Botulinum toxin for glabellar lines: a review of the efficacy and safety of currently available products. Am J Clin Dermatol. 2011;12(6):377-388. [DOI] [PubMed] [Google Scholar]
- 12. Brandt F, Swanson N, Baumann L, Huber B. Randomized, placebo-controlled study of a new botulinum toxin type a for treatment of glabellar lines: efficacy and safety. Dermatol Surg. 2009;35(12):1893-1901. [DOI] [PubMed] [Google Scholar]
- 13. Kane MA, Brandt F, Rohrich RJ, Narins RS, Monheit GD, Huber MB; Reloxin Investigational Group Evaluation of variable-dose treatment with a new U.S. botulinum toxin type A (Dysport) for correction of moderate to severe glabellar lines: results from a phase III, randomized, double-blind, placebo-controlled study. Plast Reconstr Surg. 2009;124(5):1619-1629. [DOI] [PubMed] [Google Scholar]
- 14. Moy R, Maas C, Monheit G, Huber MB; Reloxin Investigational Group Long-term safety and efficacy of a new botulinum toxin type A in treating glabellar lines. Arch Facial Plast Surg. 2009;11(2):77-83. [DOI] [PubMed] [Google Scholar]
- 15. Rubin MG, Dover J, Glogau RG, Goldberg DJ, Goldman MP, Schlessinger J. The efficacy and safety of a new U.S. botulinum toxin type A in the retreatment of glabellar lines following open-label treatment. J Drugs Dermatol. 2009;8(5):439-444. [PubMed] [Google Scholar]
- 16. Cohen JL, Schlessinger J, Cox SE, Lin X; Reloxin Investigational Group An analysis of the long-term safety data of repeat administrations of botulinum neurotoxin type A-ABO for the treatment of glabellar lines. Aesthet Surg J. 2009;29(6 Suppl):S43-S49. [DOI] [PubMed] [Google Scholar]
- 17. Schlessinger J, Dover JS, Joseph J, et al. ; Dysport Study Group. Long-term safety of abobotulinumtoxinA for the treatment of glabellar lines: results from a 36-month, multicenter, open-label extension study. Dermatol Surg. 2014;40(2):176-183. [DOI] [PubMed] [Google Scholar]
- 18. Rubin M, Dover J, Maas C, Nestor M. An analysis of safety data from five phase III clinical trials on the use of botulinum neurotoxin type A-ABO for the treatment of glabellar lines. Aesthet Surg J. 2009;29(6 Suppl):S50-S56. [DOI] [PubMed] [Google Scholar]
- 19. Rzany B, Dill-Müller D, Grablowitz D, Heckmann M, Caird D; German-Austrian Retrospective Study Group Repeated botulinum toxin A injections for the treatment of lines in the upper face: a retrospective study of 4103 treatments in 945 patients. Dermatol Surg. 2007;33(1 Spec No):S18-S25. [DOI] [PubMed] [Google Scholar]
- 20. Rzany B, Ascher B, Monheit G. Treatment of glabellar lines with botulinum toxin type A (Speywood Unit): a clinical overview. J Eur Acad Dermatol Venereol. 2010;24(Suppl 1):1-14. [DOI] [PubMed] [Google Scholar]
- 21. Monheit G, Carruthers A, Brandt F, Rand R. A randomized, double-blind, placebo-controlled study of botulinum toxin type A for the treatment of glabellar lines: determination of optimal dose. Dermatol Surg. 2007; 33(1 Spec No):S51-S59. [DOI] [PubMed] [Google Scholar]
- 22. Monheit GD, Cohen JL; Reloxin Investigational Group Long-term safety of repeated administrations of a new formulation of botulinum toxin type A in the treatment of glabellar lines: interim analysis from an open-label extension study. J Am Acad Dermatol. 2009;61(3):421-425. [DOI] [PubMed] [Google Scholar]
- 23. Nestor MS, Ablon GR. Duration of action of abobotulinumtoxina and onabotulinumtoxina: a randomized, double-blind study using a contralateral frontalis model. J Clin Aesthet Dermatol. 2011;4(9):43-49. [PMC free article] [PubMed] [Google Scholar]
- 24. Pena MA, Alam M, Yoo SS. Complications with the use of botulinum toxin type A for cosmetic applications and hyperhidrosis. Semin Cutan Med Surg. 2007;26(1):29-33. [DOI] [PubMed] [Google Scholar]
- 25. Ascher B, Rzany BJ, Grover R. Efficacy and safety of botulinum toxin type A in the treatment of lateral crow’s feet: double-blind, placebo-controlled, dose-ranging study. Dermatol Surg. 2009;35(10):1478-1486. [DOI] [PubMed] [Google Scholar]
- 26. Kiripolsky MG, Goldman MP. Safety and efficacy of administering abobotulinumtoxinA through a single injection point when treating lateral periocular rhytides. J Cosmet Dermatol. 2011;10(3):232-234. [DOI] [PubMed] [Google Scholar]
- 27. Fabi SG, Sundaram H, Guiha I, Goldman MP. A two-center, open-label, randomized, split-face study to assess the efficacy and safety of one versus three intradermal injection sites of abobotulinumtoxinA in the treatment of lateral periocular rhytides. J Drugs Dermatol. 2013;12(8):932-937. [PubMed] [Google Scholar]
- 28. Hexsel D, Brum C, Porto MD, et al. Full-face injections of variable total doses of abobotulinum toxin type A: A randomized, phase IV clinical trial of safety and efficacy. J Drugs Dermatol. 2013;12(12):1356-1362. [PubMed] [Google Scholar]
- 29. Rzany B, Fratila AA, Fischer TC, et al. Recommendations for the best possible use of botulinum neurotoxin type a (Speywood units) for aesthetic applications. J Drugs Dermatol. 2013;12(1):80-84. [PubMed] [Google Scholar]
- 30. Wortzman MS, Pickett A. The science and manufacturing behind botulinum neurotoxin type A-ABO in clinical use. Aesthet Surg J. 2009;29(6 Suppl):S34-S42. [DOI] [PubMed] [Google Scholar]
- 31. Matarasso A, Shafer D. Botulinum neurotoxin type A-ABO (Dysport): clinical indications and practice guide. Aesthet Surg J. 2009;29(6 Suppl):S72-S79. [DOI] [PubMed] [Google Scholar]
- 32. Pickett A. Dysport: pharmacological properties and factors that influence toxin action. Toxicon. 2009;54(5):683-689. [DOI] [PubMed] [Google Scholar]
- 33. Kane M, Donofrio L, Ascher B, et al. Expanding the use of neurotoxins in facial aesthetics: a consensus panel’s assessment and recommendations. J Drugs Dermatol. 2010;9(1 Suppl):s7-s22; quiz s23. [PubMed] [Google Scholar]
- 34. Hexsel C, Hexsel D, Porto MD, Schilling J, Siega C. Botulinum toxin type A for aging face and aesthetic uses. Dermatol Ther. 2011;24(1):54-61. [DOI] [PubMed] [Google Scholar]
- 35. De Boulle K, Fagien S, Sommer B, Glogau R. Treating glabellar lines with botulinum toxin type A-hemagglutinin complex: a review of the science, the clinical data, and patient satisfaction. Clin Interv Aging. 2010;5:101-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abbasi NR, Durfee MA, Petrell K, Dover JS, Arndt KA. A small study of the relationship between abobotulinum toxin A concentration and forehead wrinkle reduction. Arch Dermatol. 2012;148(1):119-121. [DOI] [PubMed] [Google Scholar]
- 37. Borodic GE, Ferrante R, Pearce LB, Smith K. Histologic assessment of dose-related diffusion and muscle fiber response after therapeutic botulinum A toxin injections. Mov Disord. 1994;9(1):31-39. [DOI] [PubMed] [Google Scholar]
- 38. Jablonka EM, Sherris DA, Gassner HG. Botulinum toxin to minimize facial scarring. Facial Plast Surg. 2012;28(5):525-535. [DOI] [PubMed] [Google Scholar]
- 39. Xiao Z, Zhang F, Cui Z. Treatment of hypertrophic scars with intralesional botulinum toxin type A injections: a preliminary report. Aesthetic Plast Surg. 2009;33(3):409-412. [DOI] [PubMed] [Google Scholar]
- 40. Chang CS, Wallace CG, Hsiao YC, Chang CJ, Chen PK. Botulinum toxin to improve results in cleft lip repair: a double-blinded, randomized, vehicle-controlled clinical trial. PLoS One. 2014;9(12):e115690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ziade M, Domergue S, Batifol D, et al. Use of botulinum toxin type A to improve treatment of facial wounds: a prospective randomised study. J Plast Reconstr Aesthet Surg. 2013;66(2):209-214. [DOI] [PubMed] [Google Scholar]
- 42. Wilson AM. Use of botulinum toxin type A to prevent widening of facial scars. Plast Reconstr Surg. 2006;117(6):1758-1766; discussion 1767. [DOI] [PubMed] [Google Scholar]
- 43. Frevert J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs R D. 2015;15(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang L, Sun Y, Yang W, Lindo P, Singh BR. Type A botulinum neurotoxin complex proteins differentially modulate host response of neuronal cells. Toxicon. 2014;82:52-60. [DOI] [PubMed] [Google Scholar]
- 45. Greene P, Fahn S, Diamond B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov Disord. 1994;9(2):213-217. [DOI] [PubMed] [Google Scholar]
- 46. Herrmann J, Geth K, Mall V, et al. Clinical impact of antibody formation to botulinum toxin A in children. Ann Neurol. 2004;55:732–735. [DOI] [PubMed] [Google Scholar]
- 47. Dressler D. Clinical presentation and management of antibody-induced failure of botulinum toxin therapy. Mov Disord. 2004;19(Suppl 8):S92-S100. [DOI] [PubMed] [Google Scholar]
- 48. Lange O, Bigalke H, Dengler R, Wegner F, deGroot M, Wohlfarth K. Neutralizing antibodies and secondary therapy failure after treatment with botulinum toxin type A: much ado about nothing? Clin Neuropharmacol. 2009;32(4):213-218. [DOI] [PubMed] [Google Scholar]
- 49. Torres S, Hamilton M, Sanches E, Starovatova P, Gubanova E, Reshetnikova T. Neutralizing antibodies to botulinum neurotoxin type A in aesthetic medicine: five case reports. Clin Cosmet Investig Dermatol. 2014;7:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Benecke R. Clinical relevance of botulinum toxin immunogenicity. BioDrugs. 2012;26(2):e1-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kranz G, Sycha T, Voller B, Kranz GS, Schnider P, Auff E. Neutralizing antibodies in dystonic patients who still respond well to botulinum toxin type A. Neurology. 2008;70(2):133-136. [DOI] [PubMed] [Google Scholar]
- 52. Stephan F, Habre M, Tomb R. Clinical resistance to three types of botulinum toxin type A in aesthetic medicine. J Cosmet Dermatol. 2014;13(4):346-348. [DOI] [PubMed] [Google Scholar]
- 53. Dressler D, Hallett M. Immunological aspects of Botox, Dysport and Myobloc/NeuroBloc. Eur J Neurol. 2006;13(Suppl 1):11-15. [DOI] [PubMed] [Google Scholar]
- 54. Borodic G. Immunologic resistance after repeated botulinum toxin type a injections for facial rhytides. Ophthal Plast Reconstr Surg. 2006;22(3):239-240. [DOI] [PubMed] [Google Scholar]
- 55. Lee SK. Antibody-induced failure of botulinum toxin type A therapy in a patient with masseteric hypertrophy. Dermatol Surg. 2007;33(1 Spec No):S105-S110. [DOI] [PubMed] [Google Scholar]
- 56. Dressler D, Wohlfahrt K, Meyer-Rogge E, Wiest L, Bigalke H. Antibody-induced failure of botulinum toxin a therapy in cosmetic indications. Dermatol Surg. 2010;36Suppl 4:2182-2187. [DOI] [PubMed] [Google Scholar]
- 57. Stengel G, Bee EK. Antibody-induced secondary treatment failure in a patient treated with botulinum toxin type A for glabellar frown lines. Clin Interv Aging. 2011;6:281-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lawrence I, Moy R. An evaluation of neutralizing antibody induction during treatment of glabellar lines with a new US formulation of botulinum neurotoxin type A. Aesthet Surg J. 2009;29(6 Suppl):S66-S71. [DOI] [PubMed] [Google Scholar]
- 59. Paul M. Controversy: botulinum toxin in pregnancy. J Cutan Aesthet Surg. 2009;2(1):4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Newman WJ, Davis TL, Padaliya BB, et al. Botulinum toxin type A therapy during pregnancy. Mov Disord. 2004;19(11):1384-1385. [DOI] [PubMed] [Google Scholar]
- 61. Morgan JC, Iyer SS, Moser ET, Singer C, Sethi KD. Botulinum toxin A during pregnancy: a survey of treating physicians. J Neurol Neurosurg Psychiatry. 2006;77(1):117-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Oliveira Monteiro E. Botulinum toxin and pregnancy. Skinmed. 2006;5(6):308. [DOI] [PubMed] [Google Scholar]
- 63. US Food and Drug Administration. FDA requires boxed warning for all botulinum toxin products. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm149574.htm Accessed March 1, 2016. [Google Scholar]
- 64. Carruthers A, Kane MA, Flynn TC, et al. The convergence of medicine and neurotoxins: a focus on botulinum toxin type A and its application in aesthetic medicine—a global, evidence-based botulinum toxin consensus education initiative: part I: botulinum toxin in clinical and cosmetic practice. Dermatol Surg. 2013;39(3 Pt 2):493-509. [DOI] [PubMed] [Google Scholar]
- 65. Carruthers A, Carruthers J. History of the cosmetic use of botulinum A exotoxin. Dermatol Surg. 1998;24(11):1168-1170. [DOI] [PubMed] [Google Scholar]
- 66. Chertow DS, Tan ET, Maslanka SE, et al. Botulism in 4 adults following cosmetic injections with an unlicensed, highly concentrated botulinum preparation. JAMA. 2006;296(20):2476-2479. [DOI] [PubMed] [Google Scholar]
- 67. Botulinum toxin. In: AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists; 2008. [Google Scholar]
- 68. Tang-Liu DD, Aoki KR, Dolly JO, et al. Intramuscular injection of 125I-botulinum neurotoxin-complex versus 125I-botulinum-free neurotoxin: time course of tissue distribution. Toxicon. 2003;42(5):461-469. [DOI] [PubMed] [Google Scholar]
- 69. Klein AW. Complications with the use of botulinum toxin. Dermatol Clin. 2004;22(2):197-205. [DOI] [PubMed] [Google Scholar]
- 70. Cox SE, Finn JC, Stetler L, Mackowiak J, Kowalski JW. Development of the facial lines treatment satisfaction questionnaire and initial results for botulinum toxin type A-treated patients. Dermatol Surg. 2003;29(5):444-449; discussion 449. [DOI] [PubMed] [Google Scholar]
- 71. Kowalski J, Kozma C, Reese PR, Slaton T, Lee J. Initial development of a patient-completed questionnaire to assess outcomes of aesthetic treatment for hyperfunctional facial lines of the upper face. [poster] American Academy of Dermatology Academy’s 2005 Annual Meeting; July 20-24, 2005; Chicago, Ill, USA. [Google Scholar]
- 72. Klassen AF, Cano SJ, Scott A, Snell L, Pusic AL. Measuring patient-reported outcomes in facial aesthetic patients: development of the FACE-Q. Facial Plast Surg. 2010;26(4):303-309. [DOI] [PubMed] [Google Scholar]
- 73. Pusic AL, Klassen AF, Scott AM, Cano SJ. Development and psychometric evaluation of the FACE-Q satisfaction with appearance scale: a new patient-reported outcome instrument for facial aesthetics patients. Clin Plast Surg. 2013;40(2):249-260. [DOI] [PubMed] [Google Scholar]
- 74. Panchapakesan V, Klassen AF, Cano SJ, Scott AM, Pusic AL. Development and psychometric evaluation of the FACE-Q aging appraisal scale and patient-perceived age visual analog scale. Aesthet Surg J. 2013;33(8):1099-1109. [DOI] [PubMed] [Google Scholar]
- 75. Fagien S, Carruthers JD. A comprehensive review of patient-reported satisfaction with botulinum toxin type a for aesthetic procedures. Plast Reconstr Surg. 2008; 122(6):1915-1925. [DOI] [PubMed] [Google Scholar]
- 76. Rivers JK, Bertucci V, McGillivray W, et al. Subject satisfaction with onabotulinumtoxinA treatment of glabellar and lateral canthal lines using a new patient-reported outcome measure. Dermatol Surg. 2015;41(8):950-959. [DOI] [PubMed] [Google Scholar]
- 77. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26. [DOI] [PubMed] [Google Scholar]
- 78. Sepehr A, Chauhan N, Alexander AJ, Adamson PA. Botulinum toxin type a for facial rejuvenation: treatment evolution and patient satisfaction. Aesthetic Plast Surg. 2010;34(5):583-586. [DOI] [PubMed] [Google Scholar]
- 79. Xie Y, Zhou J, Li H, Cheng C, Herrler T, Li Q. Classification of masseter hypertrophy for tailored botulinum toxin type A treatment. Plast Reconstr Surg. 2014;134(2):209e-218e. [DOI] [PubMed] [Google Scholar]
- 80. Dayan S, Coleman WP, 3rd, Dover JS, et al. Effects of onabotulinumtoxinA treatment for crow’s feet lines on patient-reported outcomes. Dermatol Surg. 2015;41 (Suppl 1):S67-S74. [DOI] [PubMed] [Google Scholar]
- 81. Molina B, Grangier Y, Mole B, et al. Patient satisfaction after the treatment of glabellar lines with botulinum toxin type A (Speywood unit): a multi-centre European observational study. J Eur Acad Dermatol Venereol. 2015;29(7):1382-1388. [DOI] [PubMed] [Google Scholar]
- 82. Ascher B, Zakine B, Kestemont P, Baspeyras M, Bougara A, Santini J. A multicenter, randomized, double-blind, placebo-controlled study of efficacy and safety of 3 doses of botulinum toxin A in the treatment of glabellar lines. J Am Acad Dermatol. 2004;51(2):223-233. [DOI] [PubMed] [Google Scholar]
- 83. Ascher B, Zakine B, Kestemont P, et al. Botulinum toxin A in the treatment of glabellar lines: scheduling the next injection. Aesthet Surg J. 2005;25(4):365-375. [DOI] [PubMed] [Google Scholar]
- 84. Lowe P, Patnaik R, Lowe N. Comparison of two formulations of botulinum toxin type A for the treatment of glabellar lines: a double-blind, randomized study. J Am Acad Dermatol. 2006;55(6):975-980. [DOI] [PubMed] [Google Scholar]
- 85. Kiripolsky MG, Peterson JD, Guiha I, Goldman MP. A two-phase, retrospective analysis evaluating efficacy of and patient satisfaction with abobotulinumtoxina used to treat dynamic facial rhytides. Dermatol Surg. 2011;37(10):1443-1447. [DOI] [PubMed] [Google Scholar]
- 86. Hexsel D, Brum C, Porto MD, et al. Quality of life and satisfaction of patients after full-face injections of abobotulinum toxin type A: a randomized, phase IV clinical trial. J Drugs Dermatol. 2013;12(12):1363-1367. [PubMed] [Google Scholar]
- 87. The World Health Organization, WHOQOL-BREF Group. Programme on Mental Health, December 1996. http://www.who.int/mental_health/media/en/76.pdf Accessed July 27, 2016. [Google Scholar]
- 88. Chang BL, Wilson AJ, Taglienti AJ, Chang CS, Folsom N, Percec I. Patient perceived benefit in facial aesthetic procedures: FACE-Q as a tool to study botulinum toxin injection outcomes. Aesthet Surg J. 2016;36(7):810-820. [DOI] [PubMed] [Google Scholar]