Abstract
Time to onset of response and duration of response are key measures of botulinum toxin efficacy that have a considerable influence on patient satisfaction with aesthetic treatment. However, there is no overall accepted definition of efficacy for aesthetic uses of botulinumtoxinA (BoNT-A). Mechanical methods of assessment do not lend themselves to clinical practice and clinicians rely instead on assessment scales such as the Frontalis Activity Measurement Standard, Frontalis Rating Scale, Wrinkle Severity Scale, and Subject Global Assessment Scale, but not all of these have been fully validated. Onset of activity is typically seen within 5 days of injection, but has also been recorded within 12 hours with abobotulinumtoxinA. Duration of effect is more variable, and is influenced by parameters such as muscle mass (including the effects of age and sex) and type of product used. Even when larger muscles are treated with higher doses of BoNT-A, the duration of effect is still shorter than that for smaller muscles. Muscle injection technique, including dilution of the toxin, the volume of solution injected, and the positioning of the injections, can also have an important influence on onset and duration of activity. Comparison of the efficacy of different forms of BoNT-A must be made with the full understanding that the dosing units are not equivalent. Range of equivalence studies for abobotulinumtoxinA (Azzalure; Ipsen Limited, Slough UK/Galderma, Lausanne CH/Dysport, Ipsen Biopharm Limited, Wrexham UK/Galderma LP, Fort Worth, TX) and onabotulinumtoxinA (Botox; Allergan, Parsippany, NJ) have been conducted, and results indicate that the number of units of abobotulinumtoxinA needs to be approximately twice as high as that of onabotulinumtoxinA to achieve the same effect. An appreciation of the potential influence of all of the parameters that influence onset and duration of activity of BoNT-A, along with a thorough understanding of the anatomy of the face and potency of doses, are essential to tailoring treatment to individual patient needs and expectations.
Unlike other aesthetic procedures, injections of botulinumtoxinA (BoNT-A) into the appropriate facial muscles actually address the underlying cause of wrinkles. Injections into the appropriate muscle or muscles cause temporary, reversible paralysis that softens hyperdynamic lines.1-3 Details of the mechanism of action for BoNT-A are discussed in detail elsewhere in this supplement, but a brief introduction is provided below.
Reconstituted type A complexes of the core 150 kDa neurotoxin protein, which include hemagglutinin and non-hemagglutinin proteins, appear to dissociate at physiological pH values.4 Once injected, the core protein initially binds to the presynaptic membrane, then crosses the membrane into the nerve cells, where synaptosomal- associated protein 25 kDa is cleaved. This protein plays a key role in the release of the neurotransmitter acetylcholine into the neuromuscular junction. Blocking the release of acetylcholine blocks the transmission of nerve impulses causing paralysis and/or weakness of the target muscle.4-6 Recovery of impulse transmission occurs gradually as the original nerve terminal recovers.7 This mode of action means that the effects of treatment are not seen immediately after the procedure, nor are they permanent.4-6
Time to onset of response and duration of activity are important factors that have a considerable influence on patient satisfaction with treatment. Patients want the effect of treatment to be visible as soon as possible after the procedure, and to last for as long as possible to increase the interval between procedures and hence decrease inconvenience and cost.1,2,8,9 The time to onset of response and the duration of activity are also important markers of efficacy for BoNT-A, and may be related to individual patient genetics, individual muscle mass, absolute units injected, and injection technique.10
In general, some patients are aware of an improvement in wrinkles within 1 day of treatment, and return of muscle function generally seems to occur 3 to 6 months after treatment.11-19 Patients who have had multiple treatment sessions may find that the duration of effect becomes longer, thus lengthening the interval between injections.14,20 This effect may be related or secondary to muscle atrophy, reducing the number of BoNT-A targets available and so reducing the dose requirements.
An important point to make at the outset is that interpretation and comparison of efficacy evidence for different forms of BoNT-A – and even different uses of the same form of BoNT-A – must be performed with caution. Disagreements on how to define and measure doses or improvements in efficacy (eg, what scales to use, how to measure the scale effects [live or by photographs], the timing of those measurements, and the subjective nature of many of the scales) mean that there is very little standardization in clinical studies. However, during a consensus meeting on the recommendations for treatment with abobotulinumtoxinA (ABO; Dysport; Ipsen Biopharm Limited, Wrexham UK/Galderma LP, Fort Worth, TX), Maas noted that: “Aspects of BoNT-A use were consistent across anatomic areas…suggesting that these personal preferences…are not critical for treatment success.”21 However, the actual concept of “treatment success” is not a constant, and adjusting the dose of BoNT-A based on observed muscle action, mass, facial symmetry, and desired result is considered to be important for nearly all anatomic areas.21 The original concept of “frozen” muscle has generally, but not exclusively, been replaced with a desire for the “natural look.” However, adjusting the dosing to achieve a natural look will directly affect onset and duration.22
This paper examines factors that influence time to onset and duration of response with botulinum toxin, with particular reference to ABO.
DEFINITIONS
Efficacy
Comparing onset and duration of activity data for ABO (and indeed other forms of BoNT-A), whether from real life studies or clinical trials, is complicated by the absence of an official definition of efficacy and a single, validated scale for establishing the definition.21 A regulatory definition of efficacy comes from draft Guidance to Industry issued by the United States Food and Drug Administration (FDA), which makes recommendations regarding the design of clinical trials for botulinum toxin drug products. The FDA recommends that: “Measurements at maximum contraction should be used to assess the efficacy of botulinum toxin drug products to demonstrate the paralytic effect” and that: “Success should be defined as … a two-grade improvement from the baseline, on both the [investigator’s assessment] and the [subject’s self-assessment] scales concurrently, to ensure clinical significance.”23
A number of issues have been raised against the FDA definition of efficacy. Many treating clinicians feel that such a stringent and strong definition could encourage overtreatment, resulting in the “frozen” appearance that most patients today would wish to avoid.24 In a response to the publication of the draft recommendations, Glogau et al suggested the need to be far more nuanced, taking into account more the function of each treated muscle and the effect that paralysis will have on the appearance of the face, rather than the proposed “one size fits all” approach.24 Bonaparte et al generally agree with this sentiment.10 In their systematic review and meta-analysis of safety and efficacy studies on 3 BoNT-A formulations, they found that the majority of studies defined a reduction in the Facial Wrinkle Scale of 2 points as a positive effect; however, a number of the included studies utilized a reduction of 1 point as the definition of effect.10 These studies were randomized, active- or placebo-controlled trials with ABO, incobotulinumtoxinA (INCO), or onabotulinumtoxinA (ONA), with or without other aesthetic treatments.
Rating Scales
A number of assessment scales have been developed for measuring the efficacy of BoNT-A in aesthetic treatments; however, only a minority of these have been fully validated. Table 1 shows a comparison of scales.15,25-30
Table 1.
Comparison of Scales for Assessing the Aesthetic Efficacy of Botulinum Toxins
| Study/ studies | Name | Description | Intra-observer reliability (κ) | Inter- observer reliability (κ) | Validated |
|---|---|---|---|---|---|
| 15 | Frontalis Activity Measurement Standard | Based on percentage change in frontalis height at maximum frown and at rest Partial effect = 20% difference Full effect = 33% Complete effect = 66% difference |
No | ||
| 15 | Frontalis Rating Scale | Modified form of glabellar line severity scale with 4 points instead of 5: 0 = no wrinkles 1 = mild wrinkles 2 = moderate wrinkles 3 = severe wrinkles |
Yes | ||
| 25 | Investigator’s Global Assessment of Lateral Canthal Lines | 5-point scale 0 = no wrinkles 1 = minimal wrinkles, with/without minimal etching within 1.5 cm radius of lateral canthus 2 = mild wrinkles, with minimal etching in 1.5-2.5 cm radius of lateral canthus 3 = moderately deep wrinkles with moderate etching within 1.5-2.5 cm radius of lateral canthus 4 = severe wrinkles, very long wrinkles, which may be deeply etched extending in a ≥2.5 cm radius of the lateral canthus |
Yes | ||
| 27 | Wrinkle Severity Scalesa | 5-point scale with photo guide | 0.85-0.95 | Yes | |
| 28 | Clinical severity scales for lateral canthal lines | Two 4-point scales (0 = no wrinkles to 3 = severe wrinkles), 1 for use at rest and 1 for use at maximum smile | 0.47-0.86 at rest 0.62-0.81 at max. smile | 0.60 at rest 0.58 at max. smile | No |
| 29,30 | Facial Wrinkle Scale | 4-point ordinal scale ranging from no wrinkling to severe wrinkling | 0.57-0.91 | 0.194-0.62 | No |
| 30 | Subject Global Assessment | Percentage measure assessing change in appearance from –100% to +100% | 0.443-0.992 | No |
aIndividual scales for brow positioning, lateral canthal lines, marionette lines, and forehead lines.
The FDA draft Guidance to Industry places great importance on the availability of well-defined and reliable instruments for measuring physician- and patient-reported outcomes in clinical trials.23 These should be based on qualitative research carried out in the target population and should include an assessment of the effect of drugs on outcomes that are important to that population. Scales should be ordinal, static, and include a limited number of distinct and clinically meaningful categories or grades. The FDA recommends providing a photonumeric guide for investigators and patients.23 For an assessment scale to be of use in comparing BoNT-A efficacy, the results must be reproducible whether assessed by the same person (intra-observer correlation) or by different people (inter-observer correlation).25 Kappa (κ) values are used to determine concordance and range between −1 (no agreement) and +1 (absolute agreement). Generally, a κ < 0.20 designates poor agreement, κ = 0.21 to 0.40 shows fair agreement, κ = 0.41 to 0.60 shows moderate agreement, κ0.61 to 0.80 shows good agreement, and κ = 0.81 to 1.00 shows almost perfect agreement.26
Carruthers and Carruthers later developed their wrinkle scales in association with other experts in facial aging in order to have a validated, objective and quantitative assessment method. These are 5-point photonumeric scales based on computer-simulated photographs incorporating stepwise anatomical changes caused by aging.27 From a database of photographs from 100 individuals, 50 were selected based on quality and equal distribution across each representative scale. A computer randomization program was used to select 35 images per target area, which were assessed and validated by an international group of specialists in dermatology, ophthalmology, and plastic and dermatological surgery. An important difference between this scale and similar scales (such as the Frontalis Rating Scale - FRS) is that there is a mid-point – the authors note that: “The grading of a continuous process such as aging is facilitated if there are clearly identified center and endpoints to the scale.”27
The scale for assessing lateral canthal lines described by Kane et al was developed by a group of experts over a period of 2 to 3 years. Intra-rater reliability was assessed in 5 Phase II clinical studies of BoNT-A Topical Gel (RT001) using 451 patients rather than photos. Each of the 17 investigators rated each model once and all investigators rated all models.25
Honeck et al developed a 0 to 3 score following a consensus from 28 dermatologists who had been asked to assess 50 photographs of glabellar frown lines over 2 consecutive days. The score showed good inter- and intra-observer reproducibility.29
As well as the FRS, Nestor and Ablon developed the Frontalis Activity Measurement Standard (FMS) to assess, in more detail, the effect of aesthetic treatments with BoNT-A products on a designated facial area. The FMS is designed to directly and objectively quantify changes in frontalis muscle activity by measuring the difference between the height of the frontalis at maximum elevation and at rest.15,16 The scale has the advantage of measuring the field of BoNT-A effect without the need for the Minor’s test, which has often been used to demonstrate localized and/or comparative effects of BoNT-A in areas such as the frontalis.31-33 The FMS assessment relies on a series of photographs using the same camera settings and lighting conditions with a rest period of 1 minute between photographs (Table 1). Using the frontalis muscle also allows for bilateral (split-face) comparison of different toxins, dosing and technique on a single patient.15,16
Dose Equivalence
It is well known that equivalent units of different BoNT-A products do not have equivalent potency. While all forms of type A toxins have identical mechanisms of action, the theoretical numbers of active 150 kDa molecules in a vial varies by manufactured product and this variation may have a relative relationship to the LD50 (the median lethal dose which kills 50 percent of the test population). The LD50 may be expressed, for example, in units per mL and is proprietary for each company product, defining the potency units for those products. Comparing dose equivalence can therefore only be carried out indirectly, either by comparing muscle activity on bilateral sides of individual patients or different patient populations, or by comparing other markers of BoNT activity, such as diffusion halos, which form around the injection point. This clinically delimited area, usually round or oval in shape depending on the injection angle,34 marks the toxin’s field of effect. Within this area there is an absence of voluntary muscular contraction and sweat gland activity; indeed, the absence of sweating can be used to demonstrate the size of the field of effect using the Minor’s test. Hexsel et al have demonstrated the relationship between muscle weakening halos and sweating halos in their forehead model.31-33 Because the face contains so many muscles, an injection site may overlap a non-target muscle, particularly if the toxin diffuses more widely than expected.35 Conversely, if the toxin used does not develop the same field of effect obtained with another toxin, patients may not achieve the expected results.
One randomized, split-face study investigated the difference in field of effect using 2 dose equivalence ratios for ABO to ONA.32 Patients received a total dose of 100 units/mL ONA to the frontalis on one side of the face and were randomized to receive 200 units/mL or 250 units/mL ABO to the frontalis on the other side (2:1 or 2.5:1 dose ratio). They were also randomized to determine which side of the face would be treated with ONA.32 The fields of effect were measured using clinical and photographic assessments as well as the Minor’s test, and electromyography. All patients received single injections of 0.02 mL to a depth of 3 mm into exactly the same position on each side of the face. The fields of effect for 200 units/mL ABO were equivalent to those achieved with 100 units/mL ONA, but a statistically significant larger field of effect was recorded for patients who received 250 units/mL ABO at 28 and 112 days after treatment. Both doses of ABO gave equivalent improvements on the Wrinkle Severity Scale, producing greater improvements than those observed with ONA. In a second study, which used the same methodology, patients received 2 units/0.02 mL ONA to the frontalis on one side of the face and 2 units/0.02 mL ABO on the other (1:1 dose ratio).33 The result in this study showed that the diffusion halos for ONA were significantly larger than those for ABO (P < 0.001), although there was no difference in the improvements on the Facial Wrinkle Scale for the 2 products.33 This result was due to, simply, an underdosing of ABO based on the labeled units.
Based on their systematic review of the literature in 2009, Karsai and Raulin36 suggested that dose equivalences of 2.5 units ABO to 1.0 unit ONA should be used, and that in some circumstances this ratio might be reduced to 2:1. Karsai and Raulin were not content with the level of evidence for their findings, and recommended further investigation of lower dose ratios.36 However, the findings from more recent studies are consistent with those of Karsai and Raulin.15,16,31,37,38 A study using a human abdomen, in which subjects received ABO and ONA injections at varying dose ratios into the abdomen, found that dose equivalence could be established at a ratio of 1.9:1.0.39
DRUG-RELATED FACTORS POTENTIALLY AFFECTING ONSET OF ACTIVITY
Type of Botulinum Toxin
When given at their recommended doses,40-42 the times to onset of effect for ABO and INCO are similar, but ABO has a faster onset of effect than ONA and a longer duration of activity (Table 2).6,16,19,37,43,44 Similar patterns exist for time to maximum effect and are also repeated for different target muscles.15,16,19
Table 2.
Type of Toxin and Target Muscle Can Influence Onset and Duration of Activity6
| Glabellar | Crow’s feet | |||
|---|---|---|---|---|
| ABO | ONA | ABO | ONA | |
| Na | 59 | 61 | ||
| Dose (per side, given in 3 injections), units | 20 | 8 | 30 | 10 |
| Onset of activity b | ||||
| Proportion with onset by Day 1, % | 28 | 17 | 19 | 13 |
| Proportion with onset by Day 2, % | 59 | 37 | 54 | 39 |
| Proportion with onset by Day 5, % | 100 | 100 | 100 | 100 |
| Mean difference in time to onset (ABO vs ONA), days (P-value) | 0.52 (< .0001) | 0.33 (< .0025) | ||
| Duration of activity b | ||||
| Proportion with activity at Month 3, % | 98 | 98 | 100 | 98 |
| Proportion with activity at Month 4, % | 83 | 48 | 65 | 47 |
| Proportion with activity at Month 5, % | 27 | 2 | 22 | 0 |
| Mean difference in duration (ABO vs ONA), weeks (P-value) | 2.5 (<.0001) | 1.6 (<.0001) | ||
ABO, abobotulinumtoxinA; ONA, onabotulinumtoxinA. aTotal number of patients = 93; some patients received treatment to both areas. bAssessed using photographic 4-point wrinkle severity scales.29
There are disagreements about the reasons for the differences in onset of effect with different BoNT-A products. Some groups have proposed that the different hemagglutinins and non-hemagglutinins that surround ABO and ONA in the toxin complex (and which are absent with INCO8) may influence onset of activity through their effect on the penetration profile of the individual toxins,33,44 but this view is not widely shared. Detailed research has demonstrated that the toxin complexes dissociate when the product is reconstituted (diluted) in saline prior to injection in the vial.5,43,45 Other studies have demonstrated differences in the extent of spread and diffusion with different forms of BoNT-A, but these effects have now been clearly attributed to the different doses or relative potency used in those studies.26,33,35 The effects of diffusion on toxin safety and efficacy are discussed elsewhere in this supplement and will not be discussed in detail here. In addition, when reconstituted according to the manufacturer’s instructions,40-42 the concentrations of the toxins in solution differ and this may have a role in determining the degree of efficacy.46
Antibody Formation
Botulinum toxin is a protein and as such it may be regarded by the body as foreign, causing the immune system to raise neutralizing antibodies against the molecule and resulting in loss of efficacy. This effect was observed when ONA was first introduced for therapeutic use (eg, for treating torticollis or cervical dystonia), as a result of the high doses and high administration frequency, together with the high concentration of inactive BoNT-A present in the vials.47,48 Manufacturing changes made in the late 1990s increased the purity of ONA, reduced the required quantity of toxin and toxin-related proteins that had to be administered, and reduced the incidence rates for neutralizing antibody formation to between zero (treatment for glabellar lines and neurogenic overactive bladder) and 0.3% (cervical dystonia and post-stroke spasticity).34,49-51
Patients who receive BoNT-A for aesthetic treatment have always received very small doses with longer intervals between treatments than are usually seen with therapeutic indications.52 This puts these patients overall at much lower risk of developing neutralizing antibodies; in fact, only 11 patients with neutralizing antibodies following aesthetic treatment have been reported in the literature.49,50,53,54 Only neutralizing antibodies are important; patients may develop different antibodies to BoNT-A, but only neutralizing antibodies are clinically relevant with a resulting loss of response.47
In the case of ABO, individual Phase III studies conducted to date have failed to identify any cases of neutralizing antibody formation during treatment of glabellar lines.52,55-57 Although the risk of neutralizing antibody formation after aesthetic treatments is very small, as patients are requesting treatment at younger ages (and for several different target muscles) there is a finite chance for increasing cumulative risk of antibody formation. Therefore, clinicians should be alert to this possibility and try to ensure ways of avoiding such an outcome, for example by maximizing the time between treatments.37,49,52,53
Some patients do not respond well to treatment with BoNT-A. This may be due to inadequate dosing, drug handling errors during storage or preparation, anatomical issues, or even problems with drug administration (eg, an inaccessible muscle or injection into the wrong muscle).54,58 The increasing patient expectations of treatment results over time may also lead to disappointment. In some of these cases, a “treatment holiday” may restore the level of response to BoNT-A. In patients with cervical dystonia, it is suggested that suspending injections for 12 to 18 months may optimize treatment in non-responders.59
THE INFLUENCE OF TECHNIQUE ON ABO EFFICACY
Preparation
Botulinum toxin is supplied in vials as a white lyophilized or vacuum-dried powder for reconstitution in 0.9% saline solution under aseptic conditions. Detailed instructions for accurately preparing the small volumes of toxin solution required for injection are supplied by the manufacturers.40-42 Precise reconstitution of the product is essential in order to ensure full potency when injected.43,60
The manufacturer’s guidelines for reconstituting BoNT-A recommend the use of unpreserved saline solution. One reason for this recommendation is the theoretical risk that benzyl alcohol, when used as a preservative in so-called “preserved” saline, will denature the protein and reduce the potency of the injected solution.21 As benzyl alcohol is also a mild analgesic, a number of split-face studies have compared BoNT-A solutions in preserved and unpreserved saline to investigate whether this ingredient can improve comfort for patients without compromising efficacy.61-63 All of the studies found that using preserved saline significantly (P ≤ .001) reduced pain scores without affecting the activity of the toxin. However, Maas et al pointed out that these studies were all rather small and that the pain experienced with ABO injections is mild, even using unpreserved saline solution.21
All of the toxin in the vial must be fully dissolved before use. Recommended doses are based on the concentrations achieved by dissolving the given quantity of toxin in the given volume of saline, so that if any toxin is left behind, perhaps undissolved in the vial, the patient may receive a lower dose and a suboptimal effect might result. Owing to the fragile nature of proteins in solution, vigorous agitation is not recommended to dissolve the product: gentle rolling of the vial should achieve the same aim, taking care to ensure that no powder is left around the stopper.22,60,64
Studies have demonstrated the safety of storing reconstituted toxin.65,66 An initial study by Hexsel et al found that the efficacy of ONA reconstituted in unpreserved saline and stored at 4°C for up to 6 weeks was not statistically significantly different from that of solutions prepared 24 hours before injection, without additional adverse events.65 The authors extended their work and demonstrated that ABO could be stored for up to 15 days after reconstitution without any loss of efficacy and safety.67
The latest Dysport prescribing instructions state that the reconstituted product can be stored at 2 to 8°C for 24 hours prior to use.66 The product is expensive, however, and some patients may require small top-up injections a few weeks after their initial procedure to achieve the desired effect.
Injecting
The face contains many muscles and hence is well supplied with nerves and blood vessels. A thorough understanding of facial anatomy is therefore a prerequisite for anyone undertaking aesthetic procedures with BoNT-A in order to maximize efficacy and minimize the risk of adverse events.40 In addition to information supplied by the manufacturer, which is restricted to its licensed uses,68 several guidelines for ABO exist that contain detailed discussion of facial anatomy, an indication of optimal doses and sites for injection (based on the size and shape of the muscle, the severity of the wrinkles, and the degree of immobilization required by the patient), and recommendations for the angle of the injection for optimal delivery of the toxin to the target muscle.1,2,22,37 For optimal efficacy, injections should be made into relaxed muscles.69 Erickson et al recommend that patients are seated upright during the procedure,22 although there is no consensus on this aspect of treatment.
These guidelines also contain recommendations for the size of needles and syringes to be used for both reconstitution and injection, as these can affect accuracy and patient comfort. Recent studies have demonstrated that using narrower gauge needles in various areas of the face can improve patient comfort. A narrower gauge needle (33-G) was found to be more acceptable than a larger gauge (30-G).70,71 Small syringes with graduated markings are recommended to facilitate the division of the total dose into several injections. Ascher et al recommend the use of needles marked into thirds along their length to allow the clinician to judge how deep the injection has been placed.1,2,22 A slow speed of injection,72 as well as cooling devices placed on the skin prior to injection, can minimize pain and discomfort.72
Post-Injection Procedure
There is varying advice on what patients should and/or should not do following their procedure. Among the suggestions are to remain upright for at least 4 hours, avoid massaging the treated area to prevent unwanted diffusion of the toxin, and to contract the treated muscle to encourage distribution through the whole muscle.69,73 There is only one study known at this time to support any of these recommendations, related to masseter muscle treatment only.74 The study demonstrated that activation of the masseter for a period after injection could lead to improved long-term efficacy.74
THE INFLUENCE OF MUSCLE MASS ON ABO EFFICACY
Time to onset and duration of activity vary in different muscles, and are primarily influenced by differences in muscle mass and structure.75,76 The same is also true on an inter-patient basis. In a comparison of onset and duration of effect in the glabellar region with ABO, ONA, and INCO, Rappl et al noted that subjects with the longest duration of response had mild wrinkles at baseline and very thin corrugator muscles.8
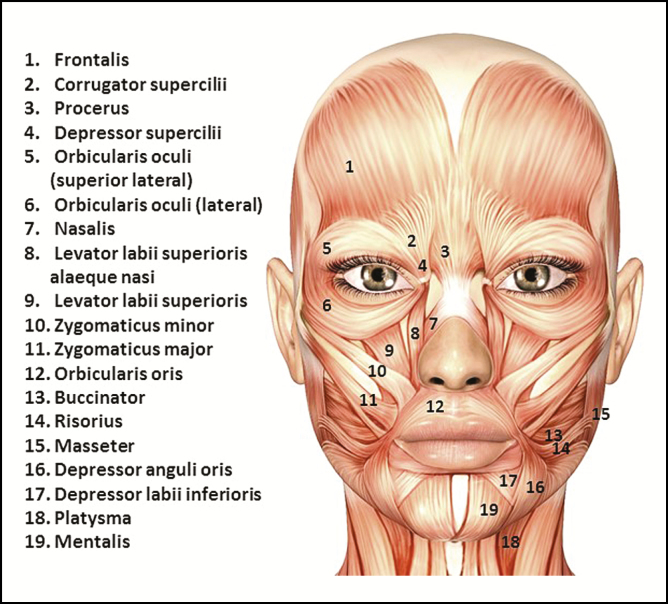
There is great variation in size, thickness, and depth below the skin among the muscles of the face (Figure 1). For example, the corrugators are strong, deep muscles, attached to bone at one end and the skin at the other, and these muscles can vary considerably in size. The frontalis is a large, thin muscle closely attached to the skin. The masseter is the largest and strongest muscle functioning in mastication, with the deepest portion originating from the inside of the zygomatic arch and inserting vertically into the ramus of the mandible. The orbicularis oculi is usually divided into 3 portions: the lacrimal portion at the medial side of the orbit, which is the smallest and the innermost portion; the palpebral portion that raises the eyelids and controls the involuntary action of blinking; and the orbital portion or pars orbicularis, which surrounds the orbit with concentric fibers, blending into the frontalis, and extending to the masseter.1,2 Although the general anatomy of the face has been known for many decades, characterization of the muscles of the face is still ongoing. The frontalis muscle has only recently been investigated in detail.77,78 The inter-patient variations are very significant, and this will influence how and where the product is injected in order to minimize side-effects and maximize the efficiency of BoNT-A.77
Figure 1.
The muscles of the face and neck (to be used merely as guidance for novice injectors).
Differences in Onset and Duration of Activity
Among the clinical studies reviewed for this paper, the shortest time to onset of effect with ABO was seen for wrinkles on the forehead and around the eyes. In 1 study, a fifth of patients saw an effect on the depth of crow’s feet wrinkles within 24 hours of treatment, with 100% of patients reporting improvement after 5 days.6 A second study found some degree of activity at Day 2 after injection and full onset at Day 6.19 For the frontalis, utilizing an objective measurement method, a single study found that the median time to onset of activity with a typical dose was 12 to 18 hours, with some patients exhibiting an effect after 6 hours (Table 3).15,16
Table 3.
Influence of Muscle on AbobotulinumtoxinA Onset and Duration
| Study/ studies | Muscle | Onset (median) | Duration | Dose (units) |
|---|---|---|---|---|
| 15 | Frontalis | 12 hours | 30-day study period | 25 |
| 18,56,79 | Glabella | 2-4 days | Median duration 85-109 days | 50-80 |
| 37 | Masseter | Initial assessment at 2 weeks | Full effect was observed for 90 days | 89 ± 27.8 |
| 19 | Orbicularis oculi |
Initial assessment at day 2 which showed onset | 6-day study period | 15 |
Not surprisingly, as the oldest registered indication, the majority of studies with onset and duration data focus on the glabella. In the studies carried out to support the approval of ABO, the median time to onset of activity with the 50 unit dose was between 2 and 4 days, with a median of 2.5 days.18,56,79 Some activity was noted in the first 24 hours after injection in some patients.6,79 Median time to onset in previously naïve patients who received up to 4 treatments at intervals of ≥85 days remained at 3 days across all cycles of treatment.80
In studies of the duration of effect in the glabella, when paralysis not relaxation was achieved, most patients maintained a near full effect of the toxin 2 months after injection; by 3 months this had declined to about a half of patients, and at 4 months to between a quarter and a third of patients.8,56,79,81 At 6 months, up to a fifth of patients continued to show an effect.8,56,81 This pattern was preserved in patients who underwent a series of injections (Table 3).80
In the study by Kassir et al, the duration of effect seemed to be longer (Table 2).6 It is interesting to note that the design of this split-face study, which compared ABO against ONA, required injections to the glabellar to be more concentrated than is usually recommended for clinical use, to mitigate against spread.
The effect of ABO on crow’s feet wrinkles, forehead lines, and masseter size seems to follow a similar pattern, with patients reliably experiencing the full effect of the toxin for at least 3 months and some patients continuing to see a good effect for as long as 5 or 6 months after injection.6,16,37,81,82 In studies that compared ABO with ONA, the proportion of patients with persistent effect from ONA at 4 months was much lower than with ABO and the effects of treatment with ONA were absent by 5 months.6,16 Specifically, in the frontalis, a bilateral comparison of ABO to ONA at a ratio of 2.5:1 showed a statistically significant overall persistence of ABO of approximately 3 weeks.16
The masseter is a large, strong muscle in an active area of the face. Doses used to treat this muscle are larger than those typically used to treat the frontalis, for example. Klein et al noted that “significant differences” in the size of the masseter were noted after 2 weeks.82
Making direct comparisons between these clinical trials is very difficult, owing to a lack of consistency in dosing and, especially, assessment measures as discussed earlier; however, there are some general trends. What is lacking is a solid explanation for these differences, even in studies in which more than 1 muscle is injected.6,81 Consensus guidelines indicate that bigger muscles require larger doses.1,2 However, this adaptation does not normalize the time to onset and duration of response – the differences still persist. Lorenc et al suggest that the volume and concentration of individual injections need to be tailored to the muscle and surrounding tissue, rather than simply the dose.83 Lorenc recommends that small, thick muscles (such as those of the glabellar) receive precisely placed, low volume, high concentration injections, whereas the broad, thin, flat geometry of the frontalis is better suited to high volume, low concentration injections that encourage diffusion across the muscle.84 For the thin, flat orbicularis oculi, precisely placed low volume, low concentration injections are required to eliminate the risk of ptosis.
Differences Between Women and Men
Studies that analyzed results by sex all found that, at equal doses, time to onset was shorter in women than in men and duration of effect was longer.8,18,56,79,80 A simple explanation for these observations is the increased muscle mass and strength in men.76,84 Two groups examined these effects in more detail.8,79 In their investigation of factors that might affect onset and duration of effect with ABO, ONA, and INCO, Rappl et al found that gender was the primary factor in determining duration of effect and a contributing factor to onset of effect with all 3 toxins.8 Kane et al used a range of doses from 50 to 80 units of ABO to treat the glabellar region. Before injecting, they classified the size of the muscle to be injected and then added an additional 10-unit “premium” for men (Table 4).79 The overall median time to onset of activity was 4 days, and median duration of activity was 107 days in line with observations from other studies. In order to compare the potential effects of muscle mass and sex, the group evaluated the proportion of responders at Day 30 (Table 5), when in most cases the toxin would have reached maximum effect.79 Overall, despite the tailored dosing, there were significantly more responders among the women than among the men (87% vs 65%; P < 0.001). As mentioned above, the proportion of responders decreased as the dose increased. Kane et al hypothesized that this was because muscles with greater mass have a higher threshold of response.79 They noted that the group of women and men receiving the highest doses for their sex (70 units and 80 units, respectively) had an overall response rate of 80%; and that the group receiving the median dose (60 units and 70 units, respectively) had an overall response rate of 88%. The authors felt that this justified making a recommendation for higher dosing in men.79
Table 4.
Totala AbobotulinumtoxinA Dose Allocation by Patient Sex and Muscle Size79
| Standard muscle mass | Larger muscle mass | Largest muscle mass | |
|---|---|---|---|
| Women (n = 475) | 50 units in 0.4 mL | 60 units in 0.5 mL | 70 units in 0.6 mL |
| Men (n = 62) | 60 units in 0.5 mL | 70 units in 0.6 mL | 80 units in 0.7 mL |
aAll doses split between 5 equal injections into the procerus, corrugator (two), and lateral corrugator/orbicularis muscles (two).
Table 5.
Proportion of Respondersa in Each Dose Group at Day 3079
| Overall | 50 units b | 60 units | 70 units | 80 units c | |
|---|---|---|---|---|---|
| Responders, % | 85 | 96 | 90 | 81 | 61 |
aAs assessed by a blinded evaluator using the 4-point glabellar lines severity scale. bWomen only. cMen only.
EFFECT OF PATIENT AGE ON ABO EFFICACY
Aging is associated with a progressive loss of muscle mass and strength, and a decline in neurophysiological functions, including loss of activity at the neuromuscular junction.84 Older skin is thinner and less elastic, and wrinkles are more likely to be caused by gravity-induced tissue sagging than muscle contraction.72 It is therefore imperative that, when using ABO in the elderly population, dilution, dosing, and frequency should be adjusted.
As mentioned previously and in other papers in this supplement, ensuring that injected toxin reaches the neuromuscular junction – the site of activity – is essential for optimizing efficacy. Gonzalez-Freire et al have summarized the findings of a range of studies into age-related changes at the neuromuscular junction. They concluded that morphological and physiological changes associated with aging result in a remodeling of the motor unit and in a decline of the number of motor neurons. This ultimately results in a loss of communication between the nerves and the muscles. However, the precise mechanism and order for these changes has not yet been elucidated.85
The effect of changes in skin quality on the efficacy of ABO has also been clearly demonstrated. Patients with thick, sebaceous skin with deep, permanent wrinkles or thin skin and hyperfunctional muscles with deep permanent wrinkles are significantly less responsive to ONA than patients with thin skin and wrinkles that are only evident when scowling.86
A post-hoc analysis of 6 clinical trials demonstrated similar efficacy and tolerability of ABO in the treatment of glabellar lines for patients with skin of color compared with white patients.87 However, the response rate 30 days after treatment was greater in patients with skin of color than for white patients.
There is limited evidence from a few clinical trials which have specifically studied efficacy of BoNT-A in the elderly, and, generally, insufficient numbers of elderly patients have been enrolled in clinical studies to make any meaningful comparisons on onset and duration of effect in this subpopulation.72 However, there is evidence from clinical trials to indicate that increasing age is associated with lower response rates.56,79,86,88
The manufacturer’s label, which is based on the finding of lower efficacy in older age groups included within the registration of clinical trials, limits the application of ABO to adults aged <65 years.40 If patients older than this are routinely to receive injections of toxin, then additional studies of efficacy and safety may be appropriate, and specific protocols for treating elderly patients need to be developed.
CONCLUSIONS
Onset and duration of effect are markers of the overall response to treatment with ABO. Significantly, they are important drivers of patient satisfaction because patients want to see both early benefits of treatment and for those benefits to last as long as possible before retreatment is required.
Factors influencing onset and duration include, for example, the product, the dose the target muscle, and the patient’s sex and age. Some of these may be inter-related and may not be universal. There is much that still needs to be elucidated about exactly how these factors exert their influence. As the literature for aesthetic uses of BoNT-A continues to grow, and more is understood about the mechanism of action in the muscles treated and about the differences between different BoNT-A products, treatment protocols can be refined to provide the best reconstitution and dosing for optimal results in individual patients. In the meantime, every patient must be treated as an individual and care must be taken to discuss all aspects of the procedure in order to manage the patient’s expectations.
Disclosures
Dr Nestor has received grants and personal fees for research and consulting from Galderma and Croma. He has also received a research grant from Evolus. Dr Pickett is a Senior Program Leader and Scientific Expert, Neurotoxins in Galderma Aesthetic and Corrective Global Business Unit. Dr Ablon declared no potential conflicts of interest with respect to the research, authorship, and publication of this article. The comments, statements and opinions expressed by A. Pickett are those of the author and Toxin Science Limited only.
Funding
This publication was supported by Galderma Laboratories, LP (Fort Worth, TX), who funded the development of this supplement. Editorial assistance was funded by Galderma Laboratories, LP and provided by Jane Tricker and MedSense, Ltd. (High Wycombe, UK). The authors did not receive compensation for writing the manuscripts.
Acknowledgments
The authors would like to acknowledge the support of Jane Tricker, freelance writer, who assisted in the writing of this paper, and MedSense, Ltd (High Wycombe, UK) for editorial support, both funded by Galderma.
REFERENCES
- 1. Ascher B, Talarico S, Cassuto D, et al. International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit)–Part I: Upper facial wrinkles. J Eur Acad Dermatol Venereol. 2010;24(11):1278-1284. [DOI] [PubMed] [Google Scholar]
- 2. Ascher B, Talarico S, Cassuto D, et al. International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit)–Part II: Wrinkles on the middle and lower face, neck and chest. J Eur Acad Dermatol Venereol. 2010;24(11):1285-1295. [DOI] [PubMed] [Google Scholar]
- 3. Nettar K, Maas C. Neuromodulators: available agents, physiology, and anatomy. Facial Plast Surg. 2011;27(6):517-522. [DOI] [PubMed] [Google Scholar]
- 4. Wortzman MS, Pickett A. The science and manufacturing behind botulinum neurotoxin type A-ABO in clinical use. Aesthet Surg J. 2009;29(6 Suppl):S34-S42. [DOI] [PubMed] [Google Scholar]
- 5. Costa A, Pegas Pereira ES, de Oliveira Pereira M, et al. Comparative study of the diffusion of five botulinum toxins type-A in five dosages of use: are there differences amongst the commercially-available products? Dermatol Online J. 2012;18(11):2. [PubMed] [Google Scholar]
- 6. Kassir R, Kolluru A, Kassir M. Triple-Blind, Prospective, Internally Controlled Comparative Study Between AbobotulinumtoxinA and OnabotulinumtoxinA for the Treatment of Facial Rhytids. Dermatol Ther (Heidelb). 2013;3(2):179-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rogozhin AA, Pang KK, Bukharaeva E, Young C, Slater CR. Recovery of mouse neuromuscular junctions from single and repeated injections of botulinum neurotoxin A. J Physiol. 2008;586(13):3163-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rappl T, Parvizi D, Friedl H, et al. Onset and duration of effect of incobotulinumtoxinA, onabotulinumtoxinA, and abobotulinumtoxinA in the treatment of glabellar frown lines: a randomized, double-blind study. Clin Cosmet Investig Dermatol. 2013;6:211-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hexsel D, Brum C, Porto MD, et al. Quality of life and satisfaction of patients after full-face injections of abobotulinum toxin type A: A randomized, phase IV clinical trial. J Drugs Dermatol. 2013;12(12):1363-1367. [PubMed] [Google Scholar]
- 10. Bonaparte JP, Ellis D, Quinn JG, Ansari MT, Rabski J, Kilty SJ. A comparative assessment of three formulations of botulinum toxin A for facial rhytides: a systematic review and meta-analyses. Syst Rev. 2013;2:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chauhan DS, Cariappa KM, Guruprasad Y. Botulinum toxin type a for the treatment of hyperkinetic lines of the face. J Maxillofac Oral Surg. 2013;12(2):173-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hexsel D, Brum C, Porto MD, et al. Full-face injections of variable total doses of abobotulinum toxin type A: A randomized, phase IV clinical trial of safety and efficacy. J Drugs Dermatol. 2013;12(12):1356-1362. [PubMed] [Google Scholar]
- 13. Jaspers GW, Pijpe J, Jansma J. The use of botulinum toxin type A in cosmetic facial procedures. Int J Oral Maxillofac Surg. 2011;40(2):127-133. [DOI] [PubMed] [Google Scholar]
- 14. Michaels BM, Csank GA, Ryb GE, Eko FN, Rubin A. Prospective randomized comparison of onabotulinumtoxinA (Botox) and abobotulinumtoxinA (Dysport) in the treatment of forehead, glabellar, and periorbital wrinkles. Aesthet Surg J. 2012;32(1):96-102. [DOI] [PubMed] [Google Scholar]
- 15. Nestor MS, Ablon GR. Comparing the clinical attributes of abobotulinumtoxinA and onabotulinumtoxinA utilizing a novel contralateral Frontalis model and the Frontalis Activity Measurement Standard. J Drugs Dermatol. 2011;10(10):1148-1157. [PubMed] [Google Scholar]
- 16. Nestor MS, Ablon GR. Duration of action of abobotulinumtoxina and onabotulinumtoxina: a randomized, double-blind study using a contralateral frontalis model. J Clin Aesthet Dermatol. 2011;4(9):43-49. [PMC free article] [PubMed] [Google Scholar]
- 17. Rzany B, Fratila AA, Fischer TC, et al. Recommendations for the best possible use of botulinum neurotoxin type a (Speywood units) for aesthetic applications. J Drugs Dermatol. 2013;12(1):80-84. [PubMed] [Google Scholar]
- 18. Schlessinger J, Monheit G, Kane MA, Mendelsohn N. Time to onset of response of abobotulinumtoxina in the treatment of glabellar lines: a subset analysis of phase 3 clinical trials of a new botulinum toxin type A. Dermatol Surg. 2011;37(10):1434-1442. [DOI] [PubMed] [Google Scholar]
- 19. Yu KC, Nettar KD, Bapna S, Boscardin WJ, Maas CS. Split-face double-blind study comparing the onset of action of onabotulinumtoxinA and abobotulinumtoxinA. Arch Facial Plast Surg. 2012;14(3):198-204. [DOI] [PubMed] [Google Scholar]
- 20. Small R. Botulinum toxin injection for facial wrinkles. Am Fam Physician. 2014;90(3):168-175. [PubMed] [Google Scholar]
- 21. Maas C, Kane MA, Bucay VW, et al. Current aesthetic use of abobotulinumtoxinA in clinical practice: an evidence-based consensus review. Aesthet Surg J. 2012;32(1 Suppl): 8S-29S. [DOI] [PubMed] [Google Scholar]
- 22. Erickson BP, Lee WW, Cohen J, Grunebaum LD. The role of neurotoxins in the periorbital and midfacial areas. Facial Plast Surg Clin North Am. 2015;23(2):243-255. [DOI] [PubMed] [Google Scholar]
- 23. FDA. Center for Drug Evaluation and Research (CDER). Guidance for industry. Upper facial lines: developing botulinum toxin drug products 2014. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM407983.pdf Accessed July 14, 2016.
- 24. Glogau R, Biesman B, Kane M. Assessment of Botulinum Toxin Aesthetic Outcomes: Clinical Study vs Real-World Practice. JAMA Dermatol. 2015;151(11):1177-1178. [DOI] [PubMed] [Google Scholar]
- 25. Kane MA, Blitzer A, Brandt FS, et al. Development and validation of a new clinically-meaningful rating scale for measuring lateral canthal line severity. Aesthet Surg J. 2012;32(3):275-285. [DOI] [PubMed] [Google Scholar]
- 26. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276-282. [PMC free article] [PubMed] [Google Scholar]
- 27. Carruthers A, Carruthers J. A validated facial grading scale: the future of facial ageing measurement tools? J Cosmet Laser Ther. 2010;12(5):235-241. [DOI] [PubMed] [Google Scholar]
- 28. Hund T, Ascher B, Rzany B; SMILE STUDY GROUP Reproducibility of two four-point clinical severity scores for lateral canthal lines (crow’s feet). Dermatol Surg. 2006;32(10):1256-1260. [DOI] [PubMed] [Google Scholar]
- 29. Honeck P, Weiss C, Sterry W, Rzany B; Gladys study group Reproducibility of a four-point clinical severity score for glabellar frown lines. Br J Dermatol. 2003;149(2):306-310. [DOI] [PubMed] [Google Scholar]
- 30. Conkling N, Bishawi M, Phillips BT, Bui DT, Khan SU, Dagum AB. Subjective rating of cosmetic treatment with botulinum toxin type A: do existing measures demonstrate interobserver validity? Ann Plast Surg. 2012;69(4):350-355. [DOI] [PubMed] [Google Scholar]
- 31. Hexsel D, Dal’Forno T, Hexsel C, Do Prado DZ, Lima MM. A randomized pilot study comparing the action halos of two commercial preparations of botulinum toxin type A. Dermatol Surg. 2008;34(1):52-59. [DOI] [PubMed] [Google Scholar]
- 32. Hexsel D, Brum C, do Prado DZ, et al. Field effect of two commercial preparations of botulinum toxin type A: a prospective, double-blind, randomized clinical trial. J Am Acad Dermatol. 2012;67(2):226-232. [DOI] [PubMed] [Google Scholar]
- 33. Hexsel D, Hexsel C, Siega C, Schilling-Souza J, Rotta FT, Rodrigues TC. Fields of effects of 2 commercial preparations of botulinum toxin type A at equal labeled unit doses: a double-blind randomized trial. JAMA Dermatol. 2013;149(12):1386-1391. [DOI] [PubMed] [Google Scholar]
- 34. Pickett A, Caird D. Discussion regarding botulinum toxin, immunologic considerations with long-term repeated use, with emphasis on cosmetic applications. Minimal risk of antibody formation after aesthetic treatment with type a botulinum toxin. Facial Plast Surg Clin North Am. 2009;17(4):633-634; discussion 634. [DOI] [PubMed] [Google Scholar]
- 35. Kerscher M, Roll S, Becker A, Wigger-Alberti W. Comparison of the spread of three botulinum toxin type A preparations. Arch Dermatol Res. 2012;304(2):155-161. [DOI] [PubMed] [Google Scholar]
- 36. Karsai S, Raulin C. Current evidence on the unit equivalence of different botulinum neurotoxin A formulations and recommendations for clinical practice in dermatology. Dermatol Surg. 2009;35(1):1-8. [DOI] [PubMed] [Google Scholar]
- 37. Lee SH, Wee SH, Kim HJ, et al. Abobotulinum toxin A and onabotulinum toxin A for masseteric hypertrophy: a split-face study in 25 Korean patients. J Dermatolog Treat. 2013;24(2):133-136. [DOI] [PubMed] [Google Scholar]
- 38. Lee JH, Park JH, Lee SK, et al. Efficacy and safety of incobotulinum toxin A in periocular rhytides and masseteric hypertrophy: side-by-side comparison with onabotulinum toxin A. J Dermatolog Treat. 2014;25(4):326-330. [DOI] [PubMed] [Google Scholar]
- 39. Kranz G, Haubenberger D, Voller B, et al. Respective potencies of Botox and Dysport in a human skin model: a randomized, double-blind study. Mov Disord. 2009;24(2):231-236. [DOI] [PubMed] [Google Scholar]
- 40. Azzalure – Summary of product characteristics (SPC) – (eMC) https://www.medicines.org.uk/emc/medicine/21985 Accessed April 22, 2016.
- 41. BOTOX 100 Units – (eMC) https://www.medicines.org.uk/emc/print-document?documentId=112 Accessed April 14, 2016
- 42. Xeomin 100 Units – Summary of product characteristics (SPC) – (eMC) https://www.medicines.org.uk/emc/medicine/20666 Accessed April 22, 2016.
- 43. Frevert J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs R D. 2015;15(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oliveira de Morais O, Matos Reis-Filho E, Vilela Pereira L, Martins Gomes C, Alves G. Comparison of four botulinum neurotoxin type A preparations in the treatment of hyperdynamic forehead lines in men: a pilot study. J Drugs Dermatol. 2012;11(2):216-219. [PubMed] [Google Scholar]
- 45. Eisele KH, Fink K, Vey M, Taylor HV. Studies on the dissociation of botulinum neurotoxin type A complexes. Toxicon. 2011;57(4):555-565. [DOI] [PubMed] [Google Scholar]
- 46. Wohlfarth K, Schwandt I, Wegner F, et al. Biological activity of two botulinum toxin type A complexes (Dysport and Botox) in volunteers: a double-blind, randomized, dose-ranging study. J Neurol. 2008;255(12):1932-1939. [DOI] [PubMed] [Google Scholar]
- 47. Borodic G. Botulinum toxin, immunologic considerations with long-term repeated use, with emphasis on cosmetic applications. Facial Plast Surg Clin North Am. 2007;15(1):11-16. [DOI] [PubMed] [Google Scholar]
- 48. Lange O, Bigalke H, Dengler R, Wegner F, deGroot M, Wohlfarth K. Neutralizing antibodies and secondary therapy failure after treatment with botulinum toxin type A: much ado about nothing? Clin Neuropharmacol. 2009;32(4):213-218. [DOI] [PubMed] [Google Scholar]
- 49. Torres S, Hamilton M, Sanches E, Starovatova P, Gubanova E, Reshetnikova T. Neutralizing antibodies to botulinum neurotoxin type A in aesthetic medicine: five case reports. Clin Cosmet Investig Dermatol. 2014;7:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stengel G, Bee EK. Antibody-induced secondary treatment failure in a patient treated with botulinum toxin type A for glabellar frown lines. Clin Interv Aging. 2011;6:281-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Naumann M, Carruthers A, Carruthers J, et al. Meta-analysis of neutralizing antibody conversion with onabotulinumtoxinA (BOTOX®) across multiple indications. Mov Disord. 2010;25(13):2211-2218. [DOI] [PubMed] [Google Scholar]
- 52. Lawrence I, Moy R. An evaluation of neutralizing antibody induction during treatment of glabellar lines with a new US formulation of botulinum neurotoxin type A. Aesthet Surg J. 2009;29(6 Suppl):S66-S71. [DOI] [PubMed] [Google Scholar]
- 53. Borodic G. Immunologic resistance after repeated botulinum toxin type a injections for facial rhytides. Ophthal Plast Reconstr Surg. 2006;22(3):239-240. [DOI] [PubMed] [Google Scholar]
- 54. Dressler D, Wohlfahrt K, Meyer-Rogge E, Wiest L, Bigalke H. Antibody-induced failure of botulinum toxin a therapy in cosmetic indications. Dermatol Surg. 2010;36(Suppl 4): 2182-2187. [DOI] [PubMed] [Google Scholar]
- 55. Monheit GD, Cohen JL; Reloxin Investigational Group Long-term safety of repeated administrations of a new formulation of botulinum toxin type A in the treatment of glabellar lines: interim analysis from an open-label extension study. J Am Acad Dermatol. 2009;61(3):421-425. [DOI] [PubMed] [Google Scholar]
- 56. Brandt F, Swanson N, Baumann L, Huber B. Randomized, placebo-controlled study of a new botulinum toxin type a for treatment of glabellar lines: efficacy and safety. Dermatol Surg. 2009;35(12):1893-1901. [DOI] [PubMed] [Google Scholar]
- 57. Moy R, Maas C, Monheit G, Huber MB; Reloxin Investigational Group Long-term safety and efficacy of a new botulinum toxin type A in treating glabellar lines. Arch Facial Plast Surg. 2009;11(2):77-83. [DOI] [PubMed] [Google Scholar]
- 58. Benecke R. Clinical relevance of botulinum toxin immunogenicity. BioDrugs. 2012;26(2):e1-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Marion MH, Humberstone M, Grunewald R, Wimalaratna S. British Neurotoxin Network recommendations for managing cervical dystonia in patients with a poor response to botulinum toxin. Pract Neurol. 2016;16(4):288-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carey WD. Incorrect reconstitution of incobotulinumtoxinA leads to loss of neurotoxin. J Drugs Dermatol. 2014;13(6):735-738. [PubMed] [Google Scholar]
- 61. Allen SB, Goldenberg NA. Pain difference associated with injection of abobotulinumtoxinA reconstituted with preserved saline and preservative-free saline: a prospective, randomized, side-by-side, double-blind study. Dermatol Surg. 2012;38(6):867-870. [DOI] [PubMed] [Google Scholar]
- 62. Garcia A, Fulton JE., Jr Cosmetic denervation of the muscles of facial expression with botulinum toxin. A dose-response study. Dermatol Surg. 1996;22(1):39-43. [DOI] [PubMed] [Google Scholar]
- 63. Alam M, Dover JS, Arndt KA. Pain associated with injection of botulinum A exotoxin reconstituted using isotonic sodium chloride with and without preservative: a double-blind, randomized controlled trial. Arch Dermatol. 2002;138(4):510-514. [DOI] [PubMed] [Google Scholar]
- 64. Dressler D, Bigalke H. Reconstituting botulinum toxin drugs: shaking, stirring or what? J Neural Transm (Vienna). 2016;123(5):523-525. [DOI] [PubMed] [Google Scholar]
- 65. Hexsel DM, De Almeida AT, Rutowitsch M, et al. Multicenter, double-blind study of the efficacy of injections with botulinum toxin type A reconstituted up to six consecutive weeks before application. Dermatol Surg. 2003;29(5):523-529. [DOI] [PubMed] [Google Scholar]
- 66. Dysport, Dysport Prescribing Information, 2016. https://www.dysport.com/pdfs/Dysport_Full_Prescribing_Information.pdf Accessed July 14, 2016.
- 67. Hexsel D, Rutowitsch MS, de Castro LC, do Prado DZ, Lima MM. Blind multicenter study of the efficacy and safety of injections of a commercial preparation of botulinum toxin type A reconstituted up to 15 days before injection. Dermatol Surg. 2009;35(6):933-939. [DOI] [PubMed] [Google Scholar]
- 68. Jabor MA, Kaushik R, Shayani P, et al. Efficacy of reconstituted and stored botulinum toxin type A: an electrophysiologic and visual study in the auricular muscle of the rabbit. Plast Reconstr Surg. 2003;111(7):2419-2426; discussion 2427. [DOI] [PubMed] [Google Scholar]
- 69. Stephan S, Wang TD. Botulinum toxin: clinical techniques, applications, and complications. Facial Plast Surg. 2011;27(6):529-539. [DOI] [PubMed] [Google Scholar]
- 70. Sezgin B, Ozel B, Bulam H, Guney K, Tuncer S, Cenetoglu S. The Effect of Microneedle Thickness on Pain During Minimally Invasive Facial Procedures: A Clinical Study. Aesthet Surg J. 2014;34(5):757-765. [DOI] [PubMed] [Google Scholar]
- 71. Alam M, Geisler A, Sadhwani D, et al. Effect of Needle Size on Pain Perception in Patients Treated With Botulinum Toxin Type A Injections: A Randomized Clinical Trial. JAMA Dermatol. 2015;151(11):1194-1199. [DOI] [PubMed] [Google Scholar]
- 72. Cheng CM. Cosmetic use of botulinum toxin type A in the elderly. Clin Interv Aging. 2007;2(1):81-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tremaine AM, McCullough JL. Botulinum toxin type A for the management of glabellar rhytids. Clin Cosmet Investig Dermatol. 2010;3:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wei J, Xu H, Dong J, Li Q, Dai C. Prolonging the duration of masseter muscle reduction by adjusting the masticatory movements after the treatment of masseter muscle hypertrophy with botulinum toxin type a injection. Dermatol Surg. 2015;41(Suppl 1:S101-S109. [DOI] [PubMed] [Google Scholar]
- 75. Lee HH, Kim ST, Lee KJ, Baik HS. Effect of a second injection of botulinum toxin on lower facial contouring, as evaluated using 3-dimensional laser scanning. Dermatol Surg. 2015;41(4):439-444. [DOI] [PubMed] [Google Scholar]
- 76. Keaney TC, Alster TS. Botulinum toxin in men: review of relevant anatomy and clinical trial data. Dermatol Surg. 2013;39(10):1434-1443. [DOI] [PubMed] [Google Scholar]
- 77. Choi YJ, Won SY, Lee JG, et al. Characterizing the Lateral Border of the Frontalis for Safe and Effective Injection of Botulinum Toxin. Aesthet Surg J. 2016;36(3):344-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Costin BR, Plesec TP, Sakolsatayadorn N, Rubinstein TJ, McBride JM, Perry JD. Anatomy and histology of the frontalis muscle. Ophthal Plast Reconstr Surg. 2015;31(1):66-72. [DOI] [PubMed] [Google Scholar]
- 79. Kane MA, Brandt F, Rohrich RJ, Narins RS, Monheit GD, Huber MB; Reloxin Investigational Group Evaluation of variable-dose treatment with a new U.S. Botulinum Toxin Type A (Dysport) for correction of moderate to severe glabellar lines: results from a phase III, randomized, double-blind, placebo-controlled study. Plast Reconstr Surg. 2009;124(5):1619-1629. [DOI] [PubMed] [Google Scholar]
- 80. Rubin MG, Dover J, Glogau RG, Goldberg DJ, Goldman MP, Schlessinger J. The efficacy and safety of a new U.S. Botulinum toxin type A in the retreatment of glabellar lines following open-label treatment. J Drugs Dermatol. 2009;8(5):439-444. [PubMed] [Google Scholar]
- 81. Dubina M, Tung R, Bolotin D, et al. Treatment of forehead/glabellar rhytide complex with combination botulinum toxin a and hyaluronic acid versus botulinum toxin A injection alone: a split-face, rater-blinded, randomized control trial. J Cosmet Dermatol. 2013;12(4):261-266. [DOI] [PubMed] [Google Scholar]
- 82. Klein FH, Brenner FM, Sato MS, Robert FM, Helmer KA. Lower facial remodeling with botulinum toxin type A for the treatment of masseter hypertrophy. An Bras Dermatol. 2014;89(6):878-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lorenc ZP, Smith S, Nestor M, Nelson D, Moradi A. Understanding the functional anatomy of the frontalis and glabellar complex for optimal aesthetic botulinum toxin type A therapy. Aesthetic Plast Surg. 2013;37(5):975-983. [DOI] [PubMed] [Google Scholar]
- 84. Yamauchi PS. Selection and preference for botulinum toxins in the management of photoaging and facial lines: patient and physician considerations. Patient Prefer Adherence. 2010;4:345-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gonzalez-Freire M, de Cabo R, Studenski SA, Ferrucci L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front Aging Neurosci. 2014;6:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sunil SM, Babu BG, Deepthi S, Veerabhadrappa AC, Vadavadagi SV, Punde P. Botulinum toxin for the treatment of hyperfunctional lines of the forehead. J Int Soc Prev Community Dent. 2015;5(4):276-282. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87. Taylor SC, Callender VD, Albright CD, Coleman J, Axford-Gatley RA, Lin X. AbobotulinumtoxinA for reduction of glabellar lines in patients with skin of color: post hoc analysis of pooled clinical trial data. Dermatol Surg. 2012;38(11):1804-1811. [DOI] [PubMed] [Google Scholar]
- 88. Ho MC, Hsu WC, Hsieh YT. Botulinum toxin type a injection for lateral canthal rhytids: effect on tear film stability and tear production. JAMA Ophthalmol. 2014;132(3):332-337. [DOI] [PubMed] [Google Scholar]