What is TPX2?
TPX2 is a microtubule-associated protein that is required for mitotic spindle assembly and function. TPX2 has a nuclear localization sequence (NLS) and is nuclear-localized during interphase; in mitosis it localizes to spindle microtubules, with an enrichment toward the spindle poles (Figure 1).
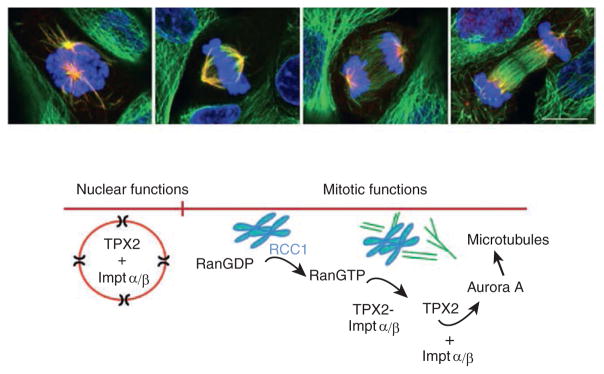
Figure 1. TPX2.
Top panel: Distribution of TPX2 in mitotic LLC-Pk1 cells. Cells are stained for tubulin (green), TPX2 (Red) and DNA (blue). Merged images of early prometaphase, metaphase, anaphase and telophase are shown. Photo credit: Marker bar = 10 um. Lower panel. Cartoon depicting TPX2 activity in promoting microtubule formation.
Where does the name TPX2 come from?
TPX2 was first characterized as a factor in mitotic egg extracts that was required for the dynein-dependent localization of a plus-end directed kinesin, Xkpl2, to the spindle poles. Based on these experiments, the protein was named Targeting Protein for Xklp2, or TPX2. Ironically, although we have learned a great deal about TPX2 since 1998, we still do not fully understand how TPX2, a microtubule-associated protein, actually targets Xklp2!
What does TPX2 do in mitosis?
TPX2 is probably best known for its role in stimulating microtubule assembly during mitotic spindle formation. For many years, how microtubule nucleation occurs in cells that lack centrosomes baffled researchers. A key advance was the demonstration that the small GTPase, Ran, is locally activated near chromosomes, and that active Ran could promote aster and spindle formation in egg extracts that lacked centrosomes or chromosomes. However, Ran doesn’t act directly on microtubules — it binds importins α and β and relieves their inhibitory effect on NLS-containing proteins, called spindle assembly factors. Following activation, spindle assembly factors promote microtubule formation near chromosomes. Recent work further shows that TPX2 is required for the formation of branched microtubules, a process that requires both the γ-tubulin ring complex and a multi-protein complex, augmin. The specific mechanism by which TPX2 nucleates spindle microtubules is now being worked out. Importantly, the critical role of TPX2 in mitosis is supported by mouse studies showing that TPX2 null embryos arrest at the morula stage with defective mitotic spindles.
Is stimulating microtubule assembly the only function of TPX2?
The choice of the phrase ‘targeting factor’ when naming TPX2 has turned out to be rather prescient — it is now clear that TPX2 targets several proteins to the spindle, rather like spindle flypaper. For example, TPX2 binds, activates and localizes the mitotic kinase Aurora A to the spindle. TPX2 is also required to localize the kinesin Eg5 to spindle fibers and to regulate motor behavior. Immunoprecipitation experiments further reveal that TPX2 interacts, directly or indirectly, with several other proteins that regulate spindle assembly and function, including microtubule-binding proteins, motors, and nucleation factors. A simple model is that TPX2 promotes microtubule nucleation, binds to the newly formed microtubule, and subsequently recruits additional binding partners.
Is TPX2 universally required for spindle assembly?
Orthologs of human TPX2 have been identified in other vertebrates and plants; proteins that share some of the features of TPX2 have also been identified in invertebrates. For example, in Drosophila, Ssp1/Mei-38 has sequence homology to TPX2 and binds microtubules in vivo and in vitro. However, Ssp1 lacks the Aurora A and kinesin-5 binding domains of vertebrate TPX2, and depletion of Ssp1 results in only minor spindle defects. In C. elegans a TPX2-like protein (TPXL-1) has been identified that activates and localizes Aurora A to the spindle, but is not required for microtubule formation in the chromosome region, a conserved feature of TPX2 in other model systems including plants. It seems likely that key functions of TPX2 are widely conserved, but depending on the organism, the functional modules may not reside within a single protein. Alternatively, other, divergent proteins may also perform some TPX2 functions.
Does TPX2 function in non-mitotic cells?
Despite its fame as a mitotic protein, evidence is accumulating to support the idea that TPX2 moonlights in non-mitotic cells. During neurogenesis in the brain, nuclei of progenitor cells migrate from one end of the cell to the other. Apical nuclear migration in G2 is promoted by TPX2, which exits the nucleus and bundles microtubules. TPX2 also contributes to process outgrowth in cultured neurons. These and other data show that TPX2 can escape from the nucleus to regulate the microtubule cytoskeleton of non-mitotic cells.
Does TPX2 function in the nucleus?
A common assumption has been that TPX2 is sequestered in the nucleus to prevent it from interacting with microtubules. However, recent work shows that TPX2 accumulates at DNA double-strand breaks and further that cells lacking TPX2 show an accumulation of ionizing radiation dependent phosphorylation of Histone 2AX. These data suggest that TPX2 is not just hiding out in the nucleus, but performs important nuclear functions.
Mutations in TPX2 are commonly observed in cancer — what is going on?
Like many other proteins that regulate mitosis, TPX2 is overexpressed in many types of cancer and TPX2 expression level correlates with poor prognosis. Overexpression of TPX2, and other mitotic regulators, could be a downstream response to other mutations. Alternatively or in addition, TPX2 overexpression could be a factor in chromosome instability, and thus contribute to carcinogenesis. Understanding how TPX2 expression contributes to cancer remains an area of active investigation. Because TPX2 is nuclear in interphase and associated with spindle microtubules in mitosis, it is a potential therapeutic target, in addition to being a useful marker for certain tumors.
What remains to be discovered about TPX2?
Expression of various truncations of TPX2 suggests that two microtubule-binding regions are present in the protein, but the domains that mediate binding to the microtubule lattice have not been precisely defined. High-resolution structural information could illuminate how the TPX2–microtubule interaction contributes to microtubule nucleation and stabilization, and how TPX2 recruits other proteins to the microtubule. Finally, precisely how Ran regulates the activities of TPX2 remain unclear — TPX2 nucleates microtubules near chromosomes where Ran levels are high, and subsequently accumulates on microtubules near spindle poles where Ran levels are low, suggesting multiple modes of TPX2 interaction with microtubules. Clearly, TPX2 continues to be the target of much deserved attention!
Where to find out more?
- 1.Wittmann T, Boleti H, Antony C, Karsenti E, Vernos I. Localization of the kinesin-like protein Xklp2 to spindle poles requires a leucine zipper, a microtubule-associated protein and dynein. J Cell Biol. 1998;143:673–685. doi: 10.1083/jcb.143.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gruss OJ, Vernos I. The mechanism of spindle assembly functions of Ran and its target TPX2. J Cell Biol. 2004;166:949–955. doi: 10.1083/jcb.200312112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tulu US, Fagerstrom C, Ferenz NP, Wadsworth P. Molecular requirements for kinetochore-associated microtubule formation in mammalian cells. Curr Biol. 2006;16:536–541. doi: 10.1016/j.cub.2006.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruss OJ, Wittmann M, Yokoyama H, Pepperkok R, Kufer T, Sillje H, Karsenti E, Mattaj IW, Vernos I. Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat Cell Biol. 2002;4:871–879. doi: 10.1038/ncb870. [DOI] [PubMed] [Google Scholar]
- 5.Petry S, Groen AC, Ishihara K, Mitchison TJ, Vale RD. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell. 2013;152:768–777. doi: 10.1016/j.cell.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scrofani J, Sardon T, Meunier S, Vernos I. Microtubule Nucleation in Mitosis by a RanGTP-Dependent Protein Complex. Curr Biol. 2015;25:131–140. doi: 10.1016/j.cub.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Aguirre-Portoles C, Bird AW, Hyman A, Canamero M, Perez de Castro I, Malumbres M. Tpx2 controls spindle integrity, genome stability, and tumor development. Cancer Res. 2012;72:1518–1528. doi: 10.1158/0008-5472.CAN-11-1971. [DOI] [PubMed] [Google Scholar]
- 8.Ma N, Titus J, Gable A, Ross JL, Wadsworth P. TPX2 regulates the localization and activity of Eg5 in the mammalian mitotic spindle. J Cell Biol. 2011;195:87–98. doi: 10.1083/jcb.201106149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumayer G, Helfricht A, Shim SY, Le HT, Lundin C, Belzil C, Chansard M, Yu Y, Lees-Miller SP, Gruss OJ, et al. Targeting protein for xenopus kinesin-like protein 2 (TPX2) regulates gamma-histone 2AX (gamma-H2AX) levels upon ionizing radiation. J Biol Chem. 2012;287:42206–42222. doi: 10.1074/jbc.M112.385674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trieselmann N, Armstrong S, Rauw J, Wilde A. Ran modulates spindle assembly by regulating a subset of TPX2 and Kid activities including Aurora A activation. J Cell Sci. 2003;16:4791–4798. doi: 10.1242/jcs.00798. [DOI] [PubMed] [Google Scholar]