Abstract
This study examines the basis of resistance and sensitivity of normal and transformed cells to histone deacetylase inhibitor (HDACi)-induced cell death, specifically the role of caspases and thioredoxin (Trx). An important attribute of HDACis is that they induce cancer cell death at concentrations to which normal cells are relatively resistant, making them well suited for cancer therapy. The mechanism underlying this selectivity has not been understood. In this study we found that the HDACi suberoylanilide hydroxamic acid (SAHA) and MS-275, a benzamide, cause an accumulation of reactive oxygen species (ROS) and caspase activation in transformed but not normal cells. Inhibition of caspases does not block HDACi-induced cell death. These studies provide a possible mechanism that can explain why normal but not certain transformed cells are resistant to HDACi-induced cell death. The HDACi causes an increase in the level of Trx, a major reducing protein for many targets, in normal cells but not in transformed cells. The SAHA-induced increase in Trx activity in normal cells is associated with no increase in ROS accumulation. Transfection of transformed cells with Trx small interfering RNA caused a marked decrease in the level of Trx protein with an increase in ROS, a decrease in cell proliferation, and an increase in sensitivity to SAHA-induced cell death. Thus, Trx, independent of the caspase apoptotic pathway, is an important determinant of resistance of cells to HDACi-induced cell death.
Keywords: apoptosis, MS-275, suberoylanilde hydroxamic acid, reactive oxygen species
The most well defined form of stress-induced cell death is apoptosis, which is mediated by the family of cysteine proteases known as caspases (1, 2). Histone deacetylase inhibitors (HDACis) cause accumulation of reactive oxygen species (ROS), activation of caspases, and cell death of transformed but not normal cells (3, 4). The basis of this selectivity has not been understood. Another intracellular system that plays an important role in response to stress stimuli that cause an increase in ROS is the thioredoxin (Trx) reduction-oxidation system. Trx protein can function as an hydrogen donor for many protein targets and a scavenger of ROS (5-7). We studied factors that might determine the sensitivity or resistance to HDACi-induced cell death of transformed and normal cells, specifically the role of the caspase pathway and the Trx reduction-oxidation pathway.
HDACis are a promising group of targeted anticancer agents that induce cell death of a broad spectrum of transformed cells, whereas normal cells are relatively resistant to these agents (8-14). The selective effects of HDACis in transformed cells compared with normal cells do not appear to be caused by a difference in the ability to inhibit HDAC activity. Accumulation of acetylated histones occurs in both normal and transformed cells (8, 12, 13). HDACs function as part of multiprotein complexes and catalyze the removal of acetyl groups from lysine residues on histones and a broad range of other proteins, altering their structure and function (14-21). Structurally diverse agents have been developed that inhibit the zinc-dependent class I and class II HDACs (22). Suberoylanilide hydroxamic acid (SAHA) is an hydroxamic acid-based inhibitor (23) that interacts directly with the catalytic site of HDAC-like protein and inhibits its enzymatic activity (24). SAHA inhibits all 11 members of the class I and II HDAC family. MS-275 is a benzamide-based HDACi with potency similar to SAHA against HDACs, except that it is a weak inhibitor of HDAC6 (25). Among HDACis, SAHA is one of the most advanced in clinical development as an anticancer agent (8-10, 14).
SAHA and MS-275 induce growth arrest and death of cancer cells in vitro and in vivo in tumor-bearing animal models. Phase I and II clinical trials with SAHA have shown that it has significant antitumor activity against various cancers at doses that are well tolerated by patients (8-10). The results of preclinical studies and clinical trials have demonstrated that SAHA can induce the death of transformed cells, whereas normal cells appear to be relatively resistant to HDACi-induced cell death (8-13).
SAHA (23) and MS-275 (25) cause an increase in ROS and activation of the caspase pathway in transformed cells, leading to cell death (3, 4, 26-28). Inhibition of caspase activation by pancaspase inhibitors does not block SAHA- or MS-275-induced death of transformed cells (3, 4, 27).
Trx functions as a hydrogen donor for many protein targets (5-7). Reduced Trx is required for activity of ribonucleotide reductase, an enzyme essential for DNA synthesis (5), and is effective in scavenging of ROS (6, 7, 29-32). There are reports that transformed cells transfected with Trx and expressing high levels of the Trx protein become resistant to anticancer drugs (31, 32). Many cancer cells have low levels of Trx, whereas others have higher levels of the protein (33). We have reported that SAHA can induce the expression of Trx binding protein 2 (TBP-2) mRNA in transformed cells (34). TBP-2 protein binds to and inactivates reduced Trx.
In the present study, we found that the HDACi SAHA and MS-275 can arrest the growth of both normal human fibroblasts (WI-38 and Hs578Bst) and transformed cells (WI-38 VA13 and ARP-1) and induce rapid cell death of the transformed but not the normal cells. SAHA induced an accumulation of ROS in the transformed but not normal cells and a marked increase in caspase activity in the transformed cells but no change in normal cells. The increase in caspase activity was completely blocked by caspase inhibitors without inhibiting HDACi-induced death of the transformed cells. SAHA induced an increase in Trx protein in normal cell lines but not in the transformed cells. Further, transfection of transformed WI-38 VA13 (to be referred to as VA13) cells with Trx small interfering RNA (siRNA) caused a decrease in the level of this protein, an increase in ROS, a decrease in proliferation of the cells, and an increase in the sensitivity of the cells to SAHA-induced death. These studies provide evidence for an important role of Trx in the resistance of normal cells and the sensitivity of transformed cells to death caused by HDACi and possibly other anticancer agents that produce ROS (31). These findings have clear implications for cancer therapy.
Materials and Methods
Cells. Normal human lung fibroblasts WI38 and the SV40-transformed derivative VA13 cell lines and the normal human breast fibroblast, Hs578Bst, cell line were obtained from the American Type Culture Collection and cultured in medium per the supplier's instructions. The human multiple myeloma cell line, ARP-1, was generously provided by J. Hardoc (Arkansas Cancer Research Center, Little Rock) and cultured as described (35). Each cell culture was performed in triplicate as follows: 5 × 104 cells were seeded in 1 ml of medium in 24-well plates. The HDACi was added in concentrations indicated 24 h after seeding and cultured for the times indicated. Cells were recovered after trypsinization and washed, and cell counts and viability were determined (36). ARP-1 cells grew in suspension culture and were not trypsinized.
Drugs and Chemicals. SAHA was synthesized as described (23), and MS-275 (25) was purchased from EMD Biosciences (San Diego). These compounds were diluted in DMSO for addition to culture medium. In all studies, an equivalent amount of DMSO without the HDACi was added to control culture medium. The pan-caspase inhibitor Z-VAD-fmk, the caspase 9 inhibitor LEHD-fmk, the caspase 8 inhibitor IETD-fmk, and the caspase 3 inhibitor DEVD-fmk were purchased from EMD Biosciences.
Cell Cycle Analysis. Cells cultured without or with HDACi for various times were assessed for DNA content by propidium iodide staining followed by flow cytometry (23).
ROS Measurements. ROS measurements were determined by the method described (4). Cells (2 × 105) were washed twice in 5 mM Hepes-buffered saline (pH 7.4) at 37°C and then resuspended in 5 mM Hepes-buffered saline alone or with 10 ng/ml of H2DCFDA dye (C-400, Molecular Probes) and incubated at 37°C for 15 min. Cells were washed with ice-cold Hepes/saline and placed on ice. Fluorescence was measured with a FACS Calibur fluorescence-activated cell sorter (Becton Dickinson) at an excitation wavelength of 480 mm and an emission wavelength of 525 nm.
Trx mRNA. The level of expression of mRNA for Trx was determined as described (34).
Western Blot Analysis. Lysates were prepared from cells, and the protein concentration of extracted lysate was determined by a Bradford protein assay (36). Protein (20-25 μg) was subjected to SDS/PAGE (BioRad) and transferred to a Hybond-poly(vinylidene difluoride) membrane (Amersham Pharmacia Biosciences), and membranes were blocked with PBS-0.1% Tween with 5% BSA and incubated with specific primary antibody for 12-15 h. Horse-radish peroxidase-labeled secondary antibody was added and visualized by using the SuperSignal West Pico detection system (Pierce) (36).
Antibodies. Antibodies for acetylated histone H3 and H4 were purchased from Upstate Biotechnology (Lake Placid, NY). Antibody for p21WAF1 protein and α-tubulin protein was purchased from EMD Biosciences. Trx goat antiserum was prepared by Arne Holmgren (37).
Histone Acetylation and P21WAF1 Protein Analyses. Acetylation of histones H3 and H4 were analyzed as described (36). The level of p21WAF1 protein was evaluated as described (38).
Trx Activity Assay. The activity of Trx in cell lysates was determined by using insulin disulfides as substrate (37). Briefly, 30 μg of sample protein in a volume of 15 μl was preincubated with 1 μl of 1 mM DTT at 37°C for 20 min. The samples were than incubated with 85 mM Hepes (pH 7.5), 660 μM NADPH, 3 mM EDTA, 0.3 mM insulin, and 0.08 unit (as defined by the manufacturer) of Trx reductase (TrxR) at 37°C for 120 min in a total volume of 50 μl. Rat recombinant TrxR, insulin, and NADPH were purchased from Sigma. The reaction was terminated by addition of 0.5 ml of 8 M guanidine hydrochloride with 0.4 mg/ml of 5,5′-dithio-bis (2-nitrobenzioic acid) in 0.2 M Tris·Cl (pH 8). Absorbance at 412 nm was measured with a Beckman DV640 spectrophotometer. For each sample, a blank containing all reagents, except TrxR, was used, and absorbance of the blank was subtracted from that of the sample.
Analysis of Caspase Activities. A previously described procedure was used to determine caspase activity (39).
siRNA for Trx. Trx siRNA was synthesized by Qiagen (Germantown, MD): r(AUG, ACU, GUC, AGG, AUG, UUG, C)d(TT) and r(GCA, ACA, UCC, UGA, CAG, UCA, U)d(CC). siRNA transfection was performed according to the manufacturer's instructions. Briefly, 106 VA13 cells were plated in a 10-cm dish. After culture overnight, the cells were washed three times with PBS, and 10 ml of medium, without antibiotics but with 10% FCS, was added to the cells. Trx siRNA (1,200 pmol) and 35 μl of X-tremeGENE Reagent (Roche Diagnostic, Penzberg, Germany) were mixed with 1 ml of serum-free medium. After 5 min, Trx siRNA and X-tremeGENE were mixed together and incubated for 20 min at room temperature. The mixture was then added to the cell culture. After overnight, the culture medium was changed with fresh complete medium. Transfection with the same amount of nonspecific siRNA (Dharmacon, Lafayette, CA) was performed as control. The sequence of the nonspecific siRNA was random and computer-generated. The cells were harvested and analyzed at 24 and 48 h after transfection for cell number, viability, and level of Trx protein by the methods described above. VA13 cells transfected with siRNA for 48 h were cultured with SAHA, in concentrations indicated for an additional 48 h, and harvested, and cell number and cell viability were determined.
Results
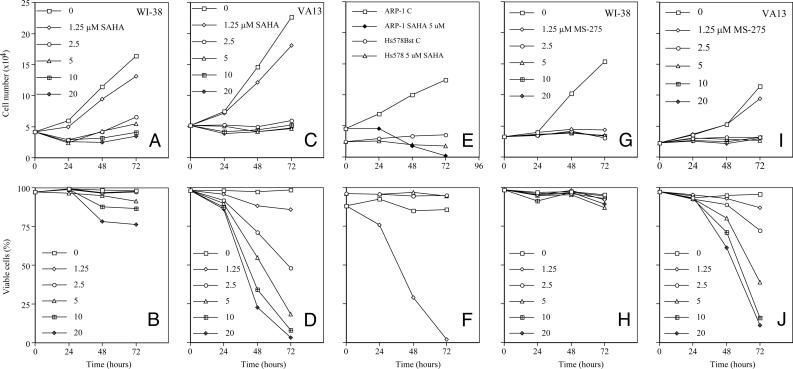
HDACi Arrests Cell Growth of Normal and Transformed Cells and Induces Apoptosis of Transformed but Not Normal Cells. We first determined the effect of SAHA on normal human lung fibroblasts, WI-38 cells, and its SV40-transformed derivative, VA13. SAHA (≥ 2.5 μM) inhibited cell growth of WI38 and VA13 cells (Fig. 1 A and C). SAHA (≥ 2.5 μM) induced marked death of the transformed but not the normal cells (Fig. 1 B and D). For example, 10 μM SAHA caused death of 95% of VA13 cells, but <10% of WI38 cells, after 72 h of culture. HDACi-induced death of various transformed cell lines has been reported (see reviews in refs. 14-17).
Fig. 1.
The effect of HDACi on cell growth and viability of normal and transformed cells in culture. (A and B) The effect of SAHA on the growth (A) and viability (B) of a normal human lung fibroblast WI-38 cell is shown. The cells were cultured without or with SAHA in μM concentrations indicated for 72 h. (C and D) The effect of SAHA on the growth (C) and viability (D) of a SV40-transformed human lung fibroblast, VA13, is shown. (E and F) Cell growth (E) and viability (F) of a normal human breast fibroblast, Hs578Bst, and human multiple myeloma cells, ARP-1, in culture without and with 5 μM SAHA are shown. (G and H) The effect of MS-275 on the growth (G) and viability (H) of WI-38 cells cultured without or with the indicated concentrations of MS-275 for 72 h is shown. (I and J) The effect of MS-275 on the growth (I) and viability (J) of VA13 cells is shown.
We next examined the effect of SAHA on another normal cell line, human breast fibroblast, Hs578Bst, and another transformed cell line, human multiple myeloma ARP-1. SAHA caused growth arrest of both cell lines and marked death of the transformed but not the normal cells (Fig. 1 E and F).
The effect of a second HDACi, MS-275, a benzamide-based HDACi (25), on WI-38 and VA13 growth and viability was determined. At 2.5 μM MS-275, proliferation of WI-38 and VA13 was inhibited within 24 h of culture (Fig. 1 G and I). This HDACi caused <10% loss of viability of WI-38 cells in concentrations as high as 20 μM after 72 h, but induced death of >90% of VA13 cells at 20 μM, by 72 h in culture (Fig. 1 H and J).
Effect of SAHA on Cell Cycle Progression and Acetylation of Histones H3 and H4 and p21WAF1 Protein Levels. Previous studies have shown that SAHA induces altered cell cycle progression, accumulation of acetylated histones H3 and H4, and increased levels of p21WAF1 protein in various transformed cells (12-20). To evaluate whether these HDACi-induced changes occur in the cell lines used in the present studies, we found that 5 μM SAHA arrested WI-38 and VA13 cells in G1 within 24 h. After 48 h of culture with SAHA, <10% of WI-38 cells were in the sub-G1 fraction (a measure of dead or apoptotic cells), whereas >45% of VA13 cells were in the sub-G1 fraction.
SAHA, at concentrations as low as 1.25 μM, induced an accumulation of acetylated histones H3 and H4 in both WI-38 and VA13 cells, which was detectable within 1 h of culture. The level of accumulation of acetylated histones H3 and H4 in VA13 cells was greater and persisted at higher levels for a longer period (24 h) than in WI38 cells (12 h). SAHA induced an increase in p21WAF1 protein levels in both cell lines, which was detectable by 1 h and reached the highest levels by 6 h in VA13 and 12-18 h in WI-38 cells (data not shown). These findings are consistent with previous reports (12-14).
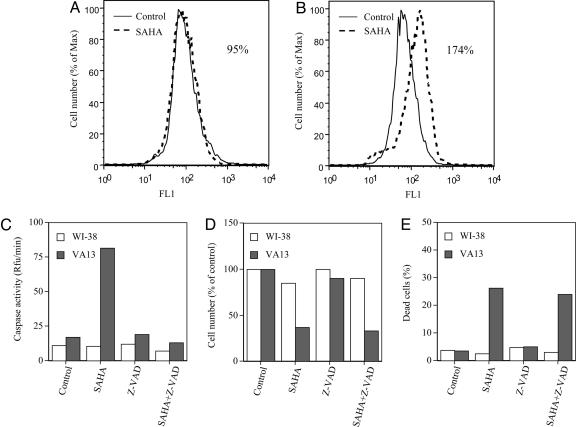
SAHA Induces Accumulation of ROS in Transformed, Not Normal, Cells. It has been reported that SAHA and MS-275 induce the production of ROS in transformed cells (4, 26, 27, 30). VA13 cells cultured with 5 μM SAHA for 48 h showed a greater accumulation of ROS than cells cultured without the inhibitor (Fig. 2B). WI-38 cells cultured with 5 μM SAHA for 48 h had no detectable increased accumulation of ROS compared with cells cultured without the HDACi (Fig. 2A).
Fig. 2.
The effect of SAHA on caspase activity, ROS accumulation, cell growth, and viability of WI-38 and VA13 cells. (A and B) Production of ROS in WI-38 cells (A) and VA13 cells (B) cultured without (control) or with 5 μM SAHA for 48 h is shown. ROS values were calculated as the percent mean fluorescence of cells cultured with SAHA compared with that of control cells, i.e., 95% for WI-38 and 174% for VA13 cells. Cells were labeled with oxidative-sensitive dye C-400 and analyzed by flow cytometer for increases in Fl-1 fluorescence. As a control, cells were labeled with C-400 in the presence of 10 μM H2O2. (C) Caspase activity in the WI-38 and VA13 cells cultured without (control) and with 5 μM SAHA, 50 μM Z-VAD-fmk, or 5 μM SAHA plus 50 μM Z-VAD-fmk is shown. (D) Cell growth-expressed percent of control cultures of WI-38 and VA13 cells is shown. (E) Dead cells in WI-38 and VA13 cells cultured without (control) and with 5 μM SAHA, 50 μM Z-VAD-fmk, or 5 μM SAHA plus 50 μM Z-VAD-fmk at 48 h are shown. All determinations were performed in duplicate, and at least two separate studies were performed for each cell line.
Caspase Inhibitors Do Not Block SAHA-Induced Cell Death of VA13 Cells. Apoptosis in mammalian cells frequently is mediated by a family of cystine proteases, the caspases (1, 2, 28, 39). Caspase activation might be triggered by various extracellular and intracellular stresses such as HDACi-induced accumulation of ROS that might, in turn, lead to cell death (2). WI-38 and VA13 cells were cultured without or with 5 μM SAHA, with 50 μM Z-VAD-fmk alone, or with 5 μM SAHA plus 50 μM Z-VAD-fmk for 48 h (Fig. 2). SAHA increased caspase activity >4-fold in VA13 cells, but had no detectable effect on caspase activity in WI-38 cells (Fig. 2C). SAHA inhibited cell growth of WI-38 and VA13 in culture without or with Z-VAD-fmk (Fig. 2D). SAHA alone or SAHA plus Z-VAD caused <5% cell death of WI-38 cells, but 25-30% cell death of VA13 cells by 48 h of culture (Fig. 2E). Z-VAD-fmk added alone had little effect on cell growth of either cells. The addition of the Z-VAD-fmk to VA13 cell cultures with SAHA completely blocked the increased caspase activity (Fig. 2C) but had essentially no effect on HDACi-induced cell death (Fig. 2E). Z-VAD-fmk added to culture without HDACi had no effect on cell death (Fig. 2E).
We next determined whether the caspase 3 inhibitor DVED-fmk, caspase 8 inhibitor IETD-fmk, or caspase 9 inhibitor LEHD-fmk blocked SAHA-induced VA13 cell death. None of the three caspase inhibitors affected the HDACi-induced cell death of the transformed cells (data not shown).
The findings that (i) SAHA induced an accumulation of ROS and caspase activation in transformed but not normal cells and (ii) inhibition of caspase activation did not block HDACi-induced death of transformed cells leads us to determine the role Trx might have on HDACi effect on normal and transformed cells.
Effect of HDACi on Trx mRNA and Protein Levels. Trx is an important regulator of redox potential with many targets and is required for activation of ribonucleotide reductase and is a scavenger of ROS (5-7, 29-32, 40).
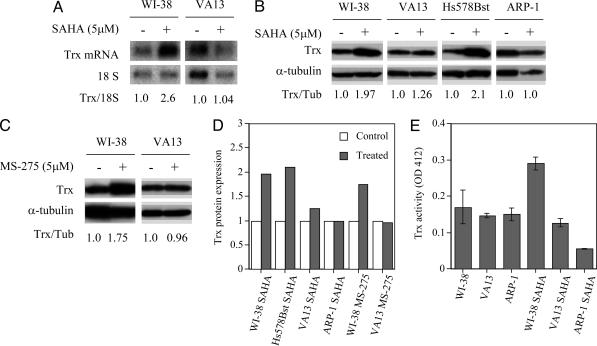
We determined the effect of SAHA and MS-275 on Trx levels in normal and transformed cells (Fig. 3). Trx mRNA levels in WI-38 cells cultured for 48 h with SAHA were higher than in VA13 cells cultured with the HDACi (Fig. 3A). The levels of Trx protein in normal cells, WI-38 and Hs578Bst, cultured with SAHA or MS-275 for 48 h were consistently higher than the Trx levels in transformed cells, VA13 or ARP-1, cultured with the HDACi (Fig. 3 B-D). These data indicate that SAHA causes an increase in accumulation of Trx protein in the WI-38 and Hs578Bst cells but not in the transformed cells, VA13 and ARP-1. To assess whether the SAHA-induced increase in Trx protein levels reflects an increase in functional activity of the redox protein, Trx activity of cells lysates was measured as the ability to reduce disulfide groups of insulin (38). In WI-38 cells cultured with SAHA, Trx activity was increased ≈2-fold by 48 h, whereas Trx activity in VA13 cells was unchanged and decreased in ARP-1 cells. These data suggested that Trx might be an important player in determining the selectivity of SAHA or MS-275 in inducing transformed but not normal cell death.
Fig. 3.
The effect of HDACi on Trx mRNA and protein levels in normal and transformed cells. (A) Trx mRNA levels in WI38 and VA13 cells cultured without or with 5 μM SAHA for 48 h are shown. 18S ribosomal RNA was assayed as a loading control. The number below the gels is the ratio of the density of the Trx mRNA band to the 18S rRNA band relative to that in cells cultured without SAHA, which is set at 1.0. (B) Trx protein levels in WI-38, VA13, Hs578Bst, and ARP-1 cells cultured without or with 5 μM SAHA for 48 h are shown. (C) Trx protein levels in WI-38 and VA13 cells cultured without or with 5 μM MS-275 for 48 h are shown; α-tubulin was assayed in each electrophoresis gel as a loading control. The numbers below the gels are ratios of the density of the Trx protein band to the α-tubulin band relative to that in cells cultured without SAHA, which is set at 1.0. (D) Expression of Trx protein as a ratio of Trx protein/α-tubulin protein relative to that in control (no SAHA or MS-275) set as 1.0 is shown. The expression of Trx protein was consistently higher in normal (WI-38 and Hs578Bst) than in transformed cells after 48 h of culture with SAHA or MS-275 (treated). (E) Trx activity in WI-38, VA13, and ARP-l cells after 48 h of culture without or with 5.0 μM SAHA for WI-38 and V13 cell cultures and 2.5 μM SAHA for ARP-1 cell cultures is shown. The range of three separate determinations is indicated for each bar.
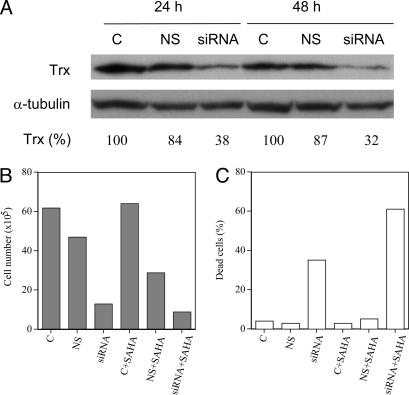
Effect of Transfection of VA13 with siRNA for Trx. If Trx protein has a role in protecting cells against stress-induced cell death, decreasing Trx protein by transfecting VA13 cells with Trx siRNA could cause cells to be more sensitive to HDACi. VA13 cells were transfected with a Trx siRNA (Fig. 4A). The siRNA caused a >60% decrease in Trx protein level by 24 h, which persisted at 48 h of culture (Fig. 4A). The nonspecific siRNA-transfected cells showed little decrease in Trx levels. The siRNA-transfected cells had somewhat higher average levels of ROS (123%) compared with nontransfected control cells (set at 100%). These transfected cells had decreased cell growth and increased proportion of dead cells compared with nontransfected control cells (Fig. 4 B and C). After 48 h of culture, control cells, cells transfected with nonspecific siRNA, and cells transfected with Trx siRNA were recovered and resuspended in medium without or with 0.5, 1.0, 1.5, or 2.5 μM SAHA for an additional 48 h of culture. SAHA (1 μM) had little or no effect on control cell growth (Fig. 4B), death (Fig. 4C), or average levels of ROS (109%) compared with nontransfected control cells. The HDACi-inhibited Trx siRNA transfected cell growth on the average 85%, induced cell death on the average 60%, and increased average levels of ROS almost 50% (148%) compared with its effect on nontransfected control cells. In these experiments, decreasing Trx protein levels in transformed cells caused an inhibition of cell growth and increased sensitivity to SAHA-induced cell death.
Fig. 4.
The effect of transfection on VA13 cells with Trx siRNA. (A) Trx protein levels in VA13 cells, nontransfected (C), transfected with nonspecific siRNA (NS), or transfected with Trx siRNA (siRNA) at 24 h and 48 h in culture (after transfection) are shown. The numbers below the gels represent the Trx band density expressed as a percent of control (set at 100). (B and C) Cell density (B) and dead cells (C) in cultures without or with 1.0 μM SAHA for 48 h are shown.
Discussion
This study has shown that Trx protein plays an important role in determining the response of normal and transformed cells to HDACi. The HDACis, SAHA and MS-275, are targeted anti-cancer agents. Among the HDACi, SAHA is one of the most advanced in clinical development as a cancer therapeutic drug (8-10, 14). SAHA has shown significant anticancer activity in tumor-bearing animals and in phases I and II clinical trials against both hematologic and solid tumors, with little evidence of adverse effects on normal cells. Normal cells in culture are relatively resistant to concentrations of SAHA or MS-275 that cause transformed cell death (11-13). This difference in sensitivity to HDACi-induced cell death of transformed cells compared with normal cells appears not to be caused by a difference in the inhibitory effects on HDACs because both agents caused the accumulation of acetylated histones in the normal and transformed cells.
Perhaps the best defined programmed cell death is apoptosis. Apoptosis in mammalian cells is mediated by the family of cysteine proteases, the caspases (1, 2, 4, 28). In the present study, we found that transformed cells but not normal cells cultured with HDACi accumulated ROS and had a marked increased in caspase activity. Caspase activation via the cytochrome c mitochondrial pathway can be induced by the production of ROS (1, 2). A striking finding in our present studies was that SAHA-induced death of SV40-transformed human fibroblast (VA13) or human multiple myeloma cells (ARP-1) was not blocked by inhibiting the activation of the caspase pathway. This finding is consistent with other reports that HDACi-induced cell death of certain (but not all) transformed cells was caspase activation-independent (4, 26, 33). Taken together, the present data suggest that HDACi can induce both caspase-dependent apoptosis and caspase-independent cell death. This caspase-independent cell death has been characterized as autophagic cell death (41).
Trx has a role in the regulation of many cellular functions, including proliferation, gene expression, and responses to oxidative stress (7, 29, 30, 42, 43). In this study we found that in two normal cell lines that are resistant to SAHA- or MS-275-induced cell death the HDACi increased Trx protein and activity. In transformed cells, SAHA and MS-275 caused marked cell death and did not cause increased levels of Trx protein or activity. Increased levels of Trx protein can account, in part at least, for preventing the accumulation of ROS and, in turn, preventing cell death of normal cells. High levels of ROS observed in transformed cells cultured with HDACi could lead to cell death independent of the activation of the caspase pathway (31, 32).
The VA13 cells transfected with Trx siRNA showed a marked decrease in Trx protein levels, increased ROS, and inhibition of cell growth. These transfected cells had increased sensitivity to SAHA-induced ROS accumulation and cell death. These effects are likely caused, in part, by low levels of Trx and, as a consequence, a loss in capacity in these transfected cells to scavenge ROS by peroxinedoxins that are Trx-dependent peroxidases (31, 32). This conclusion is supported by the recent finding that the free radical scavenger l-N-acetylcysteine attenuates HDACi-mediated ROS generation and cell death (44). The decrease in Trx was associated with inhibition of cell growth, which may reflect, in part, inhibition of DNA synthesis owing to the lack of Trx activity with ribonucleotide reductase (7).
The HDACi blocked both normal and transformed cell proliferation. SAHA-induced inhibition of cell proliferation in these cultures could be attributed to increased levels of p21WAF1 protein. P21WAF1 interacts with cyclin-dependent kinases and blocks progression of cells from G1. Our findings are consistent with previous reports that HDACi can cause G1 arrest and increased levels of p21WAF1 protein (12, 13, 36).
It has been reported that Trx levels are high in about half of the human tumors examined (33, 45, 46), whereas they are low in other malignancies (33). High levels of Trx in cancer cells were associated with resistance to cancer drugs. Those studies are consistent with the present findings that higher levels of Trx in normal cells are associated with resistance to HDACi-induced cell death, whereas transformed cells with relatively low levels of Trx were sensitive to inducer-mediated cell death.
In conclusion, Trx levels have an important role in determining HDACi-induced transformed cell death. Relatively higher levels of Trx in normal cells could account, in part at least, for the relative resistance of normal cells to the lethal cellular effects of these agents. Decreasing the levels of Trx in transformed cells increased their sensitivity to HDACi-induced cell death. Taken together, the present findings have important implications for the clinical use of HDACis as anticancer agents. Trx levels in serum have been reported to be elevated in some patients with cancer (32, 45, 46). It is possible that determining the serum level of Trx might be a useful marker for therapeutic response to HDACi. The challenge is to develop approaches to manipulate Trx levels in normal and cancer cells in a selective manner. A detailed understanding of the regulation of Trx levels is an important area of study in the pursuit of improved cancer therapeutics.
Acknowledgments
These studies were supported, in part, by grants from the National Cancer Institute (P30-CA-008748), a David H. Koch Prostate Cancer Research Award, the Susan and Jack Rudin Foundation, the Robert J. and Helen C. Kleberg Foundation, and the Swedish Medical Research Council. Y. Shao is supported by a fellowship from the Tiffany Townsend Daniels/American Brain Tumor Association. X.J. is an Alfred W. Bressler Scholar and a V Scholar.
Author contributions: J.S.U., Y. Sowa, X.J., and P.A.M. designed research; J.S.U., Y. Sowa, W.-S.X., Y. Shao, M.D., G.P., and L.N. performed research; X.J. and P.A.M. analyzed data; A.H. contributed new reagents/analytic tools; and P.A.M. wrote the paper.
Abbreviations: HDAC, histone deacetylase; HDACi, HDAC inhibitor; Trx, thioredoxin; SAHA, suberoylanilide hydroxamic acid; ROS, reactive oxygen species; siRNA, small interfering RNA.
References
- 1.Thornberry, N. A. & Lazebnik, Y. (1998) Science 281, 1312-1316. [DOI] [PubMed] [Google Scholar]
- 2.Jiang, X. & Wang, X. (2004) Annu. Rev. Biochem. 73, 87-106. [DOI] [PubMed] [Google Scholar]
- 3.Rosato, R. R., Almenara, J. A. & Grant, S. (2003) Cancer Res. 63, 3637-3645. [PubMed] [Google Scholar]
- 4.Ruefli, A. A., Ausserlechner, M. J., Bernhard, D., Sutton, V. R., Tainton, K. M., Kofler, R., Smyth, M. J. & Johnstone, R. W. (2001) Proc. Natl. Acad. Sci. USA 98, 10833-10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurent, T. C., Moore, E. C. & Reichard, P. (1964) J. Biol. Chem. 239, 3436-3444. [PubMed] [Google Scholar]
- 6.Holmgren, A. (1989) J. Biol. Chem. 264, 13963-13966. [PubMed] [Google Scholar]
- 7.Arner, E. S. & Holmgren, A. (2000) Eur. J. Biochem. 267, 6102-6109. [DOI] [PubMed] [Google Scholar]
- 8.Kelly, W. K., Richon, V. M., O'Connor, O., Curley, T., MacGregor-Curtelli, B., Tong, W., Klang, M., Schwartz, L., Richardson, S., Rosa, E., et al. (2003) Clin. Cancer Res. 9, 3578-3588. [PubMed] [Google Scholar]
- 9.Kelly, W. K., O'Connor, O. A. & Marks, P. (2002) Expert. Opin. Invest. Drugs 11, 1695-1713. [DOI] [PubMed] [Google Scholar]
- 10.Piekarz, R. & Bates, S. (2004) Curr. Pharm. Des. 10, 2289-2298. [DOI] [PubMed] [Google Scholar]
- 11.Qiu, L., Kelso, M. J., Hansen, C., West, M. L., Fairlie, D. P. & Parsons, P. G. (1999) Br. J. Cancer 80, 1252-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler, L. M., Agus, D. B., Scher, H. I., Higgins, B., Rose, A., Cordon-Cardo, C., Thaler, H. T., Rifkind, R. A., Marks, P. A. & Richon, V. M. (2000) Cancer Res. 60, 5165-5170. [PubMed] [Google Scholar]
- 13.Atadja, P., Gao, L., Kwon, P., Trogani, N., Walker, H., Hsu, M., Yeleswarapu, L., Chandramouli, N., Perez, L., Versace, R., et al. (2004) Cancer Res. 64, 689-695. [DOI] [PubMed] [Google Scholar]
- 14.Marks, P. A., Richon, V. M., Miller, T. & Kelly, W. K. (2004) Adv. Cancer Res. 91, 137-168. [DOI] [PubMed] [Google Scholar]
- 15.de Ruijter, A. J., van Gennip, A. H., Caron, H. N., Kemp, S. & van Kuilenburg, A. B. (2003) Biochem. J. 370, 737-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnstone, R. W. & Licht, J. D. (2003) Cancer Cell 4, 13-18. [DOI] [PubMed] [Google Scholar]
- 17.Marks, P., Rifkind, R. A., Richon, V. M., Breslow, R., Miller, T. & Kelly, W. K. (2001) Nat. Rev. Cancer 1, 194-202. [DOI] [PubMed] [Google Scholar]
- 18.Cress, W. D. & Seto, E. (2000) J. Cell Physiol. 184, 1-16. [DOI] [PubMed] [Google Scholar]
- 19.Jones, P. A. & Baylin, S. B. (2002) Nat. Rev. Genet. 3, 415-428. [DOI] [PubMed] [Google Scholar]
- 20.Khochbin, S., Verdel, A., Lemercier, C. & Seigneurin-Berny, D. (2001) Curr. Opin. Genet. Dev. 11, 162-166. [DOI] [PubMed] [Google Scholar]
- 21.Lehrmann, H., Pritchard, L. L. & Harel-Bellan, A. (2002) Adv. Cancer Res. 86, 41-65. [DOI] [PubMed] [Google Scholar]
- 22.Miller, T. (2004) Expert Opin. Therapeutic Patents 14, 791-804. [Google Scholar]
- 23.Richon, V. M., Webb, Y., Merger, R., Sheppard, T., Jursic, B., Ngo, L., Civoli, F., Breslow, R., Rifkind, R. A. & Marks, P. A. (1996) Proc. Natl. Acad. Sci. USA 93, 5705-5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finnin, M. S., Donigian, J. R., Cohen, A., Richon, V. M., Rifkind, R. A., Marks, P. A., Breslow, R. & Pavletich, N. P. (1999) Nature 401, 188-193. [DOI] [PubMed] [Google Scholar]
- 25.Saito, A., Yamashita, T., Mariko, Y., Nosaka, Y., Tsuchiya, K., Ando, T., Suzuki, T., Tsuruo, T. & Nakanishi, O. (1999) Proc. Natl. Acad. Sci. USA 96, 4592-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peart, M. J., Tainton, K. M., Ruefli, A. A., Dear, A. E., Sedelies, K. A., O'Reilly, L. A., Waterhouse, N. J., Trapani, J. A. & Johnstone, R. W. (2003) Cancer Res. 63, 4460-4471. [PubMed] [Google Scholar]
- 27.Budihardjo, I., Oliver, H., Lutter, M., Luo, X. & Wang, X. (1999) Annu. Rev. Cell Dev. Biol. 15, 269-290. [DOI] [PubMed] [Google Scholar]
- 28.Liu, X., Kim, C. N., Yang, J., Jemmerson, R. & Wang, X. (1996) Cell 86, 147-157. [DOI] [PubMed] [Google Scholar]
- 29.Yokomizo, A., Ono, M., Nanri, H., Makino, Y., Ohga, T., Wada, M., Okamoto, T., Yodoi, J., Kuwano, M. & Kohno, K. (1995) Cancer Res. 55, 4293-4296. [PubMed] [Google Scholar]
- 30.Sasada, T., Iwata, S., Sato, N., Kitaoka, Y., Hirota, K., Nakamura, K., Nishiyama, A., Taniguchi, Y., Takabayashi, A. & Yodoi, J. (1996) J. Clin. Invest. 97, 2268-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rundlof, A. K. & Arner, E. S. (2004) Antioxid. Redox. Signal 6, 41-52. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura, H. (2004) Antioxid. Redox. Signal 6, 15-17. [DOI] [PubMed] [Google Scholar]
- 33.Shao, L., Diccianni, M. B., Tanaka, T., Gribi, R., Yu, A. L., Pullen, J. D., Camitta, B. M. & Yu, J. (2001) Cancer Res. 61, 7333-7338. [PubMed] [Google Scholar]
- 34.Butler, L. M., Zhou, X., Xu, W.-S., Scher, H. I., Rifkind, R. A., Marks, P. A. & Richon, V. M. (2002) Proc. Natl. Acad. Sci. USA 99, 11700-11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gui, C. Y., Ngo, L., Xu, W. S., Richon, V. M. & Marks, P. A. (2004) Proc. Natl. Acad. Sci. USA 101, 1241-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richon, V. M., Zhou, X., Secrist, J. P., Cordon-Cardo, C., Kelly, W. K., Drobnjak, M. & Marks, P. A. (2004) Methods Enzymol. 376, 199-205. [DOI] [PubMed] [Google Scholar]
- 37.Arner, E. S., Zhong, L. & Holmgren, A. (1999) Methods Enzymol. 300, 226-239. [DOI] [PubMed] [Google Scholar]
- 38.Richon, V. M., Sandhoff, T. W., Rifkind, R. A. & Marks, P. A. (2000) Proc. Natl. Acad. Sci. USA 97, 10014-10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang, X., Kim, H. E., Shu, H., Zhao, Y., Zhang, H., Kofron, J., Donnelly, J., Burns, D., Ng, S. C., Rosenberg, S. & Wang, X. (2003) Science 299, 223-226. [DOI] [PubMed] [Google Scholar]
- 40.Holmgren, A. (1995) Structure (London) 3, 239-243. [DOI] [PubMed] [Google Scholar]
- 41.Shao, Y., Gao, Z., Marks, P. A. & Jiang, X. (2004) Proc. Natl. Acad. Sci. USA 101, 18030-18035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okada, H. & Mak, T. W. (2004) Nat. Rev. Cancer 4, 592-603. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura, H., Masutani, H., Tagaya, Y., Yamauchi, A., Inamoto, T., Nanbu, Y., Fujii, S., Ozawa, K. & Yodoi, J. (1992) Cancer 69, 2091-2097. [DOI] [PubMed] [Google Scholar]
- 44.Yu, C., Subler, M., Rahmani, M., Reese, E., Krystal, G., Conrad, D., Dent, P. & Grant, S. (2003) Cancer Biol. Ther. 2, 544-551. [DOI] [PubMed] [Google Scholar]
- 45.Powis, G., Mustacich, D. & Coon, A. (2000) Free Radical Biol. Med. 29, 312-322. [DOI] [PubMed] [Google Scholar]
- 46.Baker, A., Payne, C. M., Briehl, M. M. & Powis, G. (1997) Cancer Res. 57, 5162-5167. [PubMed] [Google Scholar]